Abstract
Objective
The optimal method for glycemic control in the critically burned patient is unknown. The purpose of this randomized controlled study was to determine the safety and efficacy of computer decision support software (CDSS) to control serum glucose concentration in a burn intensive care unit.
Methods
Eighteen adult burn/trauma patients receiving continuous insulin infusion were initially randomized to receive glucose management via a traditional paper-based protocol (PP) or a computer protocol (CP) for 72 hours, then crossed over to the alternate method for an additional 72 hours.
Results
Time in target glucose range (80-110 mg/dl) was higher in the CP group (47 ± 17% versus 41 ± 16.6%; p ≤ 0.05); time over target range was not significantly reduced in the CP group (49 ± 17.8% versus 54 ± 17.1; p = 0.08); and no difference was noted in time under target range of 80 mg/dl (CP 4.5 ± 2.8, PP 4.8 ± 3.3%; p = 0.8), under 60 mg/dl (p = 0.7), and under 40 mg/dl (p = 1.0). Severe hypoglycemic events (< 40 mg/dl) did not differ from the CP group compared to historical controls for patients receiving no insulin (p = 0.6). More glucose measurements were performed in the CP group (p = 0.0003), and nursing staff compliance with CP recommendations was greater (p < 0.0001).
Conclusions
Glycemic control using CDSS is safe and effective for the critically burned patient. Time in target range improved without increase in hypoglycemic events. CDSS enhanced consistency in practice, providing standardization among nursing staff.
Keywords: Glycemic control, hypoglycemia, computer decision support
Introduction
The stress response associated with severe burn incites a hyperglycemic state linked with negative outcomes for the burned patient. Glycemic control is associated with reduction in nosocomial infection [1, 2], skin graft loss [3], muscle catabolism [4], and mortality [5, 6] in the burn population. We previously showed the widespread adoption of intensive insulin therapy [7, 8] among American Burn Association burn centers [9], yet no published reports describe an efficacious method to consistently achieve tight glycemic control in the critically burned. The severely burned often undergo extended hospital courses, experience frequent bouts of septic events and become resistant to insulin over time [10]. Extremes in glycemic variability are difficult to manage, and achieving consistent time in target glucose range is a challenge.
Bedside nurses are routinely charged with managing insulin infusions in the intensive care unit (ICU) although they have varying degrees of expertise and educational backgrounds. Commonly, the manual paper protocols used within an ICU are too conservative to tightly control (80-110 mg/dl) glucose levels for seriously ill burn patients. Thus, nurses will often begin manual titration of insulin infusion based on experience in an attempt to bring the glucose levels within target range. Arbitrary glycemic management may result in improved control yet fail to be reproducible among staff members while significantly increasing risk for serious hypoglycemic events. Glycemic control is time-intensive for nursing staff and the ability to safely minimize the frequency of nursing interventions for management of insulin titration can ease nursing workload [11]. Furthermore, these “home-grown” protocols often lean to the side of safety (hyperglycemia), keeping patients out of the desired glycemic range.
The advent of computer decision support software (CDSS) offers the possibility of improved control and standardization of insulin therapy within the burn unit. CDSS systems provide the ability to predict insulin requirements based on patient-specific response to previous doses as a function of their unique insulin resistance pattern. Such systems [12-15] demonstrated safety and efficacy in achieving glycemic control in various medical, surgical, and cardiac critical care populations; yet no data supports safety or efficacy of any computerized dosing software specifically for the burn population. To control extreme glycemic excursion noted in our burn population, we selected a CDSS (EndoTool®, Hospira, Lake Forrest, IL) to conduct a performance improvement project to determine best practice for our unit. The purpose of this study was to compare our current paper-based nomogram for insulin titration to the EndoTool® CDSS to establish the safest and most effective method of managing intensive insulin therapy in the burn ICU.
Materials and Methods
Study Design
The design of this study was a prospective, paired randomization crossover trial. Approval for this study was granted by the Brooke Army Medical Center Institutional Review Board for Protection of Human Subjects, and informed consent was obtained for all participants.
Study Location
This study was conducted in a 16-bed regional adult burn center ICU responsible for the care of both military and civilian burn patients. Care in this unit was provided by nurses with a variety of educational and intensive care experience levels ranging from licensed vocational nurses (LVNs) to registered nurses (RNs). Professional educational levels ranged from certificate or associate degree for LVNs to diploma, associate, and baccalaureate degrees for RNs. All RNs have a minimum of 1 year of ICU experience; however, LVNs may come directly to the burn ICU from military training programs. Duration of burn experience also varied among the nursing staff, from new hires with no burn ICU experience to staff members who have worked in our unit for over 10 years.
Study Population
All burn ICU patients were screened for enrollment. Inclusion criteria limited eligibility to thermally injured adult patients who were expected to require exogenous insulin infusion for at least 6 days at any time during their ICU stay. Legal authorized representatives (LARs) for all patients on continuous feeds who required insulin infusion were approached for enrollment approval. Patients were excluded if they were less than 18 years of age, pregnant, a prisoner, or if informed consent was not able to be obtained.
