Abstract
Platelets occupy a central role at the interface between thrombosis and inflammation. At sites of vascular damage, adherent platelets physically and functionally interact with circulating leukocytes. Activated platelets release soluble factors into circulation that may have local and systemic effects on blood and vascular cells. Platelets can also interact with a wide variety of microbial pathogens. Emerging evidence from animal models suggests that platelets may participate in a wide variety of processes involving tissue injury, immune responses and repair that underlie diverse diseases such as atherosclerosis, autoimmune disorders, inflammatory lung and bowel disorders, host-defense responses and sepsis. In this review, we summarize the general mechanisms by which platelets may contribute to immune function, and then discuss evidence for their role in host defense responses and sepsis from preclinical and clinical studies.
Platelet–platelet interactions and platelet thrombus formation seal damaged blood vessels to prevent blood loss. Clinical studies of anti-platelet therapy in humans have unequivocally established the central role of platelets in pathologic arterial thrombosis, such as occurs in myocardial infarction and stroke1. Emerging evidence from animal models suggests that platelets may also be a critical component of the immune system 2. In this capacity, platelets may participate in a wide variety of processes involving tissue injury, immune responses and repair that underlie diverse diseases such as atherosclerosis, autoimmune disorders, inflammatory lung and bowel disorders, host-defense responses and sepsis (see Figure 1). In this review, we summarize some of the general mechanisms by which platelets may contribute to immune function, and then discuss recent advances in our understanding of their role in host defense responses and sepsis.
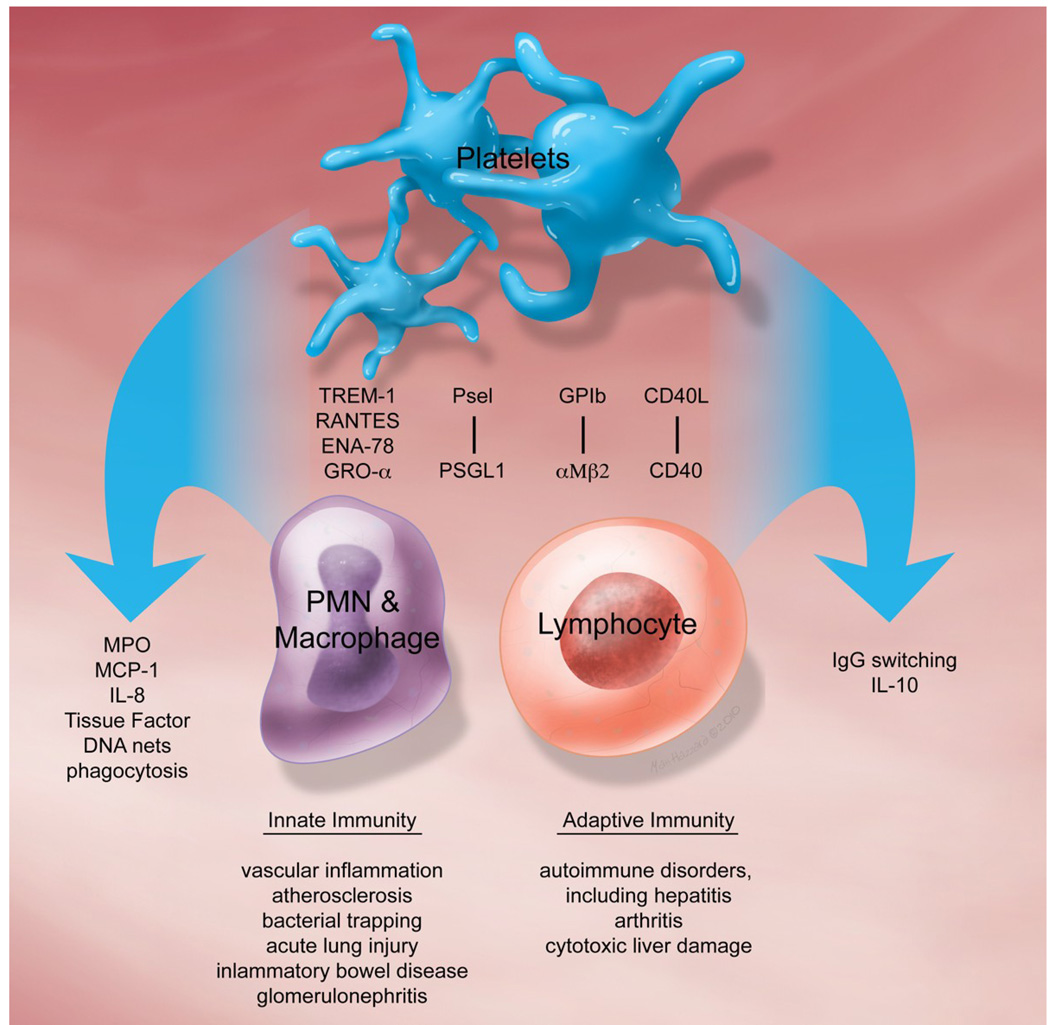
Figure 1.
