Abstract
Objectives/Hypothesis
We investigated the hypothesis that 30 minutes of raised intensity phonation alters transcript levels of vocal fold intercellular tight junction proteins and disrupts the vocal fold epithelial barrier.
Study Design
Prospective animal study.
Methods
Eighteen New Zealand white breeder rabbits were randomly assigned to receive 30 minutes of raised intensity phonation or approximation of the vocal folds without phonation. Quantitative polymerase chain reaction (qPCR) was used to investigate transcript levels of the epithelial intercellular tight junction proteins, occludin and zonula occludin-1 (Z0-1), and the adherens junction proteins β-catenin and E-cadherin. Structural alterations to the vocal fold epithelium were further examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Results
Mann Whitney U revealed significantly decreased occludin (P = .016) and β-catenin (P = .016) gene expression from rabbits undergoing raised intensity phonation, compared to control. There were no significant differences in Z0-1 and E-Cadherin gene expression between groups (P >.025). SEM revealed significant obliteration, desquamation, and evidence of microhole formation in rabbit vocal folds exposed to raised intensity phonation, compared to control, while TEM revealed dilated intercellular morphology between groups.
Conclusions
Results provide support for the hypothesis that a transient episode of raised intensity phonation alters transcript levels of vocal fold intercellular tight junction proteins and disrupts integrity of the epithelial barrier. The loss of barrier integrity may have significant consequences on epithelial defenses and compromise protection of the underlying mucosa from damage secondary to prolonged vibration exposure.
Keywords: vocal fold, phonation, phonotrauma, epithelium, tight junction proteins
INTRODUCTION
The vocal fold epithelium subserves a critical barrier function. The epithelial barrier is maintained by a series of epithelial cell layers and a circumferential arrangement of proteins referred to as the junctional complex. The surface of the outermost layer of epithelial cells consists of dense microprojections designed to increase cell surface area. These microprojections provide other important functions, including increasing the absorbance of oxygen and nutrients, and assisting in the movement of water and metabolic products across the cell membrane.1–3 The junctional complex is made up of intercellular tight junction proteins (occludin, zonula occluden (Z0-1), claudin) and subjacent adherens junctions (β-catenin and E-cadherin). Occludin, claudin and zonula-occluden proteins create the physical barrier, while β-catenin and E-cadherin are posited to maintain the integrity of the physical barrier. Together, proteins constituting the junctional complexes regulate epithelial intercellular permeability, and play a significant role in the regulation of solute and solvent flux via the intercellular pathway. In addition, the junctional complex prevents the migration of proteins and lipids between the apical and basolateral plasma membrane and restricts the passage of pathogens and pollutants into the underlying mucosa Transepithelial migration of neutrophils and altered barrier function are hallmarks of inflammatory diseases such as ulcerative colitis and chron’s disease 4, 5.
The physical barrier provided by the junctional complex may also be critical in protecting the underlying mucosa from mechanical stress during vocal fold oscillation. In cultured endothelial cells, shear stress decreases the expression of the tight junction protein, occludin within 3 hours 6. This decrease in occludin expression is associated with increased intercellular permeability 6. The vocal folds are also exposed to repeated stresses during phonation. The frequency of vibration that the vocal folds are exposed to can range from anywhere between 100–1000 Hz 7. Despite the critical importance of barrier function in health and disease, and the implications of vibration overdose and overuse in the development of pathology, currently, little is known about the effects of phonotrauma on epithelial barrier integrity. The loss of epithelial barrier integrity may have significant consequences on vocal fold pathophysiology.
