Abstract
Background and Purpose
Little is known about the role of microinfarcts in dementia and cognition. We examined microinfarcts and dementia, global cognition, and five cognitive systems in community-dwelling older persons.
Methods
425 subjects enrolled in the Religious Orders Study underwent annual clinical evaluations, including 19 neuropsychological tests and assessment for dementia, and brain autopsy (39% men; mean age-at-death 87, MMSE 21). Neuropathologic examination documented the presence, number, and location of chronic microinfarcts on 6µm hematoxylin & eosin stained sections from cortical and subcortical regions. Multiple regression analyses adjusted for age-at-death, sex, education, macroscopic infarcts, Alzheimer’s disease (AD) pathology, and Lewy bodies.
Results
Microinfarcts were present in 129/425 (30%) persons (54 cortical, 80 subcortical; 49 multiple). 58/129 (45%) of persons with microinfarcts did not exhibit macroscopic infarcts. Persons with microinfarcts had increased odds of dementia (OR=1.77; 95% CI:1.07, 2.92), especially those persons with multiple cortical microinfarcts. Microinfarcts were also associated with lower average global cognition (estimate=−0.287, SE=0.113, p=0.012), particularly for persons with multiple cortical microinfarcts. Microinfarcts were specifically associated with lower episodic memory (estimate=−0.279, SE=0.138, p=0.044), semantic memory (estimate=−0.391, SE=0.130, p=0.003), and perceptual speed (estimate=−0.400, SE=0.117, p<0.001). In addition, single, multiple, and cortical microinfarcts were associated with worse semantic memory and perceptual speed (all p<0.028). Neither macroscopic infarcts nor AD pathology modified these associations (all p>0.154).
Conclusions
Microinfarcts are common, and persons with multiple cortical microinfarcts have higher odds of dementia. Microinfarcts are also associated with lower cognition, specifically perceptual speed, semantic and episodic memory.
Keywords: microinfarct, pathology, dementia, cognition
Brain infarcts are common in older persons, and increasingly recognized with improved medical technology. While sophisticated neuroimaging is contributing to better identification of small infarcts,1 microinfarcts are, by definition, not visible to the naked eye but detected only on histologic examination. Microinfarcts have been associated with macroscopic infarcts2 and commonly coexist with Alzheimer’s disease (AD) pathology.3 The role of microinfarcts in the context of mixed pathologies (i.e., AD pathology, macroscopic infarcts, and Lewy bodies) commonly found in brains of older persons with dementia is not well understood.
Few clinical-pathologic, prospective, community studies examine associations of microinfarcts with other pathologies, cognition, or dementia. Some have found that, in spite of their small size, microinfarcts are associated with dementia or cognition, particularly if multiple and cortical.4–8 It is uncertain if microinfarcts affect specific cognitive systems or modify effects of other age-related pathologies on cognition.9
We used data from more than 400 autopsied persons enrolled in a prospective clinical-pathologic study of aging. We first describe characteristics of microinfarcts and relations to other variables. We then examine associations of microinfarcts with dementia and cognition, including global cognition and five cognitive systems, in analyses that take into account macroscopic infarcts, AD pathology, Lewy bodies, and other covariates. We also investigated whether macroscopic infarcts or AD pathology modify associations. Additional analyses examine the role of quantity and location of microinfarcts in their relation to dementia and cognition.
Methods
Cohort
Older Catholic clergy from >40 groups across the US enrolled in the Religious Orders Study, a longitudinal clinical-pathologic study of aging and dementia (IRB approved).10 Subjects agreed to evaluations and brain donation at time-of-death.
Clinical Data
Baseline uniform, structured clinical evaluations included a medical history, detailed neuropsychological testing, and neurological examination, following published procedures,11 as described elsewhere.12 Annual follow-up evaluations, performed blinded to previously-collected data, were identical to baseline in all essential details. Data were collected on laptop computers using data entry screens programmed using the Blaise system (Westat, Inc., Rockville, MD 20850).