Study Protocol
Eligible patients were sequentially enrolled and randomized in pairs to initially begin either the standard of care paper protocol (PP) or EndoTool® computer protocol (CP) for glycemic management. After 72 hours in the initial arm, insulin titration was switched to the alternate method for an additional 72 hours. To achieve paired randomization, upon enrollment a patient was randomly assigned to an initial group, and the next patient enrolled was assigned to the other group for initial management. Control of bias and reduction of threats to internal validity were achieved by using the randomized cross-over design, and paired randomization mitigated order effect. No carry-over effect is assumed because of the short half life of intravenous insulin infusion. Requirement for insulin infusion was based solely on clinical criteria and discontinued when patients were able to tolerate a regular diet. No attempt was made to maintain insulin infusion for a study patient without hyperglycemia; only data for participants who completed both arms of the trial was analyzed.
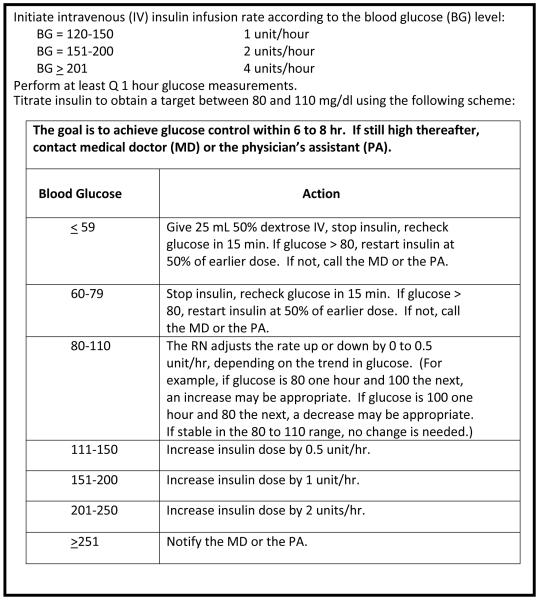
Insulin therapy was initiated if two hourly serum glucose measurements exceeded 150 mg/dl; then hourly blood glucose measurements guided insulin titration per protocol. A nurse-managed manual protocol targeting a glucose range of 80 to 110 mg/dl (Figure 1), developed by burn center staff surgeons and standard of care in our ICU since 2004, was used for the PP arm.
Figure 1.
Paper Protocol (PP) insulin titration
An EndoTool® insulin dosing CDSS package for acute glycemic control was installed on each bedside computer terminal in the burn ICU. Individual applications of the EndoTool® software were networked through the hospital information technology infrastructure, and all data were stored on a central structured query language (SQL) server database. Face-to-face training was provided by the vendor for over 90% of the nursing staff with subsequent competency validation; a 2-month limited trial period preceded prospective study enrollment. Telephonic vendor support was available to staff around the clock, and additional training for several nursing staff advanced users provided further clinical resources.
Significant flexibility exists within the software to allow the medical director to select a unit-specific target glucose range coupled with multiple dosing and safety parameters. The CDSS software was programmed for a target glucose range of 80 to 110 mg/dl, dosing of concentrated dextrose at values less than 70 mg/dl, and restriction to a maximum of hourly glucose measurements. The software will recommend more frequent measurements (30 minutes) when glucose values are low and when the application is initiated. The EndoTool® software requires entry of the current glucose value, current insulin infusion rate, and any additional caloric supplementation since the previous glucose measurement. The program calculates the appropriate insulin rate based on the course of five previous glucose values. If the associated parameter is activated, the system will recommend the time for subsequent glucose measurement of 1 to 3 hours relative to mathematical stability of the glucose trend (we selected 1 hour maximum for study purposes). If the entered glucose value is under a minimum value set by the medical director (we selected 60 mg/dl), a weight-based dose of concentrated dextrose is recommended relative to the degree of hypoglycemia. For both the PP and the CP, the nursing staff was not constrained to accept the recommended insulin infusion rate but rather to appropriately titrate according to the complete clinical scenario.
Glucose measurements were performed at bedside by nursing staff with the SureStep® Flexx® (LifeScan, Milpitas, CA) point-of-care glucometers with subsequent mathematical correction for the effect of hematocrit [16]. The nursing staff manually recorded the following on study data collection instruments: hourly glucose measurements, insulin rates recommended by the EndoTool® program or calculated with the paper nomogram, actual infused insulin rate based on the individual nurse’s clinical judgment, and nutritional intake. The research staff reviewed documented blood glucose daily to identify hypoglycemic events. A retrospective chart review of the electronic medical record (Essentris™, CliniComp International, San Diego, CA) was performed to determine demographic data and infectious processes and validate prospectively collected data.