The epithelial layer of the vocal folds provides an important defensive barrier to passive environmental and systemic stresses, however the effects of active, mechanical stress in compromising epithelial barrier function has not been examined to date. This is an important, physiologically-relevant question that can significantly impact the management of voice disorders secondary to phonotrauma. Improved understanding of the cellular and molecular events underlying acute phonotrauma is critical to the development and testing of strategies for prevention of vocal fold pathology. The identification of mechanisms involved in protection of the vocal fold lamina propria has important therapeutic implications and will allow for the direct testing of some of the most widely accepted hypotheses for which there are currently very limited empirical data to support. To address this significant need, our laboratory has developed an in vivo rabbit phonation model to investigate the cellular and molecular mechanisms underlying acute phonotrauma, as described previously.8–11
The purpose of the present study was to investigate the null hypothesis that a transient episode of raised intensity phonation does not affect epithelial barrier integrity, as measured by no significant change in messenger RNA (mRNA) levels of two intercellular tight junction proteins (occludin and Z0-1) and the subjacent adherens junctions (β-catenin and E-cadherin). These junctional complex proteins were selected because of their known roles in the protection of epithelial barrier integrity 4–6. Sufficient evidence of changes in transcript levels of the junction complex would lead to a rejection of the null hypothesis, and acceptance of the alternative hypothesis, that acute phonotrauma has an effect on epithelial barrier integrity. To investigate this hypothesis, we used quantitative polymerase chain reaction (qPCR) to investigate mRNA levels of the epithelial intercellular tight junction proteins, occludin and Z0-1, and the adherens junction proteins β-catenin and E-cadherin, following 30 minutes of experimentally induced raised intensity phonation, followed by a 30 minute period of tissue recovery. These methods are described in detail elsewhere. 11 Our rationale for the use of the above time-dose and phonatory recovery period was made a priori and driven by preliminary data collected in our laboratory showing evidence of alterations in inflammatory mediator gene expression in the underlying mucosa (lamina propria), following a corresponding time-dose and recovery period, as described previously. 11 Additionally, our decision to investigate mRNA levels of the intercellular tight junction proteins and the subjacent adherens junction proteins is supported by a growing body of evidence that implicates pro-inflammatory cytokines and their respective inflammatory signaling pathways in altered epithelial barrier function.4, 5, 12 Thus, rejection of the null hypothesis would provide evidence in the vocal fold to implicate barrier dysfunction as an early event in mucosal susceptibility for inflammation, and a rationale for the maintenance of epithelial barrier integrity as an approach for protection from phonation related injury.
Further, we recognize that transcript level changes do not necessarily correspond to structural alterations, and therefore, also investigated vocal fold epithelial ultrastructure using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The objective of these experiments was to document whether changes in junctional complex gene expression would be associated with alterations in epithelial surface and junctional complex morphology.
MATERIALS AND METHODS
Animals
Eighteen New Zealand white breeder rabbits weighing 3 to 5 kg were randomly assigned to receive 30 minutes of raised intensity phonation or approximation of the vocal folds without phonation, as described previously 10, 11. Induction of anesthesia was achieved with ketamine 35mg/kg, xylazine 5mg/kg, and acepromazine 0.75 mg/kg administered intramuscularly. Heart rate, temperature, and oxygen saturation levels were monitored throughout the experiment to monitor the animal’s state of anesthesia and general well-being. Subsequent intramuscular injections of ketamine (17.5 mg/kg) and acepromazine (0.375 mg/kg) were provided as needed to maintain a surgical plane of anesthesia. Ten animals were used for qPCR. Bilateral vocal fold specimens were averaged for each animal before assessing for group differences with five animals per group. Eight animals were used for electron microscopy (6 for TEM; 2 for SEM).
