Abstract
Rhesus is the clinically most important protein-based blood group system. It represents the largest number of antigens and the most complex genetics of the 30 known blood group systems. The RHD and RHCE genes are strongly homologous. Some genetic complexity is explained by their close chromosomal proximity and unusual orientation, with their tail ends facing each other. The antigens are expressed by the RhD and the RhCE proteins. Rhesus exemplifies the correlation of genotype and phenotype, facilitating the understanding of general genetic mechanisms. For clinical purposes, genetic diagnostics of Rhesus antigens will improve the cost-effective development of transfusion medicine.
Keywords: Rhesus, blood group, molecular genetics, molecular diagnostic, transfusion
1. Introduction
Molecular biology has been applied extensively in characterizing the genetic basis of blood group systems and developing clinical diagnostic tools for immunohematology and transfusion medicine1–4. There are now 51 antigens within the Rh system and more than 200 alleles for the RHD gene alone. RHD zygosity has been resolved, epitopes have been mapped, and many D variants with altered antigens have been identified. The relationship among the RH family members in various species contributes to our understanding of their biological importance 5.
Based on the homology of Rh polypeptides to the ammonia transporter AmpB, computational analyses have modeled the 3D structure of the RhD polypeptide to learn about additional potential functions of Rh polypeptides 6. The reason for this interest is that RHAG, a gene located on chromosome 6 (6p11–p21), shares an identical exon structure and major regions of sequence identity with RHD/RHCE. Moreover, RhAG is essential for the expression of the Rh polypeptides and was identified in 2008 as the latest blood group system, no. 30, in its own right 7.
To date, the function of RhD and RhCE appears associated with membrane integrity, and possibly transport of gases like carbon dioxide. On the other hand, the Rh-associated glycoprotein (RhAG) can transport ammonia 8, but whether it does so in red blood cells (RBCs) is debated. Also, RhAG may contribute to gas exchange across the plasma membrane, and its mutations are associated with hereditary stomatocytosis 9. Thus, expression of Rh polypeptides and associated proteins is complex, and molecular discoveries have broadened our understanding of this important blood group system. This review summarizes the progress that molecular analyses have made in furthering clinical applications for Rh.
Three clinically useful discoveries have been made since the cloning of RHCE and RHD: 1) the molecular basis underlying the common Rh-negative haplotype and the nucleotide polymorphisms associated with the common Rh antigens have been applied to predict risk for hemolytic disease of the fetus and newborn (HDFN); 2) the molecular distinction of partial D and weak D alleles, DEL, RHD-pseudogenes, and the RHD-deleted genome; and 3) the molecular basis of D antigen epitope expression on the RhCE polypeptide.
Recently efforts by a few independent research laboratories have begun to more fully characterize the molecular basis of RhCE variants and the allelic variation of RHCE. The results are applied to improve transfusion support for sickle cell disease (SCD, see a separate review in this issue) and to identify the deficiencies of monoclonal antisera in assigning antigen status accurately. It is now apparent that molecular analyses are the most accurate way to define the complex RH and other blood group systems. In a steadily increasing number of clinical settings, these molecular approaches facilitate preventing blood group incompatibilities, avoiding alloimmunizations and hemolytic transfusion reactions, and contributing to optimal RBC survival in transfusion-dependent immune disorders.
2. Molecular Basis of RH
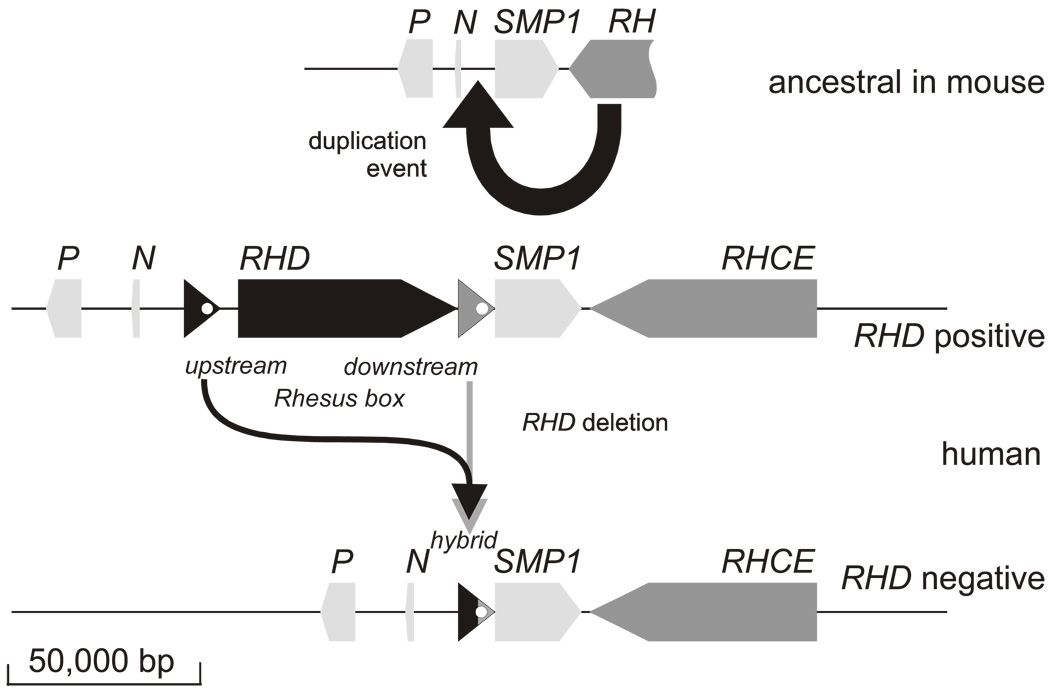
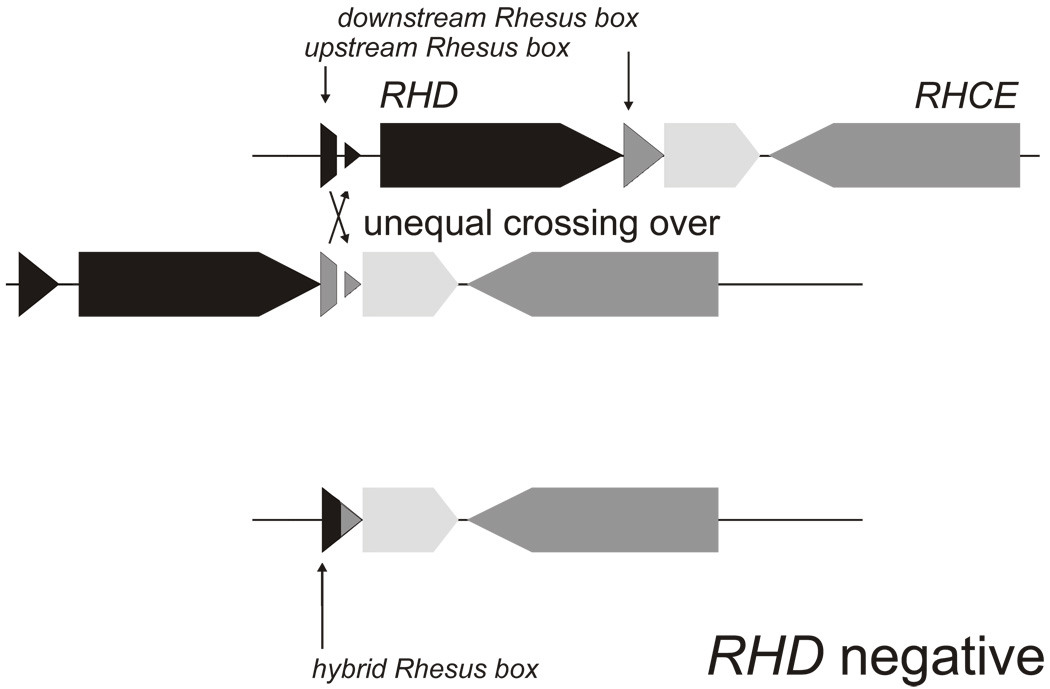
RH is a bigenic locus comprising RHD and RHCE positioned in a tail-to-tail orientation toward the end of the short arm of chromosome 1 (p34–36). Another gene, SMP1, is interspersed between both RH genes in close proximity to the 3’ end of RHCE (Figure 1) 10; this minute technical feature was instrumental in resolving the physical structure of the RH gene locus 11. Identification of the single murine equivalent in the mouse genome project provided evidence that RHCE evolved from the ancestral RH on the basis of the position and orientation of murine genes in the region (Figure 1). Therefore, RHD arose from a duplication event that predates modern humans 12. During the duplication event, and possibly associated with its cause, two approximately 9,000 base-pair-long homologous repeat sequences, termed Rhesus boxes, were likely introduced that flank the RHD gene in the genomes of modern humans. RHD was lost from the genome through unequal crossing over involving the upstream Rhesus box and downstream Rhesus box (Figure 2), an event that may have occurred more than once. RHD and RHCE share sufficient sequence homology that RBCs function normally when no RHD gene is inherited. Why the RHD-deleted genomes have persisted to this day and become more prevalent is the topic of much worthwhile debate and some esoteric speculation 13,14.
Figure 1. Duplication of the RH gene and loss of the RHD gene.
The ancestral configuration is shown as represented by the RH gene locus in mouse. The single RH gene is in close proximity to the three genes SMP1, P29-associated protein (P), and NPD014 (N). A duplication event introduced a second RH gene in reverse orientation between N and SMP1. At the two break points in front and behind the RHD gene, DNA segments of approximately 9,000 base pairs (bp) occur. Both DNA segments are flanking the RHD gene and dubbed ”upstream Rhesus box“ and ”downstream Rhesus box“. In the RHD positive haplotype, the RHD gene may have been lost by a recombination event (see Figure 3).
