Abstract
Introduction
Increased cardiovascular mortality and risk of venous thromboembolism are serious extra-pulmonary complications of chronic obstructive pulmonary disease (COPD). Previously, circulating active tissue factor (TF) and factor XIa (FXIa) have been reported to be associated with acute coronary syndromes.
Objective
To measure plasma FXIa and active TF, prothrombin fragment 1.2 (F1.2), and markers of systemic inflammation (C-reactive protein [CRP], interleukin-6 [IL-6], tumor necrosis factor α [TNFα] and matrix metalloproteinase 9 [MMP-9]) in 60 patients with documented stable COPD free of previous thromboembolic events.
Methods
In-house clotting assays using inhibitory monoclonal antibodies against FXIa and TF.
Results
FXIa was detected in 9 (15%) and TF activity in 7 (11.7%) COPD patients. Subjects positive for FXIa and/or TF (n=10; 16.7%) had higher F1.2 (median [interquartile range], 398 [216] vs 192 [42] pM, p<0.000001), fibrinogen (5.58 [2.01] vs 3.97 [2.47] g/L, p=0.0007), CRP (14.75 [1.20] vs 1.88 [2.95] mg/L, p<0.000001), IL-6 (8.14 [4.74] vs 2.45 [2.24] pg/mL, p=0.00002), and right ventricular systolic pressure (47 [15] vs 38 [12] mmHg, p=0.023), and lower vital capacity (66 [15] vs 80 [17] % predicted, p=0.04) than COPD patients without detectable FXIa and TF. COPD severity was not associated with the presence of circulating FXIa and active TF.
Conclusions
This is the first study to show that active FXIa and TF are present in stable COPD patients, who exhibit enhanced systemic inflammation and thrombin generation. Our findings suggest a new prothrombotic mechanism which might contribute to elevated risk of thromboembolic complications in COPD.
Keywords: Chronic obstructive pulmonary disease, tissue factor, factor XIa, inflammation
Chronic obstructive pulmonary disease (COPD) is mainly characterized by progressive airflow limitation associated with abnormal inflammatory response of the lung to noxious gases and particles. Recently, extra-pulmonary effects of COPD have been emphasized [1]. Exacerbations of COPD confer markedly increased risk of venous thromboembolism (VTE) [1]. Cardiovascular (CV) mortality in patients with COPD is significantly (2–4 fold) elevated, mostly as a result of coronary artery disease (CAD) [2]. A common cardiovascular risk factor, namely smoking, has been suggested as one of the causes of this elevated mortality. However, other risk factors, such as systemic inflammation, have been also implicated [3].
Initiation of blood coagulation in vivo is mainly dependent on tissue factor (TF) exposed to the blood flow upon vascular injury or expressed/exposed by some blood cells upon stimulation with inflammation-related cytokines [4]. TF binds factor (F)VIIa, normally present at a trace concentration in circulating blood, and initiates coagulation by accelerating FIX and FX activation. Activated FIX and FX combine with their respective cofactors and form the intrinsic FXase (FIXa-FVIIIa-calcium-membrane) and prothrombinase (FXa-FVa-calcium-membrane) complexes, which convert FX to FXa and prothrombin to thrombin, respectively.
It has been suggested several years ago that active TF is present on circulating microparticles in blood of individuals with diabetes mellitus [5]. Recently, TF procoagulant activity in blood of stable COPD patients has been suggested [6]. This TF activity did not correlate with thrombin generation, reflected by the thrombin-antithrombin complex (TAT) formation, although TAT levels were elevated in COPD patients. Procoagulant activity of FVIIa was not significantly higher in COPD patients and an inverse correlation between FVIIa and TF activity was observed.
Given the observations that increased levels of active FXIa are associated with higher risk of venous and arterial thrombosis [7] and that a significant proportion of patients with acute coronary syndrome (ACS), with previous myocardial infarction (MI) [8] and those with inflammatory bowel disease (IBD) [9] contain FXIa and TF activity in their plasma, we decided to investigate whether these two proteins can be detected in stable (moderate to very severe) COPD patients. Moreover, we attempted to determine a possible mechanism, which causes an occurrence of FXIa and TF activity in this patient population.
