Abstract
Introduction
There is great interest in nutritional strategies for the prevention of age-related cognitive decline, yet the best methods for nutritional assessment in populations at risk for dementia are still evolving. Our study objective was to test the reliability and validity of two common nutritional assessments (plasma nutrient biomarkers and Food Frequency Questionnaire) in people at risk for dementia.
Methods
Thirty-eight elders, half with amnestic -Mild Cognitive Impairment and half with intact cognition were recruited. Nutritional assessments were collected together at baseline and again at 1 month. Intraclass and Pearson correlation coefficients quantified reliability and validity.
Results
Twenty-six nutrients were examined and reliability was very good or better for 77% (20/26, ICC ≥ .75) of the plasma nutrient biomarkers and for 88% of the FFQ estimates. Twelve of the plasma nutrient estimates were as reliable as the commonly measured plasma cholesterol (ICC=.92). FFQ and plasma long-chain fatty acids (docosahexaenoic acid, r =.39, eicosapentaenoic acid, r = .39) and carotenoids (α-carotene, r =.49; lutein + zeaxanthin, r = .48; β-carotene, r = .43; β-cryptoxanthin, r = .41) were correlated, but no other FFQ estimates correlated with respective nutrient biomarkers. Correlations between FFQ and plasma fatty acids and carotenoids were significantly stronger after removing subjects with MCI.
Conclusion
The reliability and validity of plasma and FFQ nutrient estimates vary according to the nutrient of interest. Memory deficit attenuates FFQ estimate validity and inflates FFQ estimate reliability. Many plasma nutrient biomarkers have very good reliability over 1-month regardless of memory state. This method can circumvent sources of error seen in other less direct methods of nutritional assessment.
Keywords: reproducibility, validity, nutrient biomarkers, food frequency questionnaire, Mild Cognitive Impairment, Alzheimer, elders
INTRODUCTION
The age-strata growing most rapidly in the US include those reaching their 85th and 65th birthdays beginning this year. This wave of aging elders has implications for sporadic Alzheimer’s disease (AD) because age itself is a major risk factor.1 Prevention and delay of onset in this most common form of dementia is therefore an important public health initiative.
An Alzheimer’s prevention strategy is becoming more feasible as we better understand populations at high risk, including older adults and those with amnestic - Mild Cognitive Impairment (MCI). People at high risk for dementia experience more rapid rates of cognitive change over time, but care must be taken to not select persons with cognitive dysfunction too advanced. Several nutritional factors have been implicated in cognitive behavior and AD although specific nutrition to prevent AD has remained illusive.2–8 Nutrition that may modify the pathogenesis of AD include dietary antioxidants9–11, B vitamins12–15, saturated and polyunsaturated fatty acids16–19, and cholesterol.20, 21 Vitamin D and some minerals may be important as well.22–25
It remains unclear whether modifying these nutritional factors can reduce the incidence of age-related cognitive decline, in part because reliable nutrition assessments in people at risk are still evolving. Food frequency questionnaires (FFQ) are the best indirect method for assessing nutritional status in this setting because they reflect long-term dietary intake, the type of dietary exposure thought to be most important to neurodegeneration. FFQ also challenge more generic as opposed to episodic memory by asking usual frequency of foods consumed rather than querying specific food recall that is a more difficult cognitive task.26 However, FFQ does challenge recall memory and does not account for genetic and non-genetic factors that may modify nutrition available to the brain independently of dietary intake.27–29 Combining nutritional biomarkers with FFQ is attractive because the inherent threats to validity of each measure are mostly independent.30 Biochemical indicators of dietary intake (i.e., nutrient biomarkers) better represent nutrition with more direct access to the CNS. Some nutrient biomarkers are less reflective of long-term dietary intake. Many have not had reliability evaluated in older adults, and we are unaware of any study comparing FFQ and plasma nutrient estimates vis a vis in two groups distinctly different by cognitive status (memory state).
Reliability and validity studies of nutrient biomarkers in elder populations are limited, although more available in people under the age of 65 years.31–35 FFQ validity studies comparing with nutrient biomarkers in older persons are also limited.36–41 One report in the literature has collected dietary intake and careful classification of cognitive status, but the assessment of cognition and diet were at different time points and no nutrient biomarker data was reported.42
Establishing reliable methods of nutritional assessment in people at risk for dementia are imperative for the development of neuroprotective nutritional therapy for dementia prevention. In this pilot study we compare the reliability and validity of nutrient biomarkers and FFQ in a population at risk for dementia. We hypothesized that nutrient biomarkers would outperform FFQ. in this population well suited for primary prevention of Alzheimer’s disease.
METHODS
Subject population
Nineteen subjects with amnestic-Mild Cognitive Impairment (MCI) and 19 Non-Impaired Elderly (NIE) were evaluated in the Aging and Alzheimer’s clinic at Oregon Health & Science University after consent was obtained in accord with the Institutional Review Board for human study at OHSU.
The investigators identified MCI subjects through routine clinical contact. The NIE where recruited as part of their ongoing participation in longitudinal aging studies not collecting dietary information at the Layton Center for Aging and Alzheimer’s Research at OHSU. All MCI and NIE subjects then attended an in-clinic screening (with collateral historian for MCI) within two weeks of signing consent and brought all medications (including supplements) that they were currently taking. All enrolled subjects completed the study.
A consensus diagnosis was made at a diagnostic conference of neurologists and neuropsychologists held weekly at the Layton Center. Non-impaired elders had no evidence of cognitive or functional impairment including Mini Mental State Exam ≥ 28 and Clinical Dementia Rating = 0. Amnestic-MCI was defined by Petersen criteria.43 The assessment included interview with collateral historian, Mini Mental State Examination44, Cognistat45, Clinical Dementia Rating46, Wechsler Memory Scale-Revised (logical memory I and II)47, the Geriatric Depression Scale48, Hachinski Ischemia Score49, Clock draw, and verbal fluency (recalling as many animals or words beginning with a specified letter in 60 seconds), physical and neurological examination and review of MRI.