Figure 2. RHD deletion.
An unequal crossing over event between an upstream Rhesus box and a downstream Rhesus box caused the RHD deletion. If one of the two crossed-over chromosomal threads are resolved, an RH gene locus results that lacks the RHD gene completely and harbors a hybrid Rhesus box.
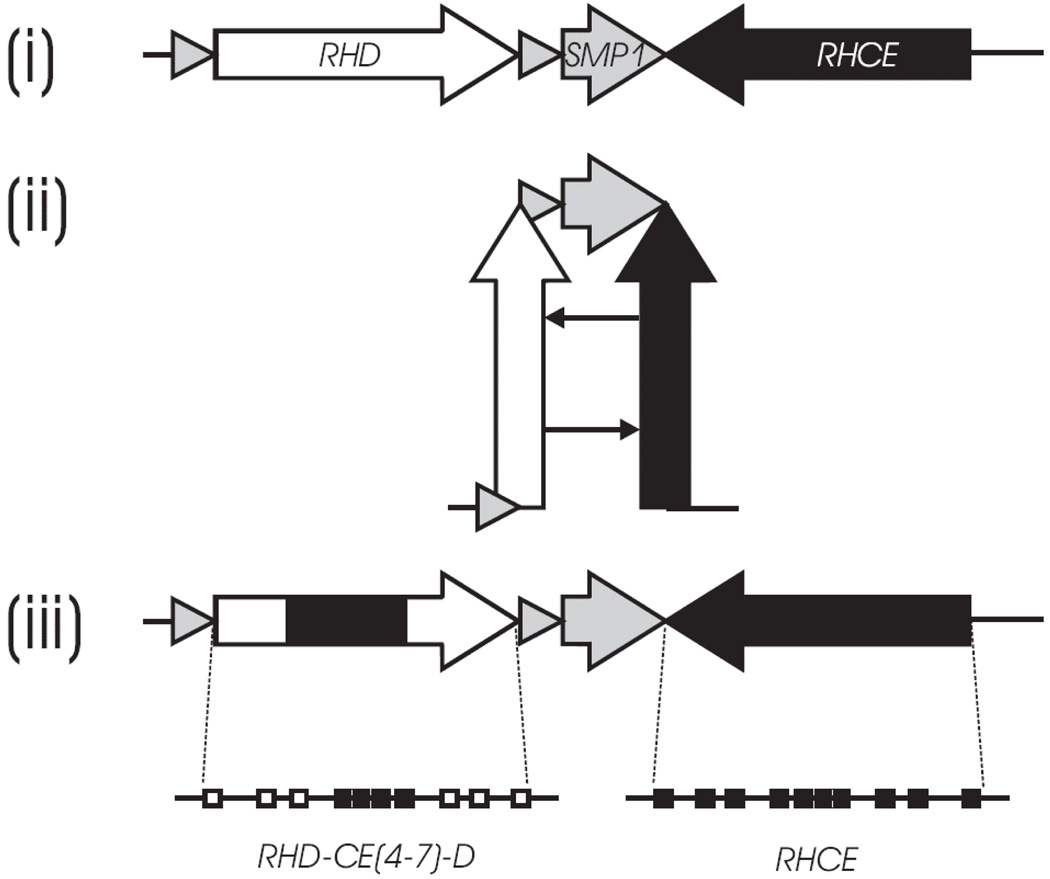
More than 200 RHD alleles have been reported and may be grouped according to serological and molecular features (Table 1). Most of the alleles harbor either single nucleotide polymorphisms (SNPs) or present as RHD/RHCE hybrid alleles. The tail-to-tail orientation (Figure 3) may facilitate the large number of alleles; the identification of corresponding nucleotides in both genes suggests that most hybrid alleles arise through gene conversion events. A clinical benefit of the molecular characterization of the RH locus is that RHD zygosity can be assigned with near certainty. In the past, Rh haplotype tables based on serological analyses were used to predict the ‘most probable genotype’ and thus RHD zygosity for Caucasians and African Americans.
Table 1.
Representative molecular changes in RHD alleles expressing distinct phenotypes of the D antigen
| classification of antigen variation |
D antigen Phenotype |
molecular basis |
representative example |
novel Rhesus antigen |
||
|---|---|---|---|---|---|---|
| protein variation | Mechanisms | RHD allele | trivial name | |||
| partial D | qualitative change | amino acid substitution on the RBC surface | missense mutation | RHD(G355S) | DNB | Unknown |
| protein segment exchange on the RBC surface | gene conversion (hybrid protein) | RHD-CE(3–6)-D | DVI type 3 | BARC | ||
| weak D | quantitative change | amino acid substitution in the membrane or intracellularly | missense mutation | RHD(V270G) | weak D type 1 | Unknown |
| DEL | major quantitative | grossly reduced translation or | missense mutation | RHD(M295I) in CDe | not applicable | unknown |
| change | protein expression | mutation at splice site | RHD(K409K) | not applicable | unknown | |
| D negative | D negative | lack of protein expression | gene deletion | RHD-Deletion | D negative | unknown |
| nonsense mutation | RHD(Y330X) | not applicable | unknown | |||
| Frame shift mutation | RHD(488del4) | not applicable | unknown | |||
| modifying gene | defect of RHAG gene | Rhnull | unknown | |||
| protein segment exchange on the RBC surface | gene conversion (hybrid protein) | RHD-CE(3–7)-D | Cdes | Unknown | ||
| antithetical antigens of the RhCE protein | expression of antigen E or antigen e | amino acid substitution on the RBC surface | missense mutation at amino acid position 226 in RHCE | RHCE allele: Ala226 coding antigen e Pro226 coding antigen E | not applicable | E versus e |
Figure 3. RHD/RHCE hairpin formation.
The schematic diagram depicts the mechanism of gene conversion at the Rhesus gene locus on one chromosome. (i) The RHD and RHCE genes are inversely orientated, which is typical for clustered genes. (ii) A hairpin formation of the chromosome would generate the close proximity of homologous segments in identical orientation. This structural feature is generally instrumental in gene conversion events in cis. (iii) Resolving the hairpin yields an RHD-CE-D hybrid gene structure, many of which have been observed to date at the RH gene locus. The RHD-CE(4–7)-D hybrid exon structure shown here is an example. Modified from Wagner et al., licensee BioMed Central Ltd. Reprinted with permission.
3. Modeling of Rh Polypeptides
The Rh proteins belong to the ammonium transport (E. coli AmtB)/methylammonium permease superfamily. Initially, molecular modeling of both Rh proteins and the RhAG protein on the crystal structure of E. coli AmpB suggested that the Rh proteins and RhAG form trimers in the red cell membrane, and may therefore function as ammonium transport proteins 15. A few independent investigators have shown that RhAG facilitates ammonium transport in yeast and Xenopus oocytes without co-transfection of RhD and RhCE polypeptides 8. Similar ammonium transport activity was shown directly in erythrocytes 16. However, RhD and RhCE do not appear to transport ammonium or carbon dioxide; key amino acid substitutions in the transmembrane channel do not appear to meet the requirements for facilitating transport 17.
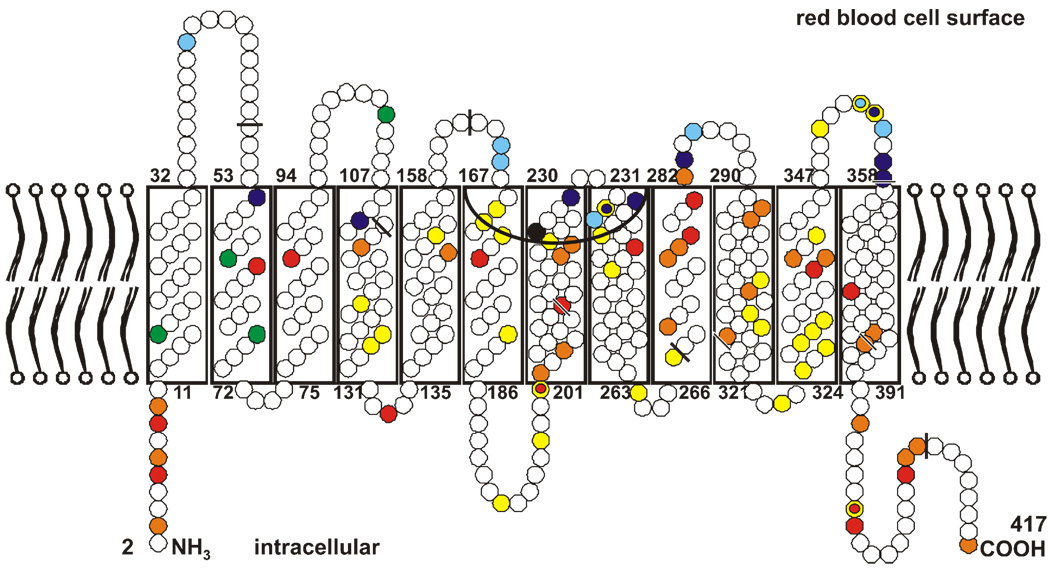
The model of red cell Rh proteins is consistent with the 6 exofacial loops that were first proposed on the basis of computational hydropathy plots 18. A critical component of the structure of RhD and RhCE is the amino acids that reside in the extracellular Rhesus vestibule of the channel formed by the transmembrane regions (Figure 4) 19. A few studies have shown that amino acid changes in the vestibule alter the molecular structure sufficiently to make persons susceptible to the formation of anti-D, although their red cells phenotype as Rh-positive 19,20. Thus, molecular studies have convincingly confirmed the hypothesis of Tippett and coworkers that some D variants lack the expression of some D antigen epitopes and can permit an immune response to the missing parts 21.