Outcomes Measured
The primary endpoint of this study was time in target glucose range of 80 to 110 mg/dl. Glucose measurements were performed every hour during the study period, and calculated time at a given glucose level was estimated as half the interval between measurements. For example, if a measurement of 80 mg/dl for 1 hour was followed by a measurement of 140 mg/dl for the second hour, 30 minutes would be considered in target range and 30 minutes as over target range. Secondary endpoints were time under and over target range and hypoglycemic events less than 60 mg/dl and 40 mg/dl (the level at which neurocognitive deficits may occur [16]). The percentage of hypoglycemic events is reported as number of events/total glucose measurements for the study group. Historic rates of hypoglycemic events were calculated for the period 2002 to 2008 for patients receiving PP (n=239) and from 2002 to 2007 for patients not receiving insulin therapy (n = 783), compared to patients receiving insulin infusions titrated with EndoTool® from January to September 2009 (n = 79). We also calculated mean glucose values with standard deviation, amount of caloric supplementation, and administered exogenous insulin. Potential confounders of glycemic control such as caloric intake, total insulin infused, and presence of infection were compared to ensure homogeneity between groups. Additionally, the number of recommendations accepted by the nursing staff was compared between groups to identify consistency in delivery of insulin therapy.
Sample Size
Sample size determination was based on historical data showing that glucose values using the paper nomogram were out of the 80- 110-mg/dl target range 50% (± 8%) of the time. Sample size for the study required a total of 24 patients; it was calculated using SAS 9.1 (SAS Institute Inc, Cary, NC) for a reduction of time spent out of range by 10% for a crossover design with no carryover effect, an alpha of 0.05, and a power of 0.8.
Randomization Procedure
A computer-generated random number list was used to ensure equal probability of assignment of patients to study group. Assignments were made by the research assistant after informed consent was obtained and immediately prior to initiating protocol.
Statistical Analysis
Serial data analyses were planned a priori to identify safety concerns and to determine the efficacy of achieving glycemic targets. On detection of statistical differences in rates of severe hypoglycemia (glucose values less than 40 mg/dl) or superiority of one method to achieve target glucose range, prospective enrollment would be halted. Statistical analysis was performed with SAS 9.1 using paired Student’s t-test for normally distributed data, Mann-Whitney U-test or Wilcoxon Signed-Ranks test for non-normal data, and chi square analysis for categorical data. Statistical significance was accepted for a two-tailed p-value ≤ 0.05. Data are reported as means ± standard deviations.
Results
From March to November 2008, 67 patients were screened for enrollment. Of these patients, 24 met eligibility requirements although two LARs declined participation on behalf of the patients (Figure 2). The remaining 22 patients were prospectively enrolled in the study. Exogenous insulin administration was discontinued for clinical indications for four patients prior to study completion; all were transferred to the step-down burn unit. Reported data represent the 18 patients who completed both study arms. Patients were enrolled within 14 ± 4 days (range 2-89 days) of admission to the burn ICU with 14 patients enrolled within 10 days of ICU admission. No protocol violations affecting study results occurred. Average length of stay in the burn ICU was 64 ± 50 days (range 15 to 227). Randomization resulted in initially starting 10 subjects on CP (EndoTool®) and 8 subjects on the PP. Order effect analysis was conducted using analysis of variance; no significant difference was noted between groups regarding which protocol was initially started.
Figure 2.
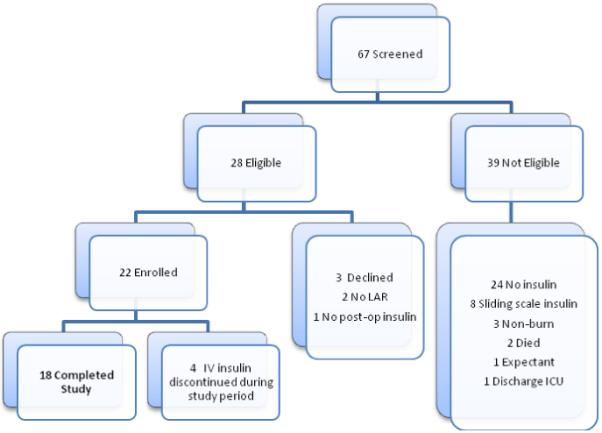
Enrollment consort diagram (LAR indicates Legal Authorized Representative; ICU, Intensive Care Unit; IV, intravenous)
Demographic data are presented in Table 1. All patients sustained thermal injury, an average of 43 ± 19% total body surface area (TBSA) burn with a mean 25 ± 20% full-thickness injury and eight inhalation injuries identified by bronchoscopy. The mean injury severity score of 25 ± 9 coupled with relatively large thermal burns demonstrates a significantly injured study cohort. Eleven active duty military and seven civilian patients were enrolled. An equal number of septic events (n = 11) occurred in the CP and PP arms, defined as hemodynamic instability requiring vasopressor support in the presence of positive blood or respiratory cultures. Four patients received exogenous corticosteroid during both arms of the study for adrenal insufficiency; two other patients developed adrenal insufficiency after completion of study period. All patients in the study were supplemented with a continuous feeding regimen of a high-protein, low-fat, semi-elemental enteric formula when tolerated. Two study patients required nutritional support with total parenteral nutrition (TPN) in the equivalent proportion of protein and fat as enteric feeds. No insulin was added to the TPN solution. Caloric content for both feeding formulas approximated 1 calorie per milliliter of solution.
