Abstract
Poly-L-lysine (PLL), a homopolymer of amino acid L-lysine (LL), has been frequently used for drug delivery. Here, we report that PLL is an effective agent to inhibit propagation of prions that cause fatal and incurable neurologic disorders in humans and animals, termed prion diseases. In our recent investigation on prion propagation facilitated by conversion of the cellular prion protein (PrP) to the misfolded, disease-associated PrP (PrPSc), we demonstrated that plasminogen stimulates PrP conversion as a cellular cofactor. In the current study, we targeted plasminogen using PLL and assessed its anti-prion efficacy. The results showed that PLL strongly inhibited PrPSc propagation in the cell-free, cell culture, and mouse models of prion disease. These results confirm the role of plasminogen in PrPSc propagation, validates plasminogen as a therapeutic target to combat prion disease, and suggests PLL as a potential anti-prion agent. Therefore, our study represents a proof-of-concept that targeting plasminogen, a cofactor for PrP conversion, using PLL results in suppression of prion propagation, which represents a successful translation of our understanding on details of prion propagation into a potential therapeutic strategy for prion diseases.
Keywords: Poly-L-lysine, prion, PrP conversion, cofactor, plasminogen, therapeutic target, translational research
1. Introduction
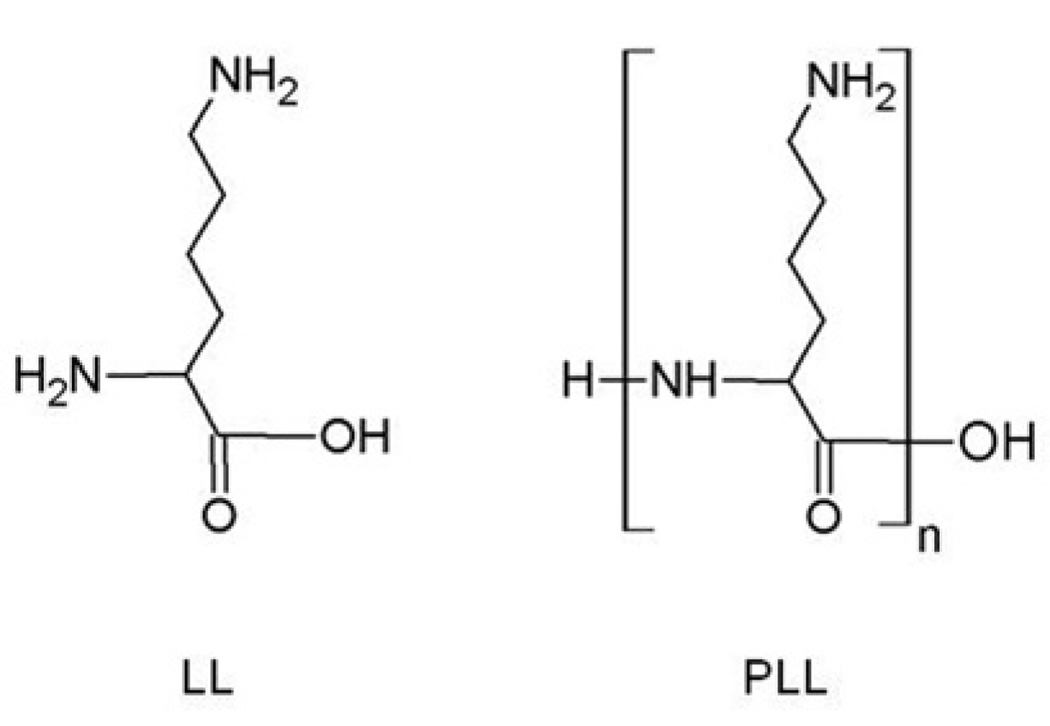
L-lysine (LL) is an essential amino acid necessary as a building block of proteins in the animal body and plays an important role in many biochemical events that occur during physiological processes [1]. Synthetic polymers of LL, termed poly-L-lysine (PLL), are the homopolymers composed of a few to hundreds of LL monomers (Fig. 1). PLL exhibits a number of biological properties such as anti-viral, anti-bacterial, and anti-tumor activities [2–5]. In addition, PLL enhances the cellular uptake of macromolecules [6]. Thus, PLL has been successfully used to develop a non-viral vehicle for delivery of pharmacologically active chemical, nucleotide, and peptide drugs [7–13].