A standardized battery of neuropsychological tests, administered at each evaluation, assessed a broad range of abilities commonly affected in aging, as previously published.10,12 Data were reviewed by a board-certified neuropsychologist blinded to previously-collected data. The Mini-Mental State Examination was administered for descriptive purposes. Nineteen other tests were summarized into composite measures of five cognitive systems and an overall score of global cognition (based on all tests). Episodic memory was based on seven tests, semantic memory on four tests, working memory on four tests, perceptual speed on two tests, and visuospatial ability on two tests.10 For each summary score, we converted raw scores of individual tests to z scores, and averaged these scores. An advantage of summary measures is that they decrease floor and ceiling artifacts and other sources of measurement error. These measures have been used in studies in this and other cohorts, adding validity to measures.13,14 For this study, we used cognitive data proximate-to-death in analyses.
Clinicians with experience in dementia reviewed clinical data from that year, blinded to previously-collected data, to determine dementia status following published recommendations.15 At time-of-death, a board-certified neurologist, blinded to pathologic data, reviewed all clinical information across study years, to render a classification of dementia status proximate-to-death.
Neuropathologic Data
Brain autopsies were conducted at pre-determined sites across the US, with a mean postmortem interval of 8.3 (SD=8.0) hours. The cerebellar and cerebral hemispheres were cut coronally into 1cm slabs. Slabs not designated for freezing were fixed for at least 48–72 hours. Neuropathologic evaluations were performed at the Rush, blinded to clinical data, and reviewed by a board-certified neuropathologist, as reported elsewhere.16,17 A uniform examination included assessment for common vascular and neurodegenerative conditions in aging. Examination for cerebral infarcts documented age (acute/subacute/chronic), size, and location (side and region) of infarcts visible to the naked eye on fixed slabs.17 All grossly visualized and suspected macroscopic infarcts were dissected for histologic confirmation. For analyses, chronic macroscopic infarcts were characterized as present or absent.17
A minimum of nine regions in one hemisphere were examined for microinfarcts on 6µm paraffin-embedded sections, stained with hematoxylin/eosin. We examined six cortical regions (midfrontal, middle temporal, entorhinal, hippocampal, inferior parietal, and anterior cingulate cortices), two subcortical regions (anterior basal ganglia, thalamus), and midbrain. Locations of microinfarcts were recorded. Because acute and subacute microinfarcts were unlikely to be related to dementia, we only considered chronic microinfarcts for this study. These included cavitated or incomplete infarcts, with few remaining macrophages and fibrillary gliosis. In primary analyses, each case was classified according to whether any chronic microinfarct was present. We created additional variables for secondary analyses. For quantity, we created a predictor with three levels: no (reference level), one, and multiple microinfarcts. For location, we created two variables: cortical (presence of any microinfarcts in any cortical region; reference = no cortical microinfarcts) and subcortical microinfarcts (presence of any microinfarcts in any subcortical region; reference = no subcortical microinfarcts). To investigate quantity and location simultaneously, we created four variables: one cortical microinfarct and multiple cortical microinfarcts (compared to no cortical microinfarcts), and one subcortical microinfarct and multiple subcortical microinfarcts (compared to no subcortical microinfarcts).
Each brain was examined for pathological markers of other common neurodegenerative conditions associated with dementia. AD pathology was assessed in fixed tissue which was paraffin embedded, cut into 6µm sections, and mounted on slides.17 Using a modified Bielschowsky silver stain, we counted neuritic plaques, diffuse plaques, and neurofibrillary tangles. Counts were scaled separately in each region and averaged across regions to create summary scores of each markers for each subject. For analyses, we created a summary score of global AD pathology, by averaging the summary scores of the three markers.16 Lewy body pathology was identified in 6µm sections of cortex and substantia nigra, using α-synuclein immunohistochemistry (Zymed, 1:100).18 For analyses, Lewy body data was dichotomized as present (if identified in any brain region) vs. absent.
