Abstract
Systemic lupus erythematosus is a chronic autoimmune disease frequently affecting the kidney. Renal involvement is characterized by glomerular immune complex deposits, and proliferative glomerulonephritis progressing to glomerulosclerosis and kidney failure. Development of systemic lupus erythematosus is genetically regulated and lupus susceptibility genes have been linked to immune hyper-responsiveness and loss of immune regulation. In addition to the systemic immune defects, recent studies in animal models show that susceptibility to lupus nephritis is influenced by intrinsic renal factors. Thus, renal cell responses to immune-mediated glomerular injury determine disease outcome. This supports the idea that future treatments for lupus nephritis need to focus on regulating end organ responses. The feasibility of this approach has been demonstrated in animal models of kidney disease. For over 50 years, the emphasis in management of lupus nephritis has been suppression of autoimmune responses and systemic control of inflammation. This review describes recently developed targeted drug delivery technologies and potential targets that can regulate glomerular cell responses offering a novel therapeutic approach for lupus nephritis.
INDEX WORDS: Mesangial cells, glomerulonephritis, immunoliposomes, glomerular targeting, mouse models, gene therapy, lupus nephritis
BACKGROUND
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the presence of circulating immune complexes and serum antibodies to nuclear and cytoplasmic antigens1. Deposition of auto-antigen-antibody complexes in different tissues and the ensuing inflammatory response is a major cause of organ damage. In the kidney, immune complex deposits are detected in the glomerular mesangium and on the basement membrane. This initiates an inflammatory cascade that leads to progressive kidney disease in susceptible individuals. Suppression of systemic auto-immune responses is the primary treatment strategy; therefore, immuno-suppressive and anti-inflammatory agents like cyclophosphamide, mycophenolate and corticosteroids have been obvious choices to induce disease remission in lupus nephritis for the past several decades2–3. However, evidence in mouse models of lupus-like glomerulonephritis (GN) suggests a critical role for the end organ in disease progression. This review will discuss the role of end organ responses in lupus-like GN and strategies for modulating these responses as a novel therapeutic approach.
CASE VIGNETTE
A 40-year-old African-American female was referred to the nephrology clinic for one month history of lower extremity edema and 5.7 grams of proteinuria. Urine microscopy showed 10–20 red blood cells per high power field, most of them dysmorphic, and a few red blood cells casts. Results of laboratory investigation are shown in Table 1. Her clinical picture was compatible with lupus nephritis so a kidney biopsy was performed. On light microscopy there was mild mesangial hypercellularity but no glomerular crescents, fibrinoid necrosis or glomerulosclerosis. There was a mild interstitial lymphocytic infiltrate, but no significant tubular atrophy, interstitial fibrosis or vascular inflammatory lesions. Immunofluorescence microscopy had a fine granular capillary loop staining and a “full-house” picture. Electron microscopy showed frequent electron dense supepithelial and rare subendothelial and paramesangial deposits. Pathologic diagnosis of class II and V lupus nephritis with no tubulointerstitial, or vascular activity, and no chronic damage was made.
Table 1.
Laboratory values and treatment provided over 5 years of follow up
| Day 1 | Month 5 |
Month 12 |
Month 17 |
Month 27 |
Month 51 |
Month 60 |
|
|---|---|---|---|---|---|---|---|
| SCr (mg/dL) | 0.8 | 1.0 | 1.8* | 1.3 | 1.4 | 1.1 | 1.1 |
| eGFR (mL/min/1.73 m2)** | 96 | 79 | 40 | 58 | 53 | 70 | 69 |
| Protein (g/dL) | 7.8 | 6.9 | 6.7 | 6.2 | 6.3 | 5.7 | |
| Albumin (g/dL) | 2.3 | 3.8 | 4.3 | 4.0 | 4.0 | 4.0 | 3.2 |
| UPCR | 5.7 | 1.9 | 0.6 | 0.5 | 0.6 | 0.3 | 1.8 |
| ANA | 1:640 | ||||||
| Anti-dsDNA | 1:640 | 1:40 | 1:40 | 1:40 | Neg | ||
| Anti-dsDNA (IU/mL, reference Range: <5) | 20 | 14 | |||||
| Anti-RNP | Neg | ||||||
| Anti-Smith | Neg | ||||||
| Anti-ENA | Pos | ||||||
| Anti-SSA/Ro | Pos | ||||||
| Anti-SSB/La | Pos | ||||||
| C3 compl (mg/dL, reference Range: 83 – 156) | 36 | 55 | 67 | 66 | 86 | 83 | 73 |
| C4 compl (mg/dL, reference Range: 10 – 38) | 4 | 6 | 9 | 8 | 13 | 11 | 8 |
| Hepatitis panel | Neg | ||||||
| HIV Antibody | Neg | ||||||
| Hb (g/dL) | 7.6 | 12 | 12.8 | 13.9 | 9.2 | ||
| Hct (%) | 24.5 | 38.2 | 40.9 | 47.0 | 29.2 | ||
| Mycophenolate | x | x | x | x | x | x | |
| Prednisone | x | x | x | x | x | x |
Note: Conversion factors for units: serum creatinine in mg/dL to µmol/L, ×88.4; eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667; protein in g/dL to g/L, ×10; albumin in g/dL to g/L, ×10; hemoglobin in g/dL to g/L, ×10. No units conversion necessary for C3 complement and C4 complement in mg/mL and g/L.
Abbreviations: SCr, serum creatinine; eGFR, estimated glomerular filtration rate, UPCR, urine protein to creatinine ratio; ANA, antinuclear antibody; dsDNA, double stranded DNA; RNP, ribonucleoprotein; ENA, extractable nuclear antigen; Neg, negative; Pos, positive; Hct, hematocrit; Hb, hemoglobin; HIV, human immunodeficiency virus.
After partial recovery from an episode of NSAID-induced AKI 4 months earlier.
calculated using the MDRD Study equation.
After discussing the risks and benefits of different options, treatment with mycophenolate mofetil (MMF) 250 mg twice a day and prednisone 60 mg once a day was initiated. The dose of MMF was increased to 500 mg twice a day, but no further increases were possible because of neutropenia. Eight months after the initial biopsy she had an episode of acute kidney injury with maximum serum creatinine of 3.4 mg/dL (300.56 µmol/L). A kidney biopsy confirmed acute tubular necrosis and interstitial nephritis due to the use of high dose non-steroidal anti-inflammatory drugs. There were no active glomerular lesions. Once her white cell count normalized, the dose of MMF was increased to 750 mg twice a day. After over 4 years of treatment and almost 6 months after increasing MMF dose, she was in remission (month 51, Table 1). However, soon after reducing MMF and prednisone doses, she had a relapse with hypocomplementemia, increased dsDNA antibody titers and proteinuria.
