Abstract
Loss of synapses and synaptic damage are the best correlates of cognitive decline identified in patients with Alzheimer’s disease (AD), and mitochondrial oxidative damage and synaptic pathology have been identified as early events in the progression of AD. The progressive accumulation of amyloid beta (Aβ) in synapses and synaptic mitochondria are hypothesized to cause synaptic degeneration and cognitive decline in patients with AD. However, the precise mechanistic link between Aβ and mitochondria is not well understood. The purpose of this study was to better understand the effects of Aβ on mitochondrial axonal transport and synaptic alterations in AD. Using mouse hippocampal neurons and Aβ25-35 peptide, we studied axonal transport of mitochondria, including mitochondrial motility, mitochondrial length and size, mitochondrial index per neurite, and synaptic alterations of the hippocampal neurons. In the PBS-treated neurons, 36.4 ± 4.7% of the observed mitochondria were motile, with 21.0 ± 1.3% moving anterograde and 15.4 ± 3.4% moving retrograde and the average speed of movement was 12.1 ± 1.8 μm/min. In contrast, in the Aβ-treated neurons, the number of motile mitochondria were significantly less, at 20.4 ± 2.6% (P<0.032), as were those moving anterograde (10.1 ± 2.6%, P<0.016) relative to PBS-treated neurons, suggesting that the Aβ25-35 peptide impairs axonal transport of mitochondria in AD neurons. In the Aβ-treated neurons, the average speed of motile mitochondria was also less, at 10.9 ±1.9 μm/min, and mitochondrial length was significantly decreased. Further, synaptic immunoreactivity was also significantly less in the Aβ-treated neurons relative to the PBS-treated neurons, indicating that Aβ affects synaptic viability. These findings suggest that, in neurons affected by AD, Aβ is toxic, impairs mitochondrial movements, reduces mitochondrial length, and causes synaptic degeneration.
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in the aged population. It is characterized by the progressive decline of memory, decline in cognitive functions such as memory, and changes in behavior and personality [1]. It is a major health concern in society mainly, especially because half of individuals 85 years of age and older are expected to develop AD [2]. AD is associated with multiple cellular changes in the brain, including the loss of synapses/synaptic pathology, mitochondrial structural/functional abnormalities, inflammatory responses, extracellular amyloid beta (Aβ) deposits, and intracellular neurofibrillary tangles [1,3–10]. Among these changes, mitochondrial oxidative damage and synaptic pathology are reported as early events in AD progression [11–15].
In AD, Aβ is a major component of neuritic plaques found in brain regions known to be responsible for learning and memory. Aβ, the 39–43 amino acid residue protein, is generated by the proteolysis of Aβ precursor protein (AβPP) by the sequential enzymatic actions β-secretase, and γ secretase. In AD, levels of Aβ are steady-state and are controlled by the production, the clearance, and the degradation of Aβ. Decreased clearance of Aβ or the overproduction of Aβ may lead to an accumulation of Aβ in subcellular compartments, including synapses, mitochondria, and may impair functions of subcellular organelles and damage neurons [4,5,16]. Thus, agents or drugs capable of increasing the clearance or decreasing the production of Aβ may be important therapeutic strategies in preventing AD development and progression.
Several researchers have reported that Aβ is responsible for damaging mitochondria and synapses in neurons affected by AD [17–24]. Monomeric and oligomeric forms of Aβ have been found in mitochondrial membranes and to interact with mitochondrial proteins, induce free radical production, alter mitochondrial enzymes, disrupt the electron transport chain, inhibit adenosine triphosphate (ATP) production, and damage mitochondria [17–19,24–26]. Although these results clearly associate Aβ with subcellular components such as mitochondria, the precise link between Aβ and mitochondria is not clear and how Aβ damages mitochondria is also not well understood.