Fig. 1.
Structure of LL (L-lysine) and PLL (poly(L-lysine)). n, numbers of LL monomer.
Prions are infectious proteins that cause fatal neurologic disorders such as sporadic Creutzfeldt-Jakob disease (CJD), familial CJD, variant CJD, iatrogenic CJD, Gerstmann–Sträussler–Scheinker syndrome, and fatal familial insomnia in humans, as well as other transmissible encephalopathies in animals [14, 15]. Prions invade the central nervous system and cause progressive degeneration of neurons [15]. Prions are devoid of nucleic acids and composed predominantly, if not exclusively, of abnormally folded prion proteins (PrP), designated PrPSc [15]. Conformational misfolding of the host-encoded normal, cellular PrP (PrPC) to PrPSc is the central biochemical process for propagation of prions [16].
Currently, effective therapy for prion diseases is unavailable. In the previous attempts to develop anti-prion therapy, a number of agents and methods have shown a certain degree of efficacy in cell culture and animal models of prion disease [17]. However, none has been proven to be clinically useful in human patients. While the major target of these approaches was either PrPC or PrPSc, no other molecular target to inhibit prion propagation has been identified.
Recently, we discovered a potential molecular target other than PrPC or PrPSc to suppress prion propagation. We identified plasminogen as a cofactor to stimulate PrP misfolding by accelerating the conversion of PrPC to PrPSc [18]. One of us (CR) and others previously reported that plasminogen interacts with PrP through lysine-binding motifs of its kringle domains [19, 20]. Furthermore, LL abrogates activity of plasminogen that enhances PrP misfolding [18] and selectively inhibits the PrP-plasminogen interaction in a concentration dependent manner [19, 20]. These studies suggested that plasminogen is a potential therapeutic target and LL can be used as a therapeutic agent for the treatment of PrP misfolding diseases [18, 21]. Nevertheless, the therapeutic application of LL in vivo appears to be impossible because the concentration of LL to effectively inhibit PrP conversion is in the millimolar range [18] and there is no method to achieve such a high local LL concentration in the body.
The objective of the study is to provide a proof-of-concept that plasminogen is a valid therapeutic target to suppress prion propagation using PLL, the synthetic polymers of LL. We hypothesized that PLL is efficacious to inhibit prion propagation through interference with PrP conversion stimulated by plasminogen. In this study, we measured efficacy of PLL in the in vitro and in vivo models of prion disease.
2. Materials and Methods
2.1. PLL and LL
PLL and LL (Fig. 1) were purchased from Sigma-Aldrich (St. Louis, MO). PLLs with various molecular weights used in this study include PLL1, PLL3, PLL10, PLL23, PLL50, PLL110, PLL225, and PLL300, where the number indicates the average molecular weight (Table 1). LL (m.w.=146) was used as a control.
Table 1.
PLLs and LL used for anti-prion assays.
| Compound | m.w. range (kDa) |
Average m.w (kDa) |
n | Conversion of concentrations |
N in 2.5 µg/ml LL and PLL |
|
|---|---|---|---|---|---|---|
| µg/ml | Molar concentrations |
|||||
| LL | 0.146 | 0.146 | 1 | 2.5 | 17.1 µM | 1.0 ×1019 |
| PLL1 | 0.5–2 | 1.25 | 8.6 | 2.5 | 2 µM | 1.0 ×1019 |
| PLL3 | 1–4 | 2.5 | 17.1 | 2.5 | 1 µM | 1.0 ×1019 |
| PLL10 | 4–15 | 10 | 68.5 | 2.5 | 0.25 µM | 1.0 ×1019 |
| PLL23 | 15–30 | 22.5 | 154.1 | 2.5 | 0.11 µM | 1.0 ×1019 |
| PLL50 | 30–70 | 50 | 342.5 | 2.5 | 0.05 µM | 1.0 ×1019 |
| PLL110 | 70–150 | 110 | 753.4 | 2.5 | 22.7 nM | 1.0 ×1019 |
| PLL225 | 150–300 | 225 | 1541.1 | 2.5 | 11.1 nM | 1.0 ×1019 |
| PLL300 | > 300 | >300 | 2054.8 | 2.5 | 8.35 nM | 1.0 ×1019 |
m.w., molecular weights; Da, Dalton; LL, L-lysine; PLL, poly(L-lysine); n, average numbers of LL monomers per molecule (multimerization degree); N, numbers of LL monomer equivalents, which represent charge densities at a given concentration.