Data collection methods
Blood sampling overview
Four 10 ml fasting venous blood specimens were obtained from each subject following an overnight fast. Serum tubes were allowed to clot at room temperature for 30 minutes prior to the centrifugation and separation of the serum. EDTA anti-coagulated vials were wrapped in foil, placed immediately on ice and centrifuged within 1 hour for plasma collection. Aliquots of 0.6 ml were collected and stored in Nunc 1.00 ml shaded cryotubes, labeled appropriately and immediately frozen for storage at −80°C. Samples were defrosted and immediately assayed following standard procedures for the following plasma nutrient biomarkers: 1) Vitamins: (ascorbic acid, alpha-and gamma-tocopherol, carotenoids, B vitamins, vitamin D) 2) Minerals: (copper, iron, magnesium, selenium, zinc) 3) Lipids: (linoleic acid, arachidonic acid, α-linolenic acid, EPA, DHA, total polyunsaturated fat, monounsaturated fat, saturated fat, and cholesterol).
Blood collection and analysis
Genetic determination of APOE allelic status was performed with the use of polymerase-chain-reaction assays.50 Plasma ascorbic acid was deproteinized with 10% metaphosphoric acid and the supernatant kept at −80°C until analysis with HPLC.51 Plasma α-tocopherol was protected from light and heat, and stored at −80°C until analyzed by HPLC with electrochemical detection after saponification in the presence of ascorbic acid and then extraction with hexane.52 Plasma carotenoids were measured as described previously.53 Briefly, plasma and lipoprotein fraction samples were protected from light and stored at −80 °C until analyzed. Echinenone in ethanol was added as an internal standard to 200 μL serum or lipoprotein fraction sample and 0.5 mL of 0.9% saline. The mixture was extracted by using 2 mL chloroform: methanol (2:1, by volume), mixed by vortex, centrifuged and the chloroform layer was removed. A second extraction was done on the mixture with the use of 3 mL hexane, which was followed by mixing by vortex and centrifugation. The hexane layer was combined with the first extraction and evaporated to dryness under nitrogen. The residue from serum was re-dissolved in 150 μL ethanol, mixed by vortex, and sonicated for 30 seconds. A 50-μL aliquot was used for the HPLC analysis. A C30 carotenoid column was used for the carotenoid measurements. Carotenoids were quantified by determining peak areas in the HPLC chromatograms and calibrated against known standards provided by DSM Nutritional Products, Parsippany, NJ. The percentage of carotenoids in each lipoprotein fraction was calculated as the absolute concentration of each fraction divided by the sum of the concentrations of all fractions. Plasma vitamin B6 (pyrodoxal 5-phosphate), folate and vitamin B12 were measured by radioimmunoassay (ALPCO, Salem, NH). Plasma fatty acids were measured by gas spectrometry with chromatography as previously described.54 Plasma total cholesterol was separated from serum by centrifugation and analyzed in the Lipid-Atherosclerosis Research Laboratory at OHSU in compliance with standards set by surveillance programs of the CDC in Atlanta, GA.55 Plasma mineral content was determined by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES; Teledyne Leeman Labs, Hudson, New Hampshire) as described previously with minor modifications.56 Briefly, 100 μl of plasma were digested in 1ml 69%–70% OmniTrace nitric acid (VWR, West Chester, VA) overnight. Following digestion, samples were diluted 10 times with chelex 100 resin (BIO-RAD, Hercules, CA) treated water and analyzed by ICP-OES against known standards.
Specimen Quality Control
Collection and storage of MCI and NIE specimens was identical. Biologic specimens were collected from fasting subjects between the hours of 0700 and 1100 h beginning in August 2006 with final collection occurring in July 2007. Laboratory staff was blinded to the clinical diagnosis and dietary intake for all study participants. Coefficient of variation was < 6% for antioxidants (ascorbic acid, tocopherols, carotenoids) and fatty acids, < 8% for B vitamins, and < 6% for cholesterol.
Diet and exercise measures
The National Cancer Institute - Diet History Questionnaire was the Food Frequency Questionnaire (FFQ) utilized as a validated measure of usual frequency of dietary intake consumption over the preceding year.57 This FFQ probes for frequency and portion of 124 food items consumed and also has dietary supplement questions. This measure takes about 1–2 hours to complete and was designed to facilitate easy completion. FFQ was utilized as opposed to 24-hour recall and food diary because it is less of a challenge to episodic memory, widely used and more reflective of long-term dietary intake. The FFQ was self-administered during baseline and 1 month visits with trained personnel introducing the questionnaire and available to answer questions as needed related to wording of the questions, portion sizes, and to assess completeness of data collection before subject dismissal. The same collateral historian accompanied MCI subjects at each visit and was available for assisting subject with the questionnaire. All study participants successfully completed the FFQ. Raw data generated from the scanned FFQ was processed using Diet*Calc version 1.4.3 software developed by the National Cancer Institute (http://riskfactor.cancer.gov/FFQ).
The Physical Activity Scale in the Elderly (PASE) has been validated for use in people aged 65 to 100 years.58 Scores range from 0 to 400 with greater activity level corresponding to a higher PASE score. The PASE is self-administered and takes about 5 to 15 minutes to complete.
Statistical methods
To assess the within subject measure variability we compared the continuous plasma and FFQ nutrient estimates collected at baseline and one month. Means and standard deviations for plasma and FFQ estimates at baseline and 1 month were calculated (data not shown). T-test of independent samples was used to determine differences between continuous variables age, BMI, physical activity and by Chi-square test for categorical variables (i.e., gender, APOE genotype).