Statistical analysis
Descriptive analyses included crude (unadjusted) associations of microinfarcts with demographic, clinical, and pathologic variables. All subsequent analyses adjusted for age-at-death, sex, and education, and macroscopic infarcts, AD pathology, and Lewy bodies. To test whether microinfarcts increase odds of dementia, we constructed a multiple logistic regression model with dementia proximate-to-death as the dichotomous outcome and presence of microinfarcts as the predictor. To test for effect modification, we augmented the model twice, first by adding a term for the interaction of microinfarcts with macroscopic infarcts, and then by adding an interaction term of microinfarcts with AD pathology. Secondary analyses tested whether associations differed by microinfarct quantity or location. For quantity, we replaced the dichotomous predictor with a three level factor. For location, we used cortical vs. no cortical microinfarcts, and subcortical vs. no subcortical microinfarcts. In additional analyses, we examined both quantity and location of microinfarcts simultaneously using the four terms (described above).
Next, we examined relations of microinfarcts to cognition, first to global cognition, then to five cognitive systems. All multiple linear regression analyses adjusted for co-variates. We then examined for effect modification. Secondary analyses examined microinfarct quantity or location.
Analyses were carried out with SAS/STAT software V9.2 (SAS Institute Inc, Cary, NC), using a Hewlitt Packard DL380Gb server with the Red Hat Enterprise Linux5 operating system. Model assumptions were evaluated and judged to be adequately met.19
Results
Subjects
Between Jan-1994 and Jan-2010, 1,147 persons enrolled and a follow-up rate is 94% of survivors (up to 16 years of annual data). Over the course of the study, 511 persons died (of whom 2 withdrew) and 478 underwent a brain autopsy (94% autopsy rate). Analyses were conducted on the first 425 persons with complete neuropathologic data. The interval between the last evaluation and death was 6.5 (SD=3.8) months. Compared to those without dementia, subjects with dementia were older and had lower MMSE (Table 1).
Table 1.
Characteristics* of subjects
| Dementia n=192 |
No dementia n=233 |
OR (95% CI)** | Total n=425 |
|
|---|---|---|---|---|
| Clinical | ||||
| Age-at-death, years | 88.7 (6.5) | 84.6 (6.8) | 1.10 (1.06, 1.13) | 86.5 (7.0) |
| Male sex, n (%) | 67 (35) | 100 (43) | 0.71 (0.48, 1.06) | 167 (39) |
| Education, years | 17.7 (3.3) | 18.2 (3.6) | 0.96 (0.91, 1.01) | 18.0 (3.5) |
| MMSE score | 14.1 (8.6) | 27.3 (3.0) | 0.60 (0.53, 0.66) | 21.4 (9.0) |
| Pathologic | ||||
| Microinfarct present, n (%) | 70 (36.5) | 59 (25.3) | 1.69 (1.12, 2.57) | 129 (30.4) |
| Number | ||||
| One, n | 41 | 39 | 1.35 (0.83, 2.20) | 80 |
| More than one, n | 29 | 20 | 1.89 (1.03, 3.47) | 49 |
| Location | ||||
| Cortical, n | 27 | 27 | 1.25 (0.71, 2.21) | 54 |
| Subcortical, n | 44 | 36 | 1.63 (0.997, 2.65) | 80 |
| Brainstem/cerebellum, n | 13 | 7 | 2.34 (0.92, 6.0) | 20 |
| Macroscopic infarct present, n (%) | 89 (46.4) | 64 (27.5) | 2.28 (1.52, 3.42) | 153 (36) |
| AD pathology score | 1.0 (0.7) | 0.5 (0.5) | 4.11 (2.82, 5.99) | 0.7 (0.7) |
| Lewy bodies present, n (%) | 54 (28.1) | 33 (14.2) | 2.37 (1.46, 3.85) | 87 (20.5) |
Mean (SD) unless otherwise indicated.
Crude (unadjusted) odds ratio (OR) for dementia, and 95% confidence interval (95% CI).