2.2. Cell culture
ScN2a cells [22], the Neuro2a neuroblastoma cell line (ATCC CCL-131) permanently infected by RML (Rocky Mountain Laboratory) prions, were cultured as described previously [23]. Under 5% CO2 and saturated humidity conditions at 37°C, ScN2a cells were cultured with Dulbecco’s Modified Eagle’s Medium (DMEM, high glucose; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% glutamax. Cell culture medium was replaced every three days.
2.3. Cell-based anti-prion activity assay
Anti-prion activity of PLL was measured in ScN2a cells by the method published previously [24]. ScN2a cells passed 1:50 dilution were incubated with PLL and vehicle (PBS) for six days Culture media containing PLL, LL, or PBS were changed once on Day 4 during the incubation. If necessary, treated cells were further cultured up to 60 days in the absence of the compounds. Cell lysate was prepared in lysis buffer (20 mM Tris, pH 8.0; 150 mM NaCl, 0.5% NP-40, 0.5% deoxycholate, sodium salt) and briefly centrifuged for 30 s at 7,000 × g. Assays to quantify total proteins were conducted using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL) as recommended by the manufacturer. Briefly, the cell lysate obtained after six-day treatment was reacted with the mixture of BCA and cupric sulfate. The colorimetric detection was performed at 562 nm. Following protein quantification, cell lysate was digested with 20 µg/ml proteinase K (PK) for 1 h at 37°C, and centrifuged at 16,000 × g for 1 h at 4°C. PK-resistant pellet containing PrPSc was analyzed by Western blotting to compare the level of PrPSc eliminated as a function of treatment with compounds. As controls, β-actin and total PrP including both PrPC and PrPSc were detected from ~ 30 µg of undigested lysate. Western blotting was performed as described elsewhere [23]. Monoclonal anti-PrP antibody, 6H4 (Prionics, Zurich, Switzerland) and anti-β-actin antibody ACTN05 (Neomark, Oviedo, FL) were used for Western blotting. Western blots were developed using ECL Plus™ Detection Reagents (Amersham Biosciences, Piscataway, NJ) and visualized after scanning in Fuji Film FLA 5000 image reader (Fuji Film, Edison, NJ). Doc-It Image Analysis Software (UVP, Upland, CA) was used for image analysis and densitometry.
2.4. Cytotoxicity assay
Cytotoxicity of PLL was measured by two independent methods: MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and protein assays. MTT assay was performed as described previously [24]. ScN2a cells were passed and incubated as described above. After treatment for six days, the cells were further incubated in the fresh DMEM media with the final concentration of 0.5 mg/ml MTT for 2 h. The formazan products dissolved in acidic alcohol (0.05 N HCl-isopropanol) were measured at 570 nm. Measurement of total protein quantity using bicinchoninic acid protein assay kit (Pierce, Rockford, IL) was performed as recommended by the manufacturer (See also Section 2.3).
2.5. PrP conversion assay
The cell-free PrP conversion assay was carried out using protein misfolding cyclic amplification (PMCA) that mimics conformational conversion of PrPC to PrPSc [25]. The PMCA procedure was performed as described previously [26] with minor modifications. For PMCA, brain homogenate (10% w/v) of RML prion-infected CD-1 mice at the terminal stage was diluted 250 fold in brain homogenate (10% w/v) of healthy CD-1 mice prepared in PMCA buffer (PBS pH 7.2 including 150 mM NaCl, 1% Triton X-100, and 4 mM EDTA) with protease inhibitors (CompleteMini, Roche, Basel, Switzerland). Additionally, 0.5 µM human plasminogen (HCPG-0130, Haematologic Technologies Inc, Essex Junction, VT) and various concentrations (0–4 µM) of PLL50 were supplemented to the reaction. PMCA underwent 94 cycles of 40 s sonication pulsed every 30 min at 37°C in a microsonicator (300 W, Misonix Model 3000, Farmingdale, NY). A pre-amplification aliquot was stored at −20°C until used for Western blotting. Pre- and post-PMCA samples (20 µl each) were digested with 100 µg/ml PK at 37°C for 1 h and analyzed by Western blotting.
