Abstract
Recent studies recognize that Hypocretin system (also known as Orexin) plays a critical role in sleep/wake disorders and feeding behaviors. However, little is known about the regulation of the Hypocretin system. It is also known that tumor necrosis factor alpha (TNF-α) is involved in regulation of sleep/wake cycle. Here, we test our hypothesis that the Hypocretin system is regulated by TNF-α. Prepro-Hypocretin and Hypocretin receptor 2 (HcrtR2) can be detected at a very low level in rat B35 neuroblastoma cells. In response to TNF-α, Prepro-Hypocretin mRNA and protein levels are down-regulated, and also HcrtR2 protein level is down-regulated in B35 cells. To investigate the mechanism, exogenous rat Prepro-Hypocretin and rat HcrtR2 were overexpressed in B35 cells. In response to TNF-α, protein and mRNA of Prepro-Hypocretin are significantly decreased (by 93% and 94%, respectively), and the half-life of Prepro-Hypocretin mRNA is decreased in a time- and dose-dependent manner. The level of HcrtR2 mRNA level is not affected by TNF-α treatment; however, HcrtR2 protein level is significantly decreased (by 86%) through ubiquitination in B35 cells treated with TNF-α. Downregulation of cellular inhibitor of apoptosis protein-1 and -2 (cIAP-1 and -2) abrogates the HcrtR2 ubiquitination induced by TNF-α. The control green fluorescent protein (GFP) expression is not affected by TNF-α treatment. These studies demonstrate that TNF-α can impair the function of the Hypocretin system by reducing the levels of both Prepro-Hypocretin and HcrtR2.
Keywords: Prepro-Hypocretin, Hypocretin, Orexin, Tumor necrosis factor, narcolepsy, sleep disorder, Hypocretin receptor, Orexin receptor, Prepro-Orexin
1. INTRODUCTION
The neuropeptides, Hypocretin-1 and Hypocretin-2 (also known as Orexin-A and Orexin-B), were first described in 1998 by two research groups [1,2]. Hypocretin-1 and Hypocretin-2 are derived from a single precursor, the Prepro-Hypocretin, through proteolytic cleavage [1,2]. Prepro-Hypocretin is expressed mainly in hypothalamic neurons, as well as in testis [1–3]. Two Hypocretin receptors, the Hypocretin receptor 1 and 2 (HcrtR1 and HcrtR2), have been identified so far, and they share 64% of homology [2,4,5]. HcrtR1 and HcrtR2 are expressed in multiple organs, such as brain, kidney, lung, and testis [3,4]. Prepro-Hypocretin and Hypocretin receptors are highly conserved among species [1,4,6,7]. Growing evidences support that homeostasis of the Hypocretin system is important for the integrity of sleep/wakefulness cycle, feeding behavior, and emotion [8–11]. Dysregulation of the Hypocretin system has been blamed for the sleep disorder, narcolepsy, as well as emotion disorders, such as depression [4,9,11,12].
Tumor necrosis factor-alpha (TNF-α) is well known as a pro-inflammation cytokine, and plays an important role in host defense and pathogenesis of various diseases [13–15]. It is a 185 amino acid glycoprotein cytokine, and was identified in 1975 [16]. TNF-α signaling cascade is propagated by binding to TNF receptors, and, upon activation, TNF receptors bind to TNF receptor-associated factors and other downstream signaling proteins, resulting in regulation of gene expression and cell functions [13–15]. TNF-α has been associate with the development of multiple brain disorders, such as depression, narcolepsy, multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease [17–20]. TNF-α is also called as adipokine due to its role in obesity and diabetes [21]. Published data indicate that TNF-α is involved in the regulation of sleep/wakefulness cycle and fatigue during infections [22]; but, the mechanism is not completely understood.
In the present study, we demonstrate that TNF-α can impair the Hypocretin system through decrease of mRNA half-life and protein ubiquitination. TNF-α treatment decreases the half-life of the Prepro-Hypocretin mRNA, resulting in downregulation of both protein and mRNA levels of Prepro-Hypocretin. TNF-α treatment decreases HcrtR2 protein level through an ubiquitination pathway mediated by cellular inhibitor of apoptosis protein-1 and -2 (cIAP-1 and cIAP-2), without affecting the mRNA level of HcrtR2. These data for the first time provide evidence that TNF-α can directly downregulate the Hypocretin system, suggesting a role of TNF-α in the development of disorders caused by the impaired Hypocretin system.
2. MATERIALS AND METHODS
2.1. Reagents
Recombinant tumor necrosis factor alpha (TNF-α) was obtained from R&D Systems (Minneapolis, MN). The following purified polyclonal antibodies were purchased: anti-Prepro-Hypocretin (Milliopore, Billerica, MA), anti- HcrtR2, anti-focal adhesion kinase (FAK), and anti-Green Fluorescent Protein (GFP) (Santa Cruz Biotechnology, Santa Cruz, CA). The following purified monoclonal antibodies (mAb) were purchased: anti-glyceraldehyde 3-phosphate dehydrogenase (G3PDH) (Research Diagnostics, Flanders, NJ), anti-human/mouse pan cellular inhibitor of apoptosis protein-1 and -2 (cIAP1 and cIAP2)-specific antibody was obtained from R&D Systems (Minneapolis, MN). All other reagents were purchased from Thermo Fisher Scientific (P-SUWANEE, GA), Sigma-Aldrich (St. Louis, MO), or Bio-Rad (Hercules, CA).
2.2 Cell culture and MSCV retroviral vectors
The B35 rat neuroblastoma cells were derived from central nervous system, and obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained and propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/ml penicillin/streptomycin/gentamycin as described [23]. Stable cell lines expressing Prepro-Hypocretin and HcrtR2 were established using the MSCV Retroviral Expression Vector System (with the Neomycin resistance gene) purchased from Clontech (Mountain View, CA) and used according to the manufacturer’s instructions. Briefly, rat Prepro-Hypocretin cDNA (gene accession #AF041241) or rat HcrtR2 cDNA (gene accession #AF041246) were first inserted into the MSCV vectors between the EcoRI site and the XhoI site of the polylinker located within the multiple cloning sites of the MSCV vector. Then, the MSCV-Prepro-Hypocretin vector or MSCV- HcrtR2 vector was transfected into the packaging cells (Clontech) by liposome-mediated transfections (Lipofectamine 2000, from Invitrogen, Carlsbad, California) to produce infectious, but replication-incompetent, retroviral particles, and the collected supernatants containing retroviral particles were used to transfect the B35 cells. Stable clones were selected by Neomycin as the MSCV vector contains the Neomycin resistance gene. The green-fluorescent-protein (GFP) protein was also subcloned into MSCV vector, and MSCV-GFP was used as a control and also used to optimize the transfection and selection conditions. GFP-positive cells were easily detected under fluorescent microscope (Nikon, Japan, Model: Eclipse, Ti).