PATHOGENESIS
Human SLE Is a Complex Autoimmune Disease
SLE affects multiple organ systems with clinically heterogeneous outcomes making it a difficult disease to study in humans. A systemic autoimmune response is the hallmark of SLE, which has been attributed to a failure of tolerance mechanisms4, aberrant T cell signaling pathways5, and reduced thresholds for immune cell activation6. Renal involvement is a major cause of morbidity and mortality in lupus. Up to 60% of all patients with SLE have involvement of the kidneys at the time of presentation or some time during the course of their disease. Individuals with lupus nephritis have lower survival rates compared to those without renal involvement 7. A study by Pollock and Pirani on 87 SLE patients showed that even within the subset of patients with lupus nephritis, the natural history of disease is significantly diverse8. During an 8 year follow up, patients with evidence of glomerulonephritis on the first biopsy showed significant deterioration of kidney function. Of the others, 27 patients showed minimal glomerular changes with only focal mesangial deposits, and endothelial hypercellularity. Most of these 27 patients had significantly better outcomes; however, 2 of these 27 showed a rapid progression to kidney failure. Thus, disease severity at presentation and rate of progression vary widely among patients and are regulated by factors not completely understood. The complexity of SLE is also reflected in the large numbers of candidate susceptibility genes identified by genome wide analyses9, yet all these account only for ~15% of SLE heritability.
Serum autoantibodies are a dominant feature associated with lupus. Studies by Yurasov et al. showed that mature, naïve autoreactive B cells persist and expand in patients even during remission10. In addition to autoantibody production, B cells are also important for antigen presentation to autoreactive T cells11. Therefore, B cell depletion would be expected to alleviate progressive SLE. Rituximab is a mouse-human chimeric antibody that recognizes CD20, a surface protein on developing and mature B cells12. It is approved for treatment of follicular B cell non-Hodgkin’s lymphoma refractory to other treatments and is well tolerated. Compared to placebo, treatment with rituximab in addition to mycophenolate moefetil induced B cell depletion and was associated with significantly greater reduction in anti-double-stranded DNA antibody titters (69% vs. 50%, p <0.01) and greater increase in C3 complement component (37.5 vs. 25.9 mg/dL, p <0.03), but failed to affect the primary outcome of the study of partial or complete remissions at week 52 (p = 0.55)13–14. These results are similar to the findings of the EXPLORER (Exploratory Phase II/III SLE Evaluation of Rituximab) trial, in which treatment of patients with moderate to severe SLE (no kidney disease) with rituximab versus placebo was associated with reduction of anti-dsDNA antibody and an increase in complement levels but no difference in clinical outcomes 15.
Experiments in mouse models showed that B cells are more sensitive to cyclophosphamide and show a rapid reduction following treatment16. However, the B cells that re-emerge after cessation of treatment are selectively enriched for the pathogenic, high affinity anti-DNA autoantibodies16. A similar effect, if present in humans, may explain the low efficacy of rituximab in lupus.
B cell reconstitution following cyclophosphamide treatment is dependent on B cell activating factor (BAFF) belonging to the TNF family Thus, inhibition of BAFF prevents B cell stimulation, proliferation and prevents autoantibody production. In mice, treatment with an anti-BAFF antibody delays disease progression17. These studies in mouse models have been successfully extended to humans and an anti-BAFF antibody (belimumab) has recently been approved by the FDA for therapy 18. This is a major breakthrough for lupus treatment and clinical use will help define the subsets of patients that will benefit from belimumab treatment 19.
As shown in the case presented here, high anti-dsDNA antibody levels, hypocomplementemia and glomerular immune complex deposits persist despite significant improvement in kidney disease. In addition, the rituximab trials show that reduction of anti-dsDNA antibody titers is not associated with a corresponding decrease in pathology. These data emphasize the dissociation between anti-dsDNA antibody and kidney disease. This feature has been replicated in several animal models and the salient features of the spontaneous and induced models of lupus-like GN are discussed in the next section.
Models of Spontaneous SLE
Animal models have provided a better understanding of SLE pathogenesis. As discussed above, they have aided the design and evaluation of new candidate therapeutic agents. Some of the most commonly used mouse strains include the New Zealand (NZ) derived NZ Black × NZ White (NZB/W) F1, NZ Mixed (NZM) 2328, NZM2410, MRL lpr/lpr, and BXSB21–23. All these strains spontaneously develop anti-dsDNA antibodies, glomerular immune complex deposits and fatal GN (figure 1). Like the human disease, the kidney pathology manifests over a long duration (5–9 months of age) and shows features of chronic progressive GN. No single mouse model can recapitulate all the characteristics of the human SLE spectrum; however, the kidney disease and immune responses in each mouse strain would represent a subpopulation of human patients. In addition to insights into pathogenesis of proliferative lupus nephritis, these murine models of spontaneous SLE have been used extensively to investigate genes dictating susceptibility to autoantibody production, proliferative GN, disease progression and mortality 22, 24, 25. The mechanistic differences between murine and human SLE, specifically with reference to the role of Fc Receptors, complement and immune complex clearance has been previously reviewed 26. However, there are also significant similarities between the spontaneous lupus nephritis-like disease in mice with human lupus nephritis (Table 2). A critical feature to note is that while studies in humans focus on systemic immune responses based on peripheral blood with or without a kidney correlate, inbred mouse strains allow dissection of factors affecting systemic autoimmunity from those causing kidney disease. Another significant advantage of mouse models over human studies is the ability to study initiation of early renal change and the role of individual pathogenic factors affecting disease progression prior to the manifestation of clinical decreased kidney function.