2.6. Animals and in vivo efficacy test
Experiments associated with animals were conducted according to the protocol approved by the Institutional Animal Care and Use Committee and the Biological Safety Office at the University of Kentucky. Groups of five week old Tg(MoPrP)4112+/− mice (hereafter referred to as Tg4112, n=3–8/group), which overexpress PrPC (~5 X) in the brain [26], were used to assess the efficacy of PLL against prion disease. Tg4112 mice were maintained as hemizygotes for the transgene by the screening methods described previously [26]. The mice were intracerebrally inoculated with 30 µl RML prion inocula using a 26-gauge needle inserted in the left parietal lobe to a depth of 2–3 mm as described previously [27]. The inocula were prepared by diluting pooled brain homogenate (10%, w/v) of FVB mice terminally ill by RML prions in PBS lacking calcium and magnesium ions to 1% (w/v) final. PLL50 was dissolved in PBS and the first dose was administered 3 h after prion inoculation through either intravenous or intraperitoneal routes. Mice in the control (n=8) group received no PLL. Mice in group I (n=6) received a weekly dose of 8 mg/kg PLL50 directly into the tail vein for four weeks. Both groups II (n=5) and III (n=3) received intraperitoneal doses of 40 mg/kg PLL50 three times a week for either four or eight weeks, respectively. During the administration period, drinking/eating pattern and weight changes of mice were monitored whether PLL50 exhibits side effects. After completion of treatment, animals were closely observed for clinical signs (ataxia, piloerection, kyphosis, cachexia, rigid tail, etc) of prion disease. The incubation time (a period from prion inoculation until appearance of a characteristic set of clinical signs) was measured from each group to compare the efficacy of treatment.
2.7. Measurement of PrPC and PrPSc in the brains of mice
Once mice were identified to exhibit multiple neurological signs consistent with prion infection, mice were humanely sacrificed and the brains were harvested. A 20 µl aliquot of brain homogenate (10% w/v) prepared in PBS was diluted in 100 µl Sarkosyl (2% final)-PBS. Five microliters of this brain material was used for analysis of the PrPC levels without PK treatment. The remainder was used to detect the level of PrPSc after treatment with 20 µg/ml PK. The samples were analyzed by Western blotting.
2.8. Ex vivo imaging of PLL
Labeling of PLL50 with the fluorephore Alexa Fluor 750 (AF750) was performed by the amine-mediated conjugation methodology. Briefly, PLL50 dissolved at 10 mg/ml in 0.1 M sodium bicarbonate buffer, pH 8.3 was mixed with a reactive form of AF750 carboxylic acid succinimidyl ester dissolved ~10 mg/ml in high-quality, anhydrous dimethyl sulfoxide by slowly adding the latter. The reaction lasted for 1 h at room temperature and stopped by the addition of 0.75 M hydroxylamine, pH 8.5. Unbound dye was removed by dialysis. After freeze drying, AF750-conjgated PLL50 (PLL50-AF750) was redissolved in PBS and utilized for injection to mice. A single injection of 10 mg/kg PLL50-AF750 was given to six week old wild type FVB mice (Harlan, Indianapolis, IN) either intravenously or intraperitoneally. The brains were extracted at 24 h post-injection and the biofluorescence was detected by the IVIS Spectrum (Caliper Life Sciences, Alameda, CA) with the near-infrared filter for AF750 (excitation and emission wavelengths at 745 and 820 nm). Regions of interest from displayed images were designated around the brain and fluorescence was quantified by total photon counting using Living Image ver3.1 software (Caliper Life Sciences).
2.9. Statistical analysis
The log-rank test was used to validate a statistically significant difference in incubation times between control and PLL-treated groups of mice. Analysis of variance (ANOVA) was used for statistical analysis of difference in the PrPSc levels of moue brains. P<0.05 was considered statistically significant.