Surgical Procedure
The neck was shaved and prepped for surgery from the level of the submentum down to the chest. Animals were placed in the supine position on an operating platform. The larynx and trachea were exposed by a midline incision extending from the hyoid bone to the sternal notch. The trachea was then transected just proximal to the sternum. Sutures were placed to suspend the lower portion of the trachea to the sternal fascia, providing a stable airway via a tracheostomy. A 3.5 cuffed endotracheal tube (RUSCh, Kernen, Germany) was then inserted into the upper portion of the bisected trachea and positioned to rest approximately two cm below the glottal aperture. The cuff of this endotracheal tube was inflated to seal off the trachea and deliver airflow through the glottis. Custom, stainless steel, hooked electrodes were prepared prior to each experiment. One electrode was inserted mediolaterally into the belly of each cricothyroid muscle in a direction perpendicular to the muscle fibers (approximately at a 45 degree angle off midline) to serve as cathodes for electrical stimulation. Two additional electrodes were then inserted into the cricothyroid membrane to serve as anodes for electrical stimulation. One electrode was placed into the membrane on each side at the intersection of a longitudinal line one millimeter lateral to midline and a transverse line one millimeter inferior to the thyroid cartilage. A Gilmont Instruments flowmeter (GF-8522-1700. Barrington, IL) and Conch Therm III humidifier (Hudson, RCI, Temecula, CA) were used to deliver compressed humidified air heated to 37 degrees Celsius to the glottis.
A Grass S-88 stimulator (SA Instrumentation, Encinitas, CA) and constant current isolation unit (Grass Telefactor, model PSIU6; West Warwick, RI) were used to provide electrical stimulation to the laryngeal apparatus. The total train duration was 10 seconds (3 seconds on; 7 seconds off), as described previously 10, 11. A 5° 2.7mm rigid endoscope (Karl Storz Endoscopy, Endoscopy–America, Inc. CA) and Telecam-C camera (Karl Storz Endoscopy, Endoscopy–America, Inc. CA) were used to obtain video-documentation of vocal fold positioning and glottal closure during phonation. To document the increase in phonation intensity during raised intensity phonation, acoustic signals were recorded using a Shure SM48 unidirectional dynamic microphone (Shure Incorporated, Niles, IL.), placed 10 cm from the opening of the laryngoscope and digitized using the Computerized Speech Lab (CSL ™ Model 4500, KayPENTAX, Lincoln Park, NJ). Three to five, 0.5–1.0 second samples were selected from the most stable portion of the acoustic waveform, and the CSL main program was used to extract mean phonation intensity and mean fundamental frequency. Raised intensity phonation was defined as a minimum 6 dB (within rabbit) increase in the intensity of phonation, based on our earlier proof of concept experiments 10, 11 and this increase in intensity from modal phonation was maintained between 5–10 dB throughout the 30 minute stimulation period. Immediately following the 30 minute phonation and recovery period, all animals were euthanized and the larynges were harvested. The lamina propria of each animal’s vocal folds was then dissected and removed with the aid of microscopic visualization. Dissection was limited to the mucosal layers of the vocal fold above the thyroarytenoid and did not include muscle. Harvested tissue was subsequently frozen and stored for later analysis in a sub-80° freezer.
Reverse Transcriptase
Vocal fold specimens were dissected away from the larynx. An ultrasonic dismembrator 150E (Fisher Scientific, PA, USA) was used to homogenize vocal fold specimens. Total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and treated with ribonuclease-free deoxyribonuclease I (QIAGEN, Valencia, CA, USA) to minimize contamination from genomic DNA. The quantity of total RNA was determined with the A260/A280 ratio using a Nanodrop ND-2000c (Thermo Scientific, MA, USA) and electrophoresis was used to evaluate the quality based on the appearance of the 18S and 28S ribosomal RNA bands. Reverse transcription was performed using TaqMan® Reverse Transcription Reagents (Applied Biosystems Inc., CA, USA) using the manufacturer’s recommended reaction protocol. Reactions were performed using a Veriti thermal cycler (Applied Biosystems Inc., CA, USA) with the following parameters: 25°C for 10 min, 37°C for 120 min, 85°C for 5 sec, and 4°C for 5 min.