Intraclass correlation coefficients (ICC) were generated to assess the reliability of plasma and FFQ estimates by the following equation: . The ρI is the ratio of between person variance (σ2a) divided by the sum of the between person and the within person variance (σ2a + σ2). It ranges from 0 to 1, with ρI = 0 indicating no reliability (i.e., large within person variability and 0 between-person variability) and ρI = 1 indicating perfect reliability (i.e., 0 within person variability and large between person variability). Classification of reliability was defined a priori into ordinal categories and described in the subsequent results section. Many nutrient variables were not normally distributed and natural log (loge) transformation was overall the most successful remedy per Shapiro-Wilk test. Residual method was used to adjust nutrients for energy intake and tocopherols for total plasma cholesterol.59, 60 Pearson correlation coefficients tested the association between baseline plasma and FFQ nutrient estimates. Three models were created: 1) without adjustment as “Crude”, 2) adjustment for total energy intake as “Calorie-adjusted”, and 3) age, gender, energy intake, ApoE genotype, and cholesterol as “Multivariate-adjusted”. Supplementation was excluded from all analyses. The variables in the multivariate model were chosen based on previously reported interaction with association between diet and plasma nutrients and differences observed in these variables among our two study groups. Scatter plots with best-fit line were generated to describe linear association between plasma and FFQ vitamin B12 and folate with plasma homocysteine. All analyses were performed using SPSS 18 for Macintosh (Chicago, Ill) and two-tailed α level was set at 0.05.
RESULTS
Baseline characteristics (Table 1)
Table 1.
Baseline characteristics of study population stratified by cognitive status (n=38).
| MCI (n=19) | NIE (n=19) | Sig. level | |
|---|---|---|---|
| Age, y, (SD) | 73 (9) | 75 (6) | .39 |
| Male, (%) | 13 (68) | 6 (32) | .02* |
| Body Mass Index | 27 (3) | 26 (5) | .48 |
| Physical Activity Scale in Elderly | 155 (70) | 125 (47) | .15 |
| Blood pressure, Systolic | 121 (16) | 126 (15) | .31 |
| Blood pressure, Diastolic | 63 (11) | 63 (8) | 1.0 |
| APOE-ε4 carrier, (%) | 8 (42) | 5 (26) | .32 |
| Albumin, serum, g/dL | 4.0 (0.3) | 3.9 (0.3) | .18 |
| Creatinine, serum, g/dL | 1.0 (0.2) | 1.0 (0.2) | .50 |
| Homocysteine, plasma, μmol/L | 8.6 (2.5) | 8.6 (2.5) | .96 |
| Cholesterol, plasma, mg/dL | 188 (32) | 201 (32) | .05 |
| Calories consumed, total kCal | 1549 (566) | 1679 (505) | .46 |
| Mini Mental State Exam | 26 (2.0) | 29 (1.0) | <.001* |
| Neurobehavioral Cognitive Status Exam, Total | 69 (5) | 77 (2) | <.001* |
| Clinical Dementia Rating | .35 (0.2) | .0 (0.0) | <.001* |
| WMS-R, Delayed Paragraph Recall | 5 (6) | 12 (5) | .01* |
| Geriatric Depression Scale | 2 (1) | 1 (2) | .54 |
| Hachinski Ischemia Score | 1 (1) | 1 (0) | .08 |
| Acetylcholine esterase inhibitor use, (%) | 74 | 0 | <.001* |
| Statin use, (%) | 63 | 11 | <.001* |
| Multivitamin use, (%) | 58 | 68 | .51 |
MCI, amnestic – Mild Cognitive Impairment; NIE, Non-impaired elders
Mean and SD unless labeled otherwise,
Significance level set at P ≤ .05
Multivitamin use derived by interview with subject (and collateral historian for MCI), including examination of all bottles from which subject was regularly taking pills; WMS-R, Wechsler Memory Scale – Revised
The average age of the study population was 74 years, half were women (n=19) and half with amnestic - mild cognitive impairment (MCI, n=19). Thirty-four percent of the population carried the apolipoprotein E 4 allele (42% in MCI and 26% in NIE). The two groups were similar in age, BMI, cerebrovascular risk, activity level, kidney function, total energy intake, multivitamin supplementation, and freedom from depression. As expected, lower MMSE and higher acetylcholinesterase inhibitor use was observed in the MCI group. This group also had more men, statin users and borderline lower plasma cholesterol (Table 1).
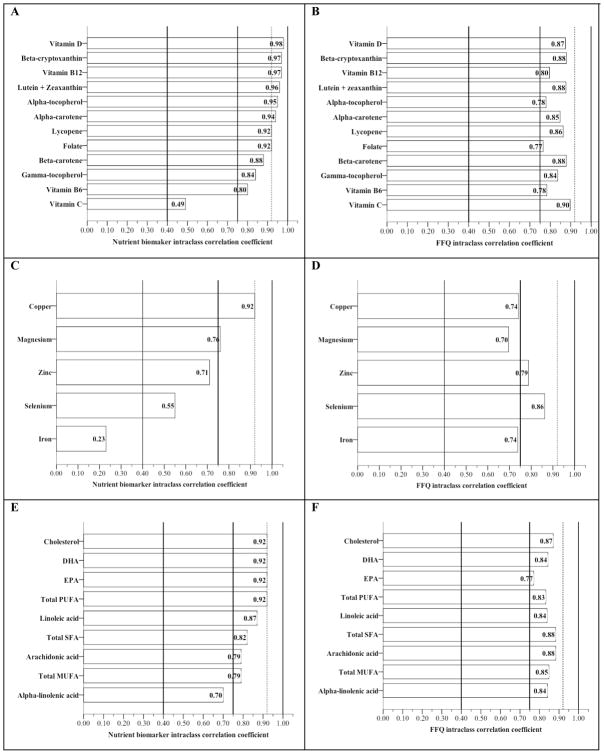
Reliability of plasma and FFQ estimates for vitamins, minerals and lipids in the total population (Figure 1)
Figure 1.
