Abstract
The goal of this study was to determine if parameters of gait are related to the path integration deficits in some people with vestibular disorders. We tested normals, and two groups of vestibularly impaired people, with unilateral benign paroxysmal positional vertigo and with unilateral weakness. Each group had 20 subjects. They walked straight ahead for 7.62 m, with eyes open or closed, to the beat of a metronome at 60 beats/min, 120 beats/min and 176 beats/min. When adjusted for age and sex, normals veered significantly less and walked significantly further before veering than the unilateral weakness group; all groups veered significantly less at 120 beats/min than the slower or faster cadences. Older patients walked slightly but significantly slower than younger patients at the faster speeds. Step length did not differ among diagnostic groups. These data confirm the involvement of vestibular function in path integration, suggest a differentiation by type of vestibular impairment, and suggest some differences by cadence.
Introduction
Path integration is the ability to keep track of one’s position in space with reference to the starting point (1). This skill is a function of the vestibular system (2–4). Normal humans can perform a path integration task when walking along a linear path but patients with acute vestibular lesions are significantly impaired on task performance (5, 6). Evidence from one study suggests that path integration skill may be speed dependent (7). The goal of the present study was to learn more about the influence of walking speed and vestibular loss on path integration skill.
Methods
Subjects
Subjects were adults, including 19 males, and 41 females. The 3 groups of subjects were a) 20 normal volunteers recruited from among staff and visitors to the Center for Balance Disorders (mean age 52.5), b) 20 patients with unilateral benign paroxysmal positional vertigo (BPPV) of the posterior semicircular canal who had been referred to the senior author for vestibular rehabilitation who were tested prior to being treated (mean age 58.0), and c) 20 patients with unilateral vestibular weakness (mean age 53.9). Normal volunteers were screened by taking a health history to rule out otologic, neurologic and musculoskeletal disorders after which they were tested with head thrusts (8, 9), head shaking and Dix-Hallpike maneuvers. Each BPPV patient had a unilateral, positive response to the Dix-Hallpike maneuver with classical nystagmus (10) while eye movements were recorded with infrared video-oculography, and they had no other significant otologic or neurologic problems. The unilateral weakness patients were of two subtypes: either resection of an acoustic neuroma more than 3 months prior to testing or a weakness of 20% or more on bi-thermal caloric testing with water (10) while eye movements were recorded with infrared video-oculography.
Testing
Subjects were tested in a well-lighted room, with unobstructed walking space approximately 11.5 m X 3.5 m. The floor is covered by 30.48 cm2 vinyl tiles. Subjects were asked to walk straight ahead for 7.62 m with eyes either opened or closed (5), to the beat of a metronome at three different speeds -- 60 beats/min (slow), 120 beats/min (medium), 176 beats/min (fast) –- for a total of 6 conditions which were given in randomized order. Dependent measures were angle of veering from the center line, time to complete the task as timed with a stopwatch, distance walked before veering, step length, and number of steps. To calculate the angle of veering and distance walked before veering the grid on the floor, created by the lines between the vinyl tiles. was used to determine when and how far the subject drifted off course. The subject’s forward and sideward drift were recorded, from which the angle was calculated. See Figure 1. The number of steps was counted by direct observation. Step length was calculated from the number of steps divided by the distance walked.
Figure 1.
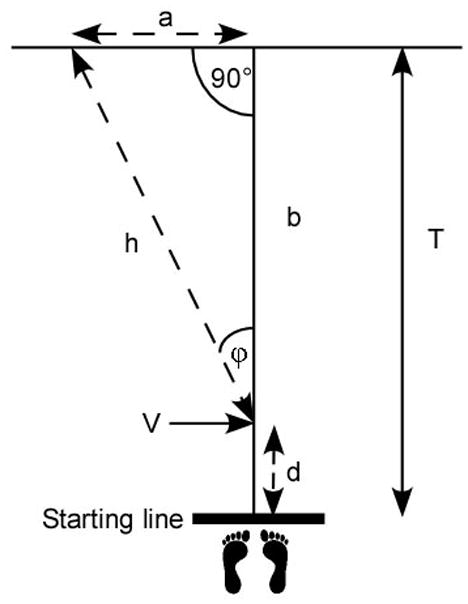
Diagram of the task for a subject who veered to the left. Subjects were instructed to start at the starting line and walk this distance, T (T=7.62 m.). (Feet are not to scale.) Subjects who veered to either side walked the distance, d, to point, V, at which they veered some angle, φ, continuing along the trajectory, h, to cross the finish line at point a, h, having moved laterally through the distance, a. (From (5) and used by permission of IOS press.)
