Abstract
Rationale
Despite overwhelming evidence of the importance of circadian rhythms in cardiovascular health and disease, little is known regarding the circadian regulation of intracellular signaling pathways controlling cardiac function and remodeling.
Objective
To assess circadian changes in processes dependent on the protein phosphatase calcineurin, relative to changes in phosphorylation of cardiac proteins, in normal, hypertrophic, and failing hearts.
Methods and Results
We found evidence of large circadian oscillations in calcineurin-dependent activities in the left ventricle of healthy C57BL/6 mice. Calcineurin-dependent transcript levels and nuclear occupancy of the nuclear factor of activated T cells (NFAT) regularly fluctuated as much as 20-fold over the course of a day, peaking in the morning when mice enter a period of rest. Phosphorylation of the protein phosphatase 1 inhibitor 1 (I-1), a direct calcineurin substrate, and phospholamban (PLB), an indirect target, oscillated directly out of phase with calcineurin-dependent signaling. Using a surgical model of cardiac pressure overload, we found that, although calcineurin-dependent activities were markedly elevated, the circadian pattern of activation was maintained, whereas, oscillations in PLB and I-1 phosphorylation were lost. Changes in the expression of fetal gene markers of heart failure did not mirror the rhythm in calcineurin/NFAT activation suggesting that these may not be direct transcriptional target genes. Cardiac function in mice subjected to pressure overload was significantly lower in the morning than in the evening when assessed by echocardiography.
Conclusions
Normal, opposing circadian oscillations in calcineurin-dependent activities and phosphorylation of proteins that regulate contractility are disrupted in heart failure.
Keywords: Calcineurin, circadian rhythms, heart failure, RCAN1/MCIP1
Introduction
Circadian rhythms are self-sustaining, 24-hour cycles in molecular, biochemical, and behavioral parameters that help an organism prepare for anticipated changes in physiological demand. Many important cardiovascular factors, including metabolism, heart rate, blood pressure, and hormone release, oscillate over a 24-hour period.1 In humans, the incidence of adverse cardiac events, such as myocardial infarction, ventricular tachycardia, and death from ischemic heart disease, vary according to the time of day.2 Despite overwhelming evidence of the importance of circadian rhythms in cardiovascular health and disease, little is known regarding the circadian regulation of intracellular signaling pathways in the heart.
The molecular basis of the circadian clock consists of cell-autonomous, positive and negative transcriptional and posttranscriptional feedback loops.3 The “master clock”, located in the suprachiasmatic nucleus within the hypothalamus, influences the phase of independent molecular clocks found in peripheral organs, including the heart. Many cells and tissues also display circadian fluctuations in cytoplasmic Ca2+ levels, although the source of these Ca2+ oscillations and their relationship to the transcriptional clock mechanism is not fully understood.4
Dysregulation of Ca2+ handling is a hallmark of heart disease. Several Ca2+-responsive signaling pathways, including the protein phosphatase calcineurin, have been causally linked to the progression of heart failure.5 Sustained activation of calcineurin is sufficient to drive pathological hypertrophic remodeling of the myocardium with subsequent heart failure and premature death.6 Calcineurin is activated by an elevation in intracellular Ca2+ and binding of a Ca2+/calmodulin complex. In a healthy heart, calcineurin is thought to be inactive and unresponsive to high amplitude, high frequency, waves of Ca2+ that drive contraction. Calcineurin is activated in response to stress presumably when either diastolic resting Ca2+ or Ca2+ in sub-cellular domains exceed a required threshold.
Calcineurin has numerous substrates including the transcription factor nuclear factor of activated T cells (NFAT) through which calcineurin influences long term changes in gene expression associated with pathological cardiac remodeling.6 When NFAT is dephosphorylated, it translocates to the nucleus where it binds to and activates calcineurin-responsive genes. Among target genes is the exon 4 isoform of the Regulator of Calcineurin 1 (Rcan1.4), previously called MCIP1, DSCR1 or calcipressin. RCAN1 proteins are potent inhibitors of calcineurin activity.7 Expression of the mouse Rcan1.4 gene is extremely responsive to changes in calcineurin activity in vivo,8 thus, altered Rcan1.4 transcript levels have been used as a sensitive indicator of changes in calcineurin activity in the heart and other tissues.
Both Ca2+ handling and cardiac myocyte contractility are regulated by changes in phosphorylation of key proteins.9 β-adrenergic stimulation activates the cAMP-dependent protein kinase (PKA), which increases cardiac output by phosphorylating a number of proteins including phospholamban (PLB). This releases inhibition of the sarcoplasmic reticulum Ca2+ ATPase (SERCA2), thereby enhancing relaxation rate and contractility. PLB and other regulatory proteins are dephosphorylated by the protein phosphatase 1 (PP1). Phosphorylation of the PP1 inhibitor-1 (I-1) by PKA at threonine 35 (I-1Thr35) prolongs β-adrenergic responses by inhibiting PP1, thus slowing dephosphorylation of PLB.10 Changes in I-1 levels and/or phosphorylation have been implicated in human heart failure and chronic atrial fibrillation.9, 11 I-1 can be phosphorylated at other sites including Ser67.12 The in vivo consequence of phosphorylation at these various sites remains controversial, however, calcineurin can dephosphorylate both I-1Ser67 and I-1Thr35. 13, 12 In vitro and in vivo studies suggest that calcineurin activity can promote dephosphorylation of PLB via regulation of I-1.14, 15
Given the need for the heart to adapt to daily changes in cardiac demand and the potentially antagonistic roles of PKA and calcineurin, we asked whether calcineurin activity and/or PLB phosphorylation change over the course of 24 hours in a healthy heart. We found circadian oscillations in both these parameters that were directly out of phase with each other. We then tested what happens to these rhythms when both β-adrenergic activity and calcineurin activity increase in the pressure stressed myocardium.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org that provides expanded details for in situ hybridization, immunohistochemistry, chromatin immunoprecipitation, quantitative RT-PCR and western blot analysis.
Animal Procedures
Male C57BL/6 mice were housed and fed under standard laboratory conditions with a strict 12:12 hour light:dark cycle with lights turning on at 6AM, circadian time 0 (CT0), and off at 6PM (CT12). For pressure overload experiments, mice were subjected to thoracic aortic constriction (TAC) or severe TAC (sTAC) for three weeks as described previously.16 Mice were shifted to constant darkness at the end of the normal light cycle for 24 hours prior to harvesting. Hearts were removed and the ventricles flash frozen within 30 seconds of sacrifice to preserve phosphorylation. A minimum of three animals was analyzed for each time point. A VisualSonics Vevo 770 imaging system was used to assess cardiac function in unanesthetized animals. The αMHC-RCAN1 mice were described previously17 and wild type littermates used as controls. Surgically implanted mini-osmotic pump (Alzet, Palo Alto, CA) were used to deliver cyclosporine at a rate of 50 µg per hour per 25 kg of body weight.