Schematic representation of some of the molecules involved in promoting platelet-leukocyte interactions, mediators produced by these interactions, and disorders in which they may play a pathologic role.
Involvement of platelets in inflammation
Following exposure to certain stimuli, cargo that platelets hold inside their granules is released into the surrounding environment and/or becomes incorporated in their plasma membrane. Platelets contain three types of granules: protein-containing α-granules, dense granules rich in ADP and serotonin, and lysosomes. Proteomics studies indicate that platelet releasate contains at least 300 proteins, some of which regulate inflammation and tissue repair processes3. Many more small molecules that can affect immune function, such as RANTES, interleukin (IL)1-β, monocyte chemoattactant factor (MCP-1), platelet factor 4 (PF4), and platelet activating factor (PAF), are released or produced by activated platelets. Exocytosis also results in surface expression of P-selectin, which is important for the initial tethering of leukocytes to activated platelets. With platelet activation, CD40L appears on the platelet surface where it can be shed into the circulation. Platelet-derived CD40L, through interactions with CD40 on immune cells, can influence antibody class switching, dendritic cell maturation, and may promote platelet–immune cell adhesion 4, 5.
Platelet–leukocyte interactions have been proposed to be a crucial link between the inflammatory and thrombotic systems 6. The interaction of leukocytes with platelet thrombi was first described in 1882 by the Italian scientist, Bizzozero 7. Indeed, leukocytes incorporate into platelet thrombi and can form a layer along the surface of thrombi. In addition, platelets that adhere to damaged endothelium and/or the subendothelial matrix can recruit leukocytes to sites of injury or inflammation. In animal models, leukocytes associate with adherent or aggregated platelets within hours of vascular injury. The physical and functional interactions between platelets and leukocytes can have important consequences for leukocyte function. Leukocytes activated by interactions with platelets release granular contents such as myeloperoxidase. Circulating polymorphonuclear leukocytes (PMNs) with attached platelets display a more adhesive phenotype and have an enhanced propensity for phagocytosis. Coincubation of platelets and leukocytes generates tissue factor activity, partly through P-selectin–PSGL-1 interactions. Platelet–leukocyte aggregates may affect the generation of tissue factor and fibrin formation8, and may contribute to downstream microcirculatory damage. For example, the microcirculatory endothelium may be damaged by platelet-leukocyte aggregates, by leukocytes activated systemically by activated platelets, and perhaps, by microparticles derived from activated platelets and/or leukocytes. Transcellular metabolism and the generation of novel lipid byproducts occur as a consequence of platelet–leukocyte interactions. Arachidonic acid released by platelets can be metabolized by leukocytes to generate leukotrienes and lipoxins that promote inflammation or its resolution, respectively.
Immunomodulatory effects of anti-platelet therapy
Anti-platelet therapy, by attenuating platelet activation and their vascular accumulation, may reduce release of inflammation and immunomodulatory mediators and decrease leukocyte recruitment to sites of injury. Use of clopidogrel, which targets the P2Y12 receptor involved in ADP-activation of platelets, has been associated with reductions in CRP levels and decreased expression of CD40L and P-selectin in a variety of disease states, including cardiovascular disease, cerebrovascular disease, diabetes, and renal transplantation9. In animal models, clopidogrel pre-treatment can decrease platelet-leukocyte interactions and reduce neutrophil production of reactive oxygen species10. It is important to note that not all studies have demonstrated an effect of platelet therapy on inflammatory markers. Recent studies have found that clopidogrel may in fact increase expression of chemokines by monocytes11. In some studies, dual anti-platelet therapy with clopidogrel and aspirin has had additional benefit on inflammatory markers.