Microinfarcts were found in 30% (129/425), and more common in those with dementia (36.5%) than without (25.3%). For persons with microinfarct(s), the mean number identified was 1.6 (SD=0.9). Eighty persons had a single and 49 had multiple microinfarcts. Microinfarcts were subcortical but not cortical in 62 subjects, cortical but not subcortical in 36, and both subcortical and cortical in 18. Thirteen persons had only brainstem and/or cerebellar microinfarcts, without cortical or subcortical lesions. About half of persons with cortical (26/54 or 48%) and, separately subcortical microinfacts (39/80 or 49%), had multiple microinfarcts. Microinfarcts were not associated with age-at-death (p=0.369) or sex (p=0.882), but were associated with lower education (p=0.027). While microinfarcts were related to macroscopic infarcts (OR=3.19; 95%CI:2.08, 4.91; p<0.0001), nearly half of persons with microinfarcts (58/129 or 45%) had microinfarcts without evidence of macroscopic infarcts. Of persons with cortical macroscopic infarcts, 24/41 (59%) had microinfarcts, and of those with subcortical macroscopic infarcts, 49/108 (45%) had microinfarcts. AD pathology did not differ by presence of microinfarcts (p=0.918). Microinfarcts were not related to Lewy bodies (OR=0.63, 95%CI:0.36, 1.09; p=0.094).
Microinfarcts and Dementia
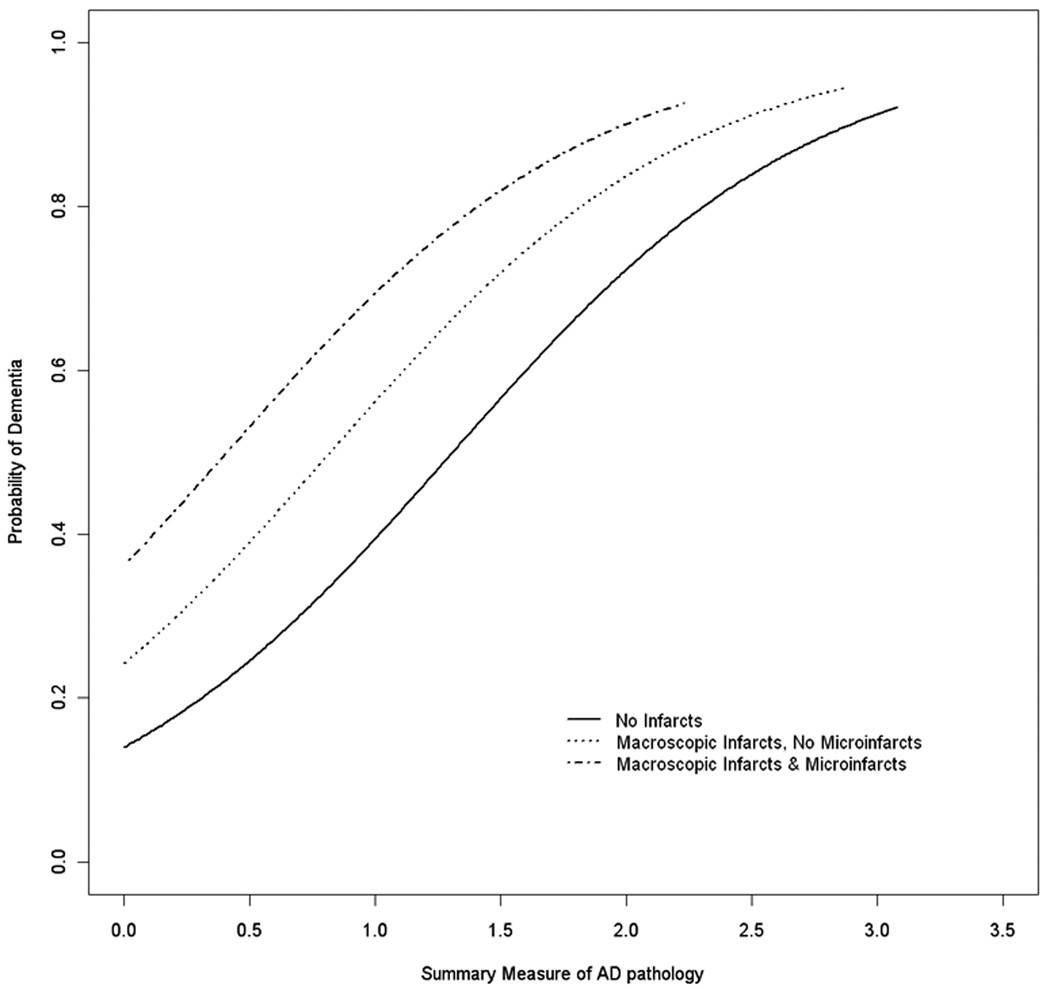
We constructed a logistic regression model with seven terms, including variables known to be related to dementia8 and microinfarcts. As shown in Table 2, controlling for age-at-death, sex, and education, and macroscopic infarcts, AD pathology, and Lewy bodies, microinfarcts were associated with a 77% increased odds of dementia. In separate models illustrated in Figure 1, we found no evidence of interactions of microinfarcts with macroscopic infarcts (p=0.371) or AD pathology (p=0.154).