Statistical methods
Multilevel analysis (11) and mixed effect models were used to describe changes in primary outcomes of interest (angle of veering from the center line, time to complete the task, distance walked before veering, and number of steps) at various speeds (slow, medium, fast) and eye conditions (open and closed) and to compare the 3 study groups (healthy controls, BPPV, unilateral weakness) on these parameters. A separate model was produced and fitted to each outcome. Within each model, we examined significance of within subject effect (within subject over speed and over eye conditions) and between subjects (between the 3 study groups). Interaction effects were included in each model and tested. Changes over conditions were compared between groups by using a likelihood ratio statistic which follows a chi-square distribution. Adjustments were made for multiple comparisons. P <.05 was considered as statistically significant. All analyses were performed using SAS Statistical software (SAS, Carry, NC).
Results
Tables 1 to 3 show descriptive statistics for all dependent measures by test conditions These measures include angle of veering from the center, step length, distance the subject walked before veering, velocity of walking, and the number of steps taken.
Table 1.
Mean angle of veering (deg) and mean distance walked before veering (m), (standard deviations and ranges in parentheses) (N = normal, UW = unilateral weakness; EO – eyes opened, EC = eyes closed)
| Angle (deg) | Distance (m) | ||||||
|---|---|---|---|---|---|---|---|
| Slow | Medium | Fast | Slow | Medium | Fast | ||
| N | EO | 0 | 0 | 0 | 7.62 (0) | 7.62 (0) | 7.62 (0) |
| EC | 5.06 (5.0, 0–12.3) | 3.0 (3.6, 0–11.5) | 4.7 (5.3, 0–19.1) | 5.5 (2.2,1.5–7.6) | 5.7 (2.1, 2.1–7.62) | 5.4 (2.3, 1.5–7.62) | |
| BPPV | EO | 0 | 0 | 0 | 7.62 (0) | 7.62 (0) | 7.62 (0) |
| EC | 6.3 (5.6, 0–17.2) | 6.7 (4.8, 0–17.2) | 8.3 (5.9, 0–22.9) | 4.2 (2.7, 0.6–7.62) | 4.3 (2.2, 1.6–7.62) | 4.1 (2.2, 1.2–7.62) | |
| UW | EO | 0 | 0 | 0 | 7.62 (0) | 7.62 (0) | 7.62 (0) |
| EC | 10.3 (7.0, 0–28.7) | 6.0 (5.3, 0–16.4) | 8.4 (8.6, 0–32.7) | 3.40 (1.9, 1.2–7.62) | 4.5 (2.5, 0.9–7.62) | 5.3 (2.2, 1.5–7.62) | |
Table 3.
Mean velocity (standard deviations and ranges in parentheses) and median number of steps (ranges in parentheses) (N = normal, UW = unilateral weakness; EO – eyes opened, EC = eyes closed; Vel= velocity)
| Velocity (m/s) | Steps | ||||||
|---|---|---|---|---|---|---|---|
| Slow | Medium | Fast | Slow | Medium | Fast | ||
| N | EO | 2.0 (0.5, 1.5–3.1) | 4.2(0.7, 2.5–5.6) | 4.7(1.1, 1.7–7.0) | 14 (11–17) | 12 (9–18) | 13 (9–18) |
| EC | 1.7 (0.3, 1.2–2.5) | 3.4(0.6, 2.5–4.7) | 4.3(0.8, 2.9–5.9) | 16 (11–19) | 14 (10–23) | 15 (12–22) | |
| BPPV | EO | 1.8 (0.4, 0.8–2.6) | 3.8 (0.9, 2.2–5.4) | 4.7 (1.1, 3.1–7.4) | 15 (11–32) | 13 (10–32) | 14 (10–22) |
| EC | 1.6 (0.4, 1.0–2.6) | 3.0 (0.8, 2.0–4.5) | 3.7 (1.4, 1.5–6.4) | 17 (11–27) | 14 (10–22) | 16 (11–35) | |
| UW | EO | 2.0 (0.5, 1.2–3.5) | 3.8 (0.7, 2.8–5.4) | 4.8 (1.1, 3.1–7.8) | 15 (11–22) | 14 (10–18) | 14 (10–25) |
| EC | 1.6 (0.5, 0.9–3.4) | 3.2 (1.0, 1.8–6.3) | 3.5 (1.2, 1.6–5.7) | 17 (5–32) | 16 (11–26) | 18 (12–47) | |
With eyes closed, normals veered significantly less than BPPV (p=0.048) and unilateral weakness patients (p=0.0006). When adjusted for age and sex, normals still veered significantly less than unilateral weakness subjects (p< 0.005). The difference between normals and BPPV subjects was no longer significant (p=0.1) and BPPV subjects did not differ significantly from unilateral weakness subjects (p=0.5). Angle of veering was related to speed, even when adjusted for age and sex (p=0.02). In general with groups collapsed subjects veered less at the medium speed (5.2°) than the slow (7.2°) or fast speeds (7.1°). See Table 1.