Figure 4. Model of Rhesus proteins in the red blood cell membrane.
Both Rhesus proteins comprise 417 amino acids, shown here as circles. Mature proteins in the membrane lack the first amino acid. The amino acid substitutions that distinguish the RhCE from the RhD protein are marked in yellow, with the 4 amino acids that code for the C antigen in green and the one that codes for the E antigen in black. The single amino acids substitutions which code for partial D are in blue, and those that code for weak D are in red. The mutations that had been identified at the Ulm Institute since 1999 are in light blue and orange. The extracellular Rh vestibule is depicted by the inverted black arc and bordered in part by amino acids of loops 3 and 4. The nine exon boundaries in the RHD cDNA, as reflected in the amino acid sequence, are indicated by black bars.
4. Molecular Classification of Rh Phenotypes
RHD and RHCE share regions of identity, with the translated RhD polypeptide differing at up to 36 amino acid positions depending on which RhCE polypeptide it is compared with. Both Rh polypeptides comprise 12 transmembraneous protein segments and 6 extracellular /intracellular loops (Figure 4). Historically, serologic studies classified the D antigen into six major categories (DII through DVII, with DI being obsolete). Three epitope models were proposed comprising 9-epitopes or 37-epitopes or the combination of both based on the serological reaction pattern of > 80 monoclonal anti-D antibodies 22,23. Many variants express altered D antigen, but no absolute correlation exists between phenotypic expression and clinical relevance of RHD alleles. RHD alleles have been classified on the basis of their phenotypic relationship to the molecular variation: partial D, weak D types, DEL, and nonfunctional alleles 24,25.
partial D
The classification of partial D variants is based on the premise that certain amino acid substitutions on an extracellular loop affect linear D epitopes or, more often, the 3-dimensional conformation of that loop. Many partial Ds are identified using monoclonal antibodies that target specific domains or loops on the surface of the erythrocyte 26. The D categories (DII to DVII) represent a subset of all partial Ds. DII and DVII are caused by single extracellular amino acid substitutions, while DIII, DIV, DV, and DVI are caused by RHD-CE-D hybrid alleles and comprise several subtypes each. The classification as partial D is of clinical relevance because carriers often produce anti-D upon exposure to the normal D antigen R 27.
The inclusion of D categories among partial D makes intuitive sense, because they share the common feature of exofacial amino acid substitutions in different spatial arrangements. However, for many partial D, anti-D immunization events are apparently rare, and for several partial D there has been no observation of any patient with anti-D so far. These facts are compatible with the conclusion that carriers of several distinct partial D may be at a very low or no risk of anti-D immunization.
weak D
A weak D type is a variant of the RhD protein that comprises an amino acid substitution located in the transmembraneous or intracellular segments and expresses a reduced amount of D antigen (generally less than 5,000 D antigens per RBC) 28. A group of 16 distinct weak D types were described originally, but the total number of weak D types including their subtypes now exceeds 80. The substitutions are thought to cause folding problems during integration of the protein into the RBC membrane, which can impede protein integration, affecting palmitylation or anchoring of the polypeptide to the RBC cytoskeleton 29. Hence, the amount of D antigen expressed on the RBC surface is quantitatively reduced, but the D antigen itself remains, by-and-large, qualitatively unchanged. Therefore, the normal D antigen is not usually immunogenic 28,30–36.
Like the mentioned exception for several partial D that cannot be immunized, there is an exception for some weak D types. Anti-D immunization in weak D carriers is rare, but there are exceptions: examples include weak D type 15, weak D type 4.2, also known as DAR, and weak D type 7 37–39. The weak D types 1, 2, 3, and 4.0/4.1, which are the most prevalent in any European and Caucasian population, represent more than 95% of all weak D types. To date, more than 10 years after their molecular description, the literature has not documented any carrier of weak D types 1 through 4.1 being alloimmunized and producing allo-anti-D. Those observed produce low titer antibodies of autoantibody nature. The observation that the common weak D types fail to make allo-anti-D is particularly relevant in the prevention and management of anti-D alloimmunization in pregnancy, which we will address in detail below.
DEL
A very weakly expressed D antigen is called DEL (formerly Del) because it was originally detected only if anti-D adsorbed and then eluted from RBCs. Thus, the D antigen detection is by elution only (DEL). Typically, RBCs with DEL express 200 or fewer copies of the D antigen per RBC 40.
The most common DEL is caused by the RHD(K409K) allele harboring the C1225A nucleotide substitution in exon 9 41. Because it is very prevalent in D negative Asians, it has been dubbed the “Asian type” DEL 36. This substitution is a silent single nucleotide polymorphism (SNP), i.e. the amino acid lysine (K) at position 409 remains unchanged. However, the substitution causes missplicing mRNA such that the complete full messenger mRNA has never been documented and at most represents a very minor form of transcript for translation 42.
Other DEL alleles have underlying molecular changes that cause more pronounced effects than in weak D and strongly impede but do not completely abrogate membrane integration 41,43–45. Even combined, all DEL phenotypes are rare in Europeans. Up to 30 % of seemingly D-negative East Asian people carry the DEL RHD(K409K), but other DEL alleles are also more frequent in Asia than in Europe.
DEL is of interest worldwide because of its potential to cause anti-D alloimmunization when DEL-positive blood donors are inadvertently labeled as D negative 46. In addition, DEL alleles can cause genotype-phenotype discrepancies and should be taken into consideration when fetal blood group genotyping methods depend on the ethnicity of the parents 31,33,47,48. The fetal inheritance of DEL would not be considered a risk of hemolytic disease of the fetus and newborn (HDFN).
D negative
The most common D negative haplotype in all populations is caused by the deletion of the whole RHD gene with the concomitant presence of the hybrid Rhesus box (Figure 2). However, other D negative haplotypes exist 49,50. Some individuals who are D negative can harbor a nonfunctional RHD allele. One of the first nonfunctional RHD alleles was termed RHD pseudogene (RHDψ). Since that time, several RHD-CE-D hybrid alleles have been reported, including Cdes with its characteristic hybrid RHD/CE exon 3 (Table 1). Both nonfunctional alleles occur rather frequently in African populations. Less common D negative alleles are caused by a host of different hybrid RHD-CE-D alleles or nonsense and frame shift mutations 41,51. It is important to note that the distinction between apparent D negative and DEL phenotypes by serology may be somewhat arbitrary. But the clinical significance is not: DEL blood transfused to D negative transfusion recipients is immunogenic, and the common “Asian type” DEL is not prone to making anti-D after its carrier is transfused with normal D positive RBC units. Therefore, in Asian populations, in whom D negative blood is rare, identifying DEL transfusion recipients (approximately 1/3 of all serological D negative) could significantly reduce the demand for Rh-negative blood 42,52,53.
Rhnull
The lack of both RhD and RhCE proteins may be caused by the inheritance of two nonfunctional RHCE alleles in the background of an RHD deleted haplotype. This constellation gives rise to the amorph type Rhnull phenotype (lack of any Rh protein), in which neither D nor CE antigens are expressed 54,55. Alternatively, because the expression of either Rh protein requires the presence of RhAG for appropriate assembly on the RBC membrane, defects in RHAG alleles cause the lack of both RhD and RhCE proteins. This biological background explains why defects in RHAG alleles cause the regulator type Rhnull phenotype (lack of expression of Rh protein), in which D and CE antigens may be undetectable but are in principle expressed 56.
Rhnull alloimmunization in pregnancy can be extremely difficult to manage in the setting of HDFN, largely due to the lack of compatible allogenic blood. Maternal blood has been used as a source of blood for the fetus and neonate 57.
RhCE variants
Partial antigens have been reported for the common RhCE antigens; C, c, E, and e, although several RHCE alleles have been characterized and many other alleles may exist 58–68. Moreover, as with partial D, carriers of partial CE antigens can make antibodies to epitopes that are missing on the variant RhCE protein. Unlike RHD, RHCE is not often deleted. Therefore partial CE antigens are less obvious from serology, because they are covered by the regular RhCE protein from the second chromosome. For example, erythrocytes expressing an e variant may be assumed to be E homozygous. Few people carry these variants, which is one reason that alloimmunization is uncommon. The clinical relevance of RhCE variants may be more appreciated once molecular analysis allows deeper insight into their associated immunization events, like, for instance, in sickle cell disease (SCD) patients, in pregnancies, and in chronically transfused patients.
5. RHD Phylogeny
The phylogenic study of RHD alleles delineated 4 clusters: the Eurasian D cluster with the consensus RHD (Genbank mRNA accession NM_016124.3), the most common allele expressed in humans, and three African clusters designated DIVa, DAU, and weak D type 4. Clusters are defined by an allele that differs from the consensus RHD allele and comprise many alleles that gained additional amino acid substitutions 69. As the genetic characteristics are unraveled and we gain more information about RHCE alleles (Genbank mRNA accession NM_020485.3) and their linkages to specific RHD alleles in haplotypes, the actual phylogenic tree will become more complete and well defined 19.