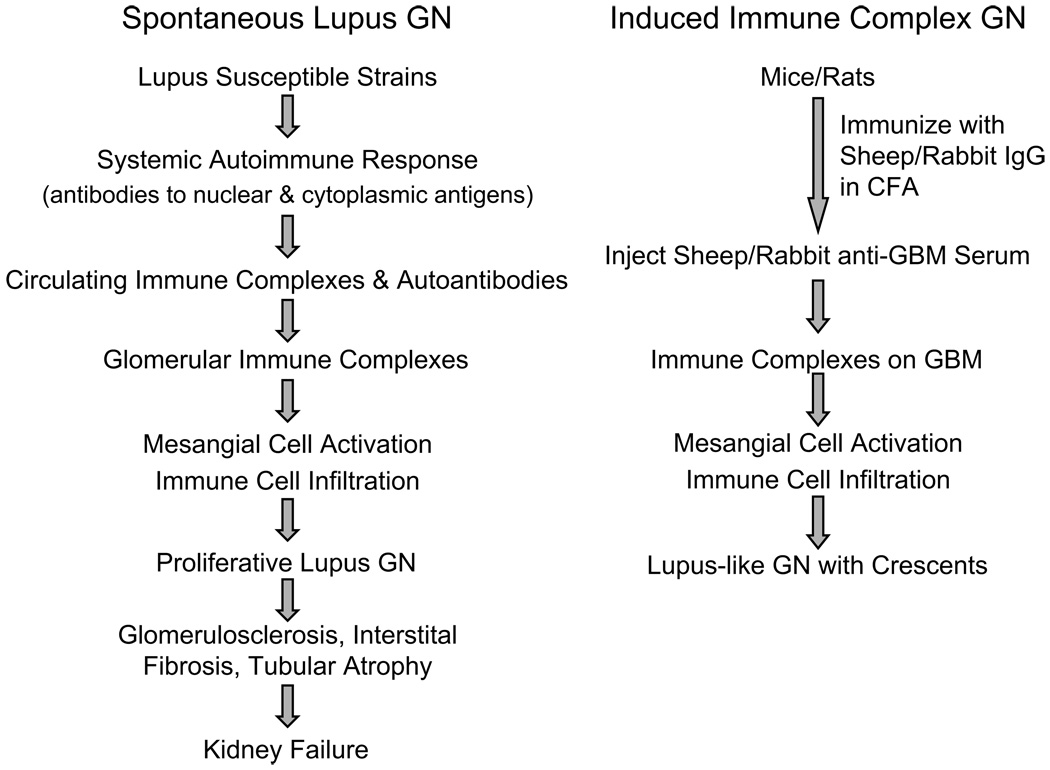
Figure 1.
A schematic comparing spontaneous and induced animal models of lupus-like glomerulonephritis (GN). Abbreviations: CFA, complete Freund adjuvant; IgG, immunoglobulin G; GBM, glomerular basement membrane.
Table 2.
A comparison of characteristics of LN in humans and mice
| Features of human LN | Spontaneous disease in lupus mice* |
|---|---|
| Female predominance | NZB/W F1, NZM2328 |
| Serum autoantibodies | |
| anti-nuclear, anti-dsDNA | All strains |
| anti-Sm | MRL/lpr |
| Kidney Pathology | |
| Glomerular Immune complexes | All strains |
| Proliferative GN | All strains |
| Type V membranous nephropathy | None** |
| Glomerulosclerosis | All strains |
| Tubulointerstitial inflammation | All strains |
| Cellular infiltrates | |
| Neutrophils | NZM2328 |
| Macrophages | All strains |
| Dendritic cells | All strains |
| NK cells | MRL/lpr |
| T cells (CD4/CD8) | MRL/lpr, NZM2328, NZB/W F1 |
| B cells | NZB/W F1 |
| Plasma cells | NZB/W F1 |
| Kidney failure*** | All strains |
| Other clinical features | |
| Hypertension | None reported |
| Systemic involvement | skin, MRL/lpr; salivary glands, NZB/W F1 |
Abbreviations: dsDNA, double-stranded DNA; LN, lupus nephritis; NZB, New Zealand Black; NZW, NZ White; NZM, NZ Mixed; NK, natural killer; GN, glomerulonephritis.
includes NZB/W F1, NZM2410, NZM2328, MRL/lpr, BXSB strains
can be induced by immuno-modulation
elevated serum creatinine, serum urea nitrogen, proteinuria
Models of Immune Complex GN
Induced models of GN in rats and mice are also a powerful resource for the study of kidney disease. Lupus nephritis is considered a prototype immune complex mediated disease. In mouse models, injection of mouse antibodies reactive with glomerular components induces a transient proteinuria, but rarely progresses to glomerular disease27. To induce glomerulonephritis, rats or mice are immunized with heterologous IgG in complete Freund adjuvant, typically from sheep or rabbit28–29 (Figure 1). This is followed by infusion of hyper-immune anti-GBM sheep or rabbit serum respectively, leading to glomerular IgG deposits. The endogenous anti-rabbit or sheep IgG responses reacting with the anti-GBM antibody leads to glomerular immune complex deposits and GN. In some models, immunization is not required and infusion of sheep anti-GBM serum alone is sufficient to induce immune-complex GN. Immune complex GN induced using these protocols causes a rapid loss of kidney function indicated by severe proteinuria, elevated serum urea nitrogen, and serum creatinine. The two major features distinguishing induced models from spontaneous lupus GN are the absence of circulating autoantibodies, and the lack of progression to chronic GN. However, a careful comparison of early glomerular changes, cellular infiltrates and cytokine profiles has established the validity of induced immune complex GN models as representatives of early pathology in proliferative lupus nephritis 28.
RECENT ADVANCES
Over the last decade, studies in animal models show that glomerular immune complex deposition alone without ensuing inflammation or susceptible genetic background is insufficient for the development of GN and its progression to kidney failure. There is a better understanding of participation by local factors including activation in regional lymph nodes, and synthesis of inflammatory mediators by the kidney. Studies demonstrating a dominant local (renal) influence on induction and progression of GN are discussed below.
Regional Immune Responses in Lupus Nephritis
Mesangial expansion and immune cell recruitment is the hallmark of proliferative lupus nephritis. A careful analysis of GN in MRL lpr/lpr mice at different ages shows that immune complex deposition is associated with a rapid increase in production of MCP1 (monocyte chemoattractant protein 1; encoded by the CCL2 gene) and RANTES (Regulated upon Activation, Normal T-cell Expressed, and Secreted; encoded by the CCL5 gene) by the glomerular mesangial cells30. Thus, the mesangial cells are the “first responders” to immune complex injury. MCP1 and RANTES are potent chemoattractants and initiate subsequent inflammatory cell infiltration. Macrophages, dendritic cells, and CD4+ T cells are the dominant cell types seen in and around the glomerulus31–33. In later stages, some mouse models and patients show CD8+ T cell, B cell and plasma cell infiltrates in the tubulointerstitial regions, correlating with prognosis of the disease.