3. Results
3.1. Decrease of PrPSc in ScN2a cells incubated with LL
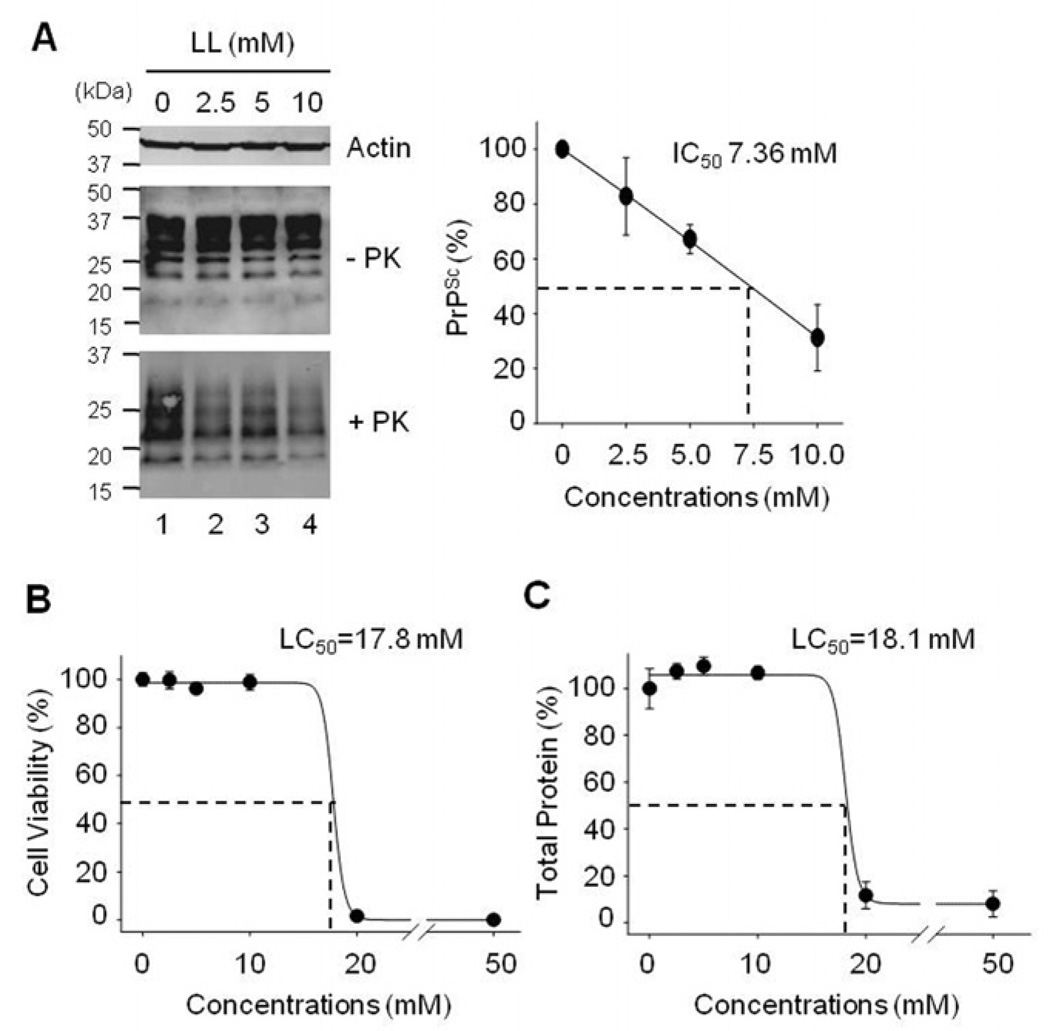
LL was tested first as a control to confirm its anti-prion activity. The level of PrPSc in ScN2a cells treated with LL was reduced in a concentration-dependent manner (Fig. 2A). The half-maximal concentration to inhibit PrPSc (IC50) was ~ 7.63 mM. However, complete elimination of PrPSc was not achieved by treatment with LL because incubation of ScN2a cells with >20 mM LL resulted in a decrease of cell numbers in the culture. Treatment with ≤ 10 mM LL did not alter the levels of actin and total PrP including PrPC. Cytotoxicity studies in ScN2a cells confirmed that higher concentrations (20 and 50 mM) of LL were toxic (Fig. 2B and C). The half-maximal lethal concentration (LC50) of LL was 17.8 – 18.1 mM, depending on the assay used.
Fig. 2.