Results
Changes in RCAN1.4 Protein and mRNA Levels Display Circadian Rhythmicity
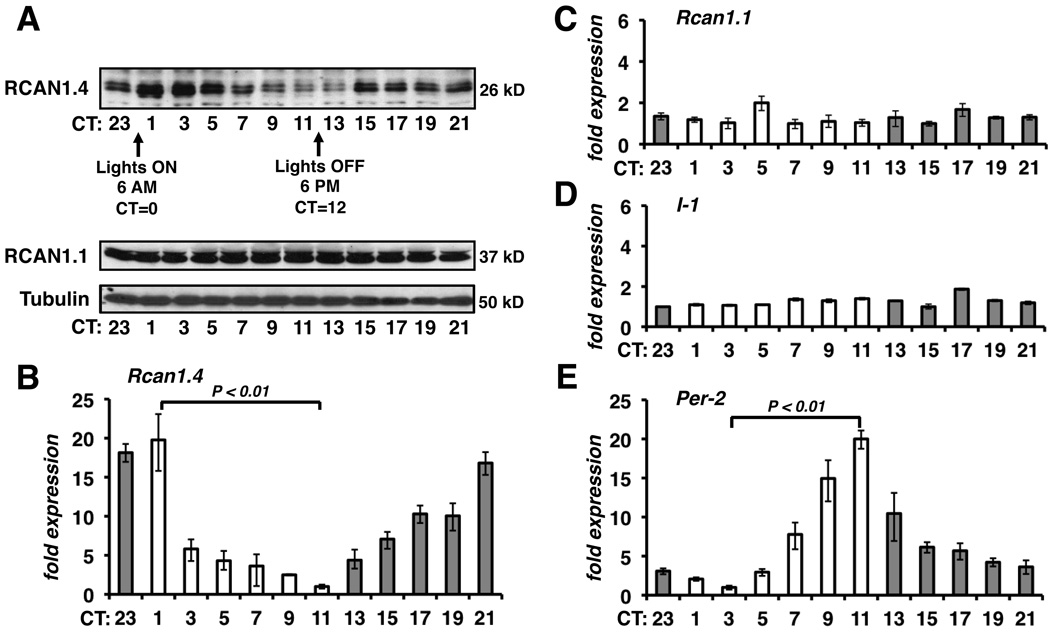
Biochemical assays of calcineurin activity are limited to measuring the potential activity of the entire cellular pool of calcineurin, rather than the fraction of the pool that was active in vivo. We therefore used multiple indirect methods to assess calcineurin activity. Initially, we quantified changes in both protein and mRNA levels of the Rcan1.4 gene, a direct target of calcineurin/NFAT. Male C57BL/6 mice were entrained to a 12:12 light:dark cycle then shifted to constant darkness at circadian time 12 (CT12) the day before samples were harvested for analysis. RCAN1.4 protein levels were highest in the heart at the beginning of the day (CT1-CT3) and lowest at the end of the day (CT11-CT13) (Figure 1A). In comparison, there were no significant changes in either the level of the exon 1 isoform of RCAN1 (RCAN1.1) or tubulin. A similar circadian pattern in RCAN1.4 protein levels was found in the hearts of 129/Sv and C3H/He inbred lines demonstrating that the oscillations were not strain dependent (data not shown). These findings are consistent with genome-wide microarray analysis identifying Rcan1 as having a circadian pattern of mRNA expression in the mouse heart.18 We found a twenty-fold oscillation in Rcan1.4 mRNA levels with a peak at CT23-CT1 and a trough at CT11 (Figure 1B) directly preceding the peak and trough in RCAN1.4 protein levels. In contrast, there were no significant circadian changes in the transcript levels of either Rcan1.1 or I-1 (Figure 1C and 1D). Thus, circadian regulation of Rcan1 expression is unique to the Rcan1.4 isoform and controlled at the level of transcript abundance. Transcription of the circadian clock gene Period 2 (Per2) oscillated with 24-hour periodicity (Figure 1E) verifying the presence of a functional clock in these samples.
Figure 1.
Rcan1.4 mRNA and protein levels oscillate with 24-hour periodicity in the hearts of healthy mice. Samples were pooled from three mice for each time point. (A) Immunoblot analysis of RCAN1.4, RCAN1.1, and tubulin are shown. Real-time RT-PCR for Rcan1.4 (B), Rcan1.1 (C), I-1 (D), and Per-2 (E) mRNA levels were normalized to 18S rRNA. Trough values for each gene were set at a value of 1. (n=3 each time point in B-E)
Calcineurin-dependent Signaling is Most Active in a Mouse Heart as the Animal Enters a Period of Decreased Physical Activity
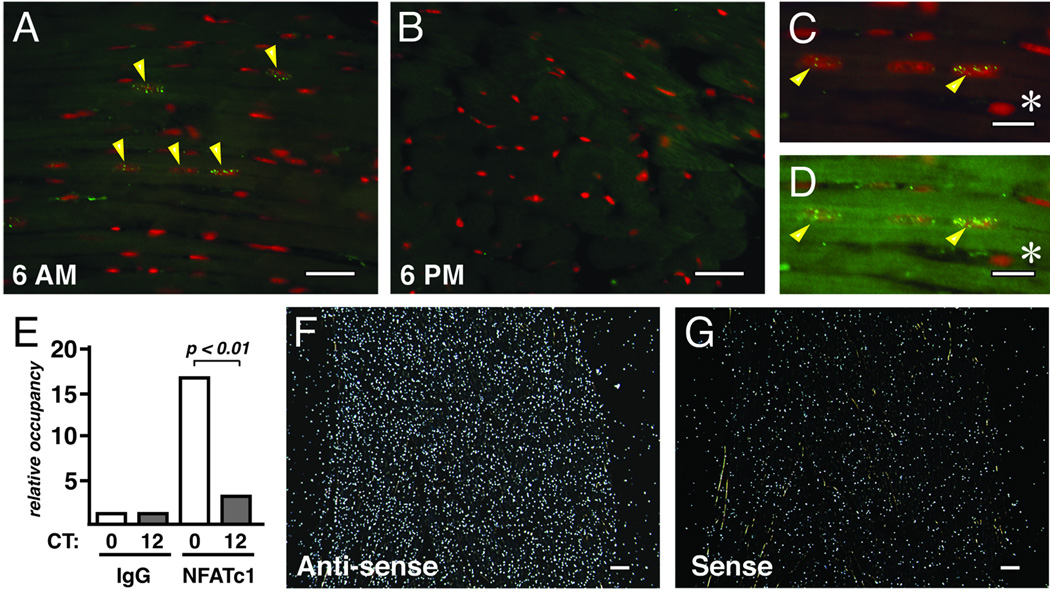
Immunohistochemical analysis for NFATc1 in the left ventricle revealed nuclear staining at 6 AM (CT0) (Figure 2A) but not at 6 PM (CT12) (Figure 2B). Although only a modest number of nuclei stained positive for NFATc1 even at the peak of activity, these positive nuclei were always embedded within sarcomere-positive cells and never observed in non-myocyte nuclei (Figure 2C and 2D).
Figure 2.