Reliability bars for plasma and FFQ vitamin, mineral and lipid estimates in the total population*
*Nutrients are presented in order of highest to lowest nutrient biomarker intraclass correlation coefficient observed for ease in comparing with coefficients observed from FFQ
Vitamins (A and B); Minerals (C and D); Lipids (E and F)
Vertical reference lines represent a priori classification of reliability: “Poor” (ICC < .40), “Good” (.40 ≤ ICC < .75), “Very good” (ICC ≥ 0.75), and “Excellent” (ICC ≥ to plasma cholesterol in this population, ICC = .92)
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PUFA, polyunsaturated fat; MUFA, monounsaturated fat; SFA, saturated fat
All variables log transformed
Reliability was classified a priori into four catagories 1) “Poor” (ICC < 0.40), 2) “Good” (.40 < ICC < .75), 3) “Very Good” (ICC ≥ 0.75)61. A fourth category of “Excellent” was defined as ICC ≥ to plasma cholesterol observed in the study population (ICC = 0.92) as a clinically relevant benchmark of reliability. Plasma nutrient biomarkers had twelve estimates rated excellent while no FFQ estimate reached this level of reliability. However, the overall range of reliability for all nutrient estimates was greater for plasma than FFQ.
Both the plasma and FFQ vitamins demonstrated at least very good reliability with the exception of plasma C, which rated good (Figure 1A and 1B). Vitamin D, beta-cryptoxanthin, vitamin B12, lutein + zeaxanthin, alpha-tocopherol, alpha-carotene, lycopene, folate and vitamin B6 demonstrated more reliable in plasma than FFQ. Plasma and FFQ beta-carotene and gamma-tocopherol had equal reliability considered “very good” by our criteria. FFQ vitamin C estimate outperformed plasma C. Plasma vitamin ICC ranged from .98 (vitamin D) to .49 (vitamin C) and 8/12 rated excellent. The range of ICC for FFQ vitamin estimates was less than plasma (.90, vitamin C − .77, folate), although no FFQ vitamin estimate rated at the level of excellent.
The range for plasma mineral reliability was large (.92, copper − .23, iron) and FFQ range was smaller (.86, selenium − .70, magnesium). Copper and magnesium were more reliable in plasma, and zinc, selenium and iron from FFQ (Figure 1C and 1D).
Plasma and FFQ lipid reliability was very good with the exception of plasma alpha-linolenic acid (Good). Docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and total polyunsaturated fat (PUFA) had excellent reliability in plasma (Figure 1E). Plasma linoleic acid, total saturated fat (SFA), arachidonic acid and total monounsaturated fat (MUFA) had very good reliability. All FFQ lipid reliability was very good (Figure 1F). Plasma cholesterol, DHA, EPA, total PUFA, and linoleic acid were more reliable in plasma, and total SFA, arachidonic acid, total MUFA, and alpha-linolenic acid performed better in FFQ. The most reliable plasma nutrients in all elders were vitamin D (ICC=.98), beta-cryptoxanthin and vitamin B12 (both ICC=.97), lutein + zeaxanthin (ICC=.96), alpha-tocopherol (ICC=.95), alpha-carotene (ICC=.94), lycopene, folate, DHA, EPA, cholesterol, total PUFA, copper (all ICC=.92), beta-carotene (ICC=.88), linoleic acid (ICC=.87), gamma-tocopherol (ICC=.84), total SFA (ICC=.82), vitamin B6 (ICC=.80), arachidonic acid and total MUFA (ICC=.79).
The most reliable FFQ nutrient estimates in all elders were vitamin C (ICC=.90), lutein + zeaxanthin, beta-cryptoxanthin, beta-carotene, total SFA, and arachidonic acid (all ICC=.88), cholesterol and vitamin D (both ICC=.86), lycopene and selenium (both ICC=.86)
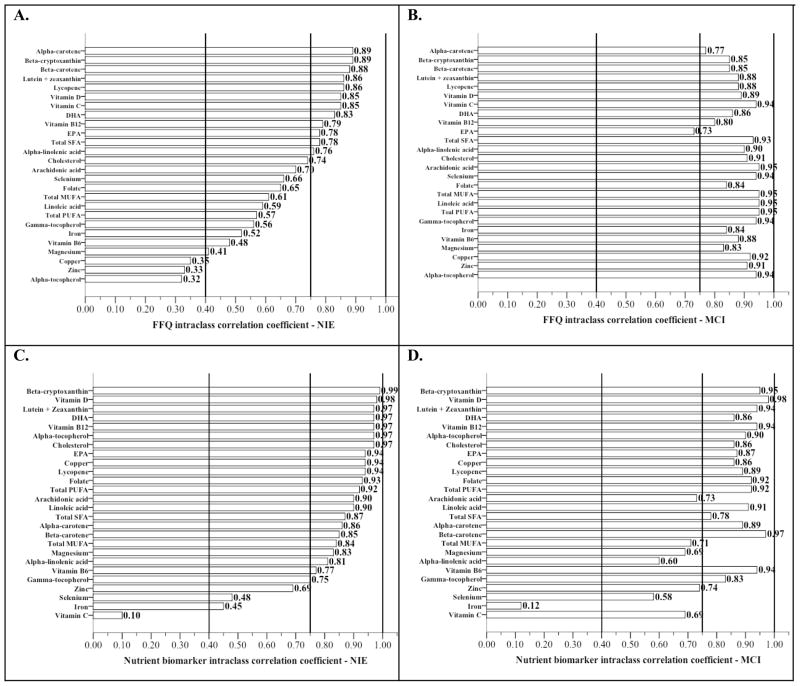
Reliability of plasma and FFQ nutrient estimates by cognitive status (Figure 2)
Figure 2.