Table 1.
Patient Demographics
(TBSA = total body surface area; ICU indicates intensive care unit; IDDM, insulin-dependent diabetes mellitus; NIDDM, non-insulin-dependent diabetes mellitus; SD, standard deviation).
| Variable | Total | Civilian | Military |
p value* |
|---|---|---|---|---|
| n = 18 mean ± SD |
n = 7 mean ± SD |
n = 11 mean ± SD |
||
| Age (years) | 32 ± 16.6 | 48 ± 16.7 | 22 ± 3 | 0.01 |
| % TBSA burn | 43 ± 19.1 | 38 ± 11.4 | 46 ± 22.8 | 0.41 |
| % TBSA full thickness | 25 ± 20.2 | 18 ± 15.2 | 30 ± 22.4 | 0.23 |
| No. of patients with positive inhalation injury |
8 (44.4%) | 2 (28.6%) | 6 (54.5%) | 0.37 |
| Injury severity score | 25 ± 9.1 | 19 ± 5.1 | 28 ± 9.6 | 0.04 |
| Body mass index | 26 ± 3.2 | 30 ± 3 | 25 ± 2.9 | 0.09 |
| ICU days | 64 ± 50 | 51 ± 34.6 | 73 ± 57.4 | 0.53 |
| Admission hemoglobin A1c | 5.6 ± 0.4 | 5.7 ± 0.5 | 5.6 ± 0.4 | 0.59 |
| No. of patients with acute adrenal insufficiency in ICU |
6 (33.3%) | 4 (57.1%) | 2 (18.2%) | 0.09 |
| No. of patients in-hospital death | 4 (22.2%) | 3 (42.9%) | 1 (9.1%) | 0.09 |
| No. of patients with a history of IDDM/NIDDM |
0 | 0 | 0 | N/A |
Civilian compared to military
A significant difference was noted in the primary endpoint of this study, time in target glucose range of 80 to 110 mg/dl. During the CP arm of the study, patients spent 47 ± 17% of time in target range compared to 41 ± 16.6% of time for the PP arm (p ≤ 0.05); time over target range was not significantly reduced in the CP group (49 ± 17.8 % versus 54 ± 17.1%; p = 0.08); and time under 80 mg/dl was similar between groups (CP 4.5 ± 2.8% versus PP 4.8 ± 3.3%; p = 0.8), as was time less than 60 mg/dl (0.6 ± 1.2% for CP and 0.5 ± 1.2% for PP; p = 0.7) (Figure 3). Three patients experienced glucose levels of less than 40 mg/dl during the study period, two of the patients died while in the ICU (one experienced hypoglycemia while on both the PP and CP; the other only while on CP) and one survivor who experienced a glucose value < 40 mg/dl during the PP. Thus, a total of 4 events of hypoglycemia (< 40 mg/dl) occurred, 2 events in each study arm. No adverse clinical events were noted for any episode of low blood glucose.
Figure 3.
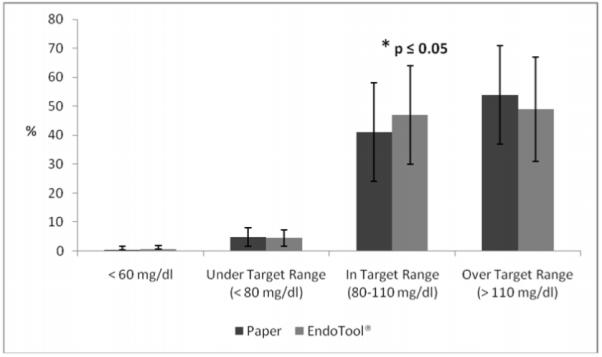
Time under, in, and over target glucose range of 80 to 110 mg/dl for each study group for CDSS and manual paper based protocol.
Comparison of EndoTool® hypoglycemic rates to historical events of glucose < 80, 60, and 40 mg/dl for patients receiving insulin titrated using traditional PP resulted in a significant difference between all measures (p < 0.001, p <0.001, and p = 0.03 respectively) (Table 2). Hypoglycemic events < 80, 40, or 60 mg/dl for patients who were managed with EndoTool® were not different from historic rates for patients receiving no insulin therapy (p = 0.1, 0.06, and 0.6 respectively). This analysis demonstrates that the CP has significantly lower incidence of glucose values < 80, 60 and 40 mg/dl than patients who require insulin managed using PP. Additionally, although a baseline incidence of hypoglycemia occurs in patients who do not receive insulin, use of the computer software confers no additional risk of low blood sugar. From January to December 2009 23/43,446 (0.05%) incidents of glucose values < 40 mg/dl were recorded using the CP.