Quantitative Polymerase Chain Reaction (qPCR)
Rabbit-specific primers for Occludin, Zonula Occludin (ZO-1), E-cadherin, β-catenin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were synthesized by Sigma-Aldrich Corp. (TX, USA). Specific primer sequences are displayed in Table 1. All primers generated a single PCR band of the expected size. DNA sequencing was used to verify the PCR products. Quantitative PCR was performed in a final volume of 20µl according to the manufacturer’s recommended protocols; the reaction mix was comprised of template cDNA, 10µl of POWER SYBR Green Master Mix (Applied Biosystems Inc., CA, USA), 0.25µM final concentration of each primer and ribonuclease-free water. Quantitative PCR was performed under the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of denaturing at 95°C for 15 seconds and annealing at 60°C for 1 minute. Fluorescence was detected using an Applied Biosystems StepOnePlus (Applied Biosystems Inc., CA, USA). The ΔΔCt method was used to determine the relative ratio of gene expression for each gene.
Table 1.
Primer Sequences.
| β-Catenin | Forward | 5'-ATGTGGATTTGGAACCCAAG-3' |
| Reverse | 5'-CCAAAGGGAGGCTTCCTAGT-3' | |
| Occludin | Forward | 5'-GCTTCTGGATCTATGTATGGCTCAC-3' |
| Reverse | 5'-TCATAGCGGTCCATCTTTCTTCGAG-3' | |
| Z0-1 | Forward | 5'-CGTAACACCAAATGCAGTAGATCGTC -3' |
| Reverse | 5'-CTGTTGCTGGATTGCTTCCTTCAAC-3' | |
| E-Cadherin | Forward | 5'-AGGACACAGACTATGTCAAGAACAG-3' |
| Reverse | 5'-GGATCGATGATGTTTATGAGCTGAG-3' | |
| GAPDH | Forward | 5'-TCGGCATTGTGGAGGGGCTC-3' |
| Reverse | 5'-TCCCGTTCAGCTCGGGGATG -3' |
Z0-1 - Zonula Occludens-1; GAPDH – Glyceraldehyde-3- phosphate dehydrogenase.
Electron microscopy
Routine methods were used for processing of tissue for scanning electron (SEM) and transmission electron (TEM) microscopy13, 14. SEM images were acquired using a Hitachi X-650 scanning electron microscope (Hitachi, Ltd., Tokyo, Japan). TEM images were acquired using a Philips CM-12 transmission electron microscope (FEI Company, Hillsboro, OR, USA).
Statistical Analysis
Gene expression ratios between raised intensity phonation and control groups were compared using the Mann Whitney U test for two independent samples to investigate differences in occludin, ZO-1, E-cadherin, and β-catenin gene expression between groups. Alpha was adjusted to .0125 to control for type I error. Analyses were performed with the use of 2-tailed p-values. Data were analyzed using PASW Statistics 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Quantitative Polymerase Chain Reaction (qPCR)
Log-transformed expression ratios were compared between the phonation and control group using the Mann Whitney U test for the following dependent variables: Z0-1, occludin, E-cadherin, and β-catenin. Results revealed significantly decreased occludin (P = .016) and β-catenin (P = .016) gene expression from rabbits receiving raised intensity phonation, compared to control (Figure 1). There were no significant differences in Z0-1 and E-Cadherin gene expression between the raised intensity phonation and control group (P >.025).
Figure 1.
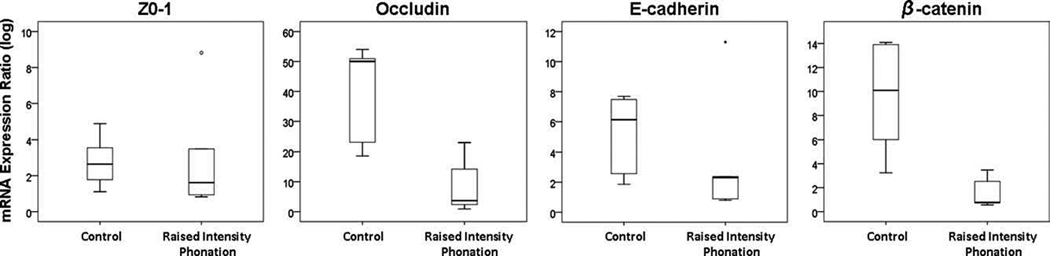
Log-transformed messenger RNA expression ratios showing significantly decreased expression of occludin and β-catenin, following a 30 minute time-dose of raised intensity phonation, compared to control. The error bars represent standard deviations from the mean. Outlier indicated by (°) and (*).