Table 2.
Odds of dementia*
| Neuropathologic variable | OR | 95%CI |
|---|---|---|
| Alzheimer’s disease pathology | 4.01 | 2.69, 5.98 |
| Macroscopic infarcts | 1.97 | 1.22, 3.18 |
| Microinfarcts | 1.77 | 1.07, 2.92 |
Simultaneous model adjusting for age-at-death, sex, education, and Lewy Bodies.
Figure 1.
Probability of dementia by AD pathology, showing additive effects of macroscopic infarcts and microinfarcts.
We examined the influence of quantity and location of microinfarcts. For quantity, only persons with multiple microinfarcts had an increased odds of dementia (OR=2.35; 95%CI:1.15, 4.80). For location, the odds of dementia in persons with cortical microinfarcts was 1.29 (95%CI:0.66, 2.51) that of the odds for persons without cortical microinfarcts, and with subcortical microinfarcts was 1.63 (95%CI:0.92, 2.89) that for persons without subcortical microinfarcts, but relationships did not reach statistical significance. However, in a model with multiple cortical compared to single cortical microinfarcts, and multiple subcortical compared to single subcortical microinfarcts, only multiple cortical microinfarcts were associated with an increased odds of dementia (OR=4.12; 95%CI:1.17, 14.53).
Microinfarcts and Global Cognition
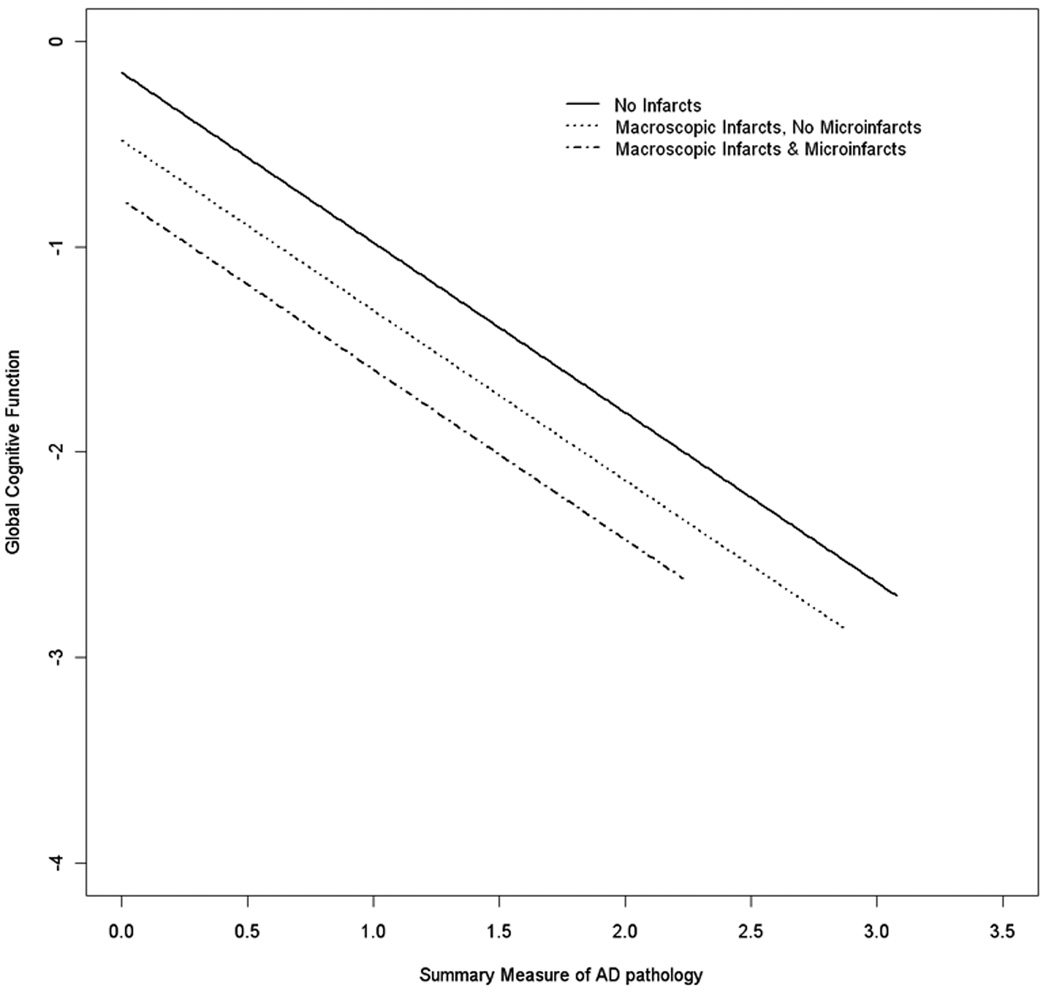
We examined the relation of microinfarcts to global cognition (Table 3), using linear regression models adjusting for demographic (age-at-death, sex, and education) and neuropathologic factors (macroscopic infarcts, AD pathology, and Lewy bodies). Persons with microinfarcts averaged about a quarter of a unit lower score on global cognition (estimate=−0.287), compared to those without microinfarcts. Similar to findings for dementia, there was no evidence of interactions of microinfarcts with macroscopic infarcts or AD pathology (Figure 2).
Table 3.
Relation of microinfarcts to global cognition and five cognitive systems*
| Cognitive Outcome | Estimate (SE), p value |
|---|---|
| Global cognition | −0.287 (0.113), 0.012 |
| Episodic memory | −0.279 (0.138), 0.044 |