Patients and Methods
Patients
We studied 60 consecutive patients with documented COPD. Inclusion criteria were age >40 years and stable disease with postbronchodilator forced expiratory volume in one second (FEV1) <80% predicted with FEV1/FVC (forced vital capacity) ratio <0.70 for at least 2 months. Exclusion criteria included the signs or symptoms of any acute illness, serum C-reactive protein (CRP) levels >25 mg/l, congestive heart failure, left ventricular ejection fraction <40%, cancer, hepatic injury, renal failure (creatinine >177 μM), a history of VTE, anticoagulant treatment and acute coronary event within the previous 3 months. Smoking was defined as current smoking of one cigarette or more daily. Patients performed spirometric tests following American Thoracic Society standards. The Jagiellonian University Ethical Committee approved the study, and patients provided written, informed consent.
Methods
Venous blood samples from fasting patients were taken into 0.13 mM trisodium citrate tubes (Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged within 60 minutes at 2540g for 20 minutes at 24°C. Platelet-poor plasma was aliquoted, frozen and stored at −80°C until analyses. To determine FXIa and TF activity, we used inhibitory monoclonal mouse antihuman FXI (αFXI-2) and mouse anti-human TF (αTF-5) antibodies (both produced in-house) at a final 0.1 mg/mL concentration and measured their effect on clotting times as previously described [8]. The quantitation limits of our TF and FXIa activity assays were 0.4 pM and 10 pM, respectively. Healthy individuals showed no detectable TF or FXIa activity. To assess thrombin generation in circulating blood, we determined prothrombin fragments 1.2 (F1.2) in citrate plasma using an ELISA assay (Dade Behring, Marburg, Germany). Fibrinogen was measured by the Clauss method, C-reactive protein (CRP) by latex nephelometry (Dade Behring), and interleukin-6 (IL-6), tumor necrosis factor α(TNFα) and matrix metalloproteinase-9 (MMP-9) by ELISA (R & D Systems, Abingdon, UK). All the intra-assay and inter-assay coefficients of variation were below 7%.
Statistical analysis
Data are expressed as mean ±SD or as median (interquartile range) unless otherwise stated. The Kolmogorov-Smirnov test was used to assess conformity with a normal distribution. Between groups comparisons were performed using the t-test for independent variables, or Mann-Whitney U-test as appropriate. Pearson or Spearman correlation coefficients were determined to assess associations between variables, as appropriate. Categorical variables were compared by the two-sided Fisher exact test. The level of significance was set at P<0.05.
Results
Characteristics of COPD patients are presented in Table 1. They were predominantly male (91.7%) aged 44–81 years, mostly at 2nd and 3rd stage of the COPD (in the 4-stage severity classification); hypertension and smoking were the most common cardiovascular risk factors. FXIa and/or TF activity data are presented in Table 2. Detectable plasma activity of FXIa was observed in 9 of 60 (15%) patients. Quantifiable amounts (>10 pM) of plasma FXIa were detected in 8 of these patients (13.3% of all COPD patients). One patient had FXIa concentration below 10 pM. The concentration of quantifiable FXIa varied from 16 to 124 pM with a median value of 42 pM . Plasma TF activity was detected in 7 of 60 (11.7%) COPD patients, 6 of which also had FXIa in their plasma. Only 3 COPD patients had TF activity above 0.4 pM in the range from 0.5 to 1.3 pM.
Table 1.
Characteristics of patients with COPD
| Parameter | COPD patients (n=60) |
|---|---|
| Age, years | 62.5±10.1 |
| Sex, M/F | 55/5 |
| BMI, kg/m2 | 25.5±4.7 |
| Current smokers, n (%) | 22 (36.7) |
| Cigarettes, pack years | 32±17 |
| Hypertension, n (%) | 28 (46.7) |
| Diabetes, n (%) | 9 (15) |
| Previous MI, n (%) | 4 (6.7) |
| Spirometry | |
| FEV1, % predicted | 55.6±15.8 |
| FEV1/FVC, % predicted | 58.1±10.7 |
| VC, % predicted | 77.7±14.3 |
| Echocardiography | |
| EF, % | 61.4±8.6 |
| RVSP, mm Hg | 40.9±9.5 |
| Laboratory tests | |
| Glucose, mM | 4.7 (1.0) |
| Creatinine, µM | 86.5±20.2 |
| Fibrinogen, g/l | 4.14±1.60 |
| CRP, mg/l | 2.87 (5.16) |
| IL-6, pg/ml | 3.07 (3.32) |
| TNFα, pg/ml | 1.93 (0.85) |
| MMP-9, ng/ml | 482.8 (266.6) |
| F1.2, pM | 195.9 (80.9) |
Results are expressed as mean ± SD or median (interquartile range). BMI, body mass index; CRP, C-reactive protein; EF, ejection fraction of left ventricle; F1.2, prothrombin fragment 1.2; FEV, forced expiratory volume in 1 second; FVC, forced vital capacity; IL-6, interleukin 6; MMP-9, matrix metalloproteinase 9; RVSP, right ventricle systolic pressure; TNFα, tumor necrosis factor α; VC, vital capacity
Table 2.