Interactions between platelets and pathogens
Given the interactions between platelets and the immune system, it is not surprising that studies have suggested a role for platelets in host defense responses. Early studies suggested that platelet accumulation along damaged endothelium served as a matrix for pathogen binding and accrual. Platelets bind to a number of different microbes, either through direct interactions, often mediated by platelet Fc receptors, or indirectly via plasma protein bridges. Many of the factors implicated in platelet interactions with different types of microbes have been discussed in an extensive review12. As occurs with agonist-induced activation of platelets, the binding of pathogens can trigger granule cargo release13 and liberation of what have been termed “platelet microbial” proteins and peptides, which include PF4, RANTES, and fibrinopeptide B. Platelets can also engulf pathogens, such as Staphylococcus aureus14, and entrapped bacteria may be resistant to immune clearance. Thus, by serving as a nidus for infection, platelets have been thought to propagate infectious endocarditis and potentially to contribute to embolic events. Platelets also contain the machinery necessary for recognition of and response to the glycolipid endotoxin, also known as lipopolysaccharide (LPS), present in the outer membrane of gram negative bacteria. LPS signals through a complex involving LPS binding protein, CD14, and Toll-like receptors (TLR) and their adaptor proteins to modulate immune cell function. Platelets express TLRs15 and the adaptor proteins MyD8816. Reports of the effects of LPS on platelet function are inconsistent16–20. The bulk of the data would support a direct effect of LPS on activating platelet function, and particularly, a potentiation of the the effects of other agonists16. TLR4 and P-selectin have been proposed as platelet for receptors LPS. LPS heightens platelet degranulation, increases P-selectin expression, releases CD40L, and thereby promotes platelet – neutrophil interactions.
Evidence for a protective role of platelets in host responses to infection
Platelets may play an important role in clearance of pathogens. Platelet interactions with blood and vascular cells may be crucial for protective host defense systems. As described above, platelet accumulation at sites of vascular injury or inflammation may sustain leukocyte recruitment and promote white blood cell tissue infiltration necessary for immunopathologic responses and pathogen clearance. In addition, platelets stimulate the formation of extracellular DNA nets by neutrophils21, 22. Nets are composed of released DNA and proteolytic activity that trap and kill gram-negative bacteria. The ability of platelets to promote DNA net formation by neutrophils is enhanced by LPS via TLR4, and is observed to occur in LPS models of sepsis. In addition to facilitating neutrophil-mediated killing of bacteria, platelets also interact with Plasmodium falciparum parasite-infected red blood cells and augment the killing of malarial parasites in mice, and thrombocytopenia or aspirin increases susceptibility to malarial infection in mice23. Thus, in several contexts, platelets may play a protective role in the host responses.
Platelets and sepsis
Thrombocytopenia in the setting of sepsis is common and appears to predict mortality24, 25. Reports place the incidence of thrombocytopenia in septic patents at 15 – 58%. Multiple studies have reported a correlation between thrombocytopenia and multi-organ failure and death in patients in the intensive care unit. Additionally, a decline in platelet count, even in the absence of frank thrombocytopenia, has recently been associated with worse outcomes in this patient population26. The reason(s) that critically ill patients with thrombocytopenia have higher mortality may be multi-factorial. Low platelet counts may reflect disease severity, underlying disseminated intravascular coagulation, and tissue/organ injury. Bleeding and transfusion requirements, both of which have been associated with mortality in other settings, are higher in patients with thrombocytopenia. If thrombocytopenia is a consequence of systemic platelet activation and clearance, then it is possible that activation-dependent events, such as platelet secretion of cytokines and other immunomodulatory molecules, influence outcomes. Disruption of the endothelial barrier occurs with profound thrombocytopenia (usually <20,000/mm3) and can result in increased vascular permeability27, which in the lung may exacerbate acute respiratory distress syndrome (ARDS). The mechanism(s) by which platelets protect the endothelial barrier may include constitutive release of barrier stabilizing factors by platelets or direct platelet interactions with the endothelium. In animal models of sepsis, platelets accumulate in lung, liver and other tissues28 where they may contribute to organ damage. Platelets have been implicated in acute lung injury29, by direct recruitment of neutrophils to injured vessels and through the release of granule contents, microparticles, and PAF. Platelet activation and sequestration in the pulmonary tissue is a key feature in inflammatory or infectious states, such as sepsis and ARDS. Thus, at the present time, it is not clear if thrombocytopenia directly contributes to death in sepsis by disrupting endothelial barrier function and protective defense systems, whether underlying platelet activation is the true culprit in sepsis-related death, or whether low platelet count is simply a marker for more extensive organ involvement. To distinguish among these possibilities, studies in which platelet number or function is pharmacologically or genetically manipulated in animals or humans are required. Below is a brief summary of several studies that have examined the consequences of pharmacologic therapy targeting platelet activation. These studies begin to provide insight into the role of platelets in immune function and the potential clinical consequences of anti-platelet therapy.