Mitochondrial shape and structure are maintained by mitochondrial fission and fusion [27–29]. Fission and fusion mechanisms are equally balanced in healthy neurons. Mitochondria alter their shape and size to move, through mitochondrial trafficking, from the cell body to the axons, dendrites and synapses via anterograde fashion, and back to the cell body via retrograde manner [30]. Synapses are the sites of high-energy demand. Mitochondria supply energy to synapses, which is required for neural communications and several synaptic functions, including the release of neurotransmitters, synaptic vesicles between pre- and post-synaptic neurons. In a healthy neuron, abundant numbers of healthy, functionally active mitochondria are present at synapses, whereas in diseased neurons, such as in AD-affected neurons, small numbers of defective mitochondria are present [31]. In the AD neuron, Aβ accumulates in the mitochondria, induces free radical production, and activates the two mitochondrial fission proteins dynamin-related protein 1 (Drp1) and mitochondrial fission 1 (Fis1) [29]. Activated Drp1 and Fis1 have been associated with excessive fragmentation of mitochondria that may not move to synapses and do not supply the necessary ATP at nerve terminals. Further, these defective mitochondria may not be able to participate in mitochondrial fusion, may not be able to synthesize healthy mitochondria, and may, ultimately, prematurely die. The continual production of excessive numbers of defective mitochondria in neurons may ultimately damage synapses and cause synaptic neurodegeneration [29,31].
Using electron and confocal microscopy, gene expression analysis, and biochemical methods, we treated mouse neuroblastoma (N2a) cells with the Aβ25-35 peptide, and studied mitochondrial structure and function; expressions of the fission genes Drp1 and Fis1 and the 3 fusion genes Mfn1, Mfn2, and Opa1; and neurite outgrowth in [32]. In the neurons treated with only Aβ, we found increased expressions of fission genes and decreased expressions of fusion genes, indicating abnormal mitochondrial dynamics in neurons treated with Aβ. Our immunocytochemistry of N2a cells treated with Aβ revealed increased immunoreactivity of Drp1 and Fis1, suggesting that Aβ elevates fission genes and fragments mitochondria. Electron microscopy of the N2a cells incubated with Aβ revealed a significantly increased number of defective mitochondria, indicating that Aβ fragments mitochondria. Biochemical analysis revealed Aβ in association with defective mitochondria. Neurite outgrowth was significantly decreased in those N2a cells that we incubated with Aβ, indicating that Aβ affects neurite outgrowth.
In the research reported here, we sought to determine the effects of Aβ on axonal transport of mitochondria in mouse hippocampal neurons. We measured the total number of mitochondria, the length and size of mitochondria, the mitochondrial index (length of the mitochondria per neurite length), and the synaptic immunoreactivity of AD neurons treated with the Aβ25-35 peptide compared to control neurons (Aβ35-25) and those treated with the vehicle (PBS).
Materials and Methods
Neuronal Culture
Hippocampal neurons were cultured, as previously described [32]. Briefly, hippocampi were dissected from C57BL/6 day 1 pups in room temperature HABG (Hibernate E medium [Brain Bits, LLC] supplemented with 1X B-27 [Invitrogen] and 0.5 mM glutamine [Invitrogen, CA]). The tissues were dissociated with 2 mg/ml papain at 30°C for 30min in a dissociation medium (Hibernate E medium without Ca++, supplemented with 0.5 mM glutamine). Digested tissue was triturated, using a fire-polished, silicon-coated Pasteur pipette in 2 ml HABG. Non-dissociated tissue was allowed to settle for 5 min, after which time the supernatant was passed through a 70-mm nylon mesh, into a 50-mL conical tube and centrifuged for 2 min at 200g. The pellet was gently resuspended in a maintenance medium (Neurobasal A medium [Invitrogen, CA] supplemented with 1X B-27 minus antioxidants, and 0.5 mM L-glutamine) and plated onto a poly-D-lysine (Sigma-Aldrich) –coated, chambered coverglass (Nunc). The medium was completely replaced after 1 hr, and then half of the medium was replaced every 3days.