CD4+ T cells are critical for lupus nephritis, and T cell depletion using anti-CD4 antibodies can prevent GN 34. In NZM2328 female mice, proliferative GN is associated with increased CD4+ T cell activation in the regional kidney draining lymph nodes 33. CD4+ T cells are also increased in the intra- and peri-glomerular regions. With disease progression, there is an antigen-specific expansion of a limited T cell repertoire preferentially in the regional lymph nodes but not in non-draining lymph nodes. Significantly, this repertoire is also expanded within the kidney. Although the antigens have not been identified, a study of one of the antigenic peptide binding regions on the T cell receptors show similar profiles in the regional lymph node and the kidney suggesting that they recognize similar, potentially local antigens. This also explains the prevention of kidney disease in MRL/lpr mice treated with FTY720, a drug that binds sphingosine-1 phosphate receptor on immune cells and prevents their egress from lymph nodes into tissues 35. Inhibition of T cell activation in severely nephritic NZB/W F1 mice also significantly delays fatal GN 36. B7.1 and B7.2 molecules on antigen presenting cells like dendritic cells and B cells bind CD28 on T cells resulting in cytokine production and proliferation. CTLA4 Ig is a recombinant fusion protein that binds B7.1 and B7.2 preventing T cell activation. NZB/W F1 mice with severe proteinuria treated with CTLA4 Ig and suboptimal doses of cyclophosphamide showed a significant increase in survival with 93% of mice surviving 13wks compared to 36% of control mice that received cyclophosphamide alone. All these studies show that local T cell activation and infiltration into the kidney are important events in disease induction and progression. Another study showed that protection by CTLA4Ig did not affect glomerular immune complex and C3 deposition37. Thus, similar to the case, lack of kidney disease progression does not lead to a corresponding reduction in immune complex deposits. A model for the pathogenesis of lupus nephritis is shown in figure 2.
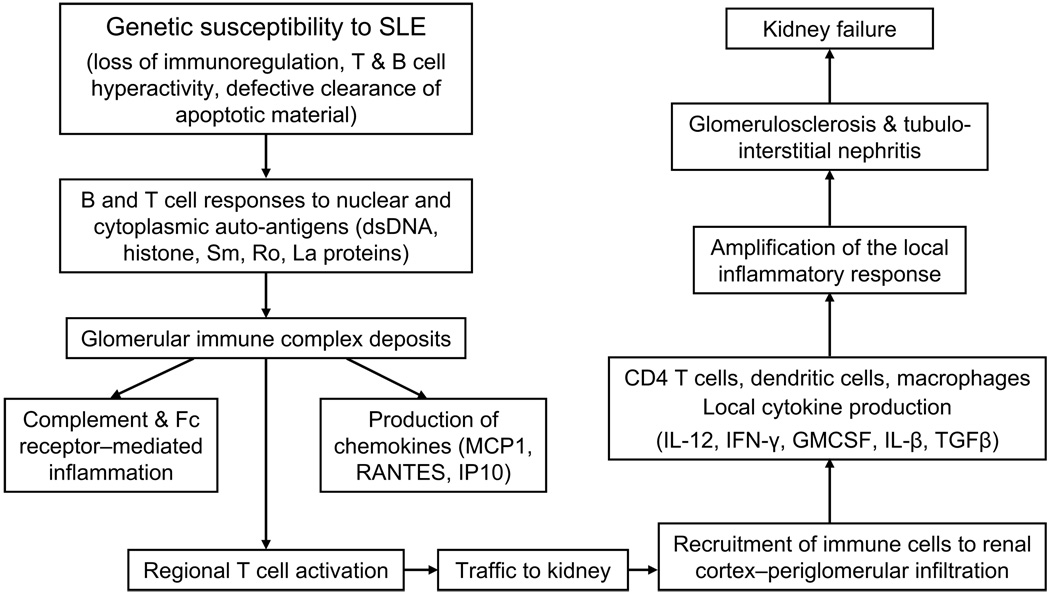
Figure 2.
A model for the pathogenesis of spontaneous lupus nephritis based on studies in mice. Abbreviations: SLE, systemic lupus erythematosus; dsDNA, double-stranded DNA; MCP1, monocyte chemoattractant protein 1; RANTES. Regulated upon Activation, Normal T-cell Expressed, and Secreted; TGFβ, transforming growth factor β; GMCSF, granulocyte-macrophage colony stimulating factor; IL, interleukin; IFN-gamma, interferon gamma.
In addition to local activation of T cells, the susceptibility of the kidney to injury is regulated by other factors. In NZM2328 mice, depletion of thymus derived CD25+ regulatory T cells led to increased autoantibody responses, glomerular immune complexes and early onset of severe proliferative GN in male and female NZM2328 mice by 20wks of age 38. However, by 30 wks only female mice progressed to chronic GN, while males did not. This suggests that besides immune responses, progression of kidney disease is determined by gender dependent end organ factors.
End Organ Responses in Immune Complex GN
Elegant studies in immune complex GN models have explored the factors dictating susceptibility to immune mediated glomerular injury. The importance of genetic susceptibility in development of GN is well established in studies using different strains in both rat and mouse models. Following injection of nephrotoxic serum, Wistar Kyoto (WKY) rats rapidly develop crescentic GN in 80% glomeruli by day 10 while Lewis rats remain resistant to GN 39. These strains share the same MHC haplotype. Bone marrow transfer from WKY into Lewis rats resulted in GN in 35% of the glomeruli suggesting that bone marrow derived cells partially regulated development of GN. To study the contribution of the kidney in disease susceptibility, (WKY × Lewis) F1 rats were transplanted with kidney from either parental strain. Immune complex GN was induced in the F1 recipients and disease severity in the parental kidney transplants was compared. WKY kidneys in the F1 recipients had greater macrophage infiltration and glomerular crescent formation compared to Lewis kidneys. There was no difference in GN in the host F1 kidneys. This difference in parental kidney disease correlated with the ability of WKY mesangial cells to produce higher amounts of MCP1 compared to Lewis mesangial cells. Thus, chemokine production by mesangial cells in response to glomerular immune complex deposits influences kidney disease.