Comparisons of COPD patients with respect to FXIa and/or TF activity
| Parameter | FXIa and/or TF activity | ||
|---|---|---|---|
| Present (n=10) | Absent (n=50) | p-value | |
| Age, years | 74.5 (20) | 63 (16) | 0.082 |
| Sex, M/F | 10/0 | 45/5 | 0.578 |
| BMI, kg/m2 | 23.7 (6.0) | 24.7 (5.1) | 0.396 |
| Current smokers, n (%) | 6 (60) | 16 (32) | 0.149 |
| Cigarettes, pack years | 36.5 (25) | 30 (22.5) | 0.943 |
| Spirometry | |||
| FEV1, % predicted | 51.5 (19) | 58 (18) | 0.293 |
| FEV1/FVC, % predicted | 64 (12) | 59.5 (16) | 0.234 |
| VC, % predicted | 66 (15) | 80 (17.2) | 0.004 |
| Echocardiography | |||
| EF, % | 60 (10) | 61 (14) | 0.331 |
| RVSP, mm Hg | 47 (15) | 38 (12) | 0.023 |
| Laboratory tests | |||
| Glucose, mM | 4.90 (0.30) | 4.65 (1.10) | 0.393 |
| Creatinine, µM | 78.5 (19) | 87 (21) | 0.525 |
| Fibrinogen, g/l | 5.58 (2.01) | 3.97 (2.47) | 0.0007 |
| CRP, mg/l | 14.75 (1.20) | 1.88 (2.95) | <10−6 |
| IL-6, pg/ml | 8.14 (4.74) | 2.45 (2.24) | 0.000003 |
| TNFα, pg/ml | 2.41 (2.48) | 1.88 (0.67) | 0.243 |
| MMP-9, ng/ml | 606 (457) | 477 (273) | 0.150 |
| F1.2, pM | 398 (216) | 192 (42) | <10−6 |
| FXIa, pM | 39.5 (81.0) | ND | - |
Results are expressed as median (interquartile range). COPD, chronic obstructive pulmonary disease; FXIa, coagulation factor XIa; TF, tissue factor; ND, not detected; other abbreviations, see table 1. FXIa was detected in 9 patients, and quantifiable in 8 subjects.
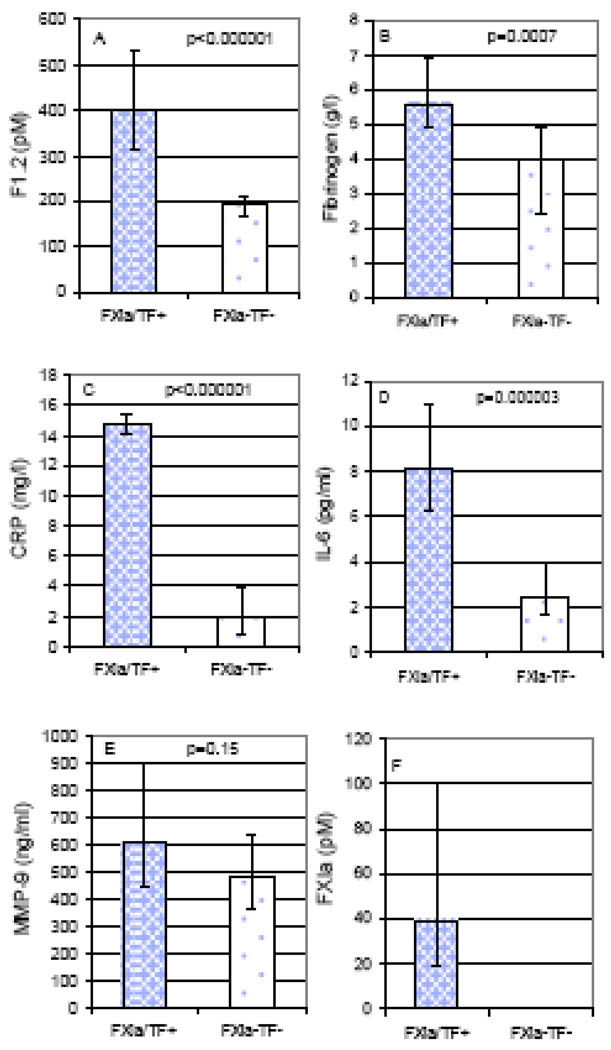
FXIa- and/or TF-positive patients did not differ from those not displaying detectable FXIa and TF activity in plasma with respect to demographics, BMI, glucose and creatinine levels, smoking and cigarette pack-years, and a COPD severity (airflow limitation) indicator. However, a trend for higher age in patients with FXIa and/or TF detectable activity was observed (Table 2). FXIa-and/or TF-positive COPD patients had significantly higher F1.2, fibrinogen, CRP, and IL-6 than those without detectable FXIa and TF activity (Figure 1). Plasma F1.2 correlated positively with fibrinogen (R=0.32, p=0.012), CRP (R=0.42, p=0.000008) and IL-6 (R=0.4, p=0.002), and negatively with VC (R= −0.31, p=0.018). COPD patients with both detectable TF and/or FXIa activity had significantly higher plasma F1.2 levels than those not displaying TF or FXIa activity (medians [IQR]: 475 [264] vs 192 [42] pM, p=0.00001). The same observation was valid for fibrinogen, CRP, IL-6 and MMP-9 (medians [IQR]: 5.58 [1.60] vs 3.97 [2.47] g/L, p=0.007; 14.55 [5.30] vs 1.88 [2.95] mg/L, p=0.000006; 6.99 [2.35] vs 2.46 [2.24] pg/mL, p=0.0008; 859 [232] vs 477 [273] ng/mL, p=0.027, respectively).