Time Lapse Photography of Mitochondrial Motility and Data Analysis
Mitochondria were labeled by transfecting pDsRed2-mito (Clontech) into the hippocampal neurons at day 2 (DIV) with lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol. DsRed-labeled neuronal mitochondria were observed in cultures up to 21 days after transfection (data not shown). Aβ25-35 Aβ35-25 (control) peptides were dissolved in 1XPBS. To increase the toxicity of Aβ peptide, we incubated Aβ25-35, and Aβ35-25 (control) peptides at 37°C for overnight, and this overnight incubation is expected to convert monomeric Aβ to oligomeric Aβ. We treated neurons with 20 μM Aβ25-35 (oligomeric) Aβ35-25 (control), and a vehicle (PBS) at 10 DIV and then imaged 24 hours later. Axonal processes were determined by morphological characteristics. Axons were identified as processes stemming from the soma that are two to three times longer than other processes [32,33]. Recordings were made on axonal segments about 20–100 μm from the soma. Series of time-lapse images were captured every 5 sec, using a Leica SP5 AOBS confocal microscope with a heated 37°C, 5% CO2 controlled stage for a total of 5min. Z-stacks for each time point were collapsed to maximum projections, and the time series were archived as avi files. ImageJ software, with a Mulitple Kymograph plug-in, was used to analyze the avi files. Mitochondrial movements (direction and speed) were determined from the kymographic images. Mitochondria were considered stationary if they did not move more than 2 μm during the entire recording period. Each series of images was recorded for at least three randomly selected Ds-Red-mito labeled cells per culture and four independent cultures per condition.
Mitochondrial Content of Neurites
After imaging the mitochondria in the neuronal cultures, the cultures were fixed with 4% paraformaldehyde for 5 min at room temperature and then washed with PBS. The plastic chambers were carefully removed, and the coverslips were mounted on slides with a ProLong Gold mounting medium. Images of cell bodies with neurites extending at least 100 μm were collected, using a Leica SP5 AOBS confocal microscope with a 63× objective. GFP and Ds-Red were analyzed using measurement tools in ImageJ to determine the neurite mitochondrial index, the average mitochondrial length, and the number of mitochondria per neurite length). Data were collected from at least six cells per culture and four independent cultures per condition
Immunofluoresence analysis of synaptic proteins
To determine the toxicity of Aβ in hippocampal neurons, we performed immunocytochemical analysis of Drp1with mitochondria-encoded protein Cytochrome b (Cyt. B), and synaptophysin, which is marker for AD, with microtubule associated protein 2 (MAP2). Briefly, we plated cells on 13-mm round coverslips coated with poly-D-lysine contained within wells of a 24-well plate. After treatment, the medium was removed, and cells were fixed with 4% paraformaldehyde in PBS for 10–15 min at room temperature. Coverslips were washed with PBS, and cell membranes were permeablized with 0.1% Triton X-100 in PBS for 5 min, after which time a blocking solution was applied (2% normal goat serum, 1% BSA in PBS). All subsequent incubations were carried out in a humidified environment. Samples were blocked for 2 hr at room temperature and then incubated with a primary antibody diluted in a blocking solution overnight at 4°C. Drp1 (1:200, rabbit polyclonal, Novus Biologicals, Inc.), and synaptophysin (1:200, mouse monoclonal, Millipore/Chemicon, Temecula, CA) were probed. After cells were incubated with the primary antibody, cells were washed three times with PBS and then incubated with either goat-anti-rabbit-Alexa488 or goat-anti-mouse-Alexa568 (both 1:500, Invitrogen/Molecular Probes) for 2 hr at room temperature. For co-labeling with Cyt. B (1:50, mouse monoclonal, Invitrogen) or MAP2 (1:1000, rabbit polyclonal, Milipore), cells were then incubated with the second primary antibody for 3 hr at room temperature. After washing three times with PBS, coverslips were incubated with the corresponding secondary antibody for 2 hr at room temperature. Antibody was removed, and DAPI (600nM in PBS) was added to the cells for 5 min. Cells were washed three times in PBS, and then coverslips were mounted on slides using a ProLong Gold antifade mounting reagent (Invitrogen). Cells were imaged, using a Zeiss Axioskop 40 FL microscope.
Statistical Analysis
Data from independent cultures were compared, using a two-tailed, unpaired student’s t-test with significance level set at p ≤ 0.05.