Mohan and colleagues have studied a large panel of inbred mouse strains to investigate the genetic susceptibility to lupus-like GN 40. Mice from a number of different strains including B6, BALB/c, A/J, DBA/1, C3H, C3HeN, C3HeJ, NZW, 129/SvJ, and SWR were immunized with rabbit IgG emulsified in complete Freund adjuvant. Anti-GBM rabbit polyclonal serum was injected and the mice studied for kidney function in the form of proteinuria, serum urea nitrogen, serum creatinine, glomerular immune complexes, renal histo-pathology and immune responses. Despite comparable responses to rabbit IgG immunization, DBA/1, NZW, 129/SvJ, C58 developed severe GN while SJL/J, SWR, NOD did not. To identify the end organ factors dictating susceptibility, 3 susceptible (DBA1, NZW, and 129/SvJ) and 2 control (BALB/c and B6) mouse strains were injected with anti-GBM antibody and kidneys harvested 10 days later 41. RNA was isolated and gene expression evaluated by microarray analyses. Of the 50 genes consistently down-regulated in all the susceptible mouse strains, 10 genes belonged to the kallekrein (Klk) family of genes. The kallekreins are a family of serine proteases with diverse physiologic processes. They act through generation of bradykinins that bind bradykinin receptors. Inhibition of bradykinin receptor activity exacerbated GN in mouse strains resistant to nephritis. The molecular pathways between bradykinin receptor activation and kidney disease are unclear. In mice, the Klk gene locus in on chromosome 7 and falls within a genetic segment linked to lupus susceptibility. In humans, the kallekrein genes are on the orthologous chromosome 19q13 locus which has been previously implicated in lupus susceptibility. Analysis of SNPs from different SLE patient cohorts showed association of SNPs between the KLK1 and KLK3 genes, possibly the KLK3 promoter region. Thus, expression of kallekreins in the kidney may be one of the factors regulating susceptibility to GN.
Targeted Delivery to Renal Glomeruli
Based on the studies discussed above, there is a need to revisit the strategy for treatment of lupus nephritis. While regulating the systemic autoimmune responses is important, increased emphasis on controlling end-organ responses would be a synergistic therapeutic approach. Targeted drug delivery increases concentrations of therapeutic agents at the site of action, maximizing efficacy while minimizing side effects 42. Liposomes are versatile carriers for targeted drug delivery (Figure 3). Liposomes are composed of cholesterols and phospholipids forming a micelle, capable of transporting water soluble compounds in the central core, and lipid soluble compounds within the hydrophobic bilayer 43. The surface of liposomes can be conjugated to antibodies or receptors to target specific cell types. In addition, liposomes can be coated with polyethylene glycols preventing uptake by macrophages and increasing half life in circulation. The unique architecture of the renal glomerulus lends itself to targeted delivery using liposomes. The glomerular capillary is lined by a layer of endothelium with 70 to 130nM fenestrations. In the central portion, the endothelium does not have a basement membrane and rests directly on the mesangium44. Thus, the vascular compartment has a direct communication with the mesangial space. At these sites, liposomes with a diameter less that 130nM can leave the vasculature and deposit in the mesangial space. Away from the center, the glomerular endothelium rests on the basement membrane. The slit diaphragm (pore size of 10–70nM) in the glomerular basement membrane prevents exit of the ~100nM liposomes into the urinary space. Modalities for glomerular delivery of drugs or oligonucleotides capable of regulating local inflammatory responses in animal models are discussed in the below.
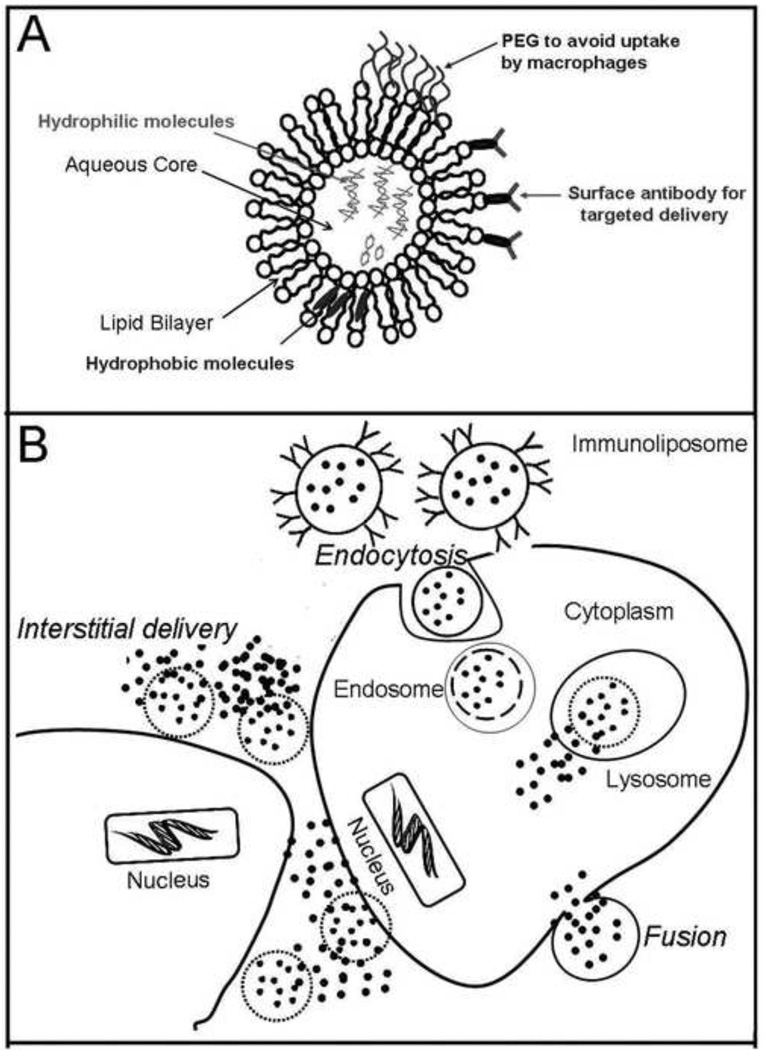
Figure 3.
(A) Illustration showing the liposome, consisting of a lipid bilayer that allows incorporation of hydrophobic molecules and a central aqueous core for hydrophilic molecules. A coat of polyethylene glycol (PEG) prevents uptake by macrophages and cells of the reticulo-endothelial system. Conjugation of antibody on the surface allows preferential targeting to cells of interest. (B) Liposomal contents are delivered into the target cells by fusion of the lipid bilayer with the cell membrane and by receptor or antibody mediated endocytosis. Liposomal contents are also released into the extracellular spaces in the vicinity of the target cell due to destabilization of the lipid bilayer in the interstitium.