Elimination of PrPSc and cytotoxicity in ScN2a cells incubated with LL. A. Western blots of β-actin, total PrP (−PK), and PK-resistant PrPSc (+PK). Densitometry analysis of the PrPSc levels shows the means of relative PrPSc levels with standard deviations (error bars, n=5). B. Cell viability measured by MTT assays (n=6). C. Total protein contents measured by BCA protein assays (n=3). The effect of LL was presented as relative values after normalization with the level of untreated control.
3.2. Elimination of PrPSc from ScN2a cells by PLLs
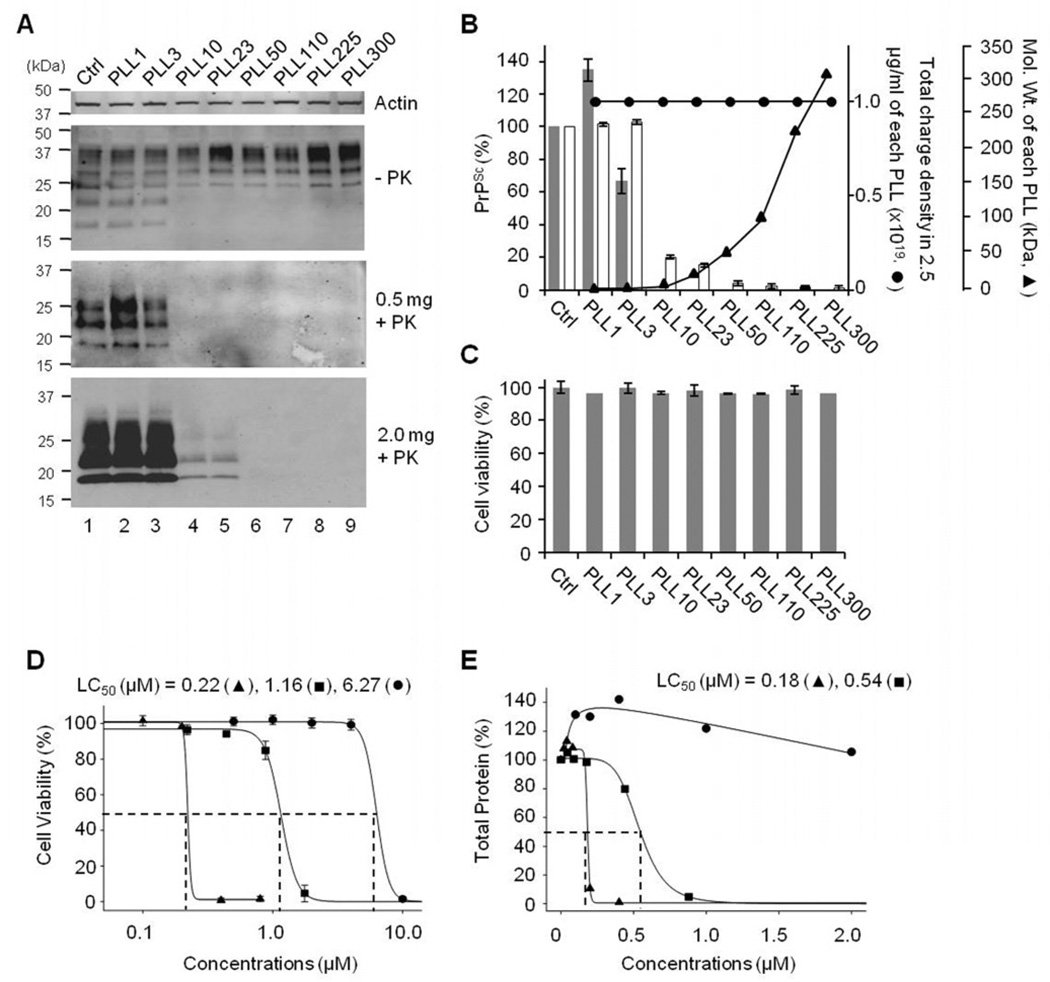
We evaluated anti-prion activity of various PLLs ranging from 1.25 to >300 kDa in the average molecular weight (Table 1) at a fixed concentration (2.5 µg/ml). This created a condition that the absolute numbers of LL monomer equivalents and the charge density supplied by PLLs were identical across the treatments, although the molar concentrations differed (Table 1). PLL showed differential efficacy in eliminating PrPSc from ScN2a cells depending on its molecular weight but not total charges provided by PLL (Fig. 3A and B). PLLs with average molecular weights > 10 kDa were effective (Fig. 3A, lanes 4–9) in eliminating PrPSc, whereas PLLs with average molecular weights ≤ 3 kDa were not able to alter the PrPSc level in ScN2a cells (Fig. 3A, lanes 2 and 3). This suggests that there is a threshold of molecular weights for PLL to exhibit anti-prion activity. The level of actin or cell viability was unaffected by treatments with various PLLs at 2.5 µg/ml (Fig. 3A and C). Cytotoxicity of PLL was proportional to its concentrations and molecular weights, suggesting that cytotoxicity is determined by the charge density of PLL (Fig. 3D and E). The LC50 of PLLs with the molecular weight above the threshold to exhibit anti-prion activity was ~6.27 µM for PLL10, 0.54–1.16 µM for PLL23, and 0.18–0.22 µM for PLL50.