Reliability bars for plasma and FFQ nutrient estimates stratified by cognitive status*
*Nutrients are presented in order from highest to lowest intraclass correlation coefficient observed in the non-impaired elders for ease in comparison with coefficients observed in mild cogntive impairment
Vertical reference lines represent a priori classification of reliability: “Poor” (ICC < .40), “Good” (.40 ≤ ICC < .75), and “Very good” (ICC ≥ 0.75) criteria for reliability
PUFA, Polyunsaturated fat; MUFA, Monounsaturated fat; DHA, Docosahexaenoic acid; EPA, Eicosapentaenoic acid
All variables log transformed
Interestingly, we observed greater intra-individual variability (lower reliability) in FFQ estimates derived from non-impaired elders (NIE) than in MCI. Ninety-six percent (25/26) of the FFQ estimate reliability rated very good or better in MCI compared to 46% (12/26) in non-impaired elders (Figure 2A and 2B). Ten of these FFQ estimates in MCI rated excellent while no ICC in NIE reached this level of reliability. The ten most reliable (least intra-individual variability) FFQ estimates in NIE were alpha-carotene and beta-cryptoxanthin (both ICC=.89), beta-carotene (ICC=.88), lutein + zeaxanthin and lycopene (both ICC=.86), vitamins D and C (both ICC=.85), DHA (ICC=.83), vitamin B12 (ICC=.79), and EPA (ICC=.78) (Figure 2A). The ten FFQ estimates with the least intra-individual variability (greatest reliability) in MCI were PUFA, MUFA, linoleic acid, arachidonic acid, and cholesterol (all ICC=.95), alpha-tocopherol, gamma-tocopherol, selenium, and vitamin C (all ICC=.94), total saturated fat (ICC=.93), and copper (ICC=.92) (Figure 2B).
The plasma nutrient biomarkers had greater reliability in the NIE compared to MCI (Figure 2C and 2D). The ten most reliable nutrient biomarkers in MCI were vitamin D (ICC=.98), beta-carotene (ICC=.97), beta-cryptoxanthin (ICC=.95), lutein + zeaxanthin, vitamins B12 and B6 (all ICC=.94), folate and PUFA (both ICC=.92), linoleic acid (ICC=.91), and alpha-tocopherol (ICC=.90) (Figure 2D). The ten most reliable nutrient biomarkers in NIE were beta-cryptoxanthin (ICC=.99), vitamin D (ICC=.98), lutein + zeaxanthin, DHA, vitamin B12, alpha-tocopherol, and cholesterol (all ICC=.97), EPA, copper and lycopene (all ICC=.94) (Figure 2C).
Correlation between FFQ nutrient estimates and respective nutrient biomarker (Table 2)
Table 2.
Pearson correlation coefficients between plasma and FFQ nutrient estimates by cognitive status*
| Crude | Calorie-adjusted1 | Multivariate-adjusted2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | MCI | NIE | Total | MCI | NIE | Total | MCI | NIE | ||
| Vitamin | Ascorbic acid | .32 | .15 | .52* | .32 | .15 | .52* | .30 | .09 | .51 |
| Alpha-tocopherol | .17 | .15 | .10 | .17 | .15 | .10 | .11 | .21 | −.04 | |
| Gamma-tocopherol | −.21 | −.20 | −.02 | −.16 | −.20 | −.02 | −.15 | −.37 | −.04 | |
| Lutein + zeaxanthin | .48** | −.19 | .72** | .47** | −.19 | .72** | .37* | −.35 | .76** | |
| Beta-cryptoxanthin | .41** | −.11 | .72** | .41** | −.11 | .72** | .35* | −.15 | .64* | |
| Alpha-carotene | .49** | .43 | .65** | .49** | .43 | .65** | .49** | .44 | .73** | |
| Beta-carotene | .43** | .45 | .48* | .43** | .35 | .49* | .34* | .22 | .71** | |
| Lycopene | .12 | −.01 | .23 | .12 | .01 | .23 | .08 | .06 | .06 | |
| Vitamin B6 | −.08 | .05 | −.28 | .01 | .09 | −.20 | .01 | .12 | −.18 | |
| Folate | .04 | −.25 | .39 | −.08 | .04 | −.28 | −.10 | .04 | −.29 | |
| Vitamin B12 | .19 | .10 | .28 | .04 | −.25 | .39 | .14 | −.07 | .42 | |
| Vitamin D | .32 | .15 | .52* | .19 | .10 | .28 | .16 | .18 | .13 | |
| Mineral | Copper | .12 | −.15 | .28 | .12 | −.15 | .28 | .06 | −.14 | .23 |
| Iron | .02 | .11 | −.04 | .02 | .13 | −.04 | .07 | .02 | .26 | |
| Magnesium | −.35* | −.52* | −.19 | −.35* | −.52* | −.19 | −.36* | −.54* | −.17 | |
| Selenium | −.33* | −.41 | −.13 | −.33* | −.41 | −.13 | −.32 | −.50 | −.14 | |
| Zinc | .01 | .09 | −.19 | .01 | .09 | −.19 | .05 | .21 | −.20 | |
| Lipid | PUFA, total | .10 | −.06 | .30 | .10 | −.06 | .30 | .00 | −.07 | −.09 |
| MUFA, total | −.15 | −.10 | −.15 | −.15 | −.10 | −.14 | −.07 | .05 | .03 | |
| SFA, total | −.13 | −.04 | −.32 | −.13 | −.04 | −.32 | −.11 | .01 | −.42 | |
| Linoleic acid | .09 | −.05 | .23 | .07 | −.05 | .23 | −.07 | −.16 | −.14 | |
| Arachidonic acid | .01 | −.05 | .10 | −.01 | −.05 | .10 | .03 | −.01 | .14 | |
| Alpha-linolenic acid | .15 | .23 | .03 | .15 | .23 | .03 | .14 | .23 | −.12 | |
| EPA | .39* | .22 | .43 | .39* | .22 | .43 | .39* | .38 | .41 | |
| DHA | .39* | .04 | .65** | .39* | .04 | .65** | .38* | .06 | .66** | |
| Cholesterol3 | −.06 | −.19 | .07 | −.06 | −.20 | .07 | −.05 | −.13 | .01 | |
Baseline nutritional assessments were utilized
Total, all subjects (n=38); MCI, amnestic - Mild Cognitive Impairment; NIE, Non-Impaired Elderly
Calorie adjustment by residual method
Multivariate adjustment includes calorie adjusted FFQ plus age, gender, APOE-4 carrier status, and plasma cholesterol
Multivariate model excludes plasma cholesterol for this variable
p < 0.01;
p ≤ .05
All variables log transformed; No supplements are included