Gene Therapy to Regulate Local Inflammation
Activation and proliferation of mesangial cells are the pathologic characteristics of lupus-like GN. Inhibition of proteins in mesangial cells that regulate these processes have been described in rodent models (Table 3). Liposomes incorporated with UV inactivated Sendai Virus or Hemagglutinating Virus of Japan (HVJ) have been used to deliver decoy oligonucleotides 53–54. Decoy oligonucleotides consist of synthetic transcription factor specific sequences. Flooding the cell with decoy oligonucleotides prevents binding of the transcription factor with specific sequences on promoter regions on target genes. E2F is a transcription factor in mesangial cells that is important for activating proliferating cell nuclear antigen and cyclin dependent kinase 2 in the cell cycle pathway 46. Thus, injection of HVJ liposomes loaded with decoy oligonucleotides to E2F prevented mesangial cell proliferation in a rat model of immune complex GN. Small interfering RNAs and anti-sense oligonucleotides that prevent translation of target proteins have also been successfully used in models of GN and are listed in Table 3. Potential target pathways for intervention in human disease include nuclear factor-κB (NFκB), platelet-derived growth factor (PDGF), and TGFβ 55.
Table 3.
Potential therapeutic targets/pathways for modulation of pathogenic glomerular responses in glomerular diseases
| MODEL | Target | Agent | Therapeutic effect in kidney |
Reference |
|---|---|---|---|---|
| Thy1.1 induced GN | EGR1 | Antisense ODN | reduced mesangial cell proliferation | 49 |
| E2F | Decoy ODN | reduced PCNA and cdk2 kinase | 50 | |
| AP1 | Decoy ODN | reduced TGFβ, PAI, mesangial cell proliferation, ECM production | 51 | |
| MAPK1 | siRNA | Reduced TGFβ, glomerulosclerosis, PAI, ECM production | 52 | |
| anti-GBM nephritis | NFκB | Decoy ODN | Reduced IL-1β, ICAM, inflammation, proteinuria | 53 |
| Anti-E selectin immunoliposomes | dexamethasone | Reduced ICAM, inflammation | 54,55 | |
| Streptozotocin induced diabetic nephropathy | TGFβ | Antisense ODN | Reduced TGFβ in urine and kidney | 56 |
| SP1 | Decoy ODN | Reduced type IV collagen, fibronectin, α-smooth muscle actin | 57 |
Abbreviations: cdk2, cyclin dependent kinase 2; ODN, oligodeoxynucleotide; PCNA, proliferating cell nuclear antigen; EGR1, early growth response 1; GN, glomerulonephritis; siRNA, small interfering RNA; TGFβ, transforming growth factor β; GBM, glomerular basement membrane; IV, intravenous; ECM, extracellular matrix; NFκB, nuclear factor-κB; MAPK, mitogen-activated protein kinase; PAI, plasminogen activator inhibitor; IL-1β, interleukin 1β.
HVJ liposomes have been used extensively for DNA delivery in gene therapy 53–54. The HVJ proteins on the liposomes bind cell surface sialic acid receptors and induce cell fusion. A disadvantage is the lack of tissue specificity in targeting. Therefore, glomerular delivery of HVJ liposomes requires cannulation of the renal artery, clamping of the proximal segment, followed by slow infusion of the DNA liposome solution. After injection, the clamps are removed to resume normal flow. Although this route of administration is shown to preferentially deliver DNA to the mesangial cells, it is currently not a realistic method for human application. However, these studies demonstrate the possibility that modulating glomerular responses is a viable therapeutic approach for kidney disease.
Immunoliposomal Delivery Systems
Antibodies to cell surface molecules can be conjugated to the surface of liposomes to make immunoliposomes. In rats, mesangial cells express Thy1.1 glycoprotein on their surface56. Therefore, liposomes with antibody to Thy1.1 on their surface (anti-Thy1.1 immunoliposomes) can target specifically to the glomerular mesangium in rats. However, no unique surface markers have been identified on murine (or human) mesangial cells to date. To develop a strategy for liposomal delivery to murine (and human) mesangium, we screened a panel of molecules expressed on the surface of mesangial cells and selected alpha 8 (α8) integrin as a potential candidate target 57. The α8 integrin combines with β1 integrin forming a heterodimer that interacts with extracellular matrix proteins 58. Unlike other integrin molecules, α8 integrin is not expressed on vascular endothelium and has a restricted tissue distribution.
Antibody to α8 integrin was conjugated to the surface of liposomes and the anti-α8 integrin immunoliposomes (anti-α8 immunoliposomes) passed through filters to obtain ~100nM particles 57. When injected into mice, anti-α8 immunoliposomes loaded with a red fluorescent dye could be seen to preferentially accumulate in the glomerular mesangial space. Localization of the fluorescent dye in the glomerulus showed uptake of liposomes into the mesangial cell cytoplasm. Thus, anti-α8 immunoliposomes can be used for delivery of drugs into the mesangial space and also into the mesangial cell cytoplasm. This strategy has also been successfully adapted to deliver proteins to the glomerular mesangium in normal mice 59. Significantly, there is no reduction in expression of α8 integrin on mesangial cells even with the development of GN. Thus, anti-α8 immunoliposomes are viable carriers of therapeutic agents to diseased glomeruli.
Another strategy used for glomerular delivery of drugs is to target immunoliposomes to adhesion molecules like E-selectins on activated endothelial cells50. Anti-E-selectin antibody conjugated to the surface of dexamethasone loaded liposomes was injected in an induced model of immune complex GN. A modification here was co-injection of recombinant TNF-α to further induce upregulation of E selectin on the endothelial cells. The dexamethasone loaded anti-E selectin ILs were detected mainly in the kidney with some accumulation in the liver and heart. Severity of kidney disease was lowered in anti-E selectin loaded with dexamethasone compared to free dexamethasone. In systemic diseases, the anti-E-selectin immunoliposomes would not be restricted to glomerular drug delivery but potentially target all other inflamed tissues associated with activated endothelium in systemic diseases.
immunoliposomes targeting tumor antigens are currently in clinical trials for delivery of cytotoxic drugs in cancer 60. However, the ability to deliver drugs to the site of inflammation in chronic kidney disease offers a novel approach. The ability for targeted glomerular delivery by anti-α8 immunoliposomes has potential application in human therapy. Local delivery will reduce the drug doses required for therapy. Significantly, this approach may be extended to all glomerular diseases.
SUMMARY
The heterogeneity of presentations and diversity in the natural course of disease makes the study and treatment of lupus nephritis challenging. Current treatments in lupus nephritis focus on treatment with anti-inflammatory and immunosuppressive drugs. Animal models have been valuable in providing insights into the underlying pathogenic mechanisms. Recent data suggest an important role for the end organ/glomerular responses in dictating disease progression. Regulation of these responses locally presents a novel therapeutic approach. Immunoliposomal systems that can be used for delivery of drugs specifically to the glomeruli have been developed. These delivery systems can potentially be adapted for human therapy and require critical evaluation.