2.3 Western Blotting and Immunoprecipitation
Essentially as described by us previously [23]. Briefly, cells were lysed in 1% NP-40 lysis buffer containing the following inhibitors, 100 μM PMSF, 10 μg/ml Aprotinin, 10 μg/ml Leupeptin, 100 μM Sodium Vanadate, and 20 μg/ml TLCK. Protein concentration of whole cell or whole lung lysate was determined by BCA kit (Pierce, Rockford, IL). Equivalent micrograms of whole cell lysates were electrophoresed on SDS PAGE, transferred to Immobilon-P membrane (Millipore Corp., Bedford, MA), probed or stripped followed by re-probing with indicated antibodies, and developed with ECL system (Pharmacia Biotech, Piscataway, NJ). Unibiquitinated HcrtR2 level was determined by Western blot under non-disulfide-reduced conditions following immunoprecipitation of equivalent cell lysates as described previously [24]. The expression of G3PDH protein was used as a loading control. Quantitative analysis (densitometry) of Western blots was performed by calculating the relative density (pixel density) of the immunoreactive bands after acquisition of the blot image (scanning) and analysis with Adobe Photoshop software as described [23]. The background of densitometric reading on the ECL-developed film was subtracted.
2.4 siRNA Studies
Transfection of siRNA was done as described previously [24]. Commercially available small interfering RNA (siRNA) duplexes directed against rat cIAP1 and cIAP2 were purchased from Invitrogen (Carlsbad, California) as described [25], and are all selected and validated Stealth siRNAs. Control siRNA duplexes were purchased from Dharmacon (Lafayette, CO). Lamin A/C siRNA (Dharmacon) was used to optimize the transfection conditions and efficiency. Additional controls of nonspecific siRNA duplex or vehicle alone were evaluated and found not to alter cIAP-1 and cIAP-2 mRNA and protein expression at the concentration of siRNA found to reduce the specific target message and protein optimally.
2.5 RT-PCR and Real-Time quantitative RT-PCR
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA), and 1μg of total RNA was reverse transcribed to cDNA for RT-PCR and real-time quantitative RT-PCR as described previously [26]. Quantitative RT-PCR analysis was carried out with the SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA) using the Biorad iQ5 Real-Time PCR Detection System, according to the manufacturer’s instructions. The quantitative RT-PCR was performed using 4 l of the synthesized cDNA, 12.5μl of SYBR® Green PCR Master Mix, 1μl of primer, and 7.5μl of water in a final 25μl volume. Samples were assayed in triplicate, and the values were normalized to the relative amounts of beta-actin mRNA level. Primer sequences were as described [3]. Primers for Prepro-Hypocretin: sense 5′-GCCGTCTCTACGAACTGTTG-3′ and anti-sense 5′-GAGGAGAGGGGAAAGTTAG-3′; for HcrtR2: sense 5′-CAATGTTGTTGGGGTGCTTA-3′ and antisense 5′-TCCCCCTCTCATAAACTTGG-3′; for rat focal adhesion kinase (FAK) (gene accession #NM_013081): sense 5′-CCTTAACAATGCGCCAGTTT-3′ and antisense 5′-CCAGATACGCGAGTGCTGTA-3′; and for Beta-actin: sense 5′-GTGGGTATGGGTCAGAAGGA-3′ and antisense 5′-AGCGCGTAACCCTCATAGAT-3′. All primers were purchased from Fisher Scientific.
2.6 mRNA Stability Assay
mRNA half-life was determined by culturing B35 cells in medium with actinomycin D (10 μg/ml) to block transcription as described by Lindholm et al. [27]. Briefly, immediately after addition of actinomycin D, B35 cells were treated with TNF-α or vehicle for the indicated time, and harvested. Total RNA was collected, and the amounts of Prepro-Hypocretin or HcrtR2 mRNA at each time point was quantified by real-time RT-PCR, and normalized to the amounts of β-actin mRNA at the same time point as described [26].
2.7 Statistical Analysis
Data were analyzed using the unpaired or paired t-test analysis (for comparisons between two groups) (Sigma Plot, SPSS Inc.), and expressed as means ± SE. Experiments were performed two to four times with duplicates. Linear regression was performed by using Sigma Plot (SPSS Inc.). A p value of < 0.05 was considered statistically significant.
3. RESULTS
3.1 Prepro-Hypocretin mRNA and protein levels, and Hypocretin receptor 2 (HcrtR2) protein levels, are significantly down-regulated in response to tumor necrosis factor-alpha (TNF-α) treatment
Expression of Prepro-Hypocretin (the common precursor of Hypocretin-1 and -2) was detected at a very low level in the B35 rat neuroblastoma cell line by RT-PCR and Western Blot analysis (Figs. 1A and 1C, respectively). In response to TNF-α treatment (2 ng/ml, for 24 hours at 37°C), both mRNA and protein of Prepro-Hypocretin were significantly down-regulated (Figs. 1B and 1C).
Figure 1. TNF-α treatment significantly decreases endogenous Prepro-Hypocretin mRNA and protein levels, as well as endogenous HcrtR2 protein level in rat B35 cells.