More NFATc1 is located in the nucleus and bound to chromatin at CT0 than at CT12. Left ventricular free-wall harvested at 6 AM (CT0) (A, C, and D) and 6 PM (CT12) (B) were stained with a FITC-labeled NFATc1 antibody (yellow/green) and propidium iodide (PI) (red). Cardiac myocytes have a high level of autofluorescence due to the abundance of mitochondrial flavins and flavoproteins, which emit in a broad band overlapping the FITC-NFATc1 signal. In image (C) the gain on the green channel has been turned down to obtain a clear outline of the nuclear PI signal. In (D) the intensity of the green overlay has been restored so that the autoflorescence of the sarcomere now obscures the myocyte-localized NFATc1-positive nuclei marked with arrows. A non-myocyte nucleus is marked with an * and is not obscured. NFATc1 occupancy of the RCAN1.4 promoter was determined by chromatin immunoprecipitation from ventricular lysates using either pre-immune IgG or NFATc1 antibodies (E) (n=4 each time point). In situ hybridization was carried out using an Rcan1.4-specific probe on the left ventricular wall harvested at 6 AM (CT12). Anti-sense probe (F) and sense probe (G) are shown. White bars denote 20 µm (A, B, F and G) or 10 µm (C and D).
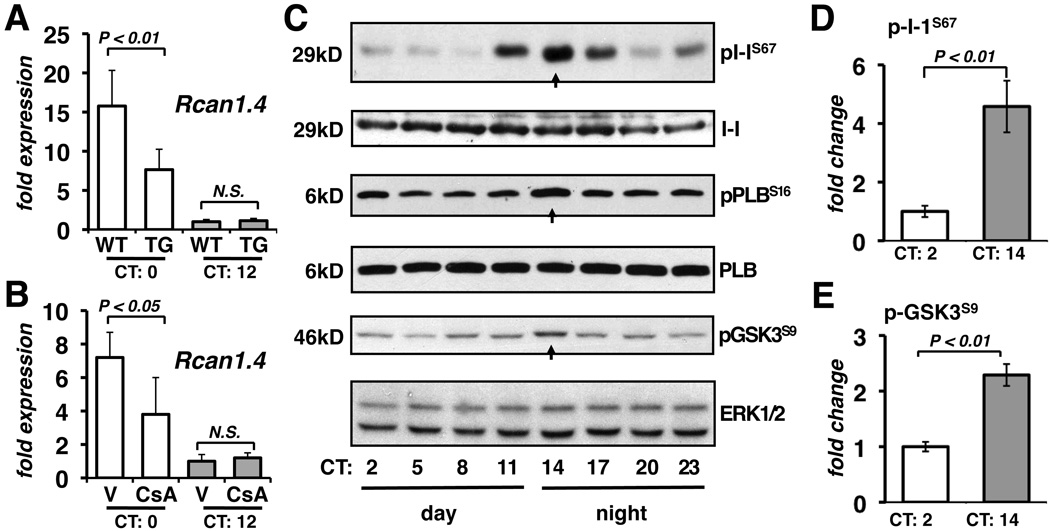
NFAT binding to the Rcan1.4 promoter was assessed by chromatin immunoprecipitation studies. A six-fold increase in NFAT occupancy of the Rcan1.4 promoter was detected at CT0 compared to at CT12 (Figure 2E) verifying that the circadian expression pattern of Rcan1.4 was driven by changes in NFAT nuclear translocation. An in situ hybridization specific for Rcan1.4 indicated that transcription was elevated uniformly across the wall of the myocardium (Figure 2F and 2G). Peak Rcan1.4 transcript levels were blunted in the hearts of mice with cardiomyocyte-specific expression of a transgene encoding RCAN1 to inhibit calcineurin (Figure 3A) or treated with the calcineurin inhibitor cyclosporine (Figure 3B). Taken as a whole, these results suggest that activation of the calcineurin/NFAT signaling pathway occurs throughout the left ventricular myocardium and is greatest when the animal is entering its rest phase and cardiac demand decreases.
Figure 3.
Calcineurin-dependent transcription oscillates out of phase with protein phosphorylation. Rcan1.4 transcript levels were quantified in the hearts of wild type (WT) and αMHC-Rcan1 (TG) mice (A) or wild type mice receiving either vehicle (V) or cyclosporine A (CsA) via a mini-osmotic pump (B) (n=4 each). Ventricular lysates collected from three different wild type hearts at the times indicated were pooled and probed for phospho-I-1Ser67, total I-1, phospho-PLBSer16, total PLB, phospho-GSK3Ser9, and total ERK1/2 (C). Signals from three experiments were quantified by densitometry (D and E).
Phosphorylation of I-1 and PLB Oscillates out of Phase with Calcineurin Activity
Immunoblot analyses were conducted to assess phosphorylation of the calcineurin substrate I-1 at Thr35 and Ser67. Unfortunately, we were not able to detect phosphorylation of Thr35 in either heart extracts or forskolin-treated cells transfected with an I-1 expression construct (data not shown). There were, however, pronounced circadian changes in Ser67 phosphorylation. Phospho-I-1Ser67 was lowest in the morning (CT1-8), increased notably at CT11 as the animals became active, and peaked at CT14 directly opposed to circadian changes in calcineurin activity (Figure 3C and 3D). In contrast, total I-1 protein (Figure 3C) and transcript levels (Figure 1D) did not change.
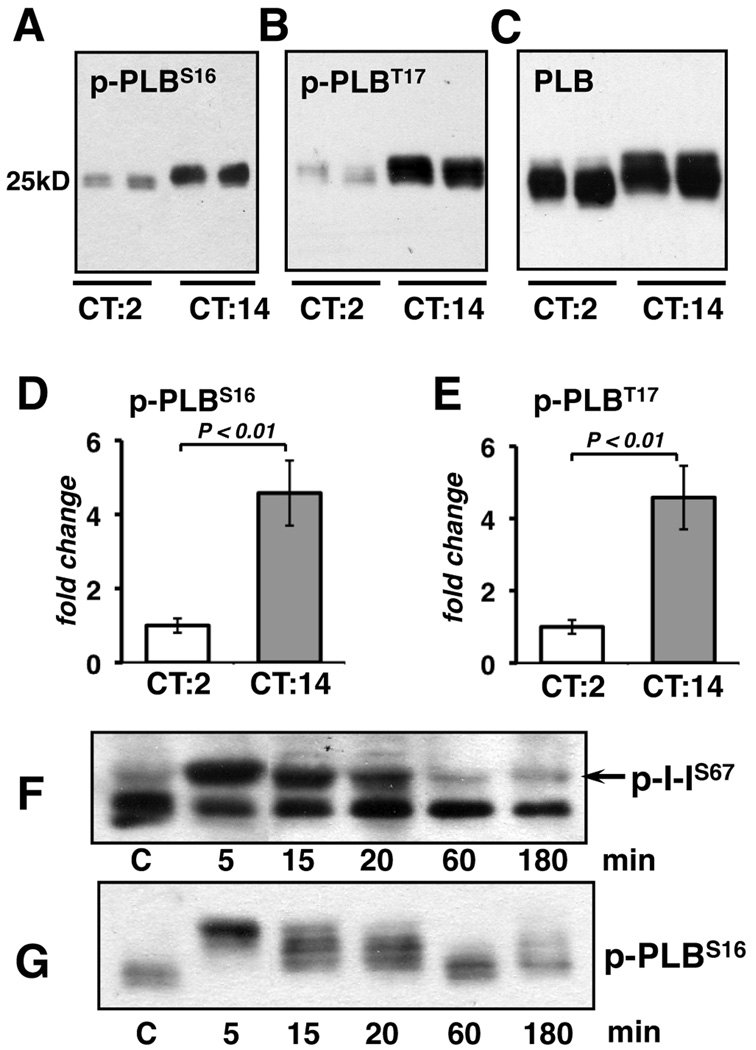
Cardiac contractility and β-adrenergic drive are both higher at night in the hearts of nocturnal rodents.19, 20 Antibodies specific for phospho-PLBSer16 showed a peak at CT14, coincident with the peak in I-1Ser67 phosphorylation (Figure 3C). The change in phosphorylation was even more pronounced when protein extracts were run such that PLB was maintained as a pentameric complex. Phospho-PLBSer16 and phospho-PLBThr17 were both elevated at CT14 compared to CT2 (Figure 4A, 4B, 4D, and 4E). This was evident in the slower electrophoretic migration of total PLB complexes from CT14 lysates (Figure 4C). Thus, the overall phosphorylation state of PLB was elevated during the period when calcineurin-dependent activities were lowest.