Results
Mitochondrial motility is less after Aβ treatment
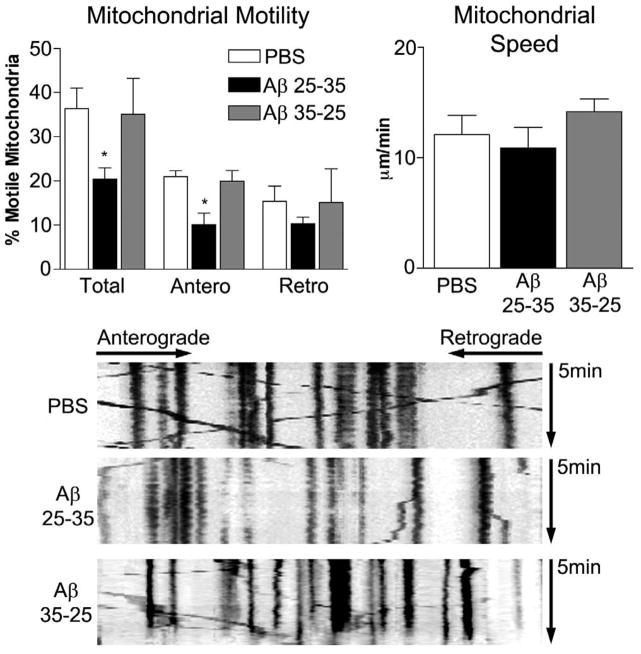
To determine the effect of Aβ peptide on mitochondrial transport, we incubated hippocampal neurons with 20 μM Aβ25-35, Aβ35-25 (control), or PBS for 24 hours, and then quantified mitochondrial motility in axons. The average speed of mitochondrial movement was 12.1 ± 1.8 mm/min. In the Aβ25-35-treated cultures, we observed significantly decreased total mitochondrial motility (20.4 ± 2.6% – mean ± SE, P<0.032) and mitochondria moving anterograde (10.1 ± 2.6% – mean ± SE, P<0.016) relative to the PBS vehicle-treated neurons. A decrease in retrograde-moving mitochondria was also observed (10.3 ± 1.5% – mean ± SE) in the Aβ25-35-treated neurons relative to the PBS vehicle-treated neurons. However, this decrease did not reach statistical significance (P=0.234). The average speed of mitochondria undergoing the Aβ25-35 treatment was only slightly decreased (10.9 ±1.9μm/min – mean ± SE, P = 0.66) compared to the mitochondria from the vehicle-treated neurons. In the PBS vehicle-treated neurons, 36.4 ± 4.7% (mean ± SE) of the observed mitochondria were motile, with 21.0 ± 1.3% moving anterograde and 15.4 ± 3.4% moving retrograde.
As shown in Fig. 1, the Aβ35-25-treated neurons (the controls) did not show any change in total mitochondrial motility – neither anterograde nor retrograde– relative to the PBS vehicle-treated neurons, indicating that mitochondrial alterations are specific to Aβ25-35 specific.
Figure 1. Amyloid beta treatment reduces mitochondrial movements.
Axons from mature hippocampal neurons transfected with DsRed-mito and GFP, then treated for 24 hours with vehicle, Aβ, or reverse peptide were imaged to evaluate mitochondrial movements. Total proportion of moving mitochondria, proportion of mitochondria moving anterograde, and proportion of mitochondria moving retrograde were calculated (A). The speed of motion was also calculated for all moving mitochondria (B). Calculations were based on analysis of kymographs. Representative kymographs are shown for the three experimental groups (C). N= 4 independent cultures. * p < 0.05 compared to vehicle treated, and statistical variation is shown as mean±SE.
Mitochondrial distribution is altered after Aβ treatment
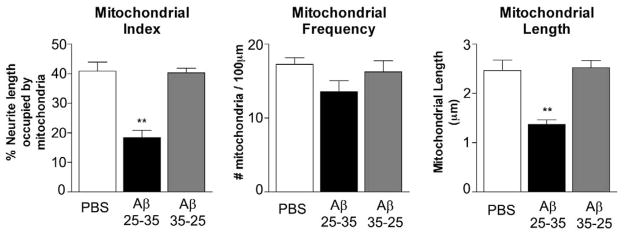
Following our observations of the decrease in overall anterograde mitochondrial motility, we examined whether Aβ treatment also induced changes in mitochondrial distribution within neurites. Cultures treated for 24 hours with the PBS vehicle or the Aβ25-35 were harvested, and the neuritic mitochondrial content was analyzed. We found that Aβ treatment significantly decreased the mitochondrial index in neurons treated with Aβ (40.1 ± 3.1%) relative to the PBS-treated (PBS, 40.1 ± 3.1% – mean ± SE; Aβ, 18.4 ± 2.5% – mean ± SE, P < 0.001) (Figs.2 and 3). We found the mitochondrial index did not change in neurons treated with Aβ35-25 peptide, indicating that the decrease in the mitochondrial index that we found in neurons treated Aβ25-35 peptide is, indeed, associated with Aβ.