Fig. 1.
Prothrombin fragment 1.2 (F1.2; A), fibrinogen (B), C-reactive protein (CRP; C), interleukin 6 (IL-6; D), matrix metalloproteinase 9 (MMP-9; E), and FXIa (F) in COPD patients with detectable FXIa and/or TF activity (FXIa/TF+; n=10) as compared to patients without detectable FXIa and/or TF activity (FXIa-TF-; n=50). Medians and interquartile ranges are shown.
All COPD patients with detectable activities of FXIa and/or TF have CRP levels above 3 mg/L. These and other patients with CRP levels above 3 mg/L (n=28) have significantly higher levels of fibrinogen, F1.2, and IL-6, and lower VC than remaining COPD subjects (medians [IQR]: 4.92 [1.85] vs 3.02 [2.01] g/L, p=0.00036; 236 [126] vs 181 [37] pM; p = 0.0001; 4.16 [4.55] vs 2.32 [2.00] pg/mL, p=0.0008; 73 [22.5] vs 83 [18] % predicted, p=0.01, respectively). Coagulant and inflammatory markers were not related to COPD severity (classification based on decrease of FEV1; data not shown). Interestingly, there were FXIa- and TF-related differences in pulmonary function tests and an echocardiographic measure of pulmonary artery pressure (right ventricular systolic pressure [RVSP]). FXIa- and/or TF-positive patients had significantly lower vital capacity (VC; 65±14 vs. 80±13% predicted; p=0.004) and higher RVSP (49±13 vs. 39±17 mm Hg; p=0.023) than FXIa- and TF-negative COPD subjects (Table 2).
Discussion
To our knowledge, this study is the first to demonstrate detectable activity of FXIa in COPD patients, mostly accompanied by the detectable TF activity. Previously we showed that circulating FXIa was observed in 96% of patients with ACS, in 76% of subjects with a history of MI [8] and in 66% of patients with IBD [9]. 38% of ACS patients and only 6% of stable CAD patients showed detectable TF activity [8]. Similar to ACS, 34% of IBD and 37% of heart failure patients had detectable TF activity in their plasma [9,10]. In all three groups of patients, all individuals with detectable TF activity contained circulating FXIa.