Figure 4.
Ventricular lysates isolated at CT2 and CT14 were probed for phospho-PLBSer16 (A: 2 µg of protein per lane), phospho-PLBThr17 (B: 0.5 µg of protein) or total PLB (C: 0.5 µg of protein) per lane.Phospho-PLBSer16 and phospho-PLBThr17 were quantified by densitometry (D and E) (n=6 each). In (Fand G) wild type mice were injected with 8 mg/kg body weight of isoproterenol at CT10. Hearts were harvested at the indicated time points after injection.
Although we were not able to detect phosphorylation of I-1 at Thr35, we predict that phosphorylation of this site by PKA should parallel PLBSer16 phosphorylation. The kinase responsible for I-1Ser67 phosphorylation in the heart has not yet been identified definitively. To test whether I-1Ser67 is phosphorylated in response to β-adrenergic stimulation, we injected wild type mice with 200ng of isoproteronol. Phospho-I-1Ser67 was maximal 5 minutes after injection and dissipated within an hour (Figure 4F). Phospho-PLBSer16 followed a similar time course (Figure 4G) suggesting that, in response to β-adrenergic stimulation, the kinetics of I-1Ser67 phosphorylation and dephosphorylation may be similar to that of PLBSer16.
It is likely that the flux through many kinase/phosphatase signaling pathways display circadian rhythmicity in the heart. Glycogen synthase kinase 3-beta (GSK3β) can antagonize calcineurin activity by rephosphorylating NFAT.21 We found a mild increase in GSK3β phosphorylation indicating inactivation of GSK3β at CT14 (Figure 3C and 3E), consistent with insulin activation of Akt kinase when the mouse begins to feed. Therefore, circadian changes in GSK3β activity are not responsible for the observed changes in NFAT localization. We also assayed for changes in Nfatc1 and Nfatc3 transcript levels which did not change significantly (data not shown), and thus, are unlikely to account for the magnitude of the changes observed in Rcan1.4 mRNA and NFAT nuclear localization. Taken as a whole, we document an inverse correlation between calcineurin-dependent activities and the phosphorylation of proteins that either inhibit PP1 or promote contractility.
Calcineurin Activity is Elevated but Maintains Rhythmicity in Hypertrophic and Failing Hearts
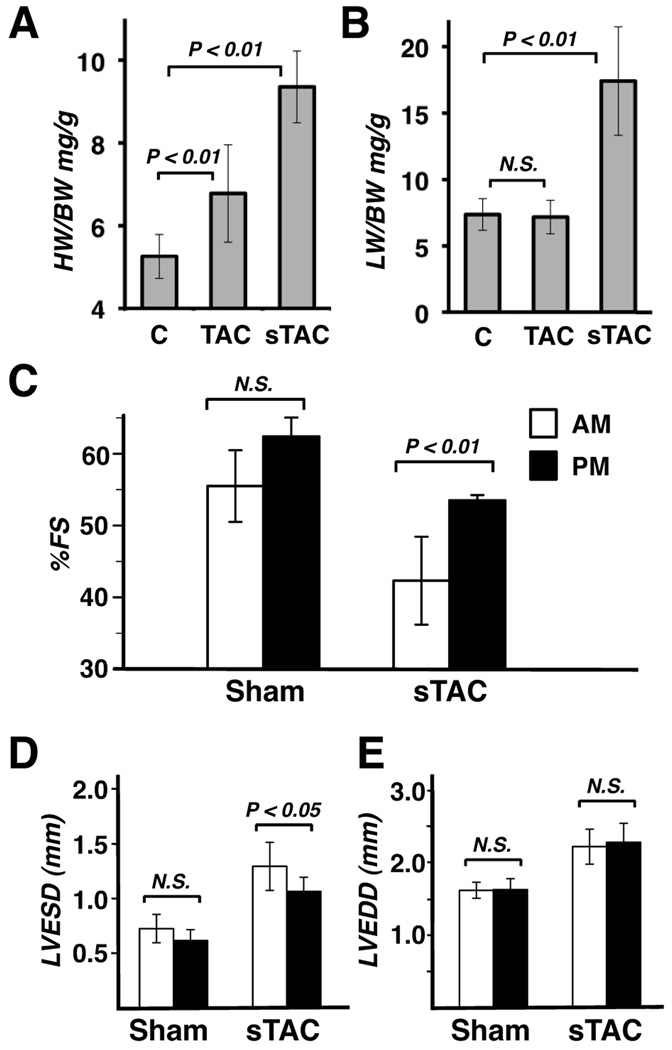
To determine whether cardiac hypertrophy or failure alters the temporal relationship between changes in calcineurin activity and protein phosphorylation, mice were subjected to either TAC surgery to induce stable compensated hypertrophy or severe sTAC to induce decompensated heart failure.16 Three weeks after surgery hearts from TAC mice showed a 29% increase in heart weight to body weight ratio compared to sham operated controls (Figure 5A). Cardiac function was preserved in these mice as assessed by echocardiography (data not shown) and lung weight (Figure 5B). In sTAC mice there was a 78% increase in heart weight and a doubling of lung weight compared to sham controls indicating that these mice had progressed to heart failure. Cardiac function was significantly reduced after sTAC (Figure 5C). Remarkably, the decline in function was more pronounced when echocardiography was performed in the morning (AM) between 5AM and 7AM than if function was assessed in the evening (PM) between 5PM and 7PM at the end of the light period, regardless of whether the initial echo was performed in the morning or evening. The time of day differences in function in the sTAC mice were primarily systolic (Figure 5D), whereas, the decline in diastolic function was similar regardless of the time of day (Figure 5E). In the control sham mice there was also a trend toward improved function if the mice were echoed in the evening, but the difference did not reach statistical significance.
Figure 5.