Table 2a.
Historic review of hypoglycemic rates among patients managed with EndoTool (ET), manual paper protocol (PP), and no insulin therapy. (CI indicates 95% confidence interval; IV, intravenous; RR, risk ratio)
| Glucose mg/dl | ET n = 79 |
Insulin IV n = 239 |
No Insulin n = 783 |
|---|---|---|---|
| < 80, No.(%) | 905 (4.6%) | 2187 (8.1%) | 212 (4.1%) |
| < 60, No.(%) | 134 (0.68%) | 344 (1.3%) | 23 (0.4%) |
| < 40, No.(%) | 18 (0.09%) | 45 (0.17%) | 3 (0.06%) |
| b: | |||
|---|---|---|---|
| Glucose | ET v. PP | ET v. No Insulin | PP v. No Insulin |
| < 80 mg/dl | p<0.001 RR 0.56 (CI 0.52, 0.61) |
p=0.13 RR 1.12 (CI 0.97, 1.3) |
p<0.001 RR 0.51 (CI 0.44, 0.58) |
| < 60 mg/dl | p<0.001 RR 0.53 (CI 0.44, 0.65) |
p=0.058 RR 1.53 (CI 0.99, 2.37) |
p<0.001 RR 0.35 (CI 0.23, 0.53) |
| < 40 mg/dl | p=0.03 RR 0.55 (CI 0.38, 0.94) |
p=0.6 RR 1.57 (CI 0.5, 4.99) |
p=0.06 RR 0.35 (CI 0.11, 1.05) |
The mean glucose for the CP arm was significantly lower than that of the PP arm (113 ± 10.2 mg/dl versus 119 ± 14 mg/dl, p = 0.02). By patient, standard deviations were not different in the CP and PP groups representing non-significant glycemic excursion, 23 ± 7 mg/dl versus 27 ± 10.7 mg/dl; p = 0.1). Analysis of glycemic control by patient revealed that 13 of 18 patients had better control in the CP arm than in the paper arm (p = 0.02) (Figure 4). Furthermore, eight patients maintained mean glucose in the target 80 to 110 mg/dl range during the CP arm compared to only four patients in the alternate arm. No difference was noted in the amount of insulin or total calories between the two study arms (Table 3).
Figure 4.
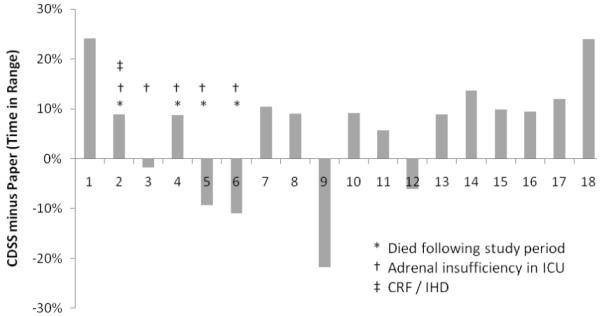
Percentage of time each subject spent in target glucose range of 80 to 110 mg/dl; CDSS time percent in range minus paper protocol time percent in range. Most patients (n = 13) had more time in target range using CDSS. (CRF indicates chronic renal failure; IHD, intermittent hemodialysis)
Table 3.
Comparison of septic events, caloric intake, infused insulin, and number of glucose measurements between study groups
[Expressed as a number or mean ± SD (u indicates units; ml, milliliter; feed, parenteral and enteral; hr, hours)]
| Variable | Computer Protocol (CP) | Paper Protocol (PP) | P Value |
|---|---|---|---|
| No. of septic events | 11 | 11 | 1.0 |
| Insulin rate / subject (unit/hr) | 6.6 ± 6.7 | 6.2 ± 5.6 | 0.71 |
| Unit of insulin infused/subject | 470 ± 484 | 437 ± 402 | 0.61 |
| ml feed/subject | 7054 ± 3442 | 7303 ± 2759 | 0.70 |
| Units of insulin : ml feed / subject* | 0.08 ± 0.09 | 0.06 ± 0.04 | 0.29 |
| No. of glucose measurements/72 hr | 73 ± 3 | 67 ± 3.7 | <0.001 |
Ratio expressed is total units of insulin infused divided by the total amount of feed administered.
More glucose measurements per patient were performed in the CP group (73 ± 3, n = 1319) than in the PP group (67 ± 3.8, n = 1210; p = 0.0003). Finally, the nursing staff complied with the insulin infusion calculation from the PP 39 ± 15% of the time and accepted the CDSS recommendations 82 ± 17.2% of the time (p < 0.0001). To ensure compliance was not due to treatment effect all CP glucose measurements from January to December 2009 were reviewed revealing 5,769/43,446 (86.7%) recommendations were accepted by the nursing staff.