For example, it is expected that a few distinct RHD alleles are associated with RHCE alleles lacking hrS or hrB expression, and it may be possible to identify specific non-sister chromatid exchanges between haplotypes through such studies. On the other hand, there are examples of similar alleles that arose from independent gene conversion events within the Eurasian D cluster. Some DV alleles appear to have been caused by random or independent molecular events, and for these reasons, the DV group of partial D is not defined as a cluster 70.
6. Population Studies
It is apparent from systematic studies in African populations as well as sporadic observations in alloimmunized patients, as in those with SCD, that the allelic variation among Africans is much larger and more variable than in any other population. The reason for the presence of so many RH alleles in Africans remains unknown. Identifying a potential selective pressure or advantage may shed light on the function of Rhesus. The prevalence of distinct alleles in South Africans and West Africans also differs, and has not been fully evaluated, while studies from East Africa are largely lacking.
Europeans and East Asians share a small and overlapping subset of the African alleles called the Eurasian D cluster. The primordial alleles of this cluster are of African origin and are still fairly prevalent in African populations. The Eurasian D cluster may have more known alleles than the other 3 clusters combined but this almost certainly represents an observation bias and hints to the host of yet-to-be discerned alleles in all populations. Arab and Indian peoples represent the largest populations for which Rhesus gene polymorphisms have hardly been explored.
More clusters may be characterized, because two “orphan” alleles are known, which may represent the primordial alleles for two novel D clusters. Other topics for further research are the nucleotide sequence diversity beyond the coding region of RH alleles and the linkage of RHCE alleles to RHD alleles.
The prevalence of alleles in the Eurasian D cluster 69 differs widely between the European and East Asian populations. For example, weak D type 15 and 17 are common in East Asians and rare in Europeans, while the prevalent “European” weak D types 1 to 4.1 are rarely encountered in East Asians. A random survey has identified additional diversity within exon 5 of RHD, which seems to be the region with the largest allelic variation, but this may be another observation bias. Prudently devised population studies have proved to be instrumental and worthwhile for research in Rhesus and much of its current clinical application, but they are not often chosen for funding.
7. Clinical Applications
Evaluation of anti-D alloimmunization in pregnancies
It is important to recognize that, in spite of our efforts in the last 50 years, anti-D alloimmunization still occurs in 1:2000 D negative pregnancies, a number that seems to defy further reduction. Apart from the lack of appropriate prophylaxis, the reasons for anti-D alloimmunization include the inappropriate administration of prophylaxis, pre-alloimmunization due to maternal-to-fetal transmission of D positive blood, early transplacental passage of fetal cells in the pregnant woman, and administration of DEL positive RBC units. But the source of a potential immunizing event often is not explored and, hence, is unlikely to be recognized. Advances in preventing anti-D alloimmunizations can be realized by ongoing surveillance of anti-D immunization in D positive recipients and identification of the root cause 71.
Prenatal diagnostics
Fetal RHD genotyping is performed routinely as part of the management of HDFN. Most of these cases are still caused by anti-D in D-negative women, although it may occur in women with partial D as well 72,73. The use of amniotic fluid-derived DNA to predict of fetal D and other blood group antigens is very reliable and has been the method of first choice for more than 10 years 74. Obtaining fetal tissue by amniocentesis avoids the high-risk procedure of taking blood from fetal cord for blood group testing. However, amniocentesis will likely be abandoned in favor of an even safer procedure, testing of fetal DNA derived from mother’s peripheral blood 75,76.
Cell-free fetal DNA in maternal plasma
The sensitivity of quantitative or real-time PCR in detecting cell-free fetal DNA in maternal peripheral blood was proposed in 1998 77,78. This fetal DNA represents rather small DNA fragments 79,80 found in maternal plasma, derived from fetal cells that are exposed to the maternal circulation. The advantage of cell-free fetal DNA is that this DNA is essentially cleared from maternal blood within hours after birth 81. Any fetal cell with its cellular DNA that may remain in the mother’s circulation for years is not tested at all. Several laboratories in various European countries have successfully implemented this technology to identify pregnancies at risk of HDFN, and algorithms have been devised to withhold RhIg prophylaxis for the RHD gene negative pregnancy 82–85.
Decision to administer RhIg in pregnancy 86
Pregnant women with the prevalent weak D types 1 to 4.1 may be transfused with D positive blood, and there is no indication for RhIg prophylaxis. In fact, the risk of alloimmunization of these weak D types is so low that the potential risks of unknown infectious agents or other adverse effects of RhIg prophylaxis should be considered as equally risky; this limited exposure approach has been taken with neonatal transfusion and to a lesser extent in adults. In terms of cost, RHD genotyping performed early in the first pregnancy may spare woman several RhIg exposures and may be implemented at a cost-neutral endpoint 73,87. Furthermore, utilizing RHD genotyping tests would identify women with rare weak D types who are prone to anti-D immunization and would benefit from RhIg prophylaxis. Transfusion medicine guidelines currently do not address this issue in any health care system, but a handy decision tree has been proposed to address this problem from a practical standpoint 88.
Monoclonal anti-D as therapeutics
Recombinant engineering has been used to design anti-D with the same variable region genes, i.e. identical epitope specificity, and Fc portions that do not elicit red cell sequestration, in effect to producing potential ‘drugs’ to prevention immune destruction of red cells in utero by the fetus 89. Potentially, these or similar anti-D can be produced with recombinant technology and substitute for human blood-derived RhIg preparation 90. It would be worthwhile to use such drugs in a clinical setting in the not-too-distant future. Further, the use of these molecular techniques is not limited to Rh antigens.
RHD zygosity in HDFN
The accurate determination of zygosity is important in the perinatal care of anti-D alloimmunized women and the study of Rhesus variants. Given today’s world migration patterns and inter-racial marriage rates, it is more accurate to evaluate RHD zygosity than to rely on limited ‘most probable genotype’ tables or calculations. Zygosity can be determined on the basis of 1) detection of the hybrid Rhesus box harboring the chromosomal breakpoint for the RHD deletion 11,74,91,92 and 2) quantitative PCR to determine RHD dosage. Both techniques are complementary and require proper controls. Altered hybrid Rhesus boxes can confound zygosity as can the presence of non-functional RHD alleles. Long-range high-fidelity nucleotide sequencing encompassing a long stretch across the RHD breakpoint 11 produces the least error, but it is technically challenging and hardly ever used in clinical applications.
The serological approach used the expression of the common Rh antigens to determine the ‘most probable genotype’ on haplotype tables published up to the 1970s. This approach should be abandoned, because exact empirical data for local populations are largely lacking and any molecular technique is more specific. Thus, this limitation of accurately assigning RHD zygosity was overcome by the genetic characterization of the RHD-deleted genome and the discovery of Rhesus boxes 11.
Methods that identify the RHD deletion, either directly or indirectly, can be used to determine the RHD heterozygous father. Therefore, a mother with an allo-anti-D can be assigned either a 50 % or 100% chance of conceiving a D-negative fetus 93. This genetic information can be used to determine whether to apply either invasive or non-invasive tests to predict fetal inheritance of RHD.
Exalted D antigen expression
The lack of expression of the RhCE polypeptide 94 can cause an exalted expression of the D antigen. Expression of D epitopes by hybrid RHCE-D-CE alleles is another mechanism. Such RBC represent excellent reagents for anti-D antibody screening. Moreover, when exalted D antigen expression is found serendipitously, the nucleotide change leading to the RHCE null allele should be evaluated to gain a better understanding of the types of molecular changes leading to nonfunctional alleles or hybrid RHCE-D-CE alleles.
Molecular identification of partial D versus weak D types in patients
Problems with determining Rh status are most often associated with a restricted number of prevalent RHD alleles. D discrepancies observed in the transfusion service laboratory include carriers of RHD alleles who can be immunized by the normal D antigen 30, but also include alleles that are not known to make anti-D. Therefore, the distinction is clinically relevant. It is important to realize that monoclonal anti-D reagents have variable reactivity with both partial D and weak D types, so they cannot reliably distinguish partial D from weak D types. Generally, the reagents detect most D category and partial D RBC in the direct agglutination phase of testing. Molecular techniques not only prove limitations of serology with polyclonal and monoclonal anti-D, they also meet the clinical need to distinguish partial D from weak D types and normal D from D negative.
Antibody investigations in the 1960s identified D category VI (DVI) as the most important allele at risk for D antigen alloimmunization in D positive patients. Later on a strategy was developed to deem DVI as D negative among Europeans 95. In the late 1980s, monoclonal anti-D reagents developed for use in direct hemagglutination tests allowed separation of DVI RBC from normal D positive RBC, a discovery that was not part of a purposeful design. By 1995, monoclonal anti-D reagents that do not detect DVI were widely accepted for use in routine D typing 95. Using this strategy, the DVI transfusion recipient and pregnant woman are typed “false negative” to avoid transfusion with D positive blood and anti-D immunization is prevented 96. Many other partial D variants, like DIV, could benefit from the same serological reagent design strategy 97. Moreover, some oligoclonal anti-D (mix of two or more monoclonal anti-D) reagents are very useful for donor typing, but they should be used judiciously in the transfusion laboratory and prenatal testing. We do not recommend using human polyclonal anti-D or polyclonal/monoclonal anti-D for any routine serological test.