Figure 2. Amyloid beta treatment causes a reduction in mitochondrial mass within neurites.
Mitochondria from DsRed-mito transfected hippocampal neurons were analyzed after vehicle, A-beta, or reverse peptide treatment. Mitochondrial index was calculated as the percent of neuritic length occupied by mitochondria. Aβ treated cultures showed pronounced reduction in mitochondrial index (A). The distribution of mitochondria was evaluated as number of mitochondria per neuritic length (B). Mitochondrial length was reduced by Aβ treatment (C). N = 4 independent cultures. ** p < 0.01. Statistical variation is shown as mean±SE.
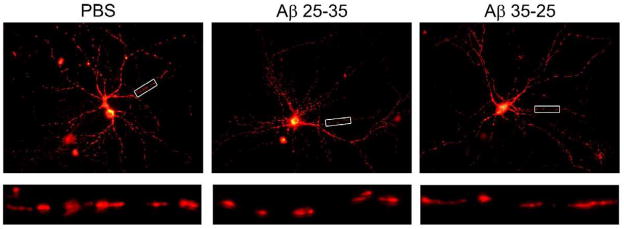
Figure 3. Mitochondria are more fragmented after Amyloid beta treatment.
DsRed-mito transfected hippocampal neurons were imaged after vehicle, Aβ, or reverse peptide treatment. Representative images are shown in upper panels. Enlargements of neurites are shown in the lower panels.
The mitochondrial index reflects the total mass of mitochondria per unit length of a neurite and can be affected by changes in either the number or the size of mitochondria. We therefore analyzed mitochondrial length and density (the number of mitochondria per length of). As shown in Fig. 2, the average mitochondrial length was significantly decreased in neurons with the Aβ peptide relative to the PBS-treated neurons (PBS, 2.47 ± 0.21 μm – mean ± SE; Aβ, 1.37 ± 0.10 μm – mean ± SE, P < 0.001) (Fig. 2). Mitochondrial density decreased from 17.3 ± 0.9 mitochondria/100 mm in the PBS-treated neurons to 13.6 ± 1.5 mitochondria/100mm relative to the Aβ-treated neurons (P = 0.054). Overall, we found that mitochondrial mass was greatly reduced after receiving Aβ treatment relative PBS vehicle treated control neurons, and that this effect is largely due to decreased mitochondrial size (Figs. 2 and 3). As shown in Fig. 3, we found fragmented mitochondria in Ds-red labeled hippocampal neurons treated with Aβ relative to PBS treated control neurons.
Immunoreactivity of synaptic protein was less in Aβ25-35- treated neurons
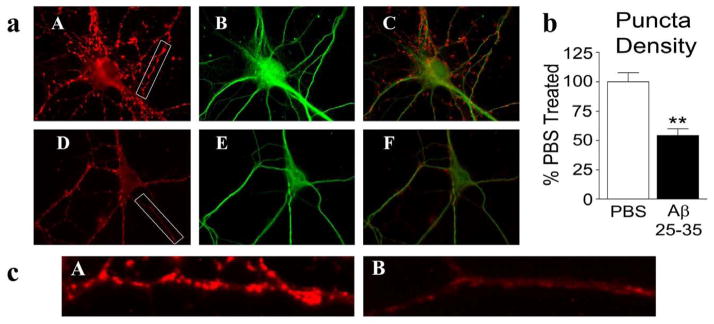
To determine the effect of Aβ25-35 treatment on the immunoreactivity of synaptophysin and Drp1in hippocampal neurons, we treated neurons with Aβ25-35 and with PBS for 24 hrs, and then performed immunostaining. As shown in Fig. 4, we found the immunoreactivity of synaptophysin significantly decreased, particularly at synapses in those neurons treated with Aβ25-35 (54.2±5.9%, mean±SE- P<0.013, Fig. 4a), relative to the synapses in neurons treated with the PBS vehicle (100±5.9%, mean±SE).
Figure 4. Double-labeling analysis of Synaptophysin and MAP2 in neurons treated with amyloid beta.