With eyes closed normals walked farther before veering (5.3 m) than unilateral weakness patients (4.2 m); this trend remained significant after adjustment for age and sex (was significant for age (F=20.7, p<0.0001), sex (F=8.45, p=0.005) and group (F=3.7, p=0.3). When adjusted for age and sex the normal and unilateral weakness groups differed significantly (p<0.03), indicating that normals walked further than patients before veering but the BPPV group did not differ significantly from either group. See Table 1.
The distance walked before veering might have been a reflection of differences in step length. The diagnostic groups did not differ significantly by step length. Step length was significantly greater at the medium speed compared to the slow (p= 002) or fast (p=0.56) speeds. Step length at slow and fast speeds did not differ significantly. Not surprisingly, step length was larger with eyes open than with eyes closed (p<0.0001). See Table 2.
Table 2.
Mean step length (m), (standard deviations and ranges in parentheses) (N = normal, UW = unilateral weakness; EO – eyes opened, EC = eyes closed; Vel= velocity)
| Slow length | Medium length | Fast length | ||
|---|---|---|---|---|
| N | EO | 0.55 (0.07, 0.44–0.69) | 0.63 (0.10, 0.42–0.85) | 0.58 (0.12, 0.42–0.85) |
| EC | 0.49 (0.08, 0.40–0.69) | 0.54 (0.09, 0.33–0.76) | 0.52 (0.10, 0.35–0.64) | |
| BPPV | EO | 0.51 (0.11, 0.24–0.69) | 0.56 (0.11, 0.33–0.76) | 0.54 (0.14, 0.35–0.76) |
| EC | 0.46 (0.10, 0.28–0.69) | 0.47 (0.10, 0.30–0.64) | 0.47 (0.16, 0.22–0.69) | |
| UW | EO | 0.53 (0.08, 0.35–0.69) | 0.57 (0.09, 0.42–0.76) | 0.52 (0.12, 0.30–0.76) |
| EC | 0.50 (0.26, 0.24–1.52) | 0.47 (0.11, 0.29–0.69) | 0.44 (0.12, 0.16–0.64) | |
In general within each group and visual condition velocity of walking increased significantly with increasing speed (p<0.0001). Walking velocity was significantly higher with eyes open than eyes closed within each group and speed condition (p<0.001). These relationships remained significant after controlling for age and sex. See Table 3. With the groups collapsed velocity decreased weakly with increased age at medium (Eyes open, r=−0.3, p=0.009; Eyes closed, r=−0.3, p=0.02) and fast (Eyes open, −0.2, p=0.07, Eyes closed, r=−0.3, p<0.3) walking speeds. For normal subjects the relationship between velocity and age was nonsignificant. For the two patient groups the relationship between velocity and age was significant but only moderate, for the BPPV group at the fast speed with eyes closed, r= −0.5, p<0.03; for the unilateral weakness group at the medium speed with eyes open, r=−0.5, p<0.03. No other significant correlations between age and velocity were found.
The number of steps did not differ significantly across the three study groups when adjusted for age and sex. Subjects took significantly fewer steps with eyes open than eyes closed; regardless of group (p=0.0008). In general the number of steps increased with increasing speed (p=0.001). See Table 3.
Discussion
Patients with unilateral weakness veered more than normals, confirming previous findings that this skill is influenced by vestibular function (5, 6). Patients with BPPV are not impaired on this task, however, suggesting that they are not impaired on path integration skill. This finding extends the previous work by showing that path integration performance is affected by cadence of walking. Patients with unilateral weakness tend to walk slowly, especially in the acute phase or recovery, and they tend to complain of bumping into walls and other indications of impaired path integration. Patients with BPPV rarely complain of such problems. Thus, the type of vestibular impairment may affect this skill.
In general, this task was more easily performed at 2.0 Hz than at lower or higher frequencies. This finding supports work by other investigators that approximately 120 steps per minute, or 2.0 Hz, is the preferred walking speed for most adults (12) and path integration is more difficult at slower speeds (13). Unlike Bredin at al (13) we did not find a difference in the fast condition. Our paradigm used a defined cadence and they used a cadence defined by the subject’s preferred cadence, however. Many of our subjects commented that the task was more difficult at both the slow and fast speeds. Subjects took more steps at the fast speed regardless of group, although they were not instructed how to walk. This phenomenon may reflect the difficulty of walking at a higher cadence and the fact that preferred walking speed results from combinations of preferred cadence and preferred step length (14), so that when cadence is forced step length must be adjusted. Decreased step length may have the effect of decreasing the thoraco-pelvis relative phase (15). At all speeds the number of steps was highly variable. Many people have difficulty matching auditory metrics (16). Several subjects, including normal people, commented that they do not have musical skills or are unable to sing or dance in rhythm.