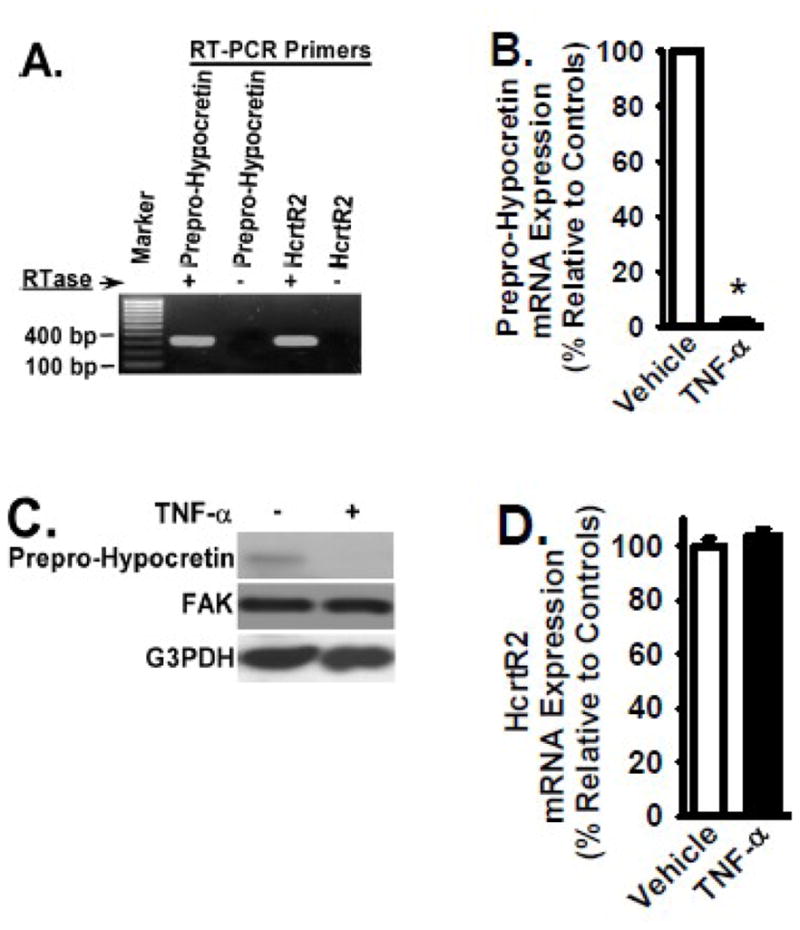
Rat B35 neuroblastoma cells were cultured in the presence and absence of TNF-α cytokine (2 ng/ml) for 24 hours at 37°C. Panel A: Low levels of endogenous Prepro-Hypocretin and HcrtR2 were detected by RT-PCR as described in the Materials and Methods in B35 cells without TNF-α treatment. Reactions without reverse transcriptase (RTase) were PCR controls. Panels B, D, and F: Total RNA in B35 cells treated with or without TNF-α was extracted to analyze the Prepro-Hypocretin mRNA level in Panel B, the HcrtR2 mRNA level in Panel D, and the control FAK mRNA level in Panel F, by quantitative real time RT-PCR (normalized to beta-actin and relative to the controls: the cells treated with vehicle only). Panels C and E: The above B35 cells were lysed, and equivalent amount of whole cell detergent lysates were Western blotted to analyze the protein level of Prepro-Hypocretin and HcrtR2 with the indicated antibodies. FAK and G3PDH expression served as controls.
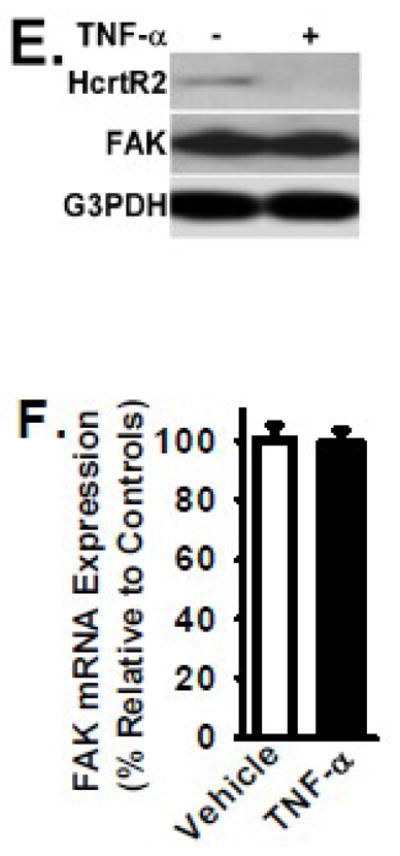
HcrtR2 expression was also detected at a very low level in the B35 rat neuroblastoma cell line by RT-PCR and Western Blot analysis (Figs. 1A and 1E, respectively). In response to TNF-α treatment (2 ng/ml, for 24 hours at 37°C), the protein level of HcrtR2 was significantly down-regulated (Fig. 1E). TNF-α had no detectable effect on the HcrtR2 mRNA level (Fig. 1D). Focal adhesion kinase (FAK) is a cytoplasmic protein kinase, and FAK is well known for its key role in cell migration [28,29]. Both mRNA and protein levels of FAK were not altered in TNF-α-treated B35 cells (Figs. 1C, 1E, and 1F), and the expression of FAK was used as a control.
3.2 Exogenous Prepro-Hypocretin and HcrtR2 expression mediated by retroviral vectors in B35 cells
As the endogenous expression of Prepro-Hypocretin and HcrtR2 are very low in the B35 rat neuroblastoma cell line (Fig. 1), exogenous rat Prepro-Hypocretin and rat HcrtR2 were overexpressed in these cells to facilitate our study to understand the mechanism. The MSCV retroviral expression system was used to generate the stable cell lines overexpressing rat Prepro-Hypocretin and HcrtR2 in B35 cells as described in the Materials and Methods. HcrtR2 was selected since animal studies demonstrate that HcrtR2 is the major receptor for the function of Hypocretins [30].
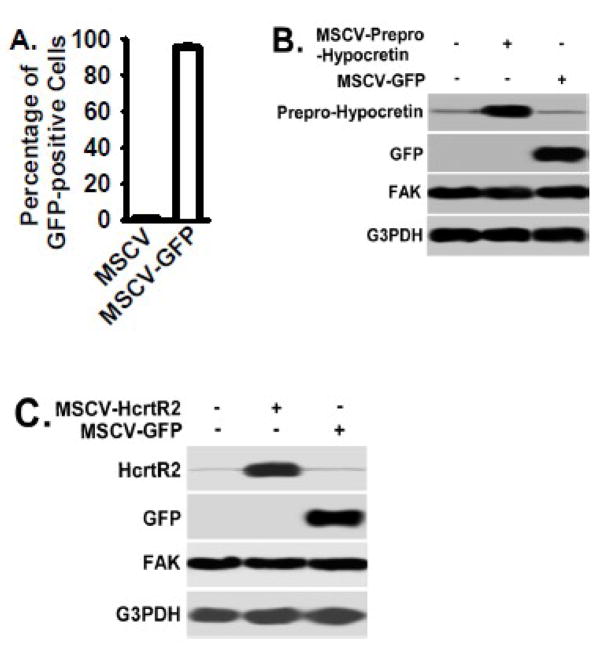
The MSCV vector containing the green-fluorescent-protein (GFP) cDNA (MSCV-GFP) and empty MSCV vector were used as controls, and MSCV-GFP was used to establish the GFP-expressing control B35 cells. Greater than 95% of B35 cells were GFP-positive after MSCV-GFP infection and selected by neomycin resistance (Fig. 2A). Prepro-Hypocretin overexpression mediated by MSCV-Prepro-Hypocretin vector was confirmed by Western Blot (Fig. 2B). HcrtR2 overexpression mediated by MSCV-HcrtR2 vector was also confirmed by Western Blot (Fig. 2C). GFP expression mediated by the MSCV-GFP vector had no effect on expression of Prepro-Hypocretin, HcrtR2, or FAK (Figs. 2B and 2C, respectively).
Figure 2. Prepro-Hypocretin and HcrtR2 overexpression mediated by MSCV vectors in rat B35 cells.