| Semantic memory | −0.391 (0.130), 0.003 |
| Working memory | −0.146 (0.099), 0.139 |
| Perceptual speed | −0.400 (0.117), <0.001 |
| Visuospatial abilities | −0.153 (0.098), 0.119 |
Each model adjusted for age-at-death, sex, education, macroscopcic infarcts, AD pathology and Lewy bodies.
Figure 2.
Relation of AD pathology to cognition, showing additive effects of macroscopic infarcts and microinfarcts.
We next investigated quantity and location of microinfarcts. Compared to those with no microinfarcts, persons with multiple microinfarcts had lower cognition (estimate=−0.339, SE=0.163, p=0.038) and there was a trend for a single microinfarct (estimate=−0.255, SE=0.133, p=0.056). We then examined location: cortical microinfarcts were associated with lower cognition (estimate=−0.400, SE=0.151, p=0.009), and no relation was found for subcortical lesions (p=0.543). Finally, in an additional model considering quantity and location simultaneously, both single (p=0.044) and multiple cortical microinfarcts (p=0.040) were associated with lower cognition, and no relations were found for single or multiple subcortical lesions.
Microinfarcts and Cognitive Systems
Because cognition is not a unitary system but is comprised of multiple inter-related systems, and vascular processes may be related to some and not other cognitive systems,20 we next examined associations of microinfarcts with function in five different cognitive systems. Microinfarcts were associated with lower levels of semantic memory and perceptual speed, with a lesser association with episodic memory. There was no relationship with working memory or visuospatial abilities (Table 3). In models adjusting for an additional summary variable of vascular risk factors (hypertension, diabetes, smoking), results were essentially unchanged (data not shown). In a series of separate models, there was no evidence for interactions of microinfarcts with macroscopic infarcts (all p>0.154) or AD pathology (all p>0.696), consistent with microinfarcts having an independent effect on cognitive systems.