Hippocampal neurons treated with PBS vehicle or Aβ were immunostained for synaptophysin and MAP2 (a). Vehicle treated cells (upper panel) showed typical strong synaptophysin (A), MAP2 (B) immunoreactivities and colocalization of synaptophysin and MAP2 (C). Aβ-treated cells (lower panel) showed reduced density of synaptophysin puncta (D), MAP2 (E) and merged (C). Image b shows quantification of synaptophysin immunoreactivity. Significantly decreased synaptophysin was found in Aβ-treated neurons (P<0.005). Image c shows enlarged portion of a neurite from PBS vehicle treated neuron (A) and Aβ-treated neuron (B).
To determine, the effect of Aβ25-35 on synapses, we performed double-labeling immunostaining analysis using synaptic marker, MAP2 and Drp1 in neurons treated with Aβ25-35 and with PBS for 24 hrs. Similar to synaptophysin, immunoreactivity of MAP2 is decreased in neurons treated with Aβ25-35 (Fig. 4a image E) relative to PBS treated neurons (image B), indicating that Aβ25-35peptide may be involved in synaptic degeneration.
To determine the effect of Aβ25-35 on mitochondria, particularly mitochondria localized at synapses, we performed double-labeling analysis of Drp1 (enriched in synapses) and Cyt.B (mitochondria-encoded protein) antibodies. As shown in Fig. 5, we found fragmented and punctuated immunoreactivity of Drp1 in neurons treated with Aβ25-35 relative to the Drp1 immunoreactivity in neurons treated with Aβ35-25 or the PBS vehicle (Fig. 5). Drp1 expression was higher in synapses, spines, and branches that were developing in reverse Aβ peptide or PBS vehicle treated neurons, whereas in Aβ25-35 peptide treated neurons, we found decreased and fragmented immunoreactivity, particularly at synapses indicating mitochondrial fragmentation, and degenerating synapses. Further, Drp1 is colocalized with Cyt. B further confirming that fragmented immunoreactivity represents mitochondrial fragmentation caused by Aβ25-35 peptide.
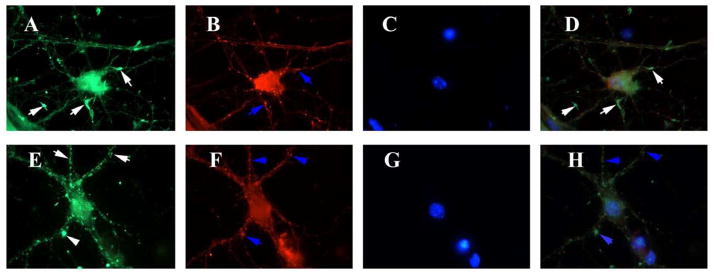
Figure 5. Drp1 distribution relocates from areas of active neurite outgrowth to neuritic processes after amyloid beta treatment.
Hippocampal neurons treated with PBS vehicle or Aβ were immunostained for Drp1, mitochondrial-encoded protein, Cyt. B and nuclear marker, DAPI. Vehicle treated cells (upper panel) showed intense immunoreactivities of Drp1 (A), Cyt. B (B), DAPI (C) and merged (D) in neurite growth cones (white arrows). Drp1 is colocalized with Cyt. B (D) (white arrows). After Aβ treatment (lower panel), Drp1 staining is reduced (E), and Drp1 is colocalzed with mitochondria in merged image (H).
Discussion
In this study, we analyzed the effect of Aβ treatment on mitochondrial motility and distribution in hippocampal neurons from mice. We found mitochondrial motility greatly reduced by the exposure of the hippocampal neurons to Aβ, and the reduction in anterograde transport greater than the reduction in retrograde movement. Overall, a reduction in mitochondrial transport in the hippocampal neurons was observed and mitochondrial transport was impaired in the distal regions of the neurites. We also found decreased synaptic branching and growth in neurons treated with Aβ. This would lead to decreased ATP production, followed by synaptic dysfunction and degeneration. These findings lead us to conclude that Aβ impairs axonal transport of mitochondria and may cause synaptic degeneration.