Changes in cardiac parameters were assessed in control mice (c) and mice subjected to TAC or sTAC. Analysis included: heart weight to body weight ratios (A), lung weight to body weight ratios (B), as well as echocardographic analysis to quantify percent fractional shortening (%FS) (C), left ventricular end systolic dimension (LVESD) (D), and left ventricular end diastolic dimension (LVEDD) (E) in Sham and sTAC mice (n=21 each in A and B; n=10 each in C, D and E).
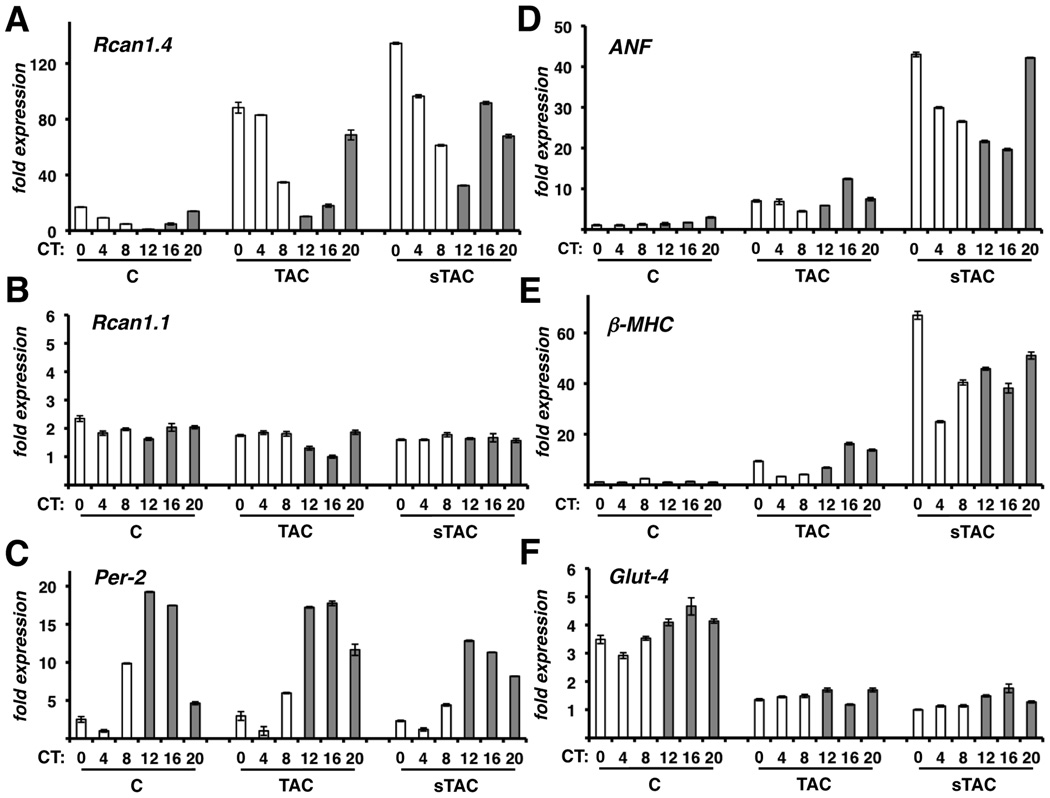
In both TAC and sTAC hearts Rcan1.4 mRNA levels were elevated at all times of the day compared to controls, however, circadian rhythmicity was maintained in both the hypertrophic and failing hearts (Figure 6A), suggesting that, although calcineurin activity increased greatly, it retained a circadian pattern of activation. Maximal Rcan1.4 expression was only slightly higher in failing hearts compared to hypertrophic hearts. However, trough expression at CT12 was much higher in the sTAC hearts than in the TAC hearts. Our results suggest that although calcineurin activity remains circadian, there is a progressive elevation in trough activity and a dampening of the fold change between peak and trough activity with increasing stress.
Figure 6.
Changes in gene expression was assessed in control mice (c) and mice subjected to TAC or sTAC. Real-time RT-PCR was carried out for RCAN1.4 mRNA (A), RCAN1.1 (B), Per2 (C), ANF (D), βMHC (E), and Glut-4 (F). mRNA was normalized to 18S rRNA. (n=3 per time point).
Rcan1.1 transcript levels remained constant throughout (Figure 6B). Per2 expression retained rhythmicity (Figure 6C) indicating maintenance of a cardiac-specific circadian clock, although Per2 oscillation was dampened slightly in sTAC hearts, consistent with previous reports.22 Expression of the fetal genes beta myosin heavy chain (βMHC) and atrial natriuretic factor (ANF) was elevated in TAC and sTAC hearts compared to sham controls indicative of cardiac stress and failure (Figure 6D and 6E). Expression of the high-affinity glucose transporter (Glut4) was depressed in TAC and sTAC hearts compared to sham controls also consistent with a failure phenotype22, 23 (Figure 6F). Importantly, there were no pronounced circadian oscillations in ANF, βMHC, and Glut4 expression in control hearts suggesting that daily activation of calcineurin in healthy hearts is not sufficient to alter expression of these genes associated with heart failure. Whether the circadian changes in βMHC and ANF expression observed in the TAC and sTAC hearts are linked to changes in calcineurin activity or other signaling pathways is not yet known.
The Circadian Pattern of PLB and I-1 Phosphorylation is Disrupted
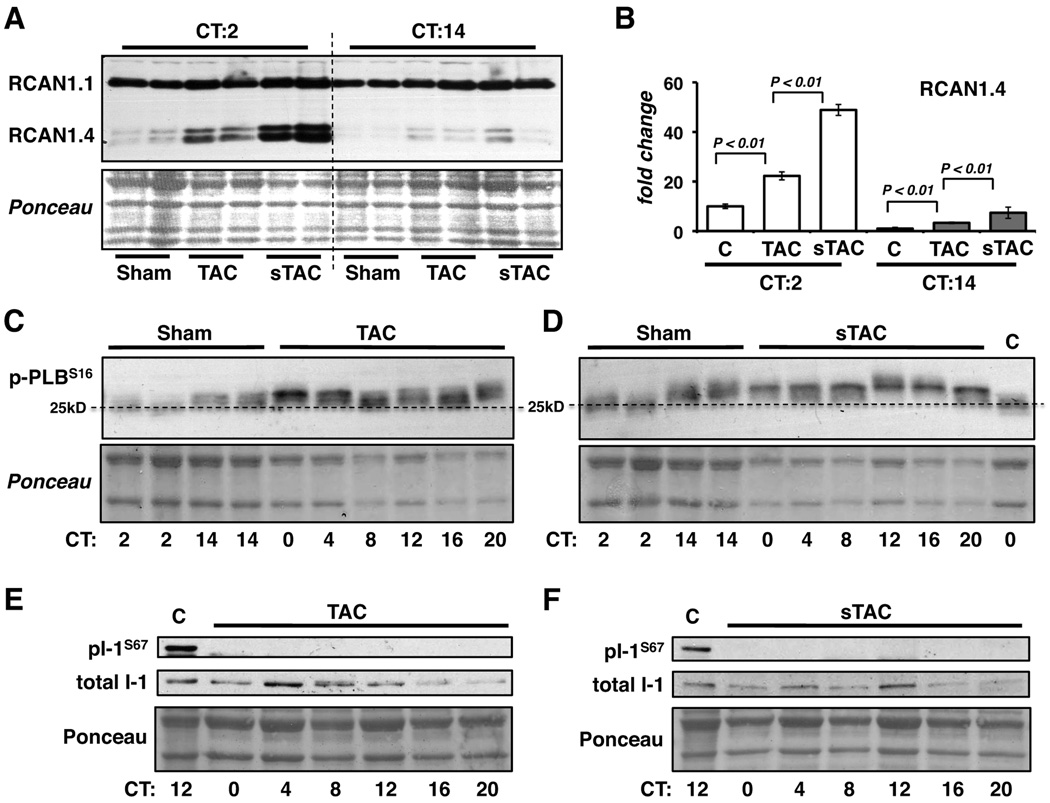
Western blot analysis of RCAN1.4 protein in control, TAC and sTAC hearts at CT2 and CT14 reflect the changes seen in Rcan1.4 mRNA (Figure 7A and 7B). Within each experimental group RCAN1.4 protein was more abundant at CT2 than at CT14. Within a given time point RCAN1.4 protein was consistently higher in the TAC and sTAC hearts than in control hearts.