Transfusion recipients
In contrast to the immunized carriers of partial D, anti-D alloimmunizations in weak D type 1 to 3 and 4.0/4.1 have not been observed. These alleles are the most common and together comprise more than 95 % of all weak D types in European or Caucasian populations. Transfusion recipients and pregnant women harboring these weak D types may be safely transfused with D positive blood. This may save up to 5% of D negative units, which are generally in short supply and should be reserved for patients who benefit from these D antigen matched transfusions 39,98,99. Of note, in African populations approximately 50 % of the weak D types are weak D type 4 subtypes. One of them, the weak D type 4.2, also known as DAR, permits anti-D immunization and requires D negative transfusion in carriers and RhIg prophylaxis in pregnancy.
Transfusion recipients with DEL
The corollary to the transfusion of D positive RBC to weak D patients is the transfusion of normal D positive RBC to DEL patients. In this setting, the risk of alloimmunization is theoretically nil. In Asian populations, where D-negative blood is in short supply, it should be safe to use D positive blood in the DEL transfusion recipient. Adopting such a policy would lessen the demand for rare D negative blood, which is found in less than 1 % of Asian populations. Transitioning a third of all patients currently classified as D negative to the group of transfusion recipients who can safely receive D positive RBC is a significant step. Additional evidence needs to be gathered, but current results are very encouraging 42,52,53. The “Asian type” DEL needs to be specifically detected as other DEL types are known or likely to be at risk of immunization.
Genetic diagnostics in specific diseases
Immunohematology investigations of transfused patients who have auto- and allo-hemolytic anemias are difficult to perform and often standard serology is not possible 74,100–102. However, genetic typing can distinguish whether anti-D is alloor auto-immune in nature or whether an apparent null allele is present instead a case of antigen masking.
Blood donors
RHD genotyping in donors is beneficial to transfusion recipients, because it can exclude weak D and DEL donors among apparent D negative blood donors 41. It is becoming obvious that determining a donor’s phenotype and genotype is a more powerful quality tool than two or more serological tests alone. Without such phenotype/genotype detection algorithms, transfusion recipients of weak D and DEL positive blood units have been anti-D immunized 31,45,48,103, an issue of significant practical relevance 47,98. Another potentially serious risk is posed by serologically D negative donors who are D positive/D negative chimeras. These individuals carry few D positive red cells, albeit with normal expression, such that a single RBC unit transfusion contains as many RhD polypeptides as 10 mL of ‘normal’ D positive blood. Therefore, these transfusions are capable of causing an anti-D immunization 41 even though the D positive RBC are not detected through routine serological methods 47.
Mass scale genotyping
No blood group system is as complex as the genetic basis of RH. The sheer number and complexity of alleles among various populations make it challenging to develop a comprehensive tool to identify all clinically relevant alleles. Mass scale genotyping may be an appropriate solution for widespread use in different clinical settings 47,98,104,105, and several such mass scale applications have been developed 106–109. Modifications to the current static high-throughput technologies will address genotyping of large donor and patient cohorts and the ‘dry matching’ of genotyped units 110. Furthermore, the computer systems to match donors and patients must be modified to present the appropriate allelic information; current commercial clinical database systems that house serological information are not particularly suitable.
8. Future Perspectives
Applications using genetic analysis of blood groups have become a reality in transfusion medicine 111–115. The way the genetic results are used is not different than with serological testing, and no new legal or ethical issues have been raised 74. Pregnant women expressing weak D type alleles or carrying D negative fetuses, which can be specifically detected by RHD genotyping, may be spared RhIg prophylaxis 52. This policy could lower their overall health care bill while avoiding potential risks associated with RhIg and be implemented at a cost equal to current practice 75,99. Such a strategy may involve some initial costs given that blood group genetic testing has yet to be implemented to a significant degree in most transfusion service laboratories. However, the genetic analysis of donor blood groups can avoid the transfusion of foreign antigens that can illicit red cell alloimmunization in transfusion recipients.
Acknowledgements
The author thanks Gregory A Denomme for his extensive editorial support in drafting the first version of this manuscript. Several figures are reprinted with permission from a previously published review (Flegel, Dtsch Aerzteblatt 2007; 104(10): A-651), which can be found online at www.aerzteblatt.de/int/article.asp?id=58088.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of disclaimer. The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Conflict-of-interest disclosure. The author receives royalties and holds patents on RHD molecular genetics.
Reference List
- 1.Denomme GA, Flegel WA. Applying molecular immunohematology discoveries to standards of practice in blood banks: now is the time. Transfusion. 2008 Nov;48(11):2461–2475. doi: 10.1111/j.1537-2995.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 2.Hillyer CD, Shaz BH, Winkler AM, Reid M. Integrating molecular technologies for red blood cell typing and compatibility testing into blood centers and transfusion services. Transfus.Med.Rev. 2008 Apr;22(2):117–132. doi: 10.1016/j.tmrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Westhoff CM. The potential of blood group genotyping for transfusion medicine practice. Immunohematology. 2008;24(4):190–195. [PubMed] [Google Scholar]
- 4.Avent ND. Large-scale blood group genotyping: clinical implications. Br.J.Haematol. 2009 Jan;144(1):3–13. doi: 10.1111/j.1365-2141.2008.07285.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang CH, Ye M. The Rh protein family: gene evolution, membrane biology, and disease association. Cell Mol.Life Sci. 2010 Apr;67(8):1203–1218. doi: 10.1007/s00018-009-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartron JP, Colin Y. Structural and functional diversity of blood group antigens. Transfus.Clin.Biol. 2001;8(3):163–199. doi: 10.1016/s1246-7820(01)00142-2. [DOI] [PubMed] [Google Scholar]
- 7.Tilley L, Green C, Poole J, Gaskell A, Ridgwell K, Burton NM, Uchikawa M, Tsuneyama H, Ogasawara K, Akkok CA, et al. A new blood group system, RHAG: three antigens resulting from amino acid substitutions in the Rh-associated glycoprotein. Vox Sang. 2010 Feb;98(2):151–159. doi: 10.1111/j.1423-0410.2009.01243.x. [DOI] [PubMed] [Google Scholar]
- 8.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat.Genet. 2000 Nov;26(3):341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 9.Bruce LJ. Hereditary stomatocytosis and cation-leaky red cells--recent developments. Blood Cells Mol.Dis. 2009 May;42(3):216–222. doi: 10.1016/j.bcmd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Wagner FF, Flegel WA. RHCE represents the ancestral RH position, while RHD is the duplicated gene. Blood. 2002 Mar 15;99(6):2272–2273. doi: 10.1182/blood-2001-12-0153. [DOI] [PubMed] [Google Scholar]
- 11.Wagner FF, Flegel WA. RHD gene deletion occurred in the Rhesus box. Blood. 2000;95(12):3662–3668. [PubMed] [Google Scholar]
- 12.Blancher A, Apoil PA. Evolution of RH genes in hominoids: characterization of a gorilla RHCE-like gene. J.Hered. 2000 May;91(3):205–210. doi: 10.1093/jhered/91.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Flegr J, Novotna M, Lindova J, Havlicek J. Neurophysiological effect of the Rh factor. Protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women. Neuro.Endocrinol.Lett. 2008 Aug;29(4):475–481. [PubMed] [Google Scholar]