MSCV retroviral vectors were used to generate stable cell lines overexpressing the Prepro-Hypocretin, HcrtR2, or control GFP protein in rat B35 neuroblastoma cells as described in the Materials and Methods. Panel A: Fluorescence microscopic analysis found that over 95% of B35 cells infected with MSCV-GFP vector were GFP positive (per high power field, n=10 per clone), indicating a high efficiency of infection. The cell clone infected with empty MSCV vector served as a control for MSCV-GFP vector. Panels B and C: MSCV-Prepro-Hypocretin and MSCV-HcrtR2 vectors were used to express Prepro-Hypocretin and HcrtR2 in B35 cells, respectively. Cells were lysed, and equivalent amount of whole cell detergent lysates were Western blotted with the indicated antibodies. FAK and G3PDH protein served as controls.
3.3 TNF-α significantly decreases the mRNA and protein levels of Prepro-Hypocretin
To examine the effect of TNF-α treatment on Prepro-Hypocretin expression in B35 cells transfected with MSCV-Prepro-Hypocretin vector, cells were cultured in the presence and absence of TNF-α cytokine (2 ng/ml) for 24 hours at 37°C. Cells were then subsequently lysed and analyzed by Western blotting for the protein level of Prepro-Hypocretin in response to TNF-α treatment. Total RNA was also extracted from these cells to analyze the mRNA level of Prepro-Hypocretin by quantitative real time RT-PCR. Prepro-Hypocretin protein level in cells treated with TNF-α was significantly decreased (by 93%, p < 0.01) when compared to that in cells treated with vehicle only (Figs. 3A and 3B). Prepro-Hypocretin mRNA level in cells treated with TNF-α was also significantly decreased (by 94%, p < 0.01) when compared to that in cells treated with vehicle only (Fig. 3C). TNF-α treatment had no effect on the control GFP expression (Fig. 3E), and hd no effect on the FAK mRAN and protein level (Figs. 3D and 3E). The data indicate that TNF-α induces a significant reduction of both mRNA and protein expression of Prepro-Hypocretin, evidenced by results obtained from both endogenous and exogenous Prepro-Hypocretin (Figs. 1 and 3, respectively).
Figure 3. TNF-α treatment significantly decreases Prepro-Hypocretin expression in B35 cells transfected with MSCV-Prepro-Hypocretin vector.
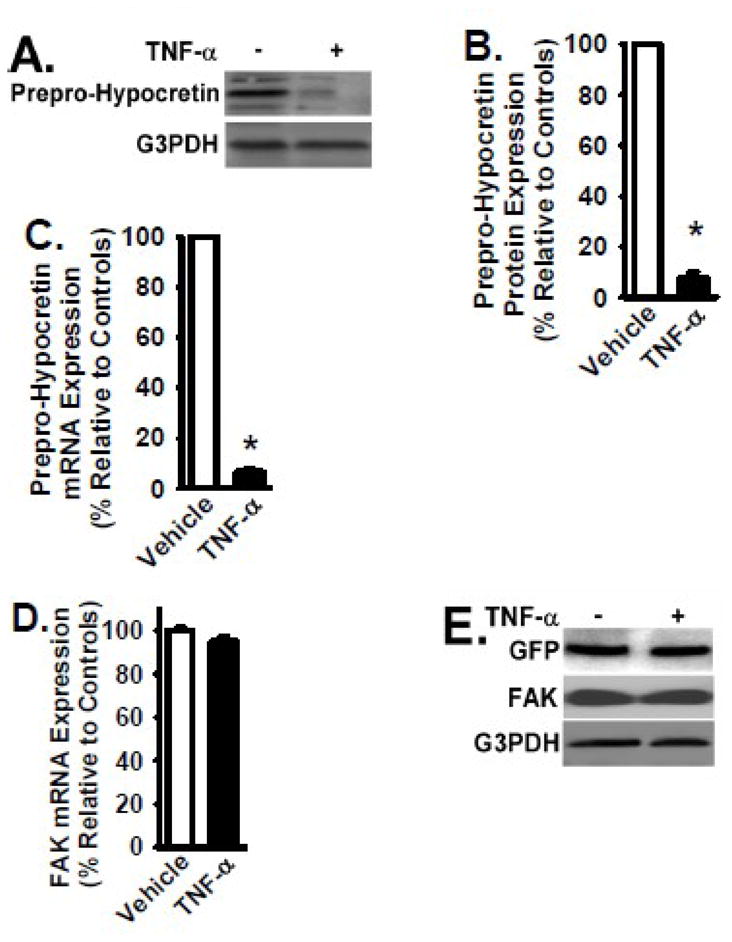
B35 cells overexpressing Prepro-Hypocretin (mediated by MSCV-Prepro-Hypocretin vector) or overexpressing GFP control protein (mediated by MSCV-GFP) were cultured in the presence and absence of TNF-α cytokine (2 ng/ml) for 24 hours at 37°C. Panels A and E: Cells were lysed, and equivalent amount of whole cell detergent lysates were Western blotted with the indicated antibodies. FAK and G3PDH protein expression served as controls. Panel B: Densitometry of Prepro-Hypocretin expression as shown in Panel A (normalized to G3PDH and relative to the controls: the cells treated with vehicle only) as described in the Materials and Methods 2.3. Panels C and D: Total RNA was extracted from the above cells to analyze the mRNA level of Prepro-Hypocretin (in Panel C) and FAK (in Panel D as a control) by quantitative real time RT-PCR. Data was normalized to beta-actin and relative to the control (cells treated with vehicle only). For Panels B and C, open bar denotes the results from control cells (vehicle only), and Black bar denotes the results from TNF-α-treated cells. Data are presented as mean ± S.D. (n = 4). * represents p < 0.01 for TNF-α-treated cells compared to vehicle-treated control group.
3.4 Stability of Prepro-Hypocretin mRNA is decreased in a time-dependent and dose-dependent manner in response to TNF-α treatment
To understand the mechanism resulting in the reduction of both mRNA and protein levels of Prepro-Hypocretin in response to TNF-α, we examined the decay/degradation of Prepro-Hypocretin mRNA in B35 cells overexpressing the Prepro-Hypocretin (mediated by MSCV-Prepro-Hypocretin vector as shown in Fig. 2). TNF-α treatment resulted in a significant reduction of the half-life of Prepro-Hypocretin mRNA when compared to that in vehicle-treated cells (Fig. 4A). The half life of Prepro-Hypocretin was about 50 minutes in TNF-α-treated cells; in contrast, it is more than 160 minutes in controls (Fig. 4A). The data also demonstrate that the decay/degradation of Prepro-Hypocretin mRNA in response to TNF-α was both time-dependent (Fig. 4A) and dose-dependent (Fig. 4B).
Figure 4. TNF-α treatment increases Prepro-Hypocretin mRNA decay.