Electron microscopy
Scanning electron microscopy revealed significant damage to the epithelial surface of the vocal folds in rabbits receiving raised intensity phonation, compared to control (Figure 2). Further examination of vocal folds undergoing raised intensity phonation showed significant obliteration, desquamation, and evidence of microhole formation along the middle one-third portion of the vocal fold (Figure 3). Transmission electron microscopy (TEM) revealed dilated intercellular morphology in rabbits receiving raised intensity phonation, compared to control (Figure 4).
Figure 2.
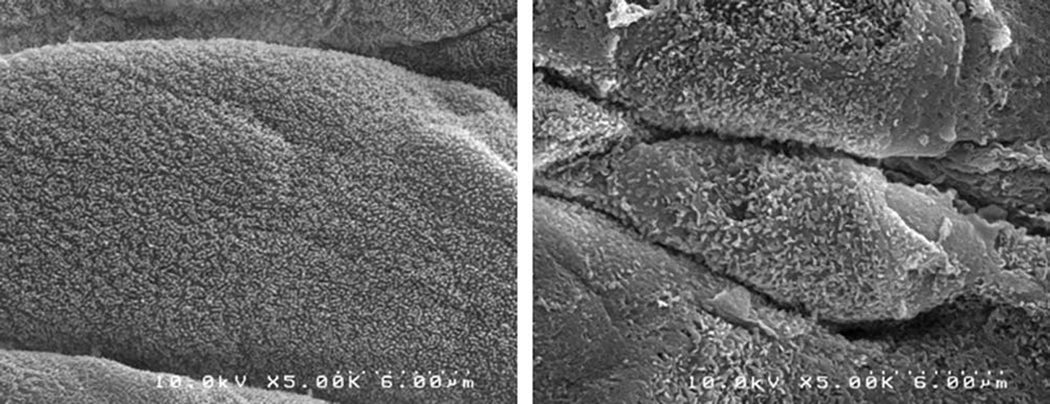
Scanning electron microscopy image (taken at microscope magnification of 5.0K), showing damage to the epithelial surface, following a 30 minute time-dose of raised intensity phonation (right panel), compared to control (left panel).
Figure 3.
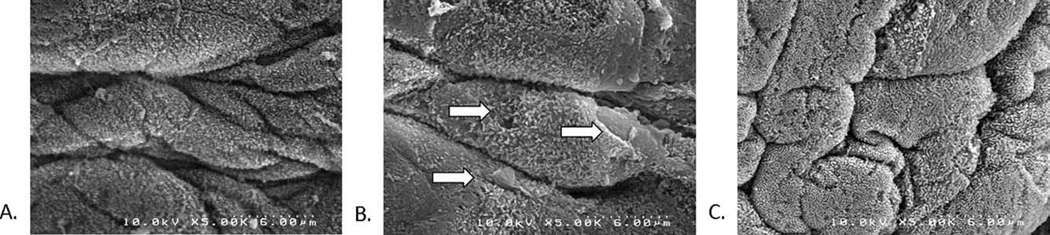
Scanning electron microscopy image (taken at microscope magnification of 5.0K) of the: A) anterior, B) middle, and C) posterior one-third portions of a rabbit vocal fold following a 30 minute time-dose of raised intensity phonation, showing significant obliteration, desquamation, and evidence of microhole formation (indicated by arrows) along the middle one-third portion of the vocal fold.
Figure 4.