Amyloid beta and impaired axonal transport of mitochondria. Aβ is generated in neurons, wherever APP and β- and γ-secreteses are present, which is in several intracellular sites, including Golgi apparatus, endoplasmic reticulum, endosomal-lysosomal systems, and multivesicular bodies. Recent studies of AD patients and transgenic AD mice found intracellular Aβ present in AD-affected brain regions and that Aβ1-42 participates in fibrillogenesis and the formation of Aβ plaques. Increasing evidence suggests that Aβ accumulates in cellular compartments, including mitochondria, and that Aβ interfere with normal functions of mitochondria and synapses. Findings from the present study help elucidate a mechanistic relationship among Aβ, synaptic damage, and mitochondrial damage in neurons affected by AD. We found a dramatic reduction (55%) in the total mitochondria in hippocampal neurons exposed to Aβ, and we found significantly reduced anterograde mitochondrial movement (cell body to synapses) in the Aβ-treated hippocampal neurons relative to the PBS-treated neurons. We also found the length of mitochondria significantly decreased in the Aβ-treated hippocampal neurons compared to the PBS-treated or the Aβ35-25-treated hippocampal neurons. Finally, we found the immunoreactivity of synaptophysin significantly decreased in the Aβ-treated neurons, compared to the PBS-treated hippocampal neurons.
Reductions in the total number of mitochondria and of anterograde-moving mitochondria are likely responsible for the synaptic degeneration observed in the AD neurons since a large number of healthy and functionally active mitochondria are necessary for ATP trafficking to synapses where energy-demand is high. Reduction in anterograde-moving mitochondria appear to reduce the supply of ATP at synapses, which may cause synaptic degeneration that marks AD neurons. Our finding pointing to Aβ-induced impaired mitochondrial transport is consistent with other studies showing decreased mitochondrial transport [23,35–36] and organelle transport [37–39] in Aβ-treated neurons.
Wang et al. [23] reported that Aβ oligomer (ADDLs)-treated hippocampal neurons from mouse showed significantly reduced mitochondrial anterograde (0.80 ± .67) and retrograde axonal transport (0.44 ±.21), compared to hippocampal neurons not so treated (5.34 ±.47 for anterograde and 3.31 ±.02 for retrograde transport). Neurons treated with Aβ42-1 exhibited a fast axonal transport of mitochondria that was similar to the axonal transport found in the control neurons.
Du and colleagues [36] found a 30% increase in the percentage of stationary mitochondria in Aβ-treated neurons relative to mitochondria in untreated neurons. Further, the total number of mitochondria moving in both directions (anterograde and retrograde) was significantly reduced in the Aβ-treated neurons compared to those treated with the PBS vehicle or the control (Aβ42-1 treated). After Aβ treatment, the anterograde moving mitochondria decreased by <23% compared to those in the PBS-treated neurons and in the control neurons (P < 0.05). In contrast, the percentage of retrograde mobile mitochondria increased those neurons treated with Aβ1-42.
The velocity of mitochondrial motility was also less in neurons treated with Aβ relative to that of the PBS-treated neurons, indicating that Aβ affects mitochondrial velocity. Our findings are consistent with those from Du et al. [34], who also reported significantly reduced mitochondrial anterograde velocity in neurons treated with Aβ1-42 relative to control and Aβ42-1-treated neurons.
Overall, findings from our study, together with those from Wang et al. [23] and Du et al. [36], indicate that the Aβ peptide is toxic and impairs axonal transport of mitochondria. Further, our findings are consistent with findings from other mitochondrial trafficking studies by Chang et al. [40] and Rui et al. [35], reporting that mitochondrial transport and distribution were affected by hippocampal neurons exposed to the Aβ peptide, glutamate and zinc.
By shifting the balance of anterograde/retrograde transport toward retrograde, a decrease in axonal and neuritic mitochondria would be expected. Indeed, we observed this effect, finding a large reduction in mitochondrial mass in the Aβ-treated cells (Fig. 2); more specifically, we found the average length of mitochondria length was decreased, but the total number of mitochondria per each length was less affected. These findings suggest that factors dictating mitochondrial distribution along an axon are unaffected by Aβ treatment. However, mitochondrial dynamics are likely shifted toward fission.
Amyloid beta and abnormal mitochondrial dynamics. We [32] and others [21,22] reported increased mitochondrial fission and decreased fusion in neurons affected by AD. In other words, abnormal mitochondrial dynamics occurs in neurons affected by AD. This may be due to the association of Aβ with mitochondria. Mitochondrial Aβ is known to induce free radical production and to activate the fission proteins Drp1 and Fis1, and to fragment mitochondria [32]. When mitochondrial fission is activated, fusion proteins decrease.