Twenty-six nutrients from both plasma and FFQ were classified into three categories 1) Vitamins (Ascorbic acid, Alpha and gamma tocopherol, carotenoids, vitamin B6, folate and B12 and vitamin D), 2) Minerals (Copper, Iron, Magnesium, Selenium, Zinc), and 3) Lipids (total PUFA, MUFA, Saturated fat, Linoleic acid, Arachidonic acid, Alpha-linolenic acid, EPA, DHA, Cholesterol).
FFQ carotenoids and long chain fatty acids correlate with plasma levels, but not in MCI. In MCI and NIE combined, FFQ estimates of lutein + zeaxanthin, beta-cryptoxanthin, alpha-carotene, beta-carotene, DHA and EPA did positively correlate with their respective plasma content (Table 2, Total). Correlation coefficients generated between FFQ and plasma levels of vitamin C, alpha-tocopherol, lycopene, folate, vitamin B12, vitamin D, copper, iron, zinc, total PUFA, linoleic acid, arachidonic acid, and α-linolenic acid all had a positive linear direction of correlation but did not reach statistical significance at our α level of .05.
In MCI 12 of the 26 correlation coefficients generated between FFQ and plasma were positive in linear direction (Table 2, MCI). By comparison, 17 of the 26 nutrients had coefficients of the appropriate direction in NIE, and 7 of these 17 did reach significance (Table 2, NIE). The calorie-and-multivariate adjusted models had no apparent advantage over the crude model that was unadjusted for energy intake and other variables (Table 2, Calorie adjusted, Multivariate-adjusted).
DISCUSSION
This pilot study provides the 1-month reliability of a comprehensive panel of nutrient biomarkers in a population at risk for Alzheimer’s disease. The results may be considered preliminary for FFQ because of the limited sample size. However, these findings suggest that reliability and validity of vitamins, minerals and lipids vary by the assessment method utilized and the nutrient understudy, but also, vary by memory state of the participant. These potential sources of error are important to consider when interpreting previous literature and designing future studies focused on the role of nutrition in cognitive aging and Alzheimer’s disease.
Nutrient biomarker reliability in our study was consistent with other reports for vitamins and lipids.34 Twelve of the nutrient biomarkers were at least as reliable as the commonly measured cholesterol and many may be relevant to neurodegeneration. These include lipophilic antioxidants, fatty acids, one-carbon metabolism participants, vitamin D and copper.
FFQ nutrient estimate reliability was higher in amnestic Mild Cognitive Impairment compared to non-impaired elders (NIE). This can be interpreted as more stable or less variability in dietary intake in MCI compared to NIE. To test this hypothesis we compared the reliability of the plasma nutrient biomarkers in the two groups. Interestingly, nutrient biomarker reliability in MCI was lower compared to NIE failing to support a more stable diet in MCI. More homogenous reporting of dietary intake in MCI is a phenomenon that may be directly related to the nature of the memory deficit seen in this population who are essentially reporting “default” eating habits.
We observed less nutrient biomarker reliability (more variability) in MCI compared to NIE. This may be representing more true variability in dietary intake in MCI, which is missed by the reporting bias associated with the FFQ. This could be explained by differences in nutrient absorption and metabolism in MCI compared to NIE. We did observe a correlation between plasma vitamin B12 and folate with plasma homocysteine and no correlation between FFQ B vitamin estimates and plasma homocysteine in this population (data not shown). However, FFQ B vitamins did not relate to plasma homocysteine in either group suggesting that absorption and metabolism are no different between these two groups for B12 and folate.
Validating FFQ with plasma nutrient biomarkers is advantageous because these two measures have unrelated measurement error. A correlation between FFQ and plasma nutrient estimate therefore provides strong documentation of qualitative FFQ validity.30 FFQ and plasma correlations were strongest for carotenoids and long chain fatty acids. All together, 19 of the 26 correlation coefficients were at least in the appropriate direction (Table 2 Crude, Total). However, after stratifying by cognitive diagnosis, the MCI generated many more negative coefficients and the FFQ – plasma correlations in NIE were strengthened. This supports the view that short-term memory deficit attenuates any association between FFQ and plasma nutrient estimates. Besides memory state, other factors could explain weak correlation between FFQ and plasma estimates including: differences in metabolism (i.e., alpha-linolenic acid conversion 18:4 n-3 or EPA), genetic factors (i.e., 5,10-methylene-tetrahydrofolate reductase 677C→T polymorphism and B vitamins), and hypo/hyper responder in plasma nutrients by dietary intake (i.e., diet and plasma cholesterol). Gender may also modify the relationship between FFQ and plasma nutrients. Although we partially controlled for gender in our analysis, our sample size restricted us from exploring effect modification by gender. Nevertheless, these data suggest that memory state of the participant has distinct affect on the reliability and validity of FFQ; reliability is falsely inflated compared to controls and validity is decreased.