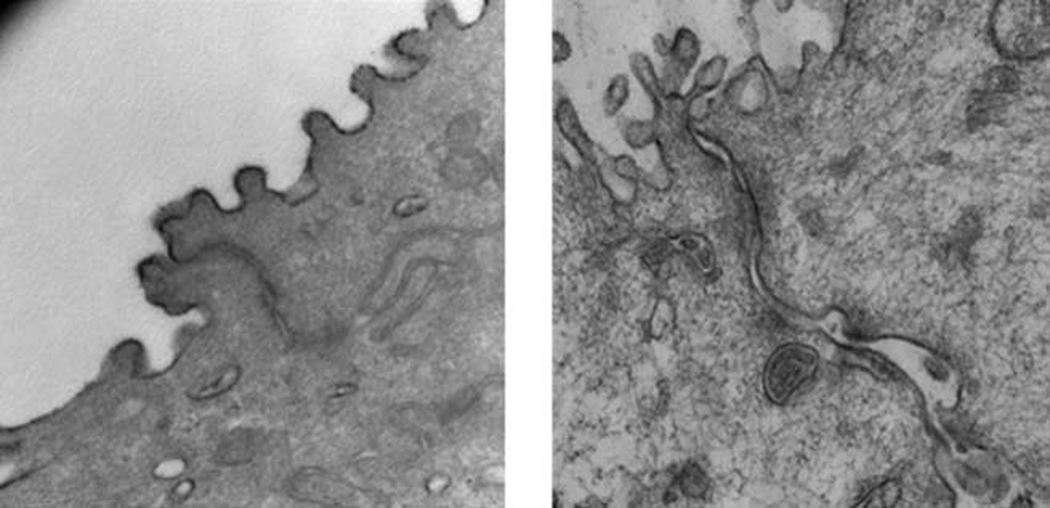
Transmission electron microscopy image (taken at microscope magnification of 53.0K) showing dilated paracellular morphology following a 30 minute time-dose of raised intensity phonation (right panel), compared to control (left panel).
DISCUSSION
To test the hypothesis that mechanical stress is detrimental to epithelial structure and function, we examined the effects of 30 minutes of raised intensity phonation on vocal fold epithelial surface features and barrier function. Morphological findings of microvilli degradation, microholes and intercellular pathway dilation following 30 minutes of raised phonation but not control condition are consistent with the hypothesis that excessive mechanical stress during raised intensity phonation is detrimental to vocal fold structure. The decreased expression of genes encoding occludin and β-catenin, two important proteins that govern the integrity of epithelial barrier is consistent with the hypothesis that mechanical trauma is detrimental to epithelial function. Disrupted epithelial barrier function may increase the likelihood of noxious airway agents entering the underlying vocal fold mucosa. It is noteworthy that the detrimental effects were observed within 30 minutes of induced mechanical stress, suggesting that even short-term vocal loading can be detrimental to both vocal fold structure and function.
Thirty-minutes of mechanical stress reduced occludin gene expression. However, transcript levels of ZO-1 were not affected. The lack of change in ZO-1 levels could be related to rapid relocalization of ZO-1. Future studies will compare the effects of acute and chronic durations of mechanical stress on the expression of junctional complex proteins. Thirty-minutes of mechanical stress reduced β–catenin but not E-cadherin mRNA expression. Immunohistochemistry of biopsy samples of patients with laryngopharyngeal reflux (LPR) demonstrate reduced E-cadherin but not β–catenin expression as compared to patients without LPR 15. The differential effects of LPR versus mechanical stress on reducing β–catenin expression are unclear but could be related to the localization of these proteins in vocal fold epithelium. E-cadherin is found in the membrane of the epithelial cells while β–catenin has been localized to both the membrane and some cellular structures in the cytoplasm of the vocal fold epithelia 16. Whether the differential distribution of these proteins accounts for the effects observed here awaits further investigation. Although the levels of junctional complex proteins were not measured in the present study, the morphologic changes appear to support the findings of alterations in gene transcript levels. Further, these morphologic and transcript level changes provide an important foundation and rationale for future investigations of post-transcriptional alterations in junctional complex protein levels and localization.