In analyses of quantity and location, persons with a single and those with multiple microinfarcts had lower semantic memory (estimate for single =−0.376, SE=0.153, p=0.015; estimate for multiple =−0.415, SE=0.187, p=0.027) and perceptual speed (estimate for single =−0.392, SE=0.138, p=0.005; estimate for multiple = −0.415, SE=0.169, p=0.015), but not other systems (all single: p>0.065, multiple p >0.220). In analyses of location, cortical microinfarcts were associated with semantic memory (estimate=−0.524, SE=0.174, p=0.003), perceptual speed (estimate=−0.449, SE=0.157, p=0.005), and visuospatial abilities (estimate=−0.326, SE=0.131, p=0.013). Subcortical microinfarcts were not associated with any of the cognitive systems (all p>0.316). Models considering both quantity and location simultaneously showed similar findings with single cortical microinfarcts associated with semantic memory (p=0.010) and perceptual speed (p=0.015) and trends for associations with multiple cortical lesions in the same systems (p for semantic memory= 0.058 and perceptual speed=0.072). Again no relations were found for single or multiple subcortical microinfarcts with cognitive systems (all p>0.165).
Discussion
In this cohort of 425 community-dwelling, older persons, microinfarcts are common and associated with increased odds of dementia. The effect on dementia appears to be driven by multiple cortical infarcts. Further, both single and multiple cortical microinfarcts are associated with lower cognition, specifically perceptual speed and semantic memory, with a lesser but significant effect on episodic memory. Analyses took into account demographic and neuropathologic covariates, including common neuropathologic causes of dementia. Associations with dementia and cognition were not modified by the presence of macroscopic infarcts or differing levels of AD pathology, suggesting effects of microinfarcts were independent of these other common neuropathologies.
Few epidemiologic, clinical-pathologic studies have examined the frequency of microinfarcts and their relation with dementia or cognition.4,6,21 Several have reported frequencies of microinfarcts in the range of 20–50%.5–8 Similarly, we found that microinfarcts are common, affecting almost a third of all subjects, and present in a third of those with dementia and a quarter of those without. Previous data have suggested a relation of microinfarcts with dementia. Because microinfarcts are associated with macroscopic infarcts and pathologies causing dementia often co-exist,3,22 analyses need to take into account a variety of vascular and non-vascular pathologies. We are aware of only four community-based cohort studies in which the role of microinfarcts in dementia has been directly addressed, while taking into account other pathologies. Three of these found a relation of microinfarcts to dementia5–7 and one found a trend,8 and relations appeared to be driven by multiple and cortical pathology. Our study confirms and extends the findings from these previous published data. We found that microinfarcts, particularly multiple cortical microinfarcts, independently increased odds of dementia even after accounting for both macroscopic infarcts and level of AD pathology, as well as other demographic and pathologic covariates. In addition, we found that microinfarcts are additive to macroscopic infarcts and AD pathology and do not modify their association to dementia or cognitive function.
Little is known about microinfarcts and global cognition or specific cognitive domains. In the Honolulu Asia Aging Study including 443 autopsied men, microvascular infarcts (microinfarcts and lacunar infarcts) were associated with a lower score on a brief screening measure of global cognition (CASI), but specific cognitive domains were not studied.21 We similarly found an association between microinfarcts and global cognition. Unlike for dementia which was related to only multiple microinfarcts, both single and multiple microinfarcts were implicated when cognition was the outcome measure; this finding may be the result of increased power using a continuous outcome rather than the dichotomous outcome of dementia. Moreover, both single and multiple cortical microinfarcts were specifically associated with lower levels of perceptual speed and semantic memory. This profile is similar to that of macroscopic infarcts. Further, we found a weaker but significant association with episodic memory impairment. While this type of impairment is most commonly associated with clinical AD, other pathologies including macroscopic infarcts have also been associated with it.22 These data further highlight that impairment of episodic memory is not specific for AD pathology and suggest that differing pathologies may result in similar cognitive phenotypes.