Some limitations of this pilot study include a relatively small sample size (n=38), homogenous ethnicity (all non-Hispanic, white), and no data beyond 1 month. The strengths include an elder population with expert classification of cognition, collection of FFQ twice over 1 month, which allows a period long enough for subjects to forget their previous responses and at the same time short enough to limit true change in diet, both factors affecting reliability30. Other strengths include the comprehensive panel of nutrient biomarkers studied and the collection of plasma, FFQ and cognitive measures at the same time point for each study participant. These factors add unique strength to our inferences regarding reliability and validity of these nutritional assessments.
Exposure reliability is particularly important in Alzheimer’s epidemiology because the neurodegenerative process is gradual, requiring large samples followed for an extensive period to assure adequate cognitive change. AD prevention trials have similar challenges. A highly reliable instrument can minimize bias and permit greater power for detecting true effects with less sample size. Many of the nutrient biomarkers tested highly reliable over 1-month. This method is less susceptible to sources of error associated with less direct methods of nutritional assessment. These results provide a strong basis for longitudinal study of the relationship between nutrition and cognitive aging and Alzheimer’s disease, and the constitution of biospecimen banks at baseline of observational and intervention studies for secondary analyses.
Acknowledgments
NCCAM K23 AT004777 Career Development Award (GLB), NCRR RR024140, Oregon Partnership for Alzheimer’s Research (GLB), NCCAM P01 AT002034 (BF), NIA P30 AG008017 (JAK), and Oregon Agricultural Experiment Station, supported in part by funds provided through the Hatch Act. The authors would like to acknowledge Dr. Clive Woffendin, Ms. Jennifer Baxter (ND candidate), Ms. Nora Mattek, MPH, Ms. Karin Hardin, Mr. Scott Leonard, and Ms. Deborah Hobbs for assistance with biospecimen collection, handling and processing.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 Aug;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002 Jun 26;287(24):3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 3.Rinaldi P, Polidori MC, Metastasio A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol Aging. 2003 Nov;24(7):915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 4.Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8(3):156–162. [PubMed] [Google Scholar]
- 5.Morris MC, Evans DA, Tangney CC, et al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005 Feb;81(2):508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 6.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006 Oct;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 7.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006 Jun;59(6):912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007 Nov 13;69(20):1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 9.Montine TJ, Neely MD, Quinn JF, et al. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic Biol Med. 2002 Sep 1;33(5):620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 10.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004 Jan;61(1):82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 11.Stuerenburg HJ, Ganzer S, Muller-Thomsen T. Plasma beta carotene in Alzheimer's disease. Association with cerebrospinal fluid beta-amyloid 1–40, (Abeta40), beta-amyloid 1–42 (Abeta42) and total Tau. Neuro Endocrinol Lett. 2005 Dec;26(6):696–698. [PubMed] [Google Scholar]
- 12.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002 Feb 14;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 13.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., 3rd High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005 Sep;82(3):627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 14.Karuppagounder SS, Xu H, Shi Q, et al. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer's mouse model. Neurobiol Aging. 2008 Apr 9; doi: 10.1016/j.neurobiolaging.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamoh S, Hashimoto M, Sugioka K, et al. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93(1):237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 17.Lim GP, Calon F, Morihara T, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005 Mar 23;25(12):3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calon F, Lim GP, Morihara T, et al. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2005 Aug;22(3):617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006 Nov;63(11):1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 20.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998 May 26;95(11):6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolsch H, Heun R, Kerksiek A, Bergmann KV, Maier W, Lutjohann D. Altered levels of plasma 24S- and 27-hydroxycholesterol in demented patients. Neurosci Lett. 2004 Sep 30;368(3):303–308. doi: 10.1016/j.neulet.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;25(6):539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006 Dec;14(12):1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 24.Quinn JF, Crane S, Harris C, Wadsworth TL. Copper in Alzheimer's disease: too much or too little? Expert Rev Neurother. 2009 May;9(5):631–637. doi: 10.1586/ern.09.27. [DOI] [PubMed] [Google Scholar]
- 25.Strozyk D, Launer LJ, Adlard PA, et al. Zinc and copper modulate Alzheimer Abeta levels in human cerebrospinal fluid. Neurobiol Aging. 2009 Jul;30(7):1069–1077. doi: 10.1016/j.neurobiolaging.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradburn NM, Rips LJ, Shevell SK. Answering autobiographical questions: the impact of memory and inference on surveys. Science. 1987 Apr 10;236(4798):157–161. doi: 10.1126/science.3563494. [DOI] [PubMed] [Google Scholar]