Discussion
The critically burned patient demonstrates benefit from tight glycemic control [1-6], yet the optimal method for achieving control has not been established. This study is the first to demonstrate benefit and safety of CDSS for glucose management specifically for the severely burned, a devastating injury with sustained profound metabolic derangement. Effective manual glycemic protocols can become extremely complicated; however, computer technology can perform complex calculations and reduce user error. Dynamics of an individual patient’s glucose variability can be incorporated into dose estimation, improving control in hyperglycemic ranges and reducing incidence of hypoglycemia. Despite the benefits and promise of CDSS technology no glucose management therapy has achieved time in target range greater than 50% of the time. Until desired glucose levels are consistently attained the benefit or risks of intensive insulin treatment cannot be fully understood.
Currently, three computerized glycemic decision support systems are approved by the U.S. Food and Drug Administration (FDA) [12-15]. For this study, we selected EndoTool®, a system based on a physiologic quadratic dosing curve and proportional control feedback mathematics. The other FDA-approved systems rely on a simple linear formula (units of insulin/hr = [blood glucose − 60] × multiplier) that is up- or down-regulated by using a simple multiplier or insulin sensitivity factor [13, 14]; the multiplier or factor is adjusted incrementally in response to the glucose trend. The benefit of the control feedback approach is that complicated variables can be entered into the formula, tailoring control more precisely to complicated patient scenarios. The EndoTool® system recommends the time for subsequent glucose measurements based on mathematical stability of previous readings, extending the frequency of measurement from hourly to every 2 or 3 hours, thus reducing nursing workload.
Our prospective randomized crossover study of acutely burned patients has shown that CDSS (EndoTool®) for glycemic control is efficacious and safe to use in this critically ill population. The computer software achieved improvement in maintaining serum glucose in the 80 to 110 mg/dl target range compared to our traditional nurse-managed paper nomogram. Patients managed via the PP spent more time over target range, yet there was no difference in time spent under target range. This finding demonstrates that computer software is adept at controlling hyperglycemic excursion while minimizing risk for hypoglycemic events. Critically ill burn patients experience significant glycemic variability, greater than the general ICU population, due to severe metabolic derangement, loss of glucose stores from muscle wasting, and liver impairment [18]. Furthermore, routine metabolic stress from frequent surgical interventions, temporarily halting continuous feeds for procedures, daily wound care, and aggressive rehabilitation significantly increase the risk of hypoglycemic events (USAISR unpublished data, 2008). This trial was halted when it was found during a planned interim analysis that glycemic control improved without increased hypoglycemic events. Thus, EndoTool® became the standard of care in our burn ICU in November 2008.
The nursing staff complied with the recommendations from the CDSS software over twice as often as compared to paper calculations for insulin titration. Because our paper protocol was conservative, achieving target glucose levels was difficult and many of the experienced staff nurses deviated from the protocol by administering higher insulin rates. The benefit of standardizing care among nurse providers is the reduction of risks associated with lack of experiential judgment. Consistency in practice is particularly critical in a specialty unit where level of burn specific knowledge and expertise varies greatly. Added variation occurs because of rapid staff turnover caused by an inherently stressful environment. Furthermore, as noted by Morris et al. [19], clinicians’ methods ultimately become a factor in experimental designs, confounding results and limiting replication of findings. The data from 2009 revealed increased acceptance of CP recommendations by the nursing staff; this effect is perhaps due to increased comfort with the aggressive dosing and low hypoglycemic rate achieved by the computer protocol. Multicenter studies evaluating intensive insulin therapy are impaired when standardized methods for glycemic management are not used.
Both military and civilian patients enrolled had similar demographic profiles except that the military patients were younger and had higher injury severity scores. As previously reported, when adjusted for age, no outcome differences exist between these groups treated at our burn center despite the difference in severity of injury and time to definitive care [20]. Homogeneity exists between groups regarding factors known to influence glycemic excursion and insulin resistance such as sepsis and exogenous steroid administration. Enrollment occurred for the majority of patients (14/18) within 10 days of ICU admission; however the potential exists for variation in glycemic requirements related to healing. Because a paired crossover, 72 hour design was utilized the effect of this phenomenon is somewhat mitigated for the relatively long ICU course (64 ± 50 days).
The number of glucose measurements differed between the study groups, with the CDSS prompting the nurse to measure glucose more frequently. In this study, the software was programmed for a minimum of hourly measurements to conform to the requirement for hourly measurements using the paper protocol and to standardize procedures for this study. The CDSS prompted the nurse to perform half-hour checks several times for most patients, generally when the system was initiated or when glucose values were low; thus, the mean number of CDSS measurements was greater than study duration (73 ± 3 for a 72-hour study period). Increasing sampling frequency in unstable patients has demonstrated improved glycemic control [21]. Because the software calculates the next time for glucose measurement based on glycemic variability, this finding supports the safety profile of CDSS over manual protocols which rely on nursing judgment for determining frequency for glucose quantification. The fact that only an average of 67± 4 measurements in the 72-hour study period was performed by the nursing staff in the PP group demonstrates that adherence to standard of care is limited by a nurse’s ability to remember to perform the point-of-care test. The CDSS software has a Medical Director feature that will decrease the frequency of measurements when mathematical stability occurs. Nursing workload may be positively impacted because CDSS software can then prompt glucose measurements every 2 or 3 hours when stable patients demonstrate sustained euglycemia.