In the current study, circulating levels of active TF in the COPD patients were significantly lower compared to those observed in acute coronary ischemia whereas concentration of active FXIa was only slightly lower than that observed in stable angina subjects who survived MI [8]. Increased levels of markers related to systemic inflammation (including IL-6) and enhanced thrombin generation determined by plasma F1.2 in FXIa-positive COPD patients is in line with earlier studies [6, 11, 12] and suggest that most likely thrombin, not the contact pathway, accounts for FXI activation in COPD patients. The presence of elevated IL-6 suggests an increased TF expression on blood and vascular cells [13]. As a consequence, an extrinsic pathway of thrombin generation is induced leading to an elevated production of this enzyme. When generated, thrombin activates zymogen FXI to its active form FXIa [14].
We have found detectable TF activity in ~12% of COPD patients. However, this does not mean that other COPD patients do not have active TF in their blood; it may simply be present at the concentrations below our detectability limit. For example, the results of our previous study showed that the concentration of active TF in blood from healthy individuals does not exceed 20 fM (if present at all) [15]. Thus, if TF present in COPD patients between 20 fM and the detectability limit, it will be not registered in our clotting assay. Largely due to the lack of validated and reliable commercial assays for TF antigen and activity [16,17], there are marked discrepancies in published concentrations of TF in blood of healthy individuals and in those with various diseases, with high (reaching sub-nanomolar) TF concentrations reported [15,18,19]. Several lines of evidence clearly indicate that such high functional TF levels would cause plasma or blood clotting within minutes (seconds) [15]. Since our assay for TF activity determination in citrated plasma has been validated and used in clinical settings [8], we believe that this analysis provides better quantitation of active TF concentrations in plasma obtained from patients.
As in the acute MI patients [8], we observed that detectable amounts of active FXIa are permanently present in plasma despite the abundance of numerous inhibitors for serine proteases (serpins) because none of these inhibitors can effectively inhibit FXIa [20]. As a consequence, 80 to 90% of active FXIa can survive in citrate blood for at least 30 min required for citrate plasma preparation [8]. There is no de novo FXIa generation observed in citrate plasma at room temperature over a period of 75 min either in the presence of contact pathway inhibition or in the absence of it [8]. These data indicate that plasma FXIa in the current study does indeed reflect the in vivo levels of this enzyme.
Our study corroborates with previous reports suggesting the role of systemic inflammation in increased CV mortality of COPD patients [3], as we have found elevated levels of CRP and IL-6 in FXIa- and TF-activity positive patients. It is not known if elevated TF and FXIa activities (in concordance with or regardless of systemic inflammation) affect structure of fibrin clots, which are composed of much denser networks and are more resistant to lysis in COPD patients [21]. Systemic inflammation might play a role in the development of pulmonary hypertension in COPD subjects since it was shown that CRP levels are associated with higher pulmonary blood pressure in these patients [22]. We were not able to replicate this observation but found higher right ventricle systolic pressures (reflecting pulmonary artery pressure) in TF- and/or FXIa-activity positive than in negative COPD patients. There was no correlation between the presence of detectable FXIa and/or TF activity and the severity of COPD, which was determined by the decrease in FEV1 (airflow limitation) indicator. No correlations between FEV1 and F1.2 or markers of systemic inflammation were observed. However, coagulation markers might be associated with pulmonary function; we found markedly decreased VC in FXIa-positive COPD patients and a significant inverse correlation between VC and F1.2. These novel observations need further investigation.
The current study has several limitations: 1) the number of patients studied was relatively small, therefore some subgroup analyses should be interpreted with caution; 2) our analyses were based on a determination of each variable at a single time point. It is likely that with time and during exacerbations, concentrations of FXIa and/or active TF will undergo changes; 3) statistical associations reported here do not necessarily infer cause-effect relationships. Further studies of COPD patients with long-term follow-up are needed to evaluate a potential impact of the presence of circulating active TF and/or FXIa on cardiovascular events beyond MI, including ischemic stroke [23, 24].
In conclusion, the current study demonstrates the presence of detectable FXIa and/or TF activity in a subset of stable COPD patients. These procoagulant activities are associated with markers of systemic inflammation and thrombin generation but not with the disease severity assessed by airflow limitation. The presence of FXIa and/or active TF in circulating blood might contribute to the risk of thrombotic complications of COPD, highlighting close links between chronic inflammation and blood coagulation. It remains to be established whether these two factors may help identify COPD patients at increased risk of myocardial infarction and possibly other thrombotic complications.
Acknowledgements
This study was supported by P01 HL46703 grant from the National Institutes of Health (SB). We would like to thank Ruhin Yuridullah and Matthew Gissel for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented at the XXII Congress of the International Society on Thrombosis and Haemostasis, July 11–16, 2009, Boston MA, USA (abstract #0602).