- 27.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005 Nov 8;65(9):1409–1414. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 28.Russell RM. Factors in aging that effect the bioavailability of nutrients. J Nutr. 2001 Apr;131(4 Suppl):1359S–1361S. doi: 10.1093/jn/131.4.1359S. [DOI] [PubMed] [Google Scholar]
- 29.Bowman GL, Dodge H, Frei B, et al. Ascorbic acid and rates of cognitive decline in Alzheimer's disease. J Alzheimers Dis. 2009 Jan;16(1):93–98. doi: 10.3233/JAD-2009-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willett W. Nutritional Epidemiology. 2. New York: Oxford University; 1998. [Google Scholar]
- 31.Tangney CC, Shekelle RB, Raynor W, Gale M, Betz EP. Intra- and interindividual variation in measurements of beta-carotene, retinol, and tocopherols in diet and plasma. Am J Clin Nutr. 1987 Apr;45(4):764–769. doi: 10.1093/ajcn/45.4.764. [DOI] [PubMed] [Google Scholar]
- 32.Smith SJ, Cooper GR, Myers GL, Sampson EJ. Biological variability in concentrations of serum lipids: sources of variation among results from published studies and composite predicted values. Clin Chem. 1993 Jun;39(6):1012–1022. [PubMed] [Google Scholar]
- 33.Ma JAF, Eckfeldt JH, Lewis L, Chambless LE. Short- and long-term repeatability of fatty acid composition of human plasma phospholipids and cholesterol esters. Am J Clinical Nutrition. 1995;62(September):572–578. doi: 10.1093/ajcn/62.3.572. [DOI] [PubMed] [Google Scholar]
- 34.Block G, Dietrich M, Norkus E, et al. Intraindividual variability of plasma antioxidants, markers of oxidative stress, C-reactive protein, cotinine, and other biomarkers. Epidemiology. 2006 Jul;17(4):404–412. doi: 10.1097/01.ede.0000220655.53323.e9. [DOI] [PubMed] [Google Scholar]
- 35.Shvetsov YB, Hernandez BY, Wong SH, Wilkens LR, Franke AA, Goodman MT. Intraindividual Variability in Serum Micronutrients: Effects on Reliability of Estimated Parameters. Epidemiology. 2008 Sep 20; doi: 10.1097/EDE.0b013e318187865e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996 Jan;126(1):129–137. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 37.Vogel S, Contois JH, Tucker KL, Wilson PW, Schaefer EJ, Lammi-Keefe CJ. Plasma retinol and plasma and lipoprotein tocopherol and carotenoid concentrations in healthy elderly participants of the Framingham Heart Study. Am J Clin Nutr. 1997 Oct;66(4):950–958. doi: 10.1093/ajcn/66.4.950. [DOI] [PubMed] [Google Scholar]
- 38.Tucker KL, Chen H, Vogel S, Wilson PW, Schaefer EJ, Lammi-Keefe CJ. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J Nutr. 1999 Feb;129(2):438–445. doi: 10.1093/jn/129.2.438. [DOI] [PubMed] [Google Scholar]
- 39.White E, Kristal AR, Shikany JM, et al. Correlates of serum alpha- and gamma-tocopherol in the Women's Health Initiative. Ann Epidemiol. 2001 Feb;11(2):136–144. doi: 10.1016/s1047-2797(00)00189-7. [DOI] [PubMed] [Google Scholar]
- 40.Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr. 2004 Apr;134(4):927–934. doi: 10.1093/jn/134.4.927. [DOI] [PubMed] [Google Scholar]
- 41.Vioque J, Weinbrenner T, Asensio L, Castello A, Young IS, Fletcher A. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. Br J Nutr. 2007 May;97(5):977–986. doi: 10.1017/S0007114507659017. [DOI] [PubMed] [Google Scholar]
- 42.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003 Dec 15;158(12):1213–1217. doi: 10.1093/aje/kwg290. [DOI] [PubMed] [Google Scholar]
- 43.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999 Mar;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Kiernan RJ, Mueller J, Langston JW, Van Dyke C. The Neurobehavioral Cognitive Status Examination: a brief but quantitative approach to cognitive assessment. Ann Intern Med. 1987 Oct;107(4):481–485. doi: 10.7326/0003-4819-107-4-481. [DOI] [PubMed] [Google Scholar]
- 46.Dooneief G, Marder K, Tang MX, Stern Y. The Clinical Dementia Rating scale: community-based validation of “profound' and “terminal' stages. Neurology. 1996 Jun;46(6):1746–1749. doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 47.Wechsler D. Wechsler Memory Scale (WMS-III) San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 48.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 49.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984 Nov;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 50.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990 Mar;31(3):545–548. [PubMed] [Google Scholar]
- 51.Kutnink MA, Hawkes WC, Schaus EE, Omaye ST. An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal Biochem. 1987 Nov 1;166(2):424–430. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- 52.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996 Apr;37(4):893–901. [PubMed] [Google Scholar]
- 53.Johnson EJ, Hammond BR, Yeum KJ, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000 Jun;71(6):1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- 54.Muskiet FAJVDJJ, Martini IA, Wolthers BG, van der Slik W. Capillary gas chromatographic profiling of total long-chain fatty acids and cholesterol in biological materials. J Chromatogr. 1983;278:231–244. doi: 10.1016/s0378-4347(00)84782-9. [DOI] [PubMed] [Google Scholar]
- 55.Manual of Laboratory Operations, Lipid and Lipoprotein Analysis. Resources DoHaH. 1982. [Google Scholar]
- 56.Song Y, Chung CS, Bruno RS, et al. Dietary zinc restriction and repletion affects DNA integrity in healthy men. Am J Clin Nutr. 2009 Aug;90(2):321–328. doi: 10.3945/ajcn.2008.27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol. 2001 Dec 15;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 58.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 59.Willett W. Epidemiologic studies in nutrition: utility and limitations. J Nutr. 1986 Dec;116(12):2557–2558. doi: 10.1093/jn/116.12.2557. [DOI] [PubMed] [Google Scholar]
- 60.Traber MG, Jialal I. Measurement of lipid-soluble vitamins--further adjustment needed? Lancet. 2000 Jun 10;355(9220):2013–2014. doi: 10.1016/S0140-6736(00)02345-X. [DOI] [PubMed] [Google Scholar]
- 61.Rosner B. Fundamentals of Biostatistics. 2006 [Google Scholar]