- 14.Hatfield JS. The genetic basis of hair whorl, handedness, and other phenotypes. Med.Hypotheses. 2006;66(4):708–714. doi: 10.1016/j.mehy.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Marini AM, Urrestarazu A, Beauwens R, Andre B. The Rh (rhesus) blood group polypeptides are related to NH4+ transporters. Trends Biochem.Sci. 1997 Dec;22(12):460–461. doi: 10.1016/s0968-0004(97)01132-8. [DOI] [PubMed] [Google Scholar]
- 16.Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK. Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J.Biol.Chem. 2002 Apr 12;277(15):12499–12502. doi: 10.1074/jbc.C200060200. [DOI] [PubMed] [Google Scholar]
- 17.Burton NM, Anstee DJ. Structure, function and significance of Rh proteins in red cells. Curr.Opin.Hematol. 2008 Nov;15(6):625–630. doi: 10.1097/MOH.0b013e328311f422. [DOI] [PubMed] [Google Scholar]
- 18.Cherif-Zahar B, Bloy C, Le Van Kim C, Blanchard D, Bailly P, Hermand P, Salmon C, Cartron JP, Colin Y. Molecular cloning and protein structure of a human blood group Rh polypeptide. Proc.Natl.Acad.Sci.USA. 1990;87(16):6243–6247. doi: 10.1073/pnas.87.16.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flegel WA, von Zabern I, Doescher A, Wagner FF, Strathmann KP, Geisen C, Palfi M, Pisacka M, Poole J, Polin H, et al. D variants at the RhD vestibule in the weak D type 4 and Eurasian D clusters. Transfusion. 2009 Jun;49(6):1059–1069. doi: 10.1111/j.1537-2995.2009.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flegel WA, von Zabern I, Doescher A, Wagner FF, Vytiskova J, Pisacka M. DCS-1, DCS-2, and DFV share amino acid substitutions at the extracellular RhD protein vestibule. Transfusion. 2008 Jan;48(1):25–33. doi: 10.1111/j.1537-2995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 21.Tippett P, Sanger R. Observations on subdivisions of the Rh antigen D. Vox Sang. 1962;7:9–13. doi: 10.1111/j.1423-0410.1962.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 22.Lomas C, Tippett P, Thompson KM, Melamed MD, Hughes-Jones NC. Demonstration of seven epitopes on the Rh antigen D using human monoclonal anti-D antibodies and red cells from D categories. Vox Sang. 1989;57(4):261–264. doi: 10.1111/j.1423-0410.1989.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 23.Scott M. Rh serology--coordinator's report. Transfus.Clin.Biol. 1996;3(6):333–337. doi: 10.1016/s1246-7820(96)80040-1. [DOI] [PubMed] [Google Scholar]
- 24.Daniels GL. Human Blood Groups. 2 ed. Oxford: Blackwell Science; 2002. [Google Scholar]
- 25.Reid ME, Lomas-Francis C. The Blood Group Antigen Facts Book. 2 ed. San Diego: Academic Press; 2003. [Google Scholar]
- 26.Lubenko A, Burslem SJ, Tandy J, Contreras M, Garner SF, Wiener E. ISBT monoclonal antibody workshop: report on group 3 (anti-Rh) antibodies. Rev.Fr.Transfus.Immunohematol. 1988 Apr;31(2):145–152. doi: 10.1016/s0338-4535(88)80099-0. [DOI] [PubMed] [Google Scholar]
- 27.Rouillac C, Colin Y, Hughes-Jones NC, Beolet M, D'Ambrosio AM, Cartron JP, Le Van KC. Transcript analysis of D category phenotypes predicts hybrid Rh D-CE-D proteins associated with alteration of D epitopes. Blood. 1995 May 15;85(10):2937–2944. [PubMed] [Google Scholar]
- 28.Wagner FF, Gassner C, Muller TH, Schonitzer D, Schunter F, Flegel WA. Molecular basis of weak D phenotypes. Blood. 1999 Jan 1;93(1):385–393. [PubMed] [Google Scholar]
- 29.Gane P, Le Van Kim C, Bony V, El Nemer W, Mouro I, Nicolas V, Colin Y, Cartron JP. Flow cytometric analysis of the association between blood group-related proteins and the detergent-insoluble material of K562 cells and erythroid precursors. Br.J.Haematol. 2001 Jun;113(3):680–688. doi: 10.1046/j.1365-2141.2001.02757.x. [DOI] [PubMed] [Google Scholar]
- 30.Denomme GA, Wagner FF, Fernandes BJ, Li W, Flegel WA, Partial D. weak D types, and novel RHD alleles among 33,864 multiethnic patients: implications for anti-D alloimmunization and prevention. Transfusion. 2005;45(10):1554–1560. doi: 10.1111/j.1537-2995.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 31.Gassner C, Doescher A, Drnovsek TD, Rozman P, Eicher NI, Legler TJ, Lukin S, Garritsen H, Kleinrath T, Egger B, et al. Presence of RHD in serologically D−, C/E+ individuals: a European multicenter study. Transfusion. 2005 Apr;45(4):527–538. doi: 10.1111/j.0041-1132.2004.04211.x. [DOI] [PubMed] [Google Scholar]
- 32.Kamesaki T, Kumada M, Omi T, Okuda H, Iwamoto S, Takahashi J, Kimura K, Hirayama H, Kamata H, Obara K, et al. A novel mutation in the RHD gene in Japanese individuals with weak D, encoding an amino acid change in the 11th transmembranous domain of the RhD protein [Letter] Vox Sang. 2003;84:141. doi: 10.1046/j.1423-0410.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 33.Kormoczi GF, Forstemann E, Gabriel C, Mayr WR, Schonitzer D, Gassner C. Novel weak D types 31 and 32: adsorption-elution-supported D antigen analysis and comparison to prevalent weak D types. Transfusion. 2005 Oct;45(10):1574–1580. doi: 10.1111/j.1537-2995.2005.00580.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin IL, Shih MC, Hsieh MH, Liu TC, Chang SE, Lin CL, Chang JG. Molecular basis of weak D in Taiwanese. Ann.Hematol. 2003 Oct;82(10):617–620. doi: 10.1007/s00277-003-0711-4. [DOI] [PubMed] [Google Scholar]
- 35.Muller TH, Wagner FF, Trockenbacher A, Eicher NI, Flegel WA, Schonitzer D, Schunter F, Gassner C. PCR screening for common weak D types shows different distributions in three Central European populations. Transfusion. 2001 Jan;41(1):45–52. doi: 10.1046/j.1537-2995.2001.41010045.x. [DOI] [PubMed] [Google Scholar]
- 36.Shao CP, Maas JH, Su YQ, Kohler M, Legler TJ. Molecular background of Rh D-positive, D-negative, D(el) and weak D phenotypes in Chinese. Vox Sang. 2002 Aug;83(2):156–161. doi: 10.1046/j.1423-0410.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 37.Ansart-Pirenne H, Asso-Bonnet M, Le Pennec P-Y, Roussel M, Patereau C, Noizat-Pirenne F. RHD variants in whites: consequences for checking clinically relevant alleles. Transfusion. 2004;44(9):1282–1286. doi: 10.1111/j.1537-2995.2004.04063.x. [DOI] [PubMed] [Google Scholar]
- 38.Hemker MB, Ligthart PC, Berger L, van Rhenen DJ, van der Schoot CE, Wijk PA. DAR, a new RhD variant involving exons 4, 5, and 7, often in linkage with ceAR, a new rhce variant frequently found in African blacks. Blood. 1999;94(12):4337–4342. [PubMed] [Google Scholar]
- 39.Legler TJ, Maas JH, Kohler M, Wagner T, Daniels GL, Perco P, Panzer S. RHD sequencing: a new tool for decision making on transfusion therapy and provision of Rh prophylaxis. Transfus.Med. 2001 Oct;11(5):383–388. doi: 10.1046/j.1365-3148.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 40.Hasekura H, Ota M, Ito S, Hasegawa Y, Ichinose A, Fukushima H, Ogata H. Flow cytometric studies of the D antigen of various Rh phenotypes with particular reference to Du and Del. Transfusion. 1990 Mar;30(3):236–238. doi: 10.1046/j.1537-2995.1990.30390194344.x. [DOI] [PubMed] [Google Scholar]
- 41.Wagner FF, Frohmajer A, Flegel WA. RHD positive haplotypes in D negative Europeans. BMC Genet. 2001;2(1):10. doi: 10.1186/1471-2156-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luettringhaus TA, Cho D, Ryang DW, Flegel WA. An easy RHD genotyping strategy for D- East Asian persons applied to Korean blood donors. Transfusion. 2006 Dec;46(12):2128–2137. doi: 10.1111/j.1537-2995.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim JY, Kim SY, Kim CA, Yon GS, Park SS. Molecular characterization of D- Korean persons: development of a diagnostic strategy. Transfusion. 2005 Mar;45(3):345–352. doi: 10.1111/j.1537-2995.2005.04311.x. [DOI] [PubMed] [Google Scholar]
- 44.Kormoczi GF, Gassner C, Shao CP, Uchikawa M, Legler TJ. A comprehensive analysis of DEL types: partial DEL individuals are prone to anti-D alloimmunization. Transfusion. 2005 Oct;45(10):1561–1567. doi: 10.1111/j.1537-2995.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 45.Wagner T, Kormoczi GF, Buchta C, Vadon M, Lanzer G, Mayr WR, Legler TJ. Anti-D immunization by DEL red blood cells. Transfusion. 2005 Apr;45(4):520–526. doi: 10.1111/j.0041-1132.2005.04256.x. [DOI] [PubMed] [Google Scholar]
- 46.Flegel WA, Gabriel C, Gassner W, Ruff H, Wagner FF. RHD genotyping of blood donors may avoid anti-D immunization. Blood. 2004;104(11):739a. [Google Scholar]