Animal models
In animals, LPS injection rapidly provokes thrombocytopenia due to trapping of platelets in the microvasculature28, 30, 31. The platelet P2Y12 receptor antagonist clopidogrel has been studied in several animal models of sepsis. In mice, pretreatment with clopidogrel prior to administration of LPS prevented thrombocytopenia, reduced by half lung fibrin accumulation, but had only a modest effect on survival32. Clopidogrel had similar beneficial effects on platelet count and fibrin deposition and also demonstrated protective effects on biomarkers of liver injury in a mouse model of polymicrobial peritoneal contamination33. In endotoxin-treated pigs, clopidogrel did not affect biomarkers of coagulation or inflammation nor did the drug reduce tissue fibrin(ogen) accumulation34. An inhibitor of the platelet integrin αIIbβ3, which is the final common pathway for platelet aggregation, improved endothelial function and integrity in rabbits treated with LPS35. Similarly, F(ab')2 fragments of the monoclonal antibody 7E3 that targets β3 integrins protected baboons from some aspects of tissue injury caused by infusion of sublethal doses of Escherichia coli and C4b binding protein36.
Clinical studies
Based on the literature reviewed above and the complex role that platelets may play in host defense responses and tissue injury, it is difficult to predict whether anti-platelet therapy would have a net beneficial or harmful effect on clinical outcomes in patients. Large clinical trials of aspirin and clopidogrel in patients at “high risk” for cardiovascular disease established a benefit in all-cause mortality that is largely driven by reduction in death due to cardiovascular disease. The mortality benefit of anti-platelet therapy in lower risk patients is less clear or not supported; however, no clear signal for an effect on incidence or outcomes of infection has been reported.
Several small scale studies have examined the impact of anti-platelet therapy on outcomes in patients with pneumonia and/or sepsis. An observational study of 224 patients hospitalized for community acquired pneumonia (CAP) indicated a reduction in ICU care in the patients receiving anti-platelet agents (aspirin, clopidogrel, or ticlopidine, n=44) for at least six months compared to unmatched controls, and the association persisted after performing age-matched analysis32. A second study in 615 patients consecutively admitted to a single ICU reported that patients who had been on anti-platelet agents (aspirin or clopidogrel) had lower mortality, despite their increased age37. In this study, premedication with anti-platelet therapy was also associated with a modest, but statically significant effect on changes in platelet count. The initial drop in platelet count in patients treated with anti-platelet agents was blunted at day 3, and the rebound in platelet count recovery at days 8 – 10 was lower. In a population based study of individuals admitted to Minnesota medical ICUs with at least one risk factor for acute lung injury (n = 161), patients that were receiving anti-platelet therapy at the time of hospital admission (49%) were less likely to develop acute lung injury or ARDS, despite being older and having more medical co-morbidities38. A retrospective analysis of the Kentucky Medicaid population examined the impact of clopiodgrel therapy on incidence of community acquired pneumonia (CAP) and its complications39. The findings suggest that patients receiving prescriptions for clopidogrel may be more likely to develop CAP but had a trend towards improved mortality if they required hospitalization. The risks associated with aspirin and clopidogrel on infection rates in the setting of cardiac surgery have also been examined40. Higher rates of post-operative infections in individuals on dual anti-platelet therapy have been reported; whether this is a direct effect on host defenses or is due to a confounding impact of increased bleeding in this setting is not clear.
In summary, the most consistently identified beneficial effect of anti-platelet therapy appears to be in the setting of critical illness and may relate to reductions in acute lung injury. It is important to note that the beneficial effects of anti-platelet therapy in critically ill patients may arise from protection from cardiovascular events and may not necessarily be related to infectious or other processes. Whether anti-platelet therapy predisposes, protects, or has no impact on incidence of infection is not clear. Additional investigation to clarify the impact of anti-platelet therapies in a variety of infectious processes is warranted to determine if there are patient populations that are likely to benefit or receive harm from this widely used class of medications.