Conflict of interest statement
None declared.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease: Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2009) www.goldcopd.com.
- 2.Sin DD, Anthonisen NR, Soriano JB, Augusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 4.Butenas S, Orfeo T, Mann KG. Tissue factor activity and function in blood coagulation. Thromb Res. 2008;122 Suppl. 1:S42–S46. doi: 10.1016/S0049-3848(08)70018-5. [DOI] [PubMed] [Google Scholar]
- 5.Diamont M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 6.Vaidyula VR, Criner GJ, Grabianowski C, Rao AK. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res. 2009;124:259–261. doi: 10.1016/j.thromres.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seligsohn U. Factor XI in haemostasis and thrombosis: Past, present and future. Thromb Haemost. 2007;98:84–89. [PubMed] [Google Scholar]
- 8.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 9.Undas A, Owczarek D, Gissel M, Salapa K, Mann KG, Butenas S. Activated factor XI and tissue factor in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1447–1448. doi: 10.1002/ibd.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zabczyk M, Butenas S, Palka I, Nessler J, Undas A. Active tissue factor and activated factor XI in circulating blood of patients with systolic heart failure due to ischemic cardiomyopathy. Pol Arch Med Wewn. 2010;120:334–340. [PMC free article] [PubMed] [Google Scholar]
- 11.Ashitani J, Mukae H, Arimura Y, Matsukura S. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Int Medicine. 2002;41:181–185. doi: 10.2169/internalmedicine.41.181. [DOI] [PubMed] [Google Scholar]
- 12.Alessandri C, Basili S, Violi F, Ferroni P, Gazzaniga PP, Cordova C. Hypercoagulability state in patients with chronic obstructive pulmonary disease. Chronic Obstructive Bronchitis and Haemostasis Group. Thromb Haemost. 1994;72:343–346. [PubMed] [Google Scholar]
- 13.Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 14.Cawthern KM, van't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91:4581–4592. [PubMed] [Google Scholar]
- 15.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 16.Parhami-Seren B, Butenas S, Krudysz-Amblo J, Mann KG. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4:1747–1755. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanov VY, Cimmino G, Tardos JG, Tunstead JR, Badimon JJ. Assessment of tissue factor activity in patients presenting with coronary artery disease: limitations of commercial assay. J Thromb Haemost. 2009;7:894–897. doi: 10.1111/j.1538-7836.2009.03315.x. [DOI] [PubMed] [Google Scholar]
- 18.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–1996. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.So AK, Varisco PA, Kemkes-Matthes B, Herkenne-Morard C, Chobaz-Peclat V, Gerster JC, Busso N. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. 2003;1:2510–2515. doi: 10.1111/j.1538-7836.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 20.Scott CF, Schapira M, James HL, Cohen AB, Colman RW. Inactivation of factor XIa by plasma protease inhibitors : predominant role of alpha 1-protease inhibitor and protective effect of high molecular weight kininogen. J Clin Invest. 1982;69:844–852. doi: 10.1172/JCI110524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Undas A, Kaczmarek P, Sładek K, Stepien E, Skucha W, Rzeszutko M, Gorkiewicz-Kot I, Tracz W. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease. Beneficial effects of simvastatin treatment. Thromb Haemost. 2009;102:1176–1182. doi: 10.1160/TH09-02-0118. [DOI] [PubMed] [Google Scholar]
- 22.Joppa P, Petrasova D, Stancak B, Tkacova R. Systemic inflammation in patients with COPD and pulmonary hypertension. Chest. 2006;130:326–333. doi: 10.1378/chest.130.2.326. [DOI] [PubMed] [Google Scholar]
- 23.Yang DT, Flanders MM, Kim H, Rodgers GM. Elevated factor XI activity levels are associated with an increased odds ratio for cerebrovascular diseases. Am J Clin Pathol. 2006;126:411–415. doi: 10.1309/QC259F09UNMKVP0R. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Hu Y, Hong M, Guo T, Wei W, Song S. Plasma thrombomodulin, fibrinogen, and activity of tissue factor as risk factors for acute cerebral infarction. Am J Clin Pathol. 2007;128:287–292. doi: 10.1309/HB6AB1YR4DQUT5AU. [DOI] [PubMed] [Google Scholar]