Our immunostaining analysis of Drp1 showed fragmented and punctuated immunoreactivity in Aβ-treated neurons, indicating increased mitochondrial fission and decreased fusion. As described elsewhere [32], in Aβ-treated neurons, mitochondrial fusion is reduced following the induction of apoptosis. Over-expression of mitofusins can reduce apoptosis. Further, it has been previously suggested that mitochondrial fusion and transport are closely linked, such that disruptions in either process affect the other. Recently, Miskow et al. [41] showed that Mfn2 interacts with the Miro-Milton complex, and this interaction is required for axonal transport of mitochondria in dorsal root ganglia neurons. Conversely, mitochondrial fragmentation induced by Pink1 knockdown can be rescued by the overexpression of Miro or Milton [42]. In this light, it is not surprising that we observed both altered mitochondrial transport and altered mitochondrial dynamics. Decreased synaptic growth in Aβ-treated neurons. Our quantitative analysis of synaptophysin immunoreactivity revealed that synaptophysin was significantly decreased in Aβ-treated neurons, indicating that Aβ affects synaptic growth, an observation supported by other studies [43–46]. Further, MAP2 immunoreactivity was decreased in Aβ-treated neurons, further supporting that synaptic degeneration is present in Aβ-treated neurons. This reduced synaptic growth was primarily due to the decrease mitochondrial trafficking, particularly the anterograde movement of mitochondria, which is known to ultimately lead to the production of ATP at synapses and to the degeneration of synapses in AD neurons.
In summary, we found reduced mitochondrial mass, reduced mitochondrial motility, and reduced mitochondrial anterograde transport in neurons exposed to the Aβ25-35 peptide. We also found a reduction in the number of mitochondria in distal regions of neurons and decreased synaptic branching and growth in the Aβ-treated neurons. These findings lead us to conclude that Aβ impairs axonal transport of mitochondria and may cause synaptic degeneration.
Acknowledgments
This research presented was supported by NIH grants AG028072, AG026051, and RR00163, Alzheimer Association grant IIRG-09-92429, and Medivation, Inc. We also thank Dr. Anda Cornea for her assistance with confocal imaging and mitochondrial transport assessment.
Abbreviations
- AβPP
Aβ precursor protein
- AD
Alzheimer’s disease
- ATP
adenosine triphosphate
- Drp1
dynamin protein 1
- Fis1
mitochondrial fission 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer Association Report. Alzheimer’s Disease Facts and Figures. 2010. [Google Scholar]
- 3.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 5.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–84. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, Davis RE, Parker WD., Jr Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 1997;49:918–25. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 12.Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Ruge W, Ulrich J. Morphological adaptive response of the synaptic junctional zones in the human dentate gyrus during aging and Alzheimer’s disease. Brain Res. 1990;517:69–75. doi: 10.1016/0006-8993(90)91009-6. [DOI] [PubMed] [Google Scholar]
- 13.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 14.Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging. 1990:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 15.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 16.Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 18.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–49. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 19.Devi L, Prabhu BM, Galati DF, Avadhani AG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegenerative Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 26.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 27.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 28.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-Relate Protein 1 and Mitochondrial Fragmentation in Neurodegenerative Diseases. Brain Res Rev. doi: 10.1016/j.brainresrev.2010.11.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alz Dis. 2010;20:S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez A, Lübke AJ, Del Río JA, Soriano E, Frotscher M. Regional variability and postsynaptic targets of chandelier cells in the hippocampal formation of the rat. J Comp Neurol. 1996;376:28–44. doi: 10.1002/(SICI)1096-9861(19961202)376:1<28::AID-CNE2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 34.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 35.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Nat Acad Sci USA. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, SaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Nat Acad Sci USA. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J Neurosci. 2010;30:9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusion 2 is necessary for transport of axonal mitochondrial and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutala RV, Reddy PH. The use of real-time PCR analysis in a gene expression study of Alzheimer’s disease post-mortem brains. J Neurosci Methods. 2004;132:101–107. doi: 10.1016/j.jneumeth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005:103–117. doi: 10.3233/jad-2005-7203. discussion 173–80. [DOI] [PubMed] [Google Scholar]
- 45.Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer’s disease. Neurobiol Aging. 2003;24:1023–1027. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]