- 47.Flegel WA. Homing in on D antigen immunogenicity. Transfusion. 2005 Apr;45(4):466–468. doi: 10.1111/j.0041-1132.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 48.Yasuda H, Ohto H, Sakuma S, Ishikawa Y. Secondary anti-D immunization by DEL red blood cells. Transfusion. 2005;45(10):1581–1584. doi: 10.1111/j.1537-2995.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 49.Daniels GL, Faas BH, Green CA, Smart E, Maaskant-van Wijk PA, Avent ND, Zondervan HA, von dem Borne AE, van der Schoot CE. The VS and V blood group polymorphisms in Africans: a serologic and molecular analysis. Transfusion. 1998;38(10):951–958. doi: 10.1046/j.1537-2995.1998.381098440860.x. [DOI] [PubMed] [Google Scholar]
- 50.Singleton BK, Green CA, Avent ND, Martin PG, Smart E, Daka A, Narter-Olaga EG, Hawthorne LM, Daniels G. The presence of an RHD pseudogene containing a 37 base pair duplication and a nonsense mutation in Africans with the Rh D- negative blood group phenotype. Blood. 2000;95(1):12–18. [PubMed] [Google Scholar]
- 51.Shao CP, Xiong W. A new hybrid RHD-positive, D antigen-negative allele. Transfus.Med. 2004 Apr;14(2):185–186. doi: 10.1111/j.0958-7578.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 52.Flegel WA. How I manage donors and patients with a weak D phenotype. Curr.Opin.Hematol. 2006 Nov;13(6):476–483. doi: 10.1097/01.moh.0000245694.70135.c3. [DOI] [PubMed] [Google Scholar]
- 53.Shao CP. Transfusion of RhD-positive blood in "Asia type" DEL recipients. N.Engl.J.Med. 2010 Feb 4;362(5):472–473. doi: 10.1056/NEJMc0909552. [DOI] [PubMed] [Google Scholar]
- 54.Carritt B, Blunt T, Avent N, Daniels G, Steers F. Rh null phenotypes are not due to a gross deletion and can occur on different Rh genetic backgrounds. Ann.Hum.Genet. 1993 Oct;57(Pt 4):273–279. doi: 10.1111/j.1469-1809.1993.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 55.Kato-Yamazaki M, Okuda H, Kawano M, Omi T, Iwamoto T, Ishimori T, Hasekura H, Kajii E. Molecular genetic analysis of the Japanese amorph rh(null) phenotype. Transfusion. 2000 May;40(5):617–618. doi: 10.1046/j.1537-2995.2000.40050617.x. [DOI] [PubMed] [Google Scholar]
- 56.Huang CH. The human Rh50 glycoprotein gene. Structural organization and associated splicing defect resulting in Rh(null) disease. J.Biol.Chem. 1998 Jan 23;273(4):2207–2213. doi: 10.1074/jbc.273.4.2207. [DOI] [PubMed] [Google Scholar]
- 57.Denomme GA, Ryan G, Seaward PG, Kelly EN, Fernandes BJ. Maternal ABO-mismatched blood for intrauterine transfusion of severe hemolytic disease of the newborn due to anti-Rh17. Transfusion. 2004 Sep;44(9):1357–1360. doi: 10.1111/j.1537-2995.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 58.Chen YX, Peng J, Novaretti M, Reid ME, Huang CH. Deletion of arginine codon 229 in the Rhce gene alters e and f but not c antigen expression. Transfusion. 2004 Mar;44(3):391–398. doi: 10.1111/j.1537-2995.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 59.Flegel WA, Wagner FF, Chen Q, Schlanser G, Frame T, Westhoff CM, Moulds MK. The RHCE allele ceCF: the molecular basis of Crawford (RH43) Transfusion. 2006;46(8):1334–1342. doi: 10.1111/j.1537-2995.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 60.Noizat-Pirenne F, Mouro I, Le Pennec PY, Ansart-Pirenne H, Juszczak G, Patereau C, Verdier M, Babinet J, Roussel M, Rouger P, et al. Two new alleles of the RHCE gene in Black individuals: the RHce allele ceMO and the RHcE allele cEMI. Br.J.Haematol. 2001 Jun;113(3):672–679. doi: 10.1046/j.1365-2141.2001.02802.x. [DOI] [PubMed] [Google Scholar]
- 61.Noizat-Pirenne F, Lee K, Pennec PY, Simon P, Kazup P, Bachir D, Rouzaud AM, Roussel M, Juszczak G, Menanteau C, et al. Rare RHCE phenotypes in black individuals of Afro-Caribbean origin: identification and transfusion safety. Blood. 2002 Dec 1;100(12):4223–4231. doi: 10.1182/blood-2002-01-0229. [DOI] [PubMed] [Google Scholar]
- 62.Noizat-Pirenne F, Ansart-Pirenne H, Menanteau C, Braddock D, Rouzaud AM, Klein MT, Patereau C, Rouger P, Le Pennec PY. Serological studies of monoclonal RH antibodies with RH1 (D), RH2 (C), RH3 (E) and RH5 (e) variant RBCs. Transfus.Clin.Biol. 2003 Oct;10(5):319–323. doi: 10.1016/s1246-7820(03)00106-x. [DOI] [PubMed] [Google Scholar]
- 63.Noizat-Pirenne F, Tournamille C, Gallon P, Juszczak G, Rouger P, Ansart-Pirenne H. ceRA: an RH allele variant producing a new rare blood. Transfusion. 2006 Jul;46(7):1232–1236. doi: 10.1111/j.1537-2995.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 64.Scharberg EA, Green C, Daniels G, Richter E, Kluter H, Bugert P. Molecular basis of the JAHK (RH53) antigen. Transfusion. 2005 Aug;45(8):1314–1318. doi: 10.1111/j.1537-2995.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 65.Strobel E, Noizat-Pirenne F, Hofmann S, Cartron JP, Bauer MF. The molecular basis of the Rhesus antigen Ew. Transfusion. 2004 Mar;44(3):407–409. doi: 10.1111/j.1537-2995.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- 66.Tax MG, van der Schoot CE, van Doorn R, Douglas-Berger L, van Rhenen DJ, Maaskant-vanWijk PA. RHC and RHc genotyping in different ethnic groups. Transfusion. 2002 May;42(5):634–644. doi: 10.1046/j.1537-2995.2002.00096.x. [DOI] [PubMed] [Google Scholar]
- 67.Tournamille C, Meunier-Costes N, Costes B, Martret J, Barrault A, Gauthier P, Galacteros F, Nzouekou R, Bierling P, Noizat-Pirenne F. Partial C antigen in sickle cell disease patients: clinical relevance and prevention of alloimmunization. Transfusion. 2010 Jan;50(1):13–19. doi: 10.1111/j.1537-2995.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 68.Westhoff CM, Storry JR, Walker P, Lomas-Francis C, Reid ME. A new hybrid RHCE gene (CeNR) is responsible for expression of a novel antigen. Transfusion. 2004 Jul;44(7):1047–1051. doi: 10.1111/j.1537-2995.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 69.Wagner FF, Ladewig B, Angert KS, Heymann GA, Eicher NI, Flegel WA. The DAU allele cluster of the RHD gene. Blood. 2002 Jul 1;100(1):306–311. doi: 10.1182/blood-2002-01-0320. [DOI] [PubMed] [Google Scholar]
- 70.Wagner FF, Ernst M, Sonneborn HH, Flegel WA. A D(V)-like phenotype is obliterated by A226P in the partial D DBS. Transfusion. 2001 Aug;41(8):1052–1058. doi: 10.1046/j.1537-2995.2001.41081052.x. [DOI] [PubMed] [Google Scholar]
- 71.Flegel WA. The Rhesus Immunization Surveillance. Ulm: DRK-Blutspendedienst Baden-Wurttemberg - Hessen; 1998. [Google Scholar]
- 72.Cannon M, Pierce R, Taber EB, Schucker J. Fatal hydrops fetalis caused by anti-D in a mother with partial D. Obstet.Gynecol. 2003 Nov;102(5 Pt 2):1143–1145. doi: 10.1016/s0029-7844(03)00709-9. [DOI] [PubMed] [Google Scholar]
- 73.Lurie S, Rotmensch S, Glezerman M. Prenatal management of women who have partial Rh (D) antigen. Br.J.Obstet.Gynaecol. 2001 Sep;108(9):895–897. doi: 10.1111/j.1471-0528.2001.00232.x. [DOI] [PubMed] [Google Scholar]
- 74.Flegel WA, Wagner FF. Molecular genetics of RH. Vox Sang. 2000;78 Suppl 2:109–115. [PubMed] [Google Scholar]
- 75.Bianchi DW, Avent ND, Costa JM, van der Schoot CE. Noninvasive prenatal diagnosis of fetal Rhesus D: ready for prime(r) time. Obstet.Gynecol. 2005 Oct;106(4):841–844. doi: 10.1097/01.AOG.0000179477.59385.93. [DOI] [PubMed] [Google Scholar]
- 76.Daniels G, Finning K, Martin P, Soothill P. Fetal blood group genotyping from DNA from maternal plasma: an important advance in the management and prevention of haemolytic disease of the fetus and newborn. Vox Sang. 2004 Nov;87(4):225–232. doi: 10.1111/j.1423-0410.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 77.Faas BH, Beuling EA, Christiaens GC, dem Borne AE, van der Schoot CE. Detection of fetal RHD-specific sequences in maternal plasma. Lancet. 1998 Oct 10;352(9135):1196. doi: 10.1016/s0140-6736(05)60534-x. [DOI] [PubMed] [Google Scholar]
- 78.Lo YMD, Hjelm NM, Fidler C, Sargent IL, Murphy MF, Chamberlain PF, Poon PMK, Redman CWG, Wainscoat JS. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N.Engl.J.Med. 1998;339(24):1734–1738. doi: 10.1056/NEJM199812103392402. [DOI] [PubMed] [Google Scholar]
- 79.Chan KC, Zhang J, Hui AB, Wong N, Lau TK, Leung TN, Lo KW, Huang DW, Lo YM. Size distributions of maternal and fetal DNA in maternal plasma. Clin.Chem. 2004 Jan;50(1):88–92. doi: 10.1373/clinchem.2003.024893. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Zimmermann B, Rusterholz C, Kang A, Holzgreve W, Hahn S. Size separation of circulatory DNA in maternal plasma permits ready detection of fetal DNA polymorphisms. Clin.Chem. 2004 Jun;50(6):1002–1011. doi: 10.1373/clinchem.2003.029835. [DOI] [PubMed] [Google Scholar]
- 81.Finning K, Martin P, Daniels G. A clinical service in the UK to predict fetal Rh (Rhesus) D blood group using free fetal DNA in maternal plasma. Ann.N.Y.Acad.Sci. 2004 Jun;1022:119–123. doi: 10.1196/annals.1318.019. 119-23. [DOI] [PubMed] [Google Scholar]