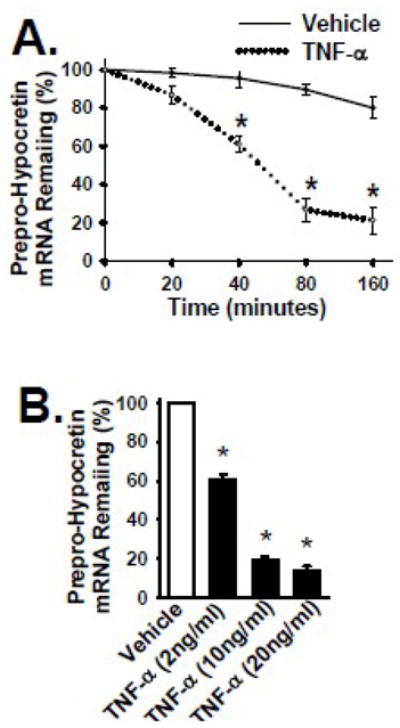
Panel A: B35 cells overexpressing the Prepro-Hypocretin were cultured in the presence and absence of TNF-α cytokine (2 ng/ml) for indicated time points at 37°C. Total RNA was collected, and the amount of Prepro-Hypocretin mRNA at each time point was quantified by real-time RT-PCR as in Fig. 2. Panel B: B35 cells overexpressing the Prepro-Hypocretin were cultured in the absence or presence of TNF-α cytokine at the indicated concentrations for 40 minutes at 37°C, and analyzed as in Panel A. Solid line in Panel A or open bar in Panel B denotes the results from control cells (vehicle only). Dotted line or Black bar denotes the results from TNF-α-treated cells. Data are presented as mean ± S.D. (n = 3 to 4). * represents p < 0.01 for TNF-α-treated cells compared to vehicle-treated control group.
3.5 TNF-α significantly decreases the protein level of HcrtR2 without affecting the level of HcrtR2 mRNA
To examine the effect of TNF-α treatment on Hypocretin receptor expression, B35 cells overexpressing the HcrtR2 (mediated by MSCV-HcrtR2 vector as shown in Fig. 2) were cultured in the absence and presence of TNF-α (2 ng/ml) for 24 hours at 37°C. Protein and mRNA levels of HcrtR2 in TNF-α-treated cells were determined by Western blot analysis and quantitative real-time RT-PCR, respectively. As shown in Figure 5, TNF-α treatment resulted in a significant reduction of HcrtR2 protein level. In response to TNF-α, HcrtR2 protein level was decreased by 86% (p < 0.01) when compared to that in vehicle-treated cells (Figs. 5A and 5B). In contrast, the mRNA level of HcrtR2 was similar between TNF-α-treated cells and vehicle-treated cells (Fig. 5C).
Figure 5. TNF-α treatment decreases HcrtR2 expression in B35 cells transfected with MSCV-HcrtR2 vector.
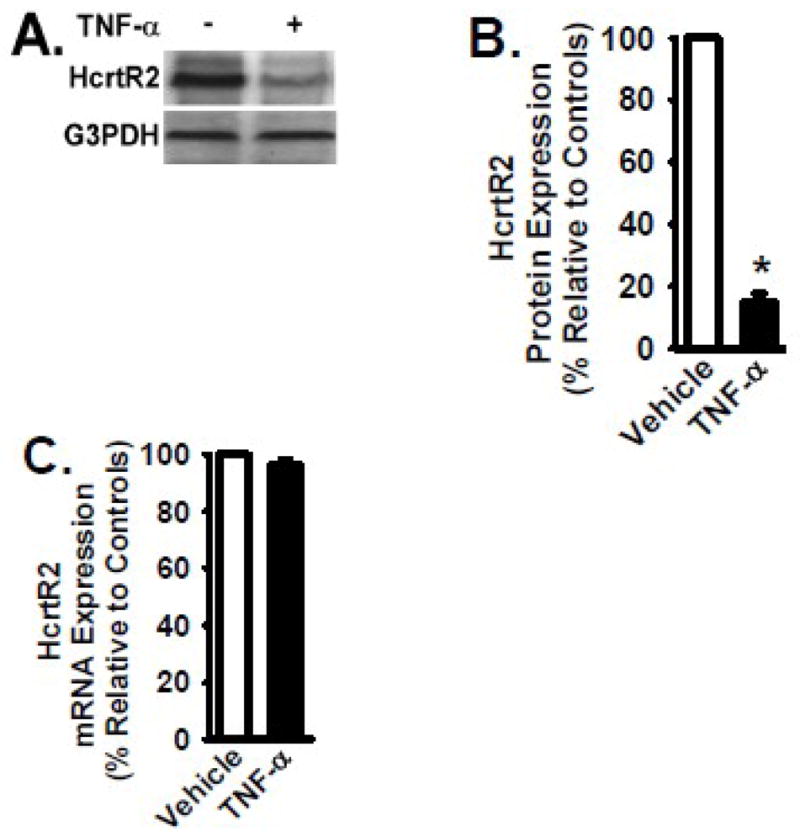
B35 cells overexpressing the HcrtR2 (mediated by MSCV-HcrtR2) were cultured in the presence and absence of TNF-α cytokine (2 ng/ml) for 24 hours at 37°C. Panel A: Cells were lysed, and equivalent amount of whole cell detergent lysates were Western blotted with the indicated antibodies. Panel B: Densitometry of HcrtR2 expression as in Fig. 2. Panel C: mRNA level of HcrtR2 was analyzed by quantitative real time RT-PCR as in Fig. 2. Data are presented as mean ± S.D. (n = 4). * represents p < 0.01 for TNF-α-treated cells compared to vehicle-treated control group.
3.6 TNF-α induces ubiquitination of HcrtR2 through a pathway mediated by cellular inhibitor of apoptosis proteins
To determine the mechanism contributing to the reduction of HcrtR2 protein level in response to TNF-α treatment, the involvement of ubiquitination was studied in B35 cells overexpressing the HcrtR2. The association of ubiquitin with HcrtR2 in TNF-α-treated cells (2ng/ml, 6 hours) was confirmed by Western blot analysis following immunoprecipitation (IP) of HcrtR2 (Fig. 6A). Cellular inhibitor of apoptosis protein-1 and -2 (cIAP1 and cIAP2) function as E3 ubiquitin-protein ligases within the TNF-α signaling pathway, and the E3 ubiquitin-protein ligase is required for the final step of the ubiquitylation cascade [25]. To determine the role of cIAPs in TNF-α-induced ubiquitination of HcrtR2, small interfering RNA (siRNA) specifically targeting cIAP1 or cIAP2 were utilized to knockdown the cIAP expression in B35 cells overexpressing the HcrtR2. The expression of cIAP1 and cIAP2 were significantly decreased by siRNAs at both protein level (Fig. 6B) and mRNA level (data not shown). Downregulation of either cIAP1 or cIAP2 expression partially restored the HcrtR2 protein level in TNF-α-treated cells (Fig. 6B). Downregulation of both cIAP1 and cIAP2 expression were required to abrogate the TNF-α-induced ubiquitination of HcrtR2, and to restore the HcrtR2 expression (Fig. 6B). Non-specific siRNA controls had no effect on HcrtR2 or cIAP expression as shown in Fig. 6B.