Figure 7.
Ventricular extracts isolated at CT2 and CT14 from sham operated control (c), TAB or sTAB mice were analyzed by western blot for RCAN1 protein (A). Ponceau stain of the corresponding region of the membrane is provided as a loading control. RCAN1.4 protein levels from 4–5 hearts per time point were quantified (B). Phospho-PLBSer16 (p-PLBS16) levels were compared in Sham, TAC (C) and sTAC (D) hearts at the time points indicated. The final lane in D contains extract from an unoperated control wild type mouse. Three times the amount of protein was loaded on the Sham lanes in C and D to facilitate obtaining a clear signal in the Sham lanes. The horizontal dashed line is drawn at mid-level migration of the Sham CT2 pentamer. Phospho-I-1Ser67 (pI-1S67) levels were compared in control Sham (c), TAC (E) and sTAC (F) hearts at the time points indicated. (Each lane in C, D, E and F contains samples from one heart selected randomly from an experiment with a sample size of n=3 per time point, per condition. PLB phosphorylation was elevated and I-1 phosphorylation was absent in all TAC and sTAC samples.)
The normal circadian pattern of I-1 and PLB phosphorylation was lost in the TAC and sTAC hearts. Phosphorylation of PLB was elevated throughout the day in both TAC and sTAC hearts (Figure 7C, Figure 7D and Online Figure I). This is most easily seen by comparing the delay in migration of the PLB pentamer in the TAC and sTAC samples to the migration of PLB in the control sham samples at CT2. Importantly, phosphorylation of I-1Ser67 could not be detected in either TAC or sTAC hearts at any time of day (Figure 7E and 7F), demonstrating that there is a loss of phosphorylation in addition to a loss of circadian changes. This was not due to loss of total I-1 protein levels as these were similar in control, TAC and sTAC hearts.
Discussion
These studies provide several important new insights into the dynamics and consequences of calcineurin signaling in the heart. First, we present evidence that there is a circadian rhythm in calcineurin activity in a normal, healthy mouse heart. This assertion is supported by five different assessments of calcineurin activity: RCAN1.4 protein levels, Rcan1.4 mRNA levels, NFATc1 nuclear translocation, NFATc1 occupancy of the Rcan1.4 promoter, and phosphorylation levels of the calcineurin substrate I-1Ser67. Second, although activation of calcineurin has been viewed primarily as a stress response driving pathological hypertrophic remodeling, these daily increases in calcineurin activity are not associated with transcriptional changes indicative of pathological remodeling. Third, calcineurin activity oscillates out of phase with phosphorylation of proteins that promote cardiac contractility. Fourth, cardiac function in failing hearts shows diurnal variation. Finally, in failing hearts, calcineurin activity is elevated above normal peak activity throughout the day. Although a pronounced circadian oscillation in calcineurin-dependent activities persists, the amplitude of circadian variation declines correlating to a decline in cardiac function. Circadian rhythmicity in the phosphorylation of the regulatory proteins I-1 and PLB is lost in hypertrophic and failing hearts. Based on our findings we propose that circadian oscillations provide temporal separation of kinase and phosphatase activities that help to regulate changes in cardiac function in response to physiological demand and that this temporal relationship is disrupted when the heart is placed under sustained hemodynamic load.
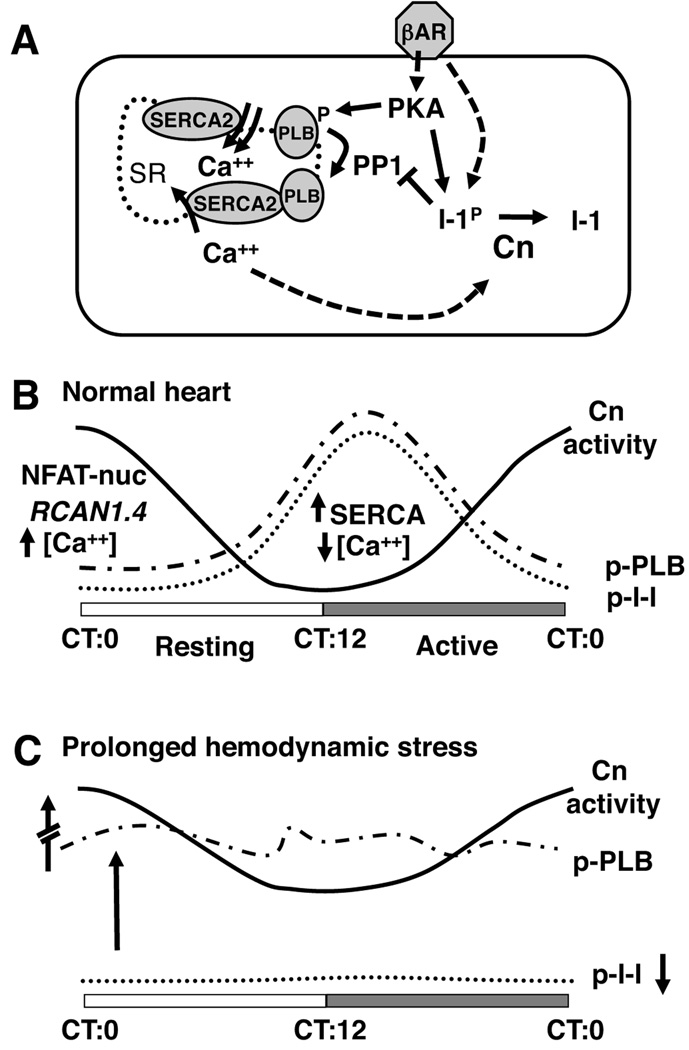
In nocturnal animals, the light to dark transition at CT12 is anticipated by a rapid increase in physical activity, blood pressure, and β-adrenergic drive,24 all factors shown to increase calcineurin activity in cardiomyocytes. We therefore expected Rcan1.4 expression to increase in parallel with these physiological parameters at dusk. Instead, there was a gradual increase in Rcan1.4 expression throughout the course of the night suggesting a progressive activation of calcineurin that peaked at dawn when β-adrenergic drive is minimal in a mouse. Figure 8A depicts a model of how calcineurin activity could crosstalk with cardiac function via dephosphorylation of I-1, thus promoting reversal of phosphorylation-driven increases in cardiac contractility as discussed in the introduction. The findings we present here document that in a normal heart, circadian control provides temporal separation of these opposing processes as schematized in Figure 8B.