Acknowledgments
This work is supported by an American Heart Association National Scientist Development Grant (Z.L.), and in part by the Centers of Biomedical Research Excellence in Obesity and Cardiovascular Disease Grant P20RR021954-01A1 from NIH/NCRR (Z.L. and S.S.S.) and by HL080166 (S.S.S). This material is the result of work supported with resources at the Lexington VA medical center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007 December 13;357(24):2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 2.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC. Platelet functions beyond hemostasis. J Thromb Haemost. 2009 November;7(11):1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 3.Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009 September;16(5):329–333. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009 August 18;54(8):669–677. doi: 10.1016/j.jacc.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 5.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogne M, Richard Y, Garraud O. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007 September;35(9):1376–1387. doi: 10.1016/j.exphem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 6.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009 February;85(2):195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 7.Mazzarello P, Calligaro AL, Calligaro A. Giulio Bizzozero: a pioneer of cell biology. Nat Rev Mol Cell Biol. 2001 October;2(10):776–781. doi: 10.1038/35096085. [DOI] [PubMed] [Google Scholar]
- 8.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, Sajer SA, Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992 October 29;359(6398):848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 9.Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Luscher TF. Clinical evidence for anti-inflammatory effects of anti-platelet therapy in patients with atherothrombotic disease. Vasc Med. 2007 May;12(2):113–122. doi: 10.1177/1358863X07077462. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista V, Manarini S, Dell'Elba G, Martelli N, Napoleone E, Di SA, Lorenzet PS. Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation. Thromb Haemost. 2005 September;94(3):568–577. [PubMed] [Google Scholar]
- 11.Waehre T, Damas JK, Pedersen TM, Gullestad L, Yndestad A, Andreassen AK, Froland SS, Semb AG, Hansteen V, Gjertsen E, Ueland T, Brosstad F, Solum NO, Aukrust P. Clopidogrel increases expression of chemokines in peripheral blood mononuclear cells in patients with coronary artery disease: results of a double-blind placebo-controlled study. J Thromb Haemost. 2006 October;4(10):2140–2147. doi: 10.1111/j.1538-7836.2006.02131.x. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006 June;4(6):445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 13.Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, Foster TJ. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol Microbiol. 2005 August;57(3):804–818. doi: 10.1111/j.1365-2958.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- 14.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002 June 1;99(11):4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 15.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005 October 1;106(7):2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009 June 15;182(12):7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cognasse F, Lafarge S, Chavarin P, Acquart S, Garraud O. Lipopolysaccharide induces sCD40L release through human platelets TLR4, but not TLR2 and TLR9. Intensive Care Med. 2007 February;33(2):382–384. doi: 10.1007/s00134-006-0488-8. [DOI] [PubMed] [Google Scholar]
- 18.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, Garraud O. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008 April;141(1):84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 19.Stahl AL, Svensson M, Morgelin M, Svanborg C, Tarr PI, Mooney JC, Watkins SL, Johnson R, Karpman D. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006 July 1;108(1):167–176. doi: 10.1182/blood-2005-08-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward JR, Bingle L, Judge HM, Brown SB, Storey RF, Whyte MK, Dower SK, Buttle DJ, Sabroe I. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005 October;94(4):831–838. [PubMed] [Google Scholar]