ACKNOWLEDGEMENTS
Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK69769 and Alliance for Lupus Research grant TIL#113300.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
REFERENCES
- 1.Cameron JS. Systemic Lupus Erythematosus. In: Neilson EG, Couser WG, editors. Immunologic Renal Diseases. 2nd ed. Philadelphia PA: Lippincott Williams and Wilkins; 2001. pp. 1057–1104. [Google Scholar]
- 2.Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenylate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong Guangzhou Nephrology Study Group. N Engl J Med. 2000;343(16):1156–1162. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 3.Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350(10):971–980. doi: 10.1056/NEJMoa031855. [DOI] [PubMed] [Google Scholar]
- 4.Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J Immunol. 2005;174(4):1775–1781. doi: 10.4049/jimmunol.174.4.1775. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. J Immunol. 2003;171(7):3325–3331. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- 6.Zielinski CE, Jacob SN, Bouzahzah F, Ehrlich BE, Craft J. Naive CD4+ T cells from lupus-prone Fas-intact MRL mice display TCR-mediated hyperproliferation due to intrinsic threshold defects in activation. J Immunol. 2005;174(8):5100–5109. doi: 10.4049/jimmunol.174.8.5100. [DOI] [PubMed] [Google Scholar]
- 7.Cervera R, Khamashta MA, Font J, et al. European Working Party on Systemic Lupus Erythematosus. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82(5):299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 8.Pollack VE, Pirani CL. Renal histologic findings in Systemic Lupus Erythematosus. Mayo Clin. Proc. 1969;44(9):630–644. [PubMed] [Google Scholar]
- 9.Crispín JC, Liossis SN, Kis-Toth K, et al. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol Med. 2010;16(2):47–57. doi: 10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yurasov S, Tiller T, Tsuiji M, et al. Persistent expression of autoantibodies in SLE patients in remission. J Exp Med. 2006;203(10):2255–2261. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189(10):1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2(1):20–27. doi: 10.1038/ncprheum0042. [DOI] [PubMed] [Google Scholar]
- 13.Furie R, Looney RJ, Rovin B, et al. efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (Ln): results from therandomized, double-blind phase iii LUNAR study. Arthritis Rheum. 2009;60(10):S1149271. [Google Scholar]
- 14.Harman C. Glomerular Disease: Lupus nephritis trials end in disappointment. Nature Reviews Nephrology. 2009;5(6):303. [Google Scholar]
- 15.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, Zhang D, Brunetta PG. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010 Jan;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawabata D, Venkatesh J, Ramanujam M, Davidson A, Grimaldi CM, Diamond B. Enhanced selection of high affinity DNA-reactive B cells following cyclophosphamide treatment in mice. PLoS One. 2010;5(1):e8418. doi: 10.1371/journal.pone.0008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 2010;62(5):1457–1468. doi: 10.1002/art.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dall'Era M, Wofsy D. Systemic lupus erythematosus clinical trials-an interim analysis. Nat Rev Rheumatol. 2009;5(6):348–351. doi: 10.1038/nrrheum.2009.79. [DOI] [PubMed] [Google Scholar]
- 19.Dall'Era M, Wofsy D. Connective tissue diseases: Belimumab for systemic lupus erythematosus: breaking through? Nat Rev Rheumatol. 2010;6(3):124–125. doi: 10.1038/nrrheum.2010.20. [DOI] [PubMed] [Google Scholar]
- 20.Waters ST, McDuffie M, Bagavant H, et al. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004;199(2):255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kono DH, Theofilopoulos AN. Genetics of SLE in mice. Springer Semin Immunopathol. 2006;28(2):83–96. doi: 10.1007/s00281-006-0030-7. [DOI] [PubMed] [Google Scholar]
- 22.Waters ST, Fu SM, Gaskin F, et al. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100(3):372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 23.Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE. Differences in expression of lupus nephritis in New Zealand mixed H-2z-homozygous inbred strains of mice derived from New Zealand Black and New Zealand White mice. Lab. Invest. 1993;68(4):419–425. [PubMed] [Google Scholar]
- 24.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1(3):219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 25.Vidal S, Kono DH, Theofilopoulous AN. Loci predisposing to autoimmunity in MRL-Fas lpr and C57BL/6-Faslpr mice. J Clin Invest. 1998;101(3):696–702. doi: 10.1172/JCI1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birmingham DJ, Rovin BH, Yu CY, Hebert LA. Of mice and men: The relevance of the mouse to the study of human SLE. Immunologic Res. 24(2):211–224. doi: 10.1385/IR:24:2:211. 200. [DOI] [PubMed] [Google Scholar]
- 27.Vlahakos DV, Foster MH, Adams S, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41(6):1690–1700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 28.Fu Y, Du Y, Mohan C. Experimental anti-GBM disease as a tool for studying spontaneous lupus nephritis. Clin Immunol. 2007;124(2):109–118. doi: 10.1016/j.clim.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Christensen M, Su AW, Snyder RW, Greco A, Lipschutz JH, Madaio MP. Simvastatin protection against acute immune-mediated glomerulonephritis in mice. Kidney Int. 2006;69(3):457–463. doi: 10.1038/sj.ki.5000086. [DOI] [PubMed] [Google Scholar]
- 30.Lema P, Maier H, Nieto E, et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol. 2001;12(7):1369–1382. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: Correlation of interstitial cells with glomerular function. Kidney Int. 1990;37(1):100–109. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- 32.Diaz Gallo C, Jevnikar AM, Brennan DC, Florquin S, Pacheco-Silva A, Kelley VR. Autoreactive kidney-infiltrating T-cell clones in murine lupus nephritis. Kid Int. 1992;42(4):851–859. doi: 10.1038/ki.1992.360. [DOI] [PubMed] [Google Scholar]
- 33.Bagavant H, Deshmukh US, Wang H, Ly T, Fu SM. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J Immunol. 2006;177(11):8258–8265. doi: 10.4049/jimmunol.177.11.8258. [DOI] [PubMed] [Google Scholar]