In stratified squamous epithelia of the esophagus, the presence of dilated intercellular pathway indicates tissue damage 17, 18. Dilated pathways have been observed after exposure to noxious acidic environments 18. The trauma from mechanical stress imposed in the current study is different from an acidic challenge but is consistent with damage to tissue. Dilated intercellular pathways are associated with increased esophageal permeability 17, 19, 19 and could increase the likelihood for increased access of luminal agents into the underlying mucosa. Future studies will be needed to assess whether dilated pathways are consistent with increased permeability of tissue as assessed by membrane resistance and permeability markers 18.
It is unclear if dilation of the intercellular pathway results directly from the mechanical stress of vocal fold impact or occurs secondary to disruption of the junctional complex. The dilation of the intercellular pathway may be a component of a restitution mechanism 20. The restitution mechanism could facilitate access of growth factors to the basement membrane zone, a region that is involved in the regeneration of epithelial cells.
The mechanical stress of vocal fold oscillation was associated with changes to the surface features of the epithelial cells. Gray and colleagues 21, 22 quantified the effects of excessive phonation on epithelial structure in canines. Induced phonation caused obliteration of surface features, blunting of microridges, and increased desquamation of epithelial cells as compared to control animals, however, these animals were phonated for durations of 2–4 hours. The extent of damage appeared to be related to the duration of phonation. The 30 minute duration of raised intensity phonation used in the present study was much shorter in time-duration than that used previously, but interestingly, induced a similar magnitude of change in surface features. These data provide evidence at the cellular and molecular levels that transient episodes of phonotrauma are damaging to vocal fold, and provides support for the alternative hypothesis proposed in the present study, that acute phonotrauma has an effect on epithelial barrier integrity. The translational utility of this line of research is that a greater understanding of the cellular and molecular events following phonotrauma may be valuable in the treatment and prevention of vocal pathology. Because our current understanding of phonotrauma is based on longstanding assumptions unsupported by data, this line of research may provide a basis for a more scientifically-grounded approach to treatment and prevention, with significant consequences for clinical practice.
The epithelial barrier is likely to be one important structural defensive mechanism to the perturbation imposed by mechanical stress. Other structural mechanisms include the presence of the surface fluid that may lubricate the lumen and reduce the impact stress of phonation. Functional mechanisms may also play a role in reducing the impact of forced oscillation including cellular defenses such as ion transport 23, volume regulation and anti-inflammatory processes.
CONCLUSION
An in-vivo rabbit phonation model was used to investigate the effects of raised intensity phonation on epithelial barrier integrity. Experimental animals were exposed to 30 minutes of raised intensity phonation, followed by a 30 minute recovery period. A separate group of animals not undergoing phonation served as controls. Results revealed significantly decreased occludin and β-catenin gene expression from rabbits receiving raised intensity phonation, compared to control. Additionally, vocal folds from animals undergoing raised intensity phonation showed significant obliteration, desquamation, and evidence of microhole formation along the middle one-third portion of the vocal fold on scanning electron microscopy and dilated intercellular morphology on transmission electron microscopy. These data demonstrate that the epithelial layer of the vocal fold, which provides an important defensive barrier to passive environmental and system stresses, is compromised after acute phonotrauma. The loss of epithelial barrier integrity may have significant consequences on barrier function and play a significant role in the development of vocal pathology.
ACKNOWLEDGEMENT
Research supported by NIH grant R03 DC 008400 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center.