- 82.Finning KM, Martin PG, Soothill PW, Avent ND. Prediction of fetal D status from maternal plasma: introduction of a new noninvasive fetal RHD genotyping service. Transfusion. 2002 Aug;42(8):1079–1085. doi: 10.1046/j.1537-2995.2002.00165.x. [DOI] [PubMed] [Google Scholar]
- 83.Legler TJ, Lynen R, Maas JH, Pindur G, Kulenkampff D, Suren A, Osmers R, Kohler M. Prediction of fetal Rh D and Rh CcEe phenotype from maternal plasma with real-time polymerase chain reaction. Transfus.Apher.Sci. 2002 Dec;27(3):217–223. doi: 10.1016/s1473-0502(02)00068-x. [DOI] [PubMed] [Google Scholar]
- 84.Rijnders RJ, Christiaens GC, de Haas M, van der Schoot CE. [Fetal DNA in maternal blood] Ned.Tijdschr.Geneeskd. 2004 Jan 24;148(4):170–174. [PubMed] [Google Scholar]
- 85.Rouillac-Le Sciellour C, Puillandre P, Gillot R, Baulard C, Metral S, Le Van KC, Cartron JP, Colin Y, Brossard Y. Large-scale pre-diagnosis study of fetal RHD genotyping by PCR on plasma DNA from RhD-negative pregnant women. Mol.Diagn. 2004;8(1):23–31. doi: 10.1007/BF03260044. [DOI] [PubMed] [Google Scholar]
- 86.Engelfriet CP, Reesink HW, Judd WJ, Ulander VM, Kuosmanen M, Koskinen S, Rouger P, Morelati F, Tantalo V, Fujii T, et al. Current status of immunoprophylaxis with anti-D immunoglobin. Vox Sang. 2003 Nov;85(4):328–337. doi: 10.1111/j.0042-9007.2003.364_1.x. [DOI] [PubMed] [Google Scholar]
- 87.Westhoff CM. The Rh blood group system in review: a new face for the next decade. Transfusion. 2004 Nov;44(11):1663–1673. doi: 10.1111/j.0041-1132.2004.04237.x. [DOI] [PubMed] [Google Scholar]
- 88.Flegel WA, Denomme GA, Yazer MH. On the complexity of D antigen typing: a handy decision tree in the age of molecular blood group diagnostics. J.Obstet.Gynaecol.Can. 2007 Sep;29(9):746–752. doi: 10.1016/s1701-2163(16)32606-8. [DOI] [PubMed] [Google Scholar]
- 89.Nielsen LK, Green TH, Sandlie I, Michaelsen TE, Dziegiel MH. In vitro assessment of recombinant, mutant immunoglobulin G anti-D devoid of hemolytic activity for treatment of ongoing hemolytic disease of the fetus and newborn. Transfusion. 2008 Jan;48(1):12–19. doi: 10.1111/j.1537-2995.2007.01474.x. [DOI] [PubMed] [Google Scholar]
- 90.Kjaersgaard M, Aslam R, Kim M, Speck ER, Freedman J, Stewart DI, Wiersma EJ, Semple JW. Epitope specificity and isotype of monoclonal anti-D antibodies dictate their ability to inhibit phagocytosis of opsonized platelets. Blood. 2007 Aug 15;110(4):1359–1361. doi: 10.1182/blood-2007-03-079848. [DOI] [PubMed] [Google Scholar]
- 91.Chiu RW, Murphy MF, Fidler C, Zee BC, Wainscoat JS, Lo YM. Determination of RhD zygosity: comparison of a double amplification refractory mutation system approach and a multiplex real-time quantitative PCR approach. Clin.Chem. 2001 Apr;47(4):667–672. [PubMed] [Google Scholar]
- 92.Li Y, Zimmermann B, Zhong XY, Gupta AK, Holzgreve W, Hahn S. Determination of RHD zygosity using real-time quantitative PCR. Swiss.Med.Wkly. 2003 Aug 9;133(31–32):442–445. doi: 10.4414/smw.2003.10164. [DOI] [PubMed] [Google Scholar]
- 93.Yu X, Wagner FF, Witter B, Flegel WA. Outliers in RhD membrane integration are explained by variant RH haplotypes. Transfusion. 2006;46(8):1343–1351. doi: 10.1111/j.1537-2995.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 94.Blunt T, Steers F, Daniels G, Carritt B. Lack of RH C/E expression in the Rhesus D--phenotype is the result of a gene deletion. Ann.Hum.Genet. 1994 Jan;58(Pt 1):19–24. doi: 10.1111/j.1469-1809.1994.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 95.Wagner FF, Kasulke D, Kerowgan M, Flegel WA. Frequencies of the blood groups ABO, Rhesus, D category VI, Kell, and of clinically relevant high-frequency antigens in south-western Germany. Infusionsther.Transfusionsmed. 1995 Oct;22(5):285–290. doi: 10.1159/000223144. [DOI] [PubMed] [Google Scholar]
- 96.Flegel WA, Wagner FF. Molecular biology of partial D and weak D: implications for blood bank practice. Clin.Lab. 2002;48(1+2):53–58. [PubMed] [Google Scholar]
- 97.Wagner FF, Eicher NI, Jorgensen JR, Lonicer CB, Flegel WA. DNB: a partial D with anti-D frequent in Central Europe. Blood. 2002 Sep 15;100(6):2253–2256. doi: 10.1182/blood-2002-03-0742. [DOI] [PubMed] [Google Scholar]
- 98.Garratty G. Do we need to be more concerned about weak D antigens? Transfusion. 2005;45(10):1547–1551. doi: 10.1111/j.1537-2995.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 99.Wagner FF, Frohmajer A, Ladewig B, Eicher NI, Lonicer CB, Muller TH, Siegel MH, Flegel WA. Weak D alleles express distinct phenotypes. Blood. 2000 Apr 15;95(8):2699–2708. [PubMed] [Google Scholar]
- 100.Legler TJ, Eber SW, Lakomek M, Lynen R, Maas JH, Pekrun A, Repas-Humpe M, Schroter W, Kohler M. Application of RHD and RHCE genotyping for correct blood group determination in chronically transfused patients. Transfusion. 1999 Aug;39(8):852–855. doi: 10.1046/j.1537-2995.1999.39080852.x. [DOI] [PubMed] [Google Scholar]
- 101.Reid ME, Rios M, Powell VI, Charles-Pierre D, Malavade V. DNA from blood samples can be used to genotype patients who have recently received a transfusion. Transfusion. 2000;40(1):48–53. doi: 10.1046/j.1537-2995.2000.40010048.x. [DOI] [PubMed] [Google Scholar]
- 102.Rios M, Hue-Roye K, Storry JR, Reiss RF. Cell typing the sensitized transfusion-dependent patient. Ann.Clin.Lab Sci. 2000 Oct;30(4):379–386. [PubMed] [Google Scholar]
- 103.Flegel WA, Khull S, Wagner FF. Primary anti-D immunization by weak D type 2 RBC. Transfusion. 2000;40(4):428–434. doi: 10.1046/j.1537-2995.2000.40040428.x. [DOI] [PubMed] [Google Scholar]
- 104.Anstee DJ. Goodbye to agglutination and all that? Transfusion. 2005 May;45(5):652–653. doi: 10.1111/j.1537-2995.2005.05052.x. [DOI] [PubMed] [Google Scholar]
- 105.Avent ND. High variability of the RH locus in different ethnic backgrounds. Transfusion. 2005 Mar;45(3):293–294. doi: 10.1111/j.1537-2995.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- 106.Beiboer SH, Wieringa-Jelsma T, Maaskant-van Wijk PA, van der Schoot CE, van Zwieten R, Roos D, den Dunnen JT, de Haas M. Rapid genotyping of blood group antigens by multiplex polymerase chain reaction and DNA microarray hybridization. Transfusion. 2005 May;45(5):667–679. doi: 10.1111/j.1537-2995.2005.04319.x. [DOI] [PubMed] [Google Scholar]
- 107.Denomme GA, Van Oene M. High-throughput multiplex single-nucleotide polymorphism analysis for red cell and platelet antigen genotypes. Transfusion. 2005 May;45(5):660–666. doi: 10.1111/j.1537-2995.2005.04365.x. [DOI] [PubMed] [Google Scholar]
- 108.Hashmi G, Shariff T, Seul M, Vissavajjhala P, Hue-Roye K, Charles-Pierre D, Lomas-Francis C, Chaudhuri A, Reid ME. A flexible array format for large-scale, rapid blood group DNA typing. Transfusion. 2005 May;45(5):680–688. doi: 10.1111/j.1537-2995.2005.04362.x. [DOI] [PubMed] [Google Scholar]
- 109.Flegel WA. Mannheim: DRK-Blutspendedienst Baden-Wurttemberg - Hessen; 2004. BloodGen consortium members, the future of blood grouping: mass genotyping for blood groups and beyond. http://www.uni-ulm.de/~wflegel/RH/SympDGTI2004/ [Google Scholar]
- 110.Denomme GA, Flegel WA. Applying molecular immunohematology discoveries to standards of practice in blood banks: now is the time. Transfusion. 2008 Nov;48(11):2461–2475. doi: 10.1111/j.1537-2995.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 111.Flegel WA, Wagner FF, Muller TH, Gassner C. Rh phenotype prediction by DNA typing and its application to practice. Transfus.Med. 1998 Dec;8(4):281–302. doi: 10.1046/j.1365-3148.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- 112.Reid ME. DNA analysis to find rare blood donors when antisera is not available. Vox Sang. 2002 Aug;83 Suppl. 1:91–93. doi: 10.1111/j.1423-0410.2002.tb05275.x. [DOI] [PubMed] [Google Scholar]
- 113.Reid ME, Lomas-Francis C. Molecular approaches to blood group identification. Curr.Opin.Hematol. 2002 Mar;9(2):152–159. doi: 10.1097/00062752-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 114.Reid ME. Molecular biology in transfusion medicine: current applications and future practice. Curr.Hematol.Rep. 2002 Nov;1(2):134–141. [PubMed] [Google Scholar]
- 115.Reid ME. Applications of DNA-based assays in blood group antigen and antibody identification. Transfusion. 2003 Dec;43(12):1748–1757. doi: 10.1111/j.0041-1132.2003.00597.x. [DOI] [PubMed] [Google Scholar]