Mechanisms by which microinfarcts relate to dementia and cognition are currently unknown. Half of persons with microinfarcts did not have macroscopic infarcts; in addition, the microinfarct effects were independent of other common pathologies. Notably, we did not find evidence that microinfarcts modified the clinical expression of macrosopic infarcts or AD pathology. A variety of plausible mechanisms linking microinfarcts to dementia and cognition may be considered. Volume of infarcts is often considered an important factor in the association of infarcts to dementia,3,6,23,24 yet microinfarcts by definition are of very small volume. One may consider that there is an unrecognized large burden of microinfarcts resulting in a large volume of tissue loss.
Alternatively, microinfarcts may represent a diffuse vascular process with deleterious tissue effects, such as diffuse hypoperfusion with hypoxia, oxidative stress, or inflammation. In addition, these processes may mediate or modify the effect of microinfarcts. Single and multiple infarcts thereby may represent proxies for small and large volumes of infarcts or mild or severe diffuse injury.
We found that cortical but not subcortical microinfarcts are related to dementia and cognitive impairment. This has also been found in previous studies.6,25 This dissociation may represent differing mechanisms for the evolution or effects of microinfarcts in the subcortical versus cortical locations. Alternatively, specific anatomical regions may need to be affected in order to have a clinical expression of dementia or cognitive impairment. Indeed, we have previously reported an association of subcortical macroscopic infarcts with cognition.26 This may be related to cohort differences, regional sampling differences, or other unrecognized factors. Further study, including specific regions within cortical and subcortical structures, with larger numbers, will be needed to tease out mechanisms underlying relations of microinfarcts to dementia and cognition.
There are limitations to this study. First, assessment for microinfarcts was performed on a relatively small number of brain regions from one hemisphere, and likely underestimated the number of microinfarcts and regional involvement. While a more comprehensive evaluation, including more regions/both hemispheres and additional staining methods,27 may provide more accurate estimates, it is important to note that our results are similar to those who performed more extensive sampling strategies, while using a comparable definition of microinfarcts.21 Also, identification of microinfarcts was based on assessment of cortical and subcortical regions, unlike in some other studies.25 Second, because of the unique demographic and life-style features of this cohort, findings may not be generalizable and will need to be replicated in a more diverse cohort.
Strengths of the study include detailed, systematic neuropathologic assessments blinded to clinical data, and availability of common neuropathologies in aging and dementia. Clinical data were available on dementia status and a detailed battery of neuropsychological tests obtained proximate-to-death, from which summary measures of five different cognitive systems were derived, minimizing sources of measurement error. Finally, this cohort comprised a large group of community-dwelling women and men with and without dementia, and the study benefits from high follow-up and autopsy-rates (both >90%), providing internal validity of findings.
Acknowledgement
The authors thank participants of the Religious Orders Study.
Sources of Funding
This work was supported by National Institute on Aging grants K23 AG23675 (ZA), P30 AG10161 (DAB), and R01 AG15819 (DAB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: None.
References
- 1.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WT, Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23:291–294. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 4.Esiri MM. Which vascular lesions are of importance in vascular dementia? Ann N Y Acad Sci. 2000;903:239–243. doi: 10.1111/j.1749-6632.2000.tb06373.x. [DOI] [PubMed] [Google Scholar]
- 5.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 6.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 7.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, Paykel E, Mukaetova-Ladinska EB, Huppert FA, O'Sullivan A, Dening T Cambridge City Over-75s Cohort Cc75c Study Neuropathology Collaboration. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18:645–658. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]
- 9.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 11.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Bienias JL, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon 2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry. 2002;73:672–677. doi: 10.1136/jnnp.73.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvanitakis Z, Bennett DA, Wilson RS, Barnes LL. Diabetes and cognitive systems in older black and white persons. Alzheimer Dis Assoc Disord. 2010;24:37–42. doi: 10.1097/WAD.0b013e3181a6bed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 17.Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collett D. Modeling Survival Data in Medical Research. Second Edition. Boca Raton, FL: Chapman & Hall; 2003. [Google Scholar]
- 20.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2010 doi: 10.1002/ana.22112. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 22.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 23.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- 26.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 27.Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, Chui HC. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]