Figure 6. TNF-α treatment induces HcrtR2 ubiquitination through a pathway mediated by cIAP-1 and cIAP-2.
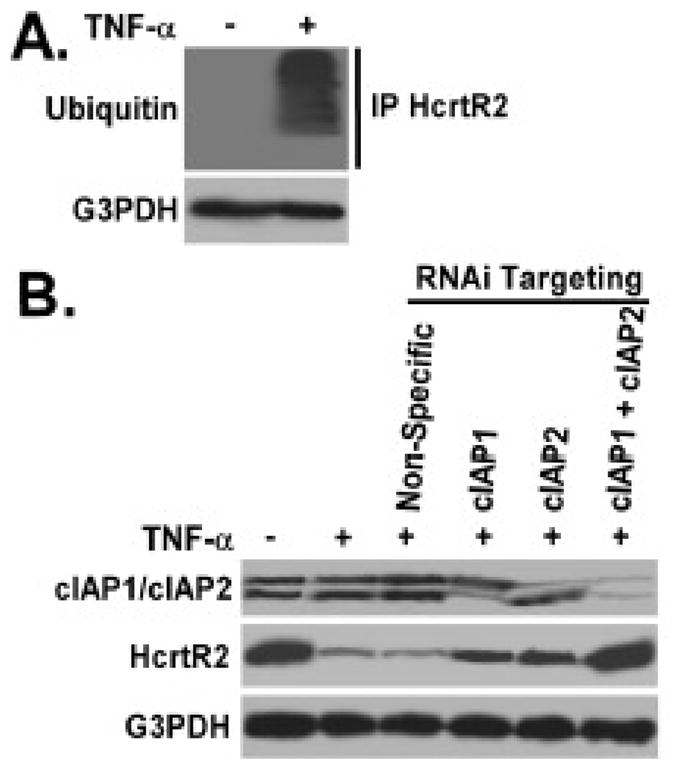
Panel A: B35 cells overexpressing the HcrtR2 were cultured in the presence and absence of TNF-α cytokine (2 ng/ml) for 6 hours at 37°C. Cells were lysed, and equivalent amount of whole cell detergent lysates were immunoprecipitated (IP) by anti-HcrtR2 IgG, followed by Western blot analysis with the indicated antibodies. Panel B: B35 cells constitutively expressing the HcrtR2 were transfection with siRNA directed toward cIAP-1 or/and cIAP-2 as described in Materials and Methods 2.4, then cultured in the presence and absence of TNF-α cytokine (2 ng/ml) for 24 hours at 37°C. Cells were harvested, and equivalent amount of whole cell detergent lysates were Western blotted with the indicated antibodies. Experiment was repeated three times, and representative images are shown.
4. DISCUSSION
4.1 The Hypocretin system is impaired by TNF-α treatment
In the present study, we describe and characterize a new function of TNF-α as an inhibitory regulator of the Hypocretin system. The effect of TNF-α treatment on Hypocretin system was studied by examining the expression (mRNA and protein levels) of two key components of the Hypocretin system, the Prepro-Hypocretin and HcrtR2. Prepro-Hypocretin is the common precursor of both Hypocretin-1 and Hypocretin-2. HcrtR2 was selected because HcrtR2 is the major Hypocretin receptor according to findings in animal studies [30].
Our data show that TNF-α treatment decreases the mRNA and protein levels of Prepro-Hypocretin through enhancing the decay of Prepro-Hypocretin mRNA. TNF-α treatment also decreases the expression of HcrtR2 through the ubiquitination pathway. cIAP-1 and cIAP-2 are two key mediators of the HcrtR2 ubiquitination pathway induced by TNF-α, and downregulation of both cIAP-1 and cIAP-2 are required to abrogate the TNF-α-induced ubiquitination of HcrtR2. These data for the first time provide evidence that TNF-α functions as a negative regulator of the Hypocretin system, suggesting a potential mechanism by which TNF-α is involved in the pathogenesis of disorders caused by an impaired Hypocretin system.
4.2 TNF-α downregulates Prepro-Hypocretin through increasing Prepro-Hypocretin mRNA degradation
In response to TNF-α treatment, both Prepro-Hypocretin protein and mRNA levels are significantly decreased (by 93% and 94%, respectively) when compared to that in cells treated with vehicle only. The TNF-α-induced Prepro-Hypocretin reduction is likely the result of the increased decay/degradation of mRNA, since the half-life of Prepro-Hypocretin mRNA is decreased in TNF-α-treated cells. It has been previously demonstrated that TNF-α can negatively regulate gene expression. TNF-α treatment (10 ng/ml) decreases the expression of both endothelial nitric oxide synthase and argininosuccinate synthase in aortic endothelial cells [31]. It is also known that TNF-α regulates mRNA stability through modulation of the expression of the AU-rich element (ARE) binding proteins [32]. ARE binding proteins, such as tristetraprolin, target the mRNAs containing an ARE in the 3′ untranslated region, and rapidly degrade mRNAs through mRNA decay machinery {616,669). It is possible that TNF-α regulates the stability of Prepro-Hypocretin mRNA through ARE-binding proteins, and the exact mechanism will be the focus of future study.
4.3 TNF-α downregulates HcrtR2 through cIAP-mediated uniquitination
Our data also demonstrate that TNF-α decreases HcrtR2 through the ubiquitination pathway, and this pathway is mediated by cIAP-1 and cIAP-2. This is evidenced by that downregulation of either cIAP-1 or cIAP-2 can only partially block the inhibitory effect of TNF-α on HcrtR2 expression. Downregulation of both cIAP-1 and cIAP-2 is required to abrogate the inhibitory effect of TNF-α on HcrtR2 expression, and to restore the HcrtR2 level. cIAP-1 and cIAP-2 are binding partners of the TNF-associated factors 1 and 2 {877}. cIAPs are important regulators of cell survival and death signaling, as cIAPs directly bind caspases and inhibit caspase-mediated cell death signaling [33]. Recent data demonstrate that cIAP-1 and cIAP-2 also function as E3 ligases, and the association of E3 ligase with target proteins is a critical step of the E ligase cascade before protein ubiquitination [25]. It has been demonstrated that cIAP-1 and cIAP-2 are required for the ubiquitination of the receptor interacting protein (RIP) involved in TNF signaling [25]. Based on these data, we speculate that cIAP-1 and cIAP-2 function as E3 ligases and mediate the HcrtR2 ubiquitination induced by TNF-α, and this remains to be elucidated in future.