Figure 8.
The model in (A) depicts interacting pathways through which PKA, PP1 and calcineurin (Cn) can influence calcium handling and contractility. Please refer to text for details. Solid arrows indicate direct interactions. Dashed arrows indicate multi-step or indirect interactions. Schematic (B) summarizes the temporal relationship between circadian oscillations in calcineurin activity, PKA-mediated phosphorylation of I-1 and PLB in the normal mouse heart. The solid line represents changes in calcineurin activity (Cn) over a 24-hour period. The dotted and broken dashed lines depict opposing changes in phosphorylation of I-1 and PLB. Schematic (C) summarizes changes observed in hypertrophic and failing hearts. Note that calcineurin activity is greatly increased but maintains circadian rhythmicity in both hypertrophic and failing hearts. In contrast, PLB phosphorylation becomes erratic and elevated throughout the day, whereas, I-1 phosphorylation can no longer be detected.
The peak in NFAT activity and Rcan1.4 transcription at dawn (CT0) implies that calcineurin activity and cytoplasmic Ca2+ levels are also maximal at this time of day. Conversely, Ca2+ levels are likely lowest at dusk (CT12) when these indicators of calcineurin activity are lowest. Oscillating out of phase with calcineurin-dependent activities is phosphorylation of I-1 and PLB in response to increased β-adrenergic drive as the animal wakes. Phosphorylation of PLB, releases inhibition of SERCA2 increasing the rate of Ca2+ uptake, consequently lowering resting cytoplasmic Ca2+ levels, and potentially helping to maintain calcineurin in an inactive state. Eventually, cardiomyocytes become desensitized to prolonged β-adrenergic stimulation leading to a decrease in I-1 and PLB phosphorylation by PKA. Restored inhibition of SERCA2 by PLB would cause a gradual rise in cytoplasmic Ca2+ consistent with a gradual increase in calcineurin activity during the second half of the night. We postulate that the peak in calcineurin activity at the dark to light transition (CT0) helps maintain PLB and other key proteins in an unphosphorylated state as the mouse transitions to a time of lower cardiac demand. Conversely, a trough in calcineurin activity around dusk (CT12) would allow maximal β-adrenergic responses when the animal enters its waking hours. Consistent with this, fractional shortening of sTAC mice was higher in the evening than in the morning (Figure 5C) potentially reflecting circadian differences in the adrenergic response of the unanaesthetized mice to handling. Likewise, a recent study demonstrated circadian differences in the response of isolated adult cardiomyocytes to β-adrenergic stimulation.25
Although in this study we used changes in Rcan1.4 transcript levels primarily as an indication of changes in calcineurin activity, the RCAN1 protein itself likely contributes to shaping the dynamics of calcineurin-dependent signaling. It is interesting to note that cardiac damage from ischemia-reperfusion (I/R) is greater in mice lacking Rcan1 than in wild type hearts.26 Furthermore, I/R damage in wild type mice has been shown to be greater when the procedure is performed at CT12 than when I/R is performed at CT0.27 This time of greater susceptibility corresponds to the time when we find RCAN1.4 protein levels in the heart are lowest.
An important short-coming of the current study is our inability to monitor changes in I-1Thr35 phosphorylation and the as yet incomplete understanding of the cumulative effect of changes in I-1 phosphorylation at other sites including Ser67. Taking these limitations into account, the model in Figure 8B suggests that daily oscillations in Ca2+ handling and calcineurin activity form interdependent positive and negative feedback loops typical of circadian rhythms that result in a separation between times of day when kinase activities predominate and times of day when phosphatase activities predominate. Clearly this simple model does not take into account many additional factors that can influence calcineurin activity, PP1 activity, and cardiac contractility. For instance, the density of β-adrenergic receptors, adenylyl cyclase activity, and phosphodiesterase activity have all been shown to undergo circadian cycling both in neurons and the heart.28
Sustained hemodynamic stress elicits increases in both calcineurin activity and β-adrenergic drive. The model in Figure 8A suggests that these are opposing processes. Our data indicates that in hypertrophy and failure the normal temporal separation of calcineurin activity and phosphorylation of contractile proteins is disrupted. PLB phosphorylation was elevated throughout the day even during the animal’s sedentary period. In contrast, I-1Ser67 phosphorylation was completely lost, consistent with increased calcineurin activity. A corresponding loss of I-1Thr35 phosphorylation would release PP1 inhibition and result in uncoupling of circadian changes in calcineurin activity from regulation of PLB phosphorylation (Figure 8C). Initially, release of I-1 inhibition would be compensatory, helping to reverse hyper-phosphorylation of regulatory proteins, however, sustained loss of a circadian pattern of PP1 inhibition could ultimately contribute to declining cardiac function. Consistent with our observations, patients with heart failure demonstrate an increase in PP1 activity coincident with a decrease in I-1 phosphorylation.29, 30
The present studies provide a deeper understanding of the dynamics of calcineurin regulation in the heart and draw attention to the need to control for normal, underlying circadian changes in the activity of intracellular signaling pathways. In a human heart, it is likely that calcineurin activity cycles with a reverse phase compared to a mouse heart although as yet this is not known. In humans there is disproportionate ischemic activity, arrhythmic activity and acute cardiovascular events in the first few hours after waking.31 Interestingly this would correspond to the time of day when calcineurin activity would be lowest allowing maximal β-adrenergic responsiveness in a normal heart. Elevation of the trough in calcineurin activity in heart failure would compromise the ability to respond appropriately. There is a growing appreciation for the potential therapeutic benefit of timing drug delivery to correlate with maximal biological need.32 Our findings highlight the importance of remaining mindful of inherent circadian oscillations in the cardiovascular system during both the study and treatment of heart disease.
Novelty and Significance
What is known?
Circadian rhythms are important for maintaining cardiovascular health.
Activation of the protein phosphatase calcineurin is known to promote cardiac hypertrophy and heart failure.
What new information does this article contribute?
In healthy hearts, there is an apparent circadian rhythm in calcineurin activity that oscillates out of phase with phosphorylation of proteins, such as phospholamban (PLB) and inhibitor I (I-1), that regulate cardiac contractility.
In heart failure, calcineurin activity increases but continues to cycle, whereas cycling of PLB and I-1 phosphorylation is lost.