The strength of this study is improved with a randomized crossover design which reduces bias inherent in the complex nature of the burn injury profile among subjects. The limited half-life of intravenous insulin eliminates a carry-over effect and facilitates use of such a design in glycemic control research when determination of an optimal management protocol is desired. Furthermore, randomizing subjects in pairs to the initial treatment group reduces an order effect that could be influenced by increasing insulin resistance during the ICU stay [10], and improvement of physiologic status over time as healing occurs. As noted, our analysis revealed no such order effect within this study.
This study is limited by inclusion of a single burn center population and a restricted number of patients. Moreover, the study period of 72 hours for each group was relatively short; however, the duration was consistent for both groups [21]. Calculation of time to reach target range for each group was not possible because many subjects were receiving continuous insulin therapy at the time of enrollment. Statistical calculation of time in range may have been enhanced with interpolation techniques rather than a simple time average. A more robust paper-based nomogram could have provided improved control and surpassed efficacy of the CP. Thus, generalizability of these findings may be restricted to units using simple paper-based nomograms similar to ours. Glycemic variability, not a component of this design, has been suggested to play an important role in glucose management, and further evaluation of this phenomenon is currently under way within our institution [21-23].
Although our results are similar to others’ reported success at achieving target glucose ranges in various ICU populations [12, 19, 24, 25], routinely achieving no greater than 50% time in target range of 80 to110 mg/dl prohibits definitive conclusions about the efficacy of intensive insulin therapy. Until reliable methods for more frequent glucose sampling and measurement (continuous glucose monitoring technology) can be performed, coupled with CDSS software tailored to appropriate targets, it is unlikely that outcomes of tight glucose control can be adequately evaluated. There is significant room for improvement in achieving target glucose levels for all ICU patients [12, 19, 24, 25] regardless of the specific range desired [26]. The surprising results of the NICE-SUGAR study suggest that general ICU patients may not benefit from tight glucose control (81 to 108 mg/dl). Yet the trauma subgroup showed the opposite effect, a trend toward improved survival was noted in this group (p=0.07) [26]. Burn patients represent a model of extreme trauma and thus, we continue to target the range of 80-110 mg/dl for glycemic control. Our rate of severe hypoglycemia using the CDSS of only 0.05% in 2009 while maintaining an aggressive glucose target is testament that computerized protocols offer a degree of safety during tight glycemic control. Thus, regardless of the specific glucose range desired, computerized protocols continue to offer the potential for reduction of hypoglycemic episodes [27-28], a potential for decreased glycemic variability, and standardization among providers and institutions [19].
We noted specific CDSS enhancements that would benefit glycemic management of the burned ICU patient. Both metabolic stress of intensive daily wound care, which significantly reduces the serum glucose concentrations in the severely burned patient (ISR unpublished data, 2008) and transient stress-induced post-surgical hyperglycemia present unique challenges to intensive insulin therapy. Frequent bouts of sepsis in the burn patient, use of exogenous corticosteroids, and adrenal insufficiency of critical illness serve to increase blood glucose variability, thus providing this patient population increased benefit from glycemic control. Additionally, feedings are frequently held for repeated surgical procedures or gastric intolerance, yet insulin infusions are continued to control underlying hyperglycemia. These clinical scenarios represent a sample of situations necessitating development of a burn-specific build created by the vendor to address wound care, feeding interruption, and steroid administration and taper. Further studies are warranted to compare the efficacy of EndoTool® for glycemic control in additional burn populations with other FDA-approved software [12-14] to guide insulin titration.
Conclusions
CDSS improved glycemic control in burn ICU patients by achieving target glucose more often compared to a conservative paper-based nomogram, and no increased risk of hypoglycemic events was identified. This finding is consistent with reported CDSS performance in other critically ill populations. Furthermore, nurses complied with CDSS recommendations more often than the calculated infusion rate from the PP, increasing uniformity in delivery of insulin therapy. Consistent glycemic management will provide increased internal validity for multi-center clinical trials evaluating insulin therapy.
Acknowledgements
Special thanks to Michelle Morrow and Bonnie Jackson for outstanding research assistance and to Nikolaos Kypreos, Orenthio Goodwin and Eric Hobbs for database item retrieval. This study was possible because of the tremendous support from our dedicated burn ICU nursing staff.