BIBLIOGRAPHY
- 1.Collin SP, Collin HB. The corneal epithelial surface in the eyes of vertebrates: environmental and evolutionary influences on structure and function. J Morphol. 2006;267(3):273–291. doi: 10.1002/jmor.10400. [DOI] [PubMed] [Google Scholar]
- 2.Beuerman RW, Pedroza L. Ultrastructure of the human cornea. Microsc Res Tech. 1996;33(4):320–335. doi: 10.1002/(SICI)1097-0029(19960301)33:4<320::AID-JEMT3>3.0.CO;2-T. 1. [DOI] [PubMed] [Google Scholar]
- 3.Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol. 2000;279(4):F698–F712. doi: 10.1152/ajprenal.2000.279.4.F698. [DOI] [PubMed] [Google Scholar]
- 4.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 5.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. 26. [DOI] [PubMed] [Google Scholar]
- 6.De MA, Torres MB, Reeves RH. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock. 2005;23(1):11–17. doi: 10.1097/01.shk.0000144134.03598.c5. [DOI] [PubMed] [Google Scholar]
- 7.Titze IR, Hitchcock RW, Broadhead K, et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37(10):1521–1529. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau B, Ge P, French LC, Zealear DL, Thibeault SL, Ossoff RH. Experimentally induced phonation increases matrix metalloproteinase-1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge PJ, French LC, Ohno T, Zealear DL, Rousseau B. Model of evoked rabbit phonation. Ann Otol Rhinol Laryngol. 2009;118(1):51–55. doi: 10.1177/000348940911800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson ER, Abdollahian D, Ohno T, Ge P, Zealear DL, Rousseau B. Characterization of raised phonation in an evoked rabbit phonation model. Laryngoscope. 2009;119(7):1439–1443. doi: 10.1002/lary.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson ER, Ohno T, Abollahian D, Garrett CG, Rousseau B. Effects of raised intensity phonation on inflammatory mediator gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. doi: 10.1016/j.otohns.2010.04.264. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9(11):778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 13.Jerome WG, Lewis JC, Taylor RG, White MS. Concurrent endothelial cell turnover and leukocyte margination in early atherosclerosis. Scan Electron Microsc. 1983;(Pt 3):1453–1459. [PubMed] [Google Scholar]
- 14.Sivasankar M, Erickson E, Rosenblat M, Branski R. Hypertonic challenge to the vocal folds: effects on barrier function. Otolaryngol Head Neck Surg. doi: 10.1016/j.otohns.2009.09.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reichel O, Mayr D, Durst F, Berghaus A. E-cadherin but not beta-catenin expression is decreased in laryngeal biopsies from patients with laryngopharyngeal reflux. Eur Arch Otorhinolaryngol. 2008;265(8):937–942. doi: 10.1007/s00405-007-0568-6. [DOI] [PubMed] [Google Scholar]
- 16.Gill GA, Buda A, Moorghen M, Dettmar PW, Pignatelli M. Characterisation of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. J Clin Pathol. 2005;58(12):1265–1270. doi: 10.1136/jcp.2004.016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111(5):1200–1205. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 18.Tobey NA, Hosseini SS, Argote CM, Dobrucali AM, Awayda MS, Orlando RC. Dilated intercellular spaces and shunt permeability in nonerosive aciddamaged esophageal epithelium. Am J Gastroenterol. 2004;99(1):13–22. doi: 10.1046/j.1572-0241.2003.04018.x. [DOI] [PubMed] [Google Scholar]
- 19.van MH, Farre R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol. 2008;103(4):1021–1028. doi: 10.1111/j.1572-0241.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 20.Mullin JM. Epithelial barriers, compartmentation, and cancer. Sci STKE. 2004;2004(216):e2. doi: 10.1126/stke.2162004pe2. 20. [DOI] [PubMed] [Google Scholar]
- 21.Gray S, Titze I. Histologic investigation of hyperphonated canine vocal cords. Ann Otol Rhinol Laryngol. 1988;97(4 Pt 1):381–388. doi: 10.1177/000348948809700410. [DOI] [PubMed] [Google Scholar]
- 22.Gray SD, Titze I, Lusk RP. Electron microscopy of hyperphonated canine vocal cords. J Voice. 1987;1(1):109–115. [Google Scholar]
- 23.Orlando RC. Pathogenesis of gastroesophageal reflux disease. Am J Med Sci. 2003;326(5):274–278. doi: 10.1097/00000441-200311000-00003. [DOI] [PubMed] [Google Scholar]