4.4 TNF-α, Hypocretin system, and sleep disorders
TNF-α is well known for its role in inflammation, host defense, and pathogenesis of various diseases. Published data indicate that TNF-α is involved in regulation of sleep-wakefulness behavior and fatigue during infections and depression [14,17,20,22]. Elevated plasma TNF-α level has been reported in patients with excessive daytime sleepiness, such as obstructive sleep apnea, idiopathic hypersomnia [18–20,34]. Elevated TNF-α level is also detected in patients with narcolepsy; but, this finding is not supported by others [14,17–19]. Narcolepsy patients have excessive daytime sleepiness [35,36]. Animal studies convincingly support a role of TNF-α signaling in regulation of sleep and wakefulness. Data obtained from rabbits or rodents demonstrate that loss of TNF-α signaling decreases or suppresses sleep [37–39], while exogenous TNF-α administration increases sleep [40,41]. Published data also demonstrate that serum TNF-α level increases by an average of 8% for each hour reduction in sleep [42], further supporting an association between TNF-α signaling and the sleep-wake cycle regulation.
Our data suggest that TNF-α can be involved in the development of sleep and other related disorders by impairing the function of the Hypocretin system. The Hypocretin system can be impaired in situations with transiently or persistently increased TNF-α level. The Hypocretin system is considered as a critical regulator of sleep/wakefulness cycle, feeding behavior, emotion, and rewarding system [8–10]. Prepro-Hypocretin knockout mice develop a phenotype similar to narcolepsy, such as fragmented sleep-wake cycles [8]. Hypocretin-1 level in cerebrospinal fluid is decreased in narcolepsy patient with cataplexy [43,44]. Higher body mass index is associated with sleep disorders (such as narcolepsy and severe obstructive sleep apnea), as well as increased plasma TNF-α level [45–49]. The potential role of Hypocretin in treating breathing disorders associated with sleep has also been proposed [50,51]. So far, very little is known about the mechanism(s) by which Hypocretin system is regulated during these disorders. Our data show that the Hypocretin system can be impaired by TNF-α. Whether this finding could be related to the excessive daytime sleepiness in patients remains to be elucidated.
Our limitation is that the majority of the work was performed in an overexpressed system. Similar results were also obtained by using the endogenous system; however, due to technical reasons, the mechanisms were further elucidated with the overexpressed system. Other publications also use this strategy (overexpression) to delineate the physiological effects of Hypocretin/Orexin receptors in human embryonic kidney (HEK) 293 cells or in Chinese hamster ovary (CHO) cells [52–54]. Using primary cells that express significant amount of Hypocretin/Orexin or receptors has been considered; however, this is a big challenge as the number of cells in brain express Hypocretin/Orexin is very limited [1,2], and these primary cells are difficult to maintain in vitro.
4.5 Conslusions
In conclusion, our work for the first time provides evidence that the Hypocretin system can be impaired by TNF-α directly. TNF-α treatment decreases the expression of both Prepro-Hypocretin and HcrtR2 either through enhancing the decay of Prepro-Hypocretin mRNA or through HcrtR2 ubiquitination mediated by cIAP-1 and cIAP-2. Our findings suggest a potential mechanism by which TNF-α is involved in the pathogenesis of disorders caused by an impaired Hypocretin system, and add a new dimension to the biological activities of TNF-α that may have important implications in health care and diseases.
Research Highlights.
This manuscript for the first time provides evidence that the Hypocretin system can be impaired by TNF-α directly. TNF-α treatment decreases the levels of both Prepro-Hypocretin and Hypocretin receptor 2, either through enhancing the mRNA decay or through protein ubiquitination. The findings suggest a potential mechanism by which TNF-α is involved in the pathogenesis of sleep disorders caused by an impaired Hypocretin system, and add a new dimension to the biological activities of TNF-α that may have important implications in health care and diseases.
Acknowledgments
This work was supported by awards from NIH/NHLBI grants HL085324 and HL095451 (Q.D.), and from American Heart Association, Greater Southeast Affiliate Research Program (G.Q.C.). We thank Dr. Susan Marie Harding Hawkins (University of Alabama at Birmingham) for insightful discussion and comment.
Abbreviations
- TNF-α
tumor necrosis factor alpha
- HcrtR2
Hypocretin receptor 2
- cIAP
cellular inhibitor of apoptosis protein
- FAK
focal adhesion kinase
- G3PDH
glyceraldehyde 3-phosphate dehydrogenase
- RT-PCR
reverse transcriptase polymerase chain reaction
- MSCV vector
murine stem cell viral vector
- GFP
green-fluorescent-protein
- mRNA
messenger RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Lecea L, Kilduff T, Peyron C, Gao X, Foye P, Danielson P, Fukuhara C, Battenberg E, Gautvik V, Bartlett F, Frankel W, van den Pol A, Bloom F, Gautvik K, Sutcliffe J. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli R, Tanaka H, Williams S, Richardson J, Kozlowski G, Wilson S, Arch J, Buckingham R, Haynes A, Carr S, Annan R, McNulty D, Liu W, Terrett J, Elshourbagy N, Bergsma D, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Johren O, Neidert S, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- 4.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 5.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamantidis A, Zhang F, Aravanis A, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez C, Sutcliffe J. Hypocretin is an early member of the incretin gene family. Neurosci Lett. 2002;324:169–172. doi: 10.1016/s0304-3940(02)00195-7. [DOI] [PubMed] [Google Scholar]
- 8.Chemelli R, Willie J, Sinton C, Elmquist J, Scammell T, Lee C, Richardson J, Williams S, Xiong Y, Kisanuki Y, Fitch T, Nakazato M, Hammer R, Saper C, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 9.Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong P, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 11.Willie J, Chemelli R, Sinton C, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 12.de Lecea L. A decade of hypocretins: past, present and future of the neurobiology of arousal. Acta Physiol (Oxf) 2010;198:203–208. doi: 10.1111/j.1748-1716.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 13.Popa C, Netea M, van Riel P, van der Meer J, Stalenhoef A. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Berthold-Losleben M, Himmerich H. The TNF-alpha system: functional aspects in depression, narcolepsy and psychopharmacology. Curr Neuropharmacol. 2008;6:193–202. doi: 10.2174/157015908785777238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger K, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carswell E, Old L, Kassel R, Green S, Fiore N, Williamson B. An endotoxin–induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himmerich H, Beitinger P, Fulda S, Wehrle R, Linseisen J, Wolfram G, Himmerich S, Gedrich K, Wetter T, Pollmacher T. Plasma levels of tumor necrosis factor alpha and soluble tumor necrosis factor receptors in patients with narcolepsy. Arch Intern Med. 2006;166:1739–1743. doi: 10.1001/archinte.166.16.1739. [DOI] [PubMed] [Google Scholar]