Grant Information: The National Institutes of Health (1 R01 GM063120-04) and The Technologies for Metabolic Monitoring (TMM)/Julia Weaver Fund, A Congressionally Directed Program Jointly Managed by the USA MRMC, NIH, NASA, and the Juvenile Diabetes Research Foundation and Combat Casualty Care Division, United States Army Medical Research and Materiel Command
Footnotes
Declaration of Competing Interests
The authors declare that they have no competing interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hemmila MR, Taddonio MA, Arbabi S, et al. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008;144:629–637. doi: 10.1016/j.surg.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pham TN, Warren AJ, Pham HH, et al. Impact of tight glycemic control in severely burned children. J Trauma. 2005;59:1148–1154. doi: 10.1097/01.ta.0000188933.16637.68. [DOI] [PubMed] [Google Scholar]
- 3.Mowlavi A, Andrews K, Milner S, et al. The effects of hyperglycemia on skin graft survival in the burn patient. Ann Plast Surg. 2000;45:629–632. doi: 10.1097/00000637-200045060-00010. [DOI] [PubMed] [Google Scholar]
- 4.Gore DC, Chinkes DL, Hart DW, et al. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med. 2002;30:2438–2442. doi: 10.1097/00003246-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gore DC, Chinkes D, Heggers J, et al. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Holm C, Horbrand F, Mayr M, et al. Acute hyperglycaemia following thermal injury: friend or foe. Resusc. 2004;60:71–77. doi: 10.1016/j.resuscitation.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 9.Mann EA, Pidcoke HF, Salinas J, et al. The impact of intensive insulin protocols and restrictive blood transfusion strategies on glucose measurement in American Burn Association (ABA) verified burn centers. J Burn Care Res. 2008;29:718–723. doi: 10.1097/BCR.0b013e3181848c74. [DOI] [PubMed] [Google Scholar]
- 10.Pidcoke HF, Salinas J, Wanek SM, et al. Patterns of exogenous insulin requirement reflect insulin sensitivity changes in trauma. Am J Surg. 2007;194:798–803. doi: 10.1016/j.amjsurg.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15:370–377. [PubMed] [Google Scholar]
- 12.Dortch MJ, Mowery NT, Ozdas A, et al. A computerized insulin infusion titration protocol improves glucose control with less hypoglycemia compared to a manual titration protocol in a trauma intensive care unit. J of Paren Enter Nutr. 2008;32:18–27. doi: 10.1177/014860710803200118. [DOI] [PubMed] [Google Scholar]
- 13.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabet Care. 2005;28:2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 14.Juneja R, Roudebush C, Kumar N, et al. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabet Tech Therap. 2007;9:232–240. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]
- 15.Saager L, Collins GL, Burnside B, et al. A randomized study in diabetic patients undergoing cardiac surgery comparing computer-guided glucose management with a standard sliding scale protocol. J Cardiothor Vascr Anesth. 2008;22:377–382. doi: 10.1053/j.jvca.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Mann EA, Salinas J, Pidcoke HF, et al. Error rates resulting from anemia can be corrected in multiple commonly used point-of-care glucometers. J Trauma. 2008;64:15–21. doi: 10.1097/TA.0b013e318160b9e4. [DOI] [PubMed] [Google Scholar]
- 17.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35:2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 18.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008;29:683–694. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris AH, Orme J, Truwit JD, et al. A replicable method for blood glucose control in critically ill patients. Crit Care Med. 2008;36:1787–1795. doi: 10.1097/CCM.0b013e3181743a5a. [DOI] [PubMed] [Google Scholar]
- 20.Wolf SE, Kauvar DS, Wade CE, et al. Comparison between civilian burns and combat burns from Operation Iraqi Freedom and Operation Enduring Freedom. Ann Surg. 2006;243:786–795. doi: 10.1097/01.sla.0000219645.88867.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Herpe T, De Brabanter J, Beullens M, et al. Glycemic penalty index for adequately assessing and comparing different blood glucose control algorithms. Crit Care. 2008;12:R24. doi: 10.1186/cc6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali NA, O’Brien JM, Dungan K, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 24.Kaukonen K-M, Rantala M, Pettila V, et al. Severe hypoglycemia during intensive insulin therapy. Acta Anaesth Scandin. 2009;53:61–65. doi: 10.1111/j.1399-6576.2008.01795.x. [DOI] [PubMed] [Google Scholar]
- 25.McMullin J, Brozek J, McDonald E, et al. Lowering of glucose in critical care: a randomized pilot trial. J Crit Care. 2007;22:112–119. doi: 10.1016/j.jcrc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 26.The NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 27.Flanders S, Juneja R, Roudebush C, et al. Glycemic control and insulin safety: the impact of computerized intravenous insulin dosing. Am J Med Qual. 2009;24:489–497. doi: 10.1177/1062860609338406. [DOI] [PubMed] [Google Scholar]
- 28.Juneja R, Roudebush C, Nasraway S, et al. Computerized intensive insulin dosing can mitigate hypoglycemia and achieve tight glycemic control when glucose measurement is performed frequently and on time. Crit Care. 2009;13:R163. doi: 10.1186/cc8129. doi:10.1186/cc8129. [DOI] [PMC free article] [PubMed] [Google Scholar]