- 34.Connolly K, Roubinian JR, Wofsy D. Development of murine lupus in CD4− depletedNZB/NZW mice. Sustained inhibition of residual CD4+ T cells is required to suppress autoimmunity. J Immunol. 1992;149(9):3083–3088. [PubMed] [Google Scholar]
- 35.Wenderfer SE, Stepkowski SM, Braun MC. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 2008;4(10):1319–1326. doi: 10.1038/ki.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daikh DI, Wofsy D. Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J. Immunol. 2001;166(5):2913–2916. doi: 10.4049/jimmunol.166.5.2913. [DOI] [PubMed] [Google Scholar]
- 37.Schiffer L, Sinha J, Wang X, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171(1):489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 38.Bagavant H, Tung KS. Failure of CD25+ T cells from lupus-prone mice to suppress lupus glomerulonephritis and sialoadenitis. J Immunol. 2005;175(2):944–950. doi: 10.4049/jimmunol.175.2.944. [DOI] [PubMed] [Google Scholar]
- 39.Smith J, Lai PC, Behmoaras J, et al. Genes expressed by both mesangial cells and bone marrow-derived cells underlie genetic susceptibility to crescentic glomerulonephritis in the rat. J Am Soc Nephrol. 2007;18(6):1816–1823. doi: 10.1681/ASN.2006070733. [DOI] [PubMed] [Google Scholar]
- 40.Xie C, Sharma R, Wang H, Zhou XJ, Mohan C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J Immunol. 2004;172(8):5047–5055. doi: 10.4049/jimmunol.172.8.5047. [DOI] [PubMed] [Google Scholar]
- 41.Liu K, Li QZ, Delgado-Vega AM, Abelson AK, et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J Clin Invest. 2009;119(4):911–923. doi: 10.1172/JCI36728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morimoto K, Kondo M, Kawahara K, et al. Advances in targeting drug delivery to glomerular mesangial cells by long circulating cationic liposomes for the treatment of glomerulonephritis. Pharma Research. 2007;24(5):946–954. doi: 10.1007/s11095-006-9213-0. [DOI] [PubMed] [Google Scholar]
- 43.Bangham A. Surrogate Cells or Trojan Horses: The discovery of liposomes. Bioessays. 1995;17(21):1081–1088. doi: 10.1002/bies.950171213. [DOI] [PubMed] [Google Scholar]
- 44.Madsen K, Tisher CC. Anatomy of the kidney. Elements of Normal Renal Structure and Function. In: Brenner BM, editor. Brenner & Rector's The Kidney. 7th ed. Philadephia PA: WB Saunders Company; 2004. pp. 3–72. [Google Scholar]
- 45.Carl MY, Akagi S, Weidner S, Isaka Y, Imai E, Rupprecht HD. Specific inhibition of Egr-1 prevents mesangial cell hypercellularity in experimental nephritis. Kidney Int. 2003;63(4):1302–1312. doi: 10.1046/j.1523-1755.2003.00865.x. [DOI] [PubMed] [Google Scholar]
- 46.Tomita N, Kim JY, Gibbons GH, et al. Gene therapy with an E2F transcription factor decoy inhibits cell cycle progression in rat anti-Thy 1 glomerulonephritis. Int J Mol Med. 2004;13(5):629–636. [PubMed] [Google Scholar]
- 47.Ahn JD, Morishita R, Kaneda Y, et al. Transcription factor decoy for AP-1 reduces mesangial cell proliferation and extracellular matrix production in vitro and in vivo. Gene Ther. 2004;11(11):916–923. doi: 10.1038/sj.gt.3302236. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu H, Hori Y, Kaname S, et al. siRNA-based therapy ameliorates glomerulonephritis. J Am Soc Nephrol. 2004;21(4):622–633. doi: 10.1681/ASN.2009030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaneda Y, Saeki Y, Morishita R. Gene therapy using HVJ liposomes: the best of both worlds. Mol Med Today. 1999;5(7):298–303. doi: 10.1016/s1357-4310(99)01482-3. [DOI] [PubMed] [Google Scholar]
- 50.Asgeirsdóttir SA, Zwiers PJ, Morselt HW, et al. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded Ab Esel liposomes. Am J Physiol Renal Physiol. 2008;294(3):554–561. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 51.Jeong HS, Park KK, Kim SP, Choi IJ, Lee IK, Kim HC. Effect of antisense TGF-beta1 oligodeoxynucleotides in streptozotocin induced diabetic rat kidney. J Korean Med Sci. 2004;19(3):374–383. doi: 10.3346/jkms.2004.19.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang JH, Chae YM, Park KK, Kim CH, Lee IS, Chang YC. Suppression of mesangial cell proliferation and extracellular matrix production in streptozotocin-induced diabetic rats by Sp1 decoy oligodeoxynucleotide in vitro and in vivo. J Cell Biochem. 2008;103(2):663–674. doi: 10.1002/jcb.21440. [DOI] [PubMed] [Google Scholar]
- 53.Tomita N, Higaki J, Morishita R, et al. Direct in vivo gene introduction into rat kidney. Biochem Biophys Res Commun. 1992;186(1):129–134. doi: 10.1016/s0006-291x(05)80784-3. [DOI] [PubMed] [Google Scholar]
- 54.Tomita N, Morishita R, Yamamoto K, et al. Targeted gene therapy for rat glomerulonephritis using HVJ-immunoliposomes. J Gene Med. 2002;4(5):527–535. doi: 10.1002/jgm.300. [DOI] [PubMed] [Google Scholar]
- 55.Scindia YM, Deshmukh US, Bagavant H. Mesangial pathology in glomerular disease: Targets for therapeutic intervention [published online ahead of print September 6, 2010] Adv Drug Deliv Rev. doi: 10.1016/j.addr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuffin G, Waelti E, Huwyler J, Hammer C, Marti HP. Immunoliposome Targeting to Mesangial Cells: A Promising Strategy for Specific Drug Delivery to the Kidney. J Am Soc Nephrol. 2005;16(11):3295–3305. doi: 10.1681/ASN.2005050485. [DOI] [PubMed] [Google Scholar]
- 57.Scindia Y, Deshmukh U, Thimmalapura PR, Bagavant H. Anti–α8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: a novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus. Arthritis Rheum. 2008;58(12):3884–3891. doi: 10.1002/art.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnapp LM, Breuss JM, Ramos DM, Sheppard D, Pytela R. Sequence and tissue distribution of the human integrin alpha subunit: beta1-associated alpha subunit expressed in smooth muscle cells. J Cell Sci. 1995;108(2):537–544. doi: 10.1242/jcs.108.2.537. [DOI] [PubMed] [Google Scholar]
- 59.Bagavant H, Scindia Y, Nandula S, Deshmukh U. Role of T cell mediated inflammation in lupus-like glomerulonephritis (GN) J. Immunol. 2010;184:93.40. [AAI abstract 93.40] [Google Scholar]
- 60.Park JW, Hong K, Kirpotin DB, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–1181. [PubMed] [Google Scholar]