Acknowledgments
Supported by NIH grant R01 DC003602. We thank the staff of the Center for Balance Disorders for technical assistance.
Footnotes
Conflict of interest statement
Neither author has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mittelstaedt H, Mittelstaedt M-L. Homing by path integration. In: Papi P, Wallraff HG, editors. Avian Navigation. Berlin Heidelberg: Springer-Verlag; 1982. pp. 290–7. [Google Scholar]
- 2.Barlow JS. Inertial navigation as a basis for animal navigation. J Theor Biol. 1964;6:76–117. doi: 10.1016/0022-5193(64)90067-0. [DOI] [PubMed] [Google Scholar]
- 3.Mayne R. A systems concept of the vestibular organs. In: Kornhuber HH, editor. Handbook of Sensory Physiology. Berlin: Springer Verlag; 1974. pp. 493–580. [Google Scholar]
- 4.Miller S, Potegal M, Abraham L. Vestibular involvement in a passive transport and return task. Physiol Psychol. 1983;11:1–10. [Google Scholar]
- 5.Cohen HS. Vestibular disorders and impaired path integration along a linear trajectory. J Vestib Res. 2000;10:7–15. [PubMed] [Google Scholar]
- 6.Cohen HS, Kimball KT. Improvements in path integration after vestibular rehabilitation. J Vestib Res. 2002;12:47–51. [PubMed] [Google Scholar]
- 7.Dickstein R, Ufaz S, Dunsky A, Nadeau S, Abulaffio N. Speed-dependent deviations from a straight-ahead path during forward locomotion in healthy individuals. Amer J Phys Med Rehab. 2005;84:330–7. doi: 10.1097/01.phm.0000159980.14702.be. [DOI] [PubMed] [Google Scholar]
- 8.Aw ST, Halmagyi GM, Black RA, Curthoys IS, Yavor RA, Todd MJ. Head impulses reveal loss of individual semicircular canal function. J Vestib Res. 1999;9:173–80. [PubMed] [Google Scholar]
- 9.Halmagyi GM, Curthoys IA. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 10.Coats AC. Electronystagmography. In: Bradford LJ, editor. Physiological Measures of the Audio-Vestibular System. New York: Academic Press; 1975. pp. 37–85. [Google Scholar]
- 11.Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- 12.Murray MP, Kory RC, Sepic SB. Walking patterns of normal women. Arch Phys Med Rehab. 1970;51:637–50. [PubMed] [Google Scholar]
- 13.Bredin J, Kerlirzin Y, Israel I. Path integration: is there a difference between athletes and non-athletes? Exp Brain Res. 2005;167:670–4. doi: 10.1007/s00221-005-0251-3. [DOI] [PubMed] [Google Scholar]
- 14.Latt MD, Menz HB, Fung VS, Lord SR. Walking speed, cadence and step length are selected to optimize the stability of head and pelvis accelerations. Exp Brain Res. 2008;184:201–9. doi: 10.1007/s00221-007-1094-x. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Meijer OG, Lin J, Bruijn SM, Wu W, Lin X, et al. The effects of stride length and stride frequency on trunk coordination in human walking. Gait Posture. 2010 doi: 10.1016/j.gaitpost.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Iversen JR, Repp BH, Patel AD. Top-down control of rhythm perception modulates early auditory responses. Annals of the New York Academy of Sciences. 2009;1169:58–73. doi: 10.1111/j.1749-6632.2009.04579.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen JL, Penhune VB, Zatorre RJ. The role of auditory and premotor cortex in sensorimotor transformations. Annals of the New York Academy of Sciences. 2009;1169:15–34. doi: 10.1111/j.1749-6632.2009.04556.x. [DOI] [PubMed] [Google Scholar]
- 18.Grahn JA, Rowe JB. Feeling the beat: premotor and striatal interactions in musicias and nonmusicians during beat perception. J Neurosci. 2009;29:7540–8. doi: 10.1523/JNEUROSCI.2018-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JL, Penhune VB, Zatorre RJ. Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex. 2008;18:2844–54. doi: 10.1093/cercor/bhn042. [DOI] [PubMed] [Google Scholar]