Summary
Despite overwhelming evidence that circadian rhythms are important in cardiovascular health and disease, little is known regarding circadian regulation of intracellular signaling pathways that control cardiac function and remodeling. Activation of calcineurin is known to promote pathological cardiac remodeling. Here we present evidence that there is a circadian rhythm in calcineurin activity in normal, healthy hearts that is not associated with transcriptional changes indicative of pathological remodeling. Calcineurin activity oscillates out of phase with phosphorylation of proteins, such as PLB and I-1 that promote cardiac contractility. In heart failure, calcineurin activity increases but continues to cycle, whereas cycling of PLB and I-1 phosphorylation is lost. We propose a model in which daily oscillations in Ca2+ handling and calcineurin activity form interdependent positive and negative feedback loops typical of circadian rhythms that result in separation between times of day when kinase activities predominate and times of day when phosphatase activities predominate. These studies provide a deeper understanding of the dynamics of calcineurin regulation in the heart and draw attention to the importance of normal, underlying circadian changes in regulating the activity of intercellular signaling pathways.
Supplementary Material
Acknowledgements
B.A.R. would like to dedicate this publication to the memory of Dr. Ruth Lyttle Satter (March 8, 1923 – August 3, 1989) a gifted teacher and friend.
Sources of Funding
This work was supported by NIH (HL072016, and HL097768 to B.A.R.; HL075173, HL080144, and HL006296 to J.A.H.; MH67777 to J.A.B) and the American Heart Association (0655202Y to B.A.R.; 0640084N to J.A.H).
Non-standard Abbreviations and Acronyms
- ANF
atrial natriuretic factor
- βMHC
beta myosin heavy chain
- CT
circadian time
- Glut4
high-affinity glucose transporter
- GSK3b
glycogen synthase kinase 3 beta
- I-1
PP1 inhibitor-1
- I/R
ischemia-reperfusion
- NFAT
nuclear factor of activated T cells
- Per2
period 2
- PKA
cAMP-dependent protein kinase
- PLB
phospholamban
- PP1
protein phosphatase 1
- RCAN1
Regulator of Calcineurin 1
- SERCA2
sarcoplasmic reticulum Ca2+ ATPase
- sTAC
severe transverse aortic constriction
- TAC
transverse aortic constriction
- TG
transgenic
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: 136, 138, 148, 155, 10
Disclosures None
References
- 1.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 2.Mitler MM, Hajdukovic RM, Shafor R, Hahn PM, Kripke DF. When people die. Cause of death versus time of death. Am J Med. 1987;82:266–274. doi: 10.1016/0002-9343(87)90067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 4.Honma S, Honma K. The biological clock: Ca2+ links the pendulum to the hands. Trends Neurosci. 2003;26:650–653. doi: 10.1016/j.tins.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann N Y Acad Sci. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 7.Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med. 2003;13:15–21. doi: 10.1016/s1050-1738(02)00188-3. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 9.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 10.El-Armouche A, Rau T, Zolk O, Ditz D, Pamminger T, Zimmermann WH, Jackel E, Harding SE, Boknik P, Neumann J, Eschenhagen T. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes. Faseb J. 2003;17:437–439. doi: 10.1096/fj.02-0057fje. [DOI] [PubMed] [Google Scholar]
- 11.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 12.Bibb JA, Nishi A, O'Callaghan JP, Ule J, Lan M, Snyder GL, Horiuchi A, Saito T, Hisanaga S, Czernik AJ, Nairn AC, Greengard P. Phosphorylation of protein phosphatase inhibitor-1 by Cdk5. J Biol Chem. 2001;276:14490–14497. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- 13.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 14.El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didie M, Dobrev D, Eschenhagen T. Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem Biophys Res Commun. 2006;346:700–706. doi: 10.1016/j.bbrc.2006.05.182. [DOI] [PubMed] [Google Scholar]
- 15.MacDonnell SM, Kubo H, Harris DM, Chen X, Berretta R, Barbe MF, Kolwicz S, Reger PO, Eckhart A, Renna BF, Koch WJ, Houser SR, Libonati JR. Calcineurin inhibition normalizes beta-adrenergic responsiveness in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2007;293:H3122–H3129. doi: 10.1152/ajpheart.00687.2007. [DOI] [PubMed] [Google Scholar]
- 16.Rothermel BA, Berenji K, Tannous P, Kutschke W, Dey A, Nolan B, Yoo KD, Demetroulis E, Gimbel M, Cabuay B, Karimi M, Hill JA. Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure. Physiol Genomics. 2005;23:18–27. doi: 10.1152/physiolgenomics.00061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 19.Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 20.Durgan DJ, Moore MW, Ha NP, Egbejimi O, Fields A, Mbawuike U, Egbejimi A, Shaw CA, Bray MS, Nannegari V, Hickson-Bick DL, Heird WC, Dyck JR, Chandler MP, Young ME. Circadian Rhythms in Myocardial Metabolism and Contractile Function; Influence of Workload and Oleate. Am J Physiol Heart Circ Physiol. 2007;293:H2385–H2393. doi: 10.1152/ajpheart.01361.2006. [DOI] [PubMed] [Google Scholar]
- 21.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 23.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 24.Lemmer B. The importance of circadian rhythms on drug response in hypertension and coronary heart disease--from mice and man. Pharmacol Ther. 2006;111:629–651. doi: 10.1016/j.pharmthera.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Collins HE, Rodrigo GC. Inotropic response of cardiac ventricular myocytes to beta-adrenergic stimulation with isoproterenol exhibits diurnal variation: involvement of nitric oxide. Circ Res. 2010;106:1244–1252. doi: 10.1161/CIRCRESAHA.109.213942. [DOI] [PubMed] [Google Scholar]
- 26.Sanna B, Brandt EB, Kaiser RA, Pfluger P, Witt SA, Kimball TR, van Rooij E, De Windt LJ, Rothenberg ME, Tschop MH, Benoit SC, Molkentin JD. Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo. Proc Natl Acad Sci U S A. 2006;103:7327–7332. doi: 10.1073/pnas.0509340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witte K, Hu K, Swiatek J, Mussig C, Ertl G, Lemmer B. Experimental heart failure in rats: effects on cardiovascular circadian rhythms and on myocardial beta-adrenergic signaling. Cardiovasc Res. 2000;47:350–358. doi: 10.1016/s0008-6363(00)00099-7. [DOI] [PubMed] [Google Scholar]
- 29.Gupta RC, Mishra S, Rastogi S, Imai M, Habib O, Sabbah HN. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 30.Sande JB, Sjaastad I, Hoen IB, Bokenes J, Tonnessen T, Holt E, Lunde PK, Christensen G. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc Res. 2002;53:382–391. doi: 10.1016/s0008-6363(01)00489-8. [DOI] [PubMed] [Google Scholar]
- 31.Martino TA, Sole MJ. Molecular Time: An Often Overlooked Dimension to Cardiovascular Disease. Circ Res. 2009;105:1047–1061. doi: 10.1161/CIRCRESAHA.109.206201. [DOI] [PubMed] [Google Scholar]
- 32.Smolensky MH, Hermida RC, Ayala DE, Tiseo R, Portaluppi F. Administration-time-dependent effects of blood pressure-lowering medications: basis for the chronotherapy of hypertension. Blood Press Monit. 2010;15:173–180. doi: 10.1097/MBP.0b013e32833c7308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.