- 21.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, len-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007 April;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 22.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008 March;6(3):415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 23.McMorran BJ, Marshall VM, de GC, Drysdale KE, Shabbar M, Smyth GK, Corbin JE, Alexander WS, Foote SJ. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009 February 6;323(5915):797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 24.Baughman RP, Lower EE, Flessa HC, Tollerud DJ. Thrombocytopenia in the intensive care unit. Chest. 1993 October;104(4):1243–1247. doi: 10.1378/chest.104.4.1243. [DOI] [PubMed] [Google Scholar]
- 25.Stephan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest. 1999 May;115(5):1363–1370. doi: 10.1378/chest.115.5.1363. [DOI] [PubMed] [Google Scholar]
- 26.Moreau D, Timsit JF, Vesin A, Garrouste-Org, de LA, Zahar JR, Adrie C, Vincent F, Cohen Y, Schlemmer B, Azoulay E. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007 June;131(6):1735–1741. doi: 10.1378/chest.06-2233. [DOI] [PubMed] [Google Scholar]
- 27.Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008 September 18;359(12):1261–1270. doi: 10.1056/NEJMra0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Ohtaki Y, Yamaguchi K, Matsushita M, Fujita T, Yokochi T, Takada H, Endo Y. LPS-induced platelet response and rapid shock in mice: contribution of O-antigen region of LPS and involvement of the lectin pathway of the complement system. Blood. 2002 November 1;100(9):3233–3239. doi: 10.1182/blood-2002-01-0252. [DOI] [PubMed] [Google Scholar]
- 29.Zarbock A, Ley K. The role of platelets in acute lung injury (ALI) Front Biosci. 2009;14:150–158. doi: 10.2741/3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006 January 15;107(2):637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 31.Jayachandran M, Brunn GJ, Karnicki K, Miller RS, Owen WG, Miller VM. In vivo effects of lipopolysaccharide and TLR4 on platelet production and activity: implications for thrombotic risk. J Appl Physiol. 2007 January;102(1):429–433. doi: 10.1152/japplphysiol.01576.2005. [DOI] [PubMed] [Google Scholar]
- 32.Winning J, Reichel J, Eisenhut Y, Hamacher J, Kohl M, Deigner HP, Claus RA, Bauer M, Losche W. Anti-platelet drugs and outcome in severe infection: clinical impact and underlying mechanisms. Platelets. 2009 February;20(1):50–57. doi: 10.1080/09537100802503368. [DOI] [PubMed] [Google Scholar]
- 33.Seidel M, Winning J, Claus RA, Bauer M, Losche W. Beneficial effect of clopidogrel in a mouse model of polymicrobial sepsis. J Thromb Haemost. 2009 June;7(6):1030–1032. doi: 10.1111/j.1538-7836.2009.03352.x. [DOI] [PubMed] [Google Scholar]
- 34.Lipcsey M, Larsson A, Olovsson M, Sjolin J, Eriksson MB. Early endotoxin-mediated haemostatic and inflammatory responses in the clopidogrel-treated pig. Platelets. 2005 November;16(7):408–414. doi: 10.1080/09537100500163168. [DOI] [PubMed] [Google Scholar]
- 35.Pu Q, Wiel E, Corseaux D, Bordet R, Azrin MA, Ezekowitz MD, Lund N, Jude B, Vallet B. Beneficial effect of glycoprotein IIb/IIIa inhibitor (AZ-1) on endothelium in Escherichia coli endotoxin-induced shock. Crit Care Med. 2001 June;29(6):1181–1188. doi: 10.1097/00003246-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Taylor FB, Coller BS, Chang AC, Peer G, Jordan R, Engellener W, Esmon CT. 7E3 F(ab')2, a monoclonal antibody to the platelet GPIIb/IIIa receptor, protects against microangiopathic hemolytic anemia and microvascular thrombotic renal failure in baboons treated with C4b binding protein and a sublethal infusion of Escherichia coli. Blood. 1997 June 1;89(11):4078–4084. [PubMed] [Google Scholar]
- 37.Winning J, Neumann J, Kohl M, Claus RA, Reinhart K, Bauer M, Losche W. Antiplatelet drugs and outcome in mixed admissions to an intensive care unit. Crit Care Med. 2010 January;38(1):32–37. doi: 10.1097/CCM.0b013e3181b4275c. [DOI] [PubMed] [Google Scholar]
- 38.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Pre-hospitalization anti-platelet therapy is associated with a reduced incidence of acute lung injury: A population-based cohort study. Chest. 2010 August 5; doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross AK, Dun SP, Feola DJ, Martin CA, Smyth SS. Effect of clopidogrel therapy on the incidence and severity of community acquired pneumonia: results of a five year cohort. Circulation. 2010 abs, in press. [Google Scholar]
- 40.Blasco-Colmenares E, Perl TM, Guallar E, Baumgartner WA, Conte JV, Alejo D, Pastor-Barriuso R, Sharrett AR, Faraday N. Aspirin plus clopidogrel and risk of infection after coronary artery bypass surgery. Arch Intern Med. 2009 April 27;169(8):788–796. doi: 10.1001/archinternmed.2009.42. [DOI] [PubMed] [Google Scholar]