- 18.Okun M, Giese S, Lin L, Einen M, Mignot E, Coussons-Read M. Exploring the cytokine and endocrine involvement in narcolepsy. Brain Behav Immun. 2004;18:326–332. doi: 10.1016/j.bbi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Vgontzas A, Papanicolaou D, Bixler E, Kales A, Tyson K, Chrousos G. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 20.Eller T, Aluoja A, Maron E, Vasar V. Soluble interleukin-2 receptor and tumor necrosis factor levels in depressed patients in Estonia. Medicina (Kaunas) 2009;45:971–977. [PubMed] [Google Scholar]
- 21.Pan W, Kastin A. Adipokines and the blood-brain barrier. Peptides. 2007;28:1317–1330. doi: 10.1016/j.peptides.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollmacher T, Schuld A, Kraus T, Haack M, Hinze-Selch D, Mullington J. Experimental immunomodulation, sleep, and sleepiness in humans. Ann N Y Acad Sci. 2000;917:488–499. doi: 10.1111/j.1749-6632.2000.tb05413.x. [DOI] [PubMed] [Google Scholar]
- 23.Ding Q, Gladson C, Wu H, Hayasaka H, Olman M. FAK-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem. 2008;283:26839–26849. doi: 10.1074/jbc.M803645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Q, Stewart J, Jr, Olman M, Klobe M, Gladson C. The pattern of enhancement of Src kinase activity on platelet-derived growth factor stimulation of blioblastoma cells is affected by the integrin engaged. Journal of Biological Chemistry. 2003;278:39882–39891. doi: 10.1074/jbc.M304685200. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand M, Milutinovic S, Dickson K, Ho W, Boudreault A, Durkin J, Gillard J, Jaquith J, Morris S, Barker P. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Ding Q, Grammer J, Nelson M, Guan J, Stewart J, Jr, Gladson C. p27(Kip1) and cyclin D1 are necessary for focal adhesion kinase (FAK) regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. Journal of Biological Chemistry. 2005;280:6802–6805. doi: 10.1074/jbc.M409180200. [DOI] [PubMed] [Google Scholar]
- 27.Mignot E, Lammers G, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 28.Parsons J. Focal adhesion kinase: the first ten years. Journal of Cell Science. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 29.Cai G, Zheng A, Tang Q, White E, Chou C, Gladson C, Olman M, Ding Q. Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp Cell Res. 2010;316:1600–1609. doi: 10.1016/j.yexcr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willie J, Chemelli R, Sinton C, Tokita S, Williams S, Kisanuki Y, Marcus J, Lee C, Elmquist J, Kohlmeier K, Leonard C, Richardson J, Hammer R, Yanagisawa M. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin B, Pendleton L, Levy M, Solomonson L, Eichler D. Tumor necrosis factor-alpha reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1115–H1121. doi: 10.1152/ajpheart.01100.2006. [DOI] [PubMed] [Google Scholar]
- 32.Stephens J, Carter B, Pekala P, Malter J. Tumor necrosis factor alpha-induced glucose transporter (GLUT-1) mRNA stabilization in 3T3-L1 preadipocytes. Regulation by the adenosine-uridine binding factor. J Biol Chem. 1992;267:8336–8341. [PubMed] [Google Scholar]
- 33.Roy N, Deveraux Q, Takahashi R, Salvesen G, Reed J. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiropoulos P, Kotsianidis I, Nena E, Tsara V, Gounari E, Hatzizisi O, Kyriazis G, Christaki P, Froudarakis M, Bouros D. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep. 2009;32:537–543. doi: 10.1093/sleep/32.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ICSD International Classification of Sleep Disorders. 2. Westchester: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 36.Overeem S, Black J, III, Lammers G. Narcolepsy: immunological aspects. Sleep Med Rev. 2008;12:95–107. doi: 10.1016/j.smrv.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang J, Wang Y, Krueger J. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci. 1997;17:5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deboer T, Fontana A, Tobler I. Tumor necrosis factor (TNF) ligand and TNF receptor deficiency affects sleep and the sleep EEG. J Neurophysiol. 2002;88:839–846. doi: 10.1152/jn.2002.88.2.839. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S, Tooley D, Kapas L, Fang J, Seyer J, Krueger J. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep. Pflugers Arch. 1995;431:155–160. doi: 10.1007/BF00410186. [DOI] [PubMed] [Google Scholar]
- 40.Shoham S, Davenne D, Cady A, Dinarello C, Krueger J. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- 41.Kapas L, Hong L, Cady A, Opp M, Postlethwaite A, Seyer J, Krueger J. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. Am J Physiol. 1992;263:R708–R715. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- 42.Patel S, Zhu X, Storfer-Isser A, Mehra R, Jenny N, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–204. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mignot E, Lammers G, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 44.Dauvilliers Y, Baumann C, Carlander B, Bischof M, Blatter T, Lecendreux M, Maly F, Besset A, Touchon J, Billiard M, Tafti M, Bassetti C. CSF hypocretin-1 levels in narcolepsy, Kleine-Levin syndrome, and other hypersomnias and neurological conditions. J Neurol Neurosurg Psychiatry. 2003;74:1667–1673. doi: 10.1136/jnnp.74.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–1275. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 46.Schuld A, Beitinger P, Dalal M, Geller F, Wetter T, Albert E, Hebebrand J, Pollmacher T. Increased body mass index (BMI) in male narcoleptic patients, but not in HLA-DR2-positive healthy male volunteers. Sleep Med. 2002;3:335–339. doi: 10.1016/s1389-9457(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Xiong K, Lian Y, Huang J, Zhao M, Li J, Liu C. Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath. 2010 doi: 10.1007/s11325-010-0337-4. [DOI] [PubMed] [Google Scholar]
- 48.Shah N, Roux F. The relationship of obesity and obstructive sleep apnea. Clin Chest Med. 2009;30:455–65. vii. doi: 10.1016/j.ccm.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Pollmacher T. TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw. 2006;17:196–201. [PubMed] [Google Scholar]
- 50.Gestreau C, Bevengut M, Dutschmann M. The dual role of the orexin/hypocretin system in modulating wakefulness and respiratory drive. Curr Opin Pulm Med. 2008;14:512–518. doi: 10.1097/MCP.0b013e32831311d3. [DOI] [PubMed] [Google Scholar]
- 51.Williams R, Burdakov D. Hypothalamic orexins/hypocretins as regulators of breathing. Expert Rev Mol Med. 2008;10:e28. doi: 10.1017/S1462399408000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang J, Chen J, Ramanjaneya M, Punn A, Conner A, Randeva H. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Holmqvist T, Akerman K, Kukkonen J. High specificity of human orexin receptors for orexins over neuropeptide Y and other neuropeptides. Neurosci Lett. 2001;305:177–180. doi: 10.1016/s0304-3940(01)01839-0. [DOI] [PubMed] [Google Scholar]
- 54.Smart D, Jerman J, Brough S, Rushton S, Murdock P, Jewitt F, Elshourbagy N, Ellis C, Middlemiss D, Brown F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]