Fig. 3.
Anti-prion activity and cytotoxicity of PLLs in ScN2a cells. A. Inhibition of PrPSc propagation in ScN2a cells incubated with 2.5 µg/ml PLLs of various molecular weights (lanes 2–9). Numbers following PLL, the average molecular weights (kDa) of each PLL; Ctrl, incubated with no PLL (lane 1); −PK, total PrP; +PK, PK-resistant PrPSc. PrPSc was analyzed from 0.5 and 2 mg of cell lysates. B. The composite plot of the PrPSc levels measured by densitometry (bars), total charge density available from added PLLs (●), and the molecular weights of each PLL (▲). The means of relative PrPSc levels and standard deviations (error bars) were determined from the analyses with 0.5 (filled bar, n=3) and 2 (open bar, n=6) mg cell lysates. The numbers of LL unit equivalent represent the charge density of PLL. C. Cytotoxicity of various PLLs at 2.5 µg/ml measured by MTT assays (n=3). Cell viability was presented as % normalized with the untreated control (Ctrl) value. For D and E, LC50 of PLL10 (▲), PLL23 (■), and PLL50 (●) in ScN2a cells. D. Cytotoxicity of PLLs measured by MTT assays (n=3). E. Total protein contents in PLL-treated cells measured by BCA protein assays (single measurement of triplicates).
3.3. In vitro anti-prion activity of PLL50
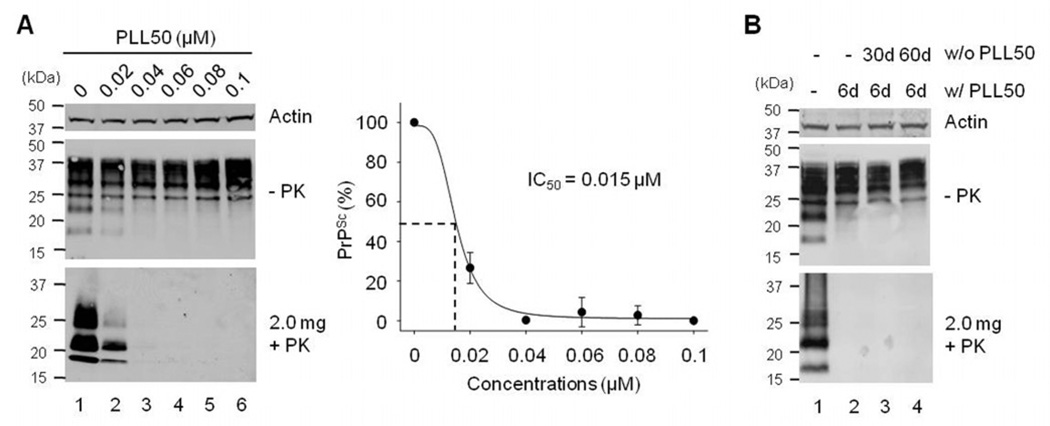
We further determined anti-prion activity of PLL50 because our results indicate that PLL50 is the optimal PLL composition as it showed the lowest cytotoxicity among PLLs that completely eliminated PrPSc from ScN2a cells (Fig. 3). On the basis of cytotoxicity studies of PLL50, we focused on concentrations below the LC50. ScN2a cells were treated with 0–0.1 µM PLL50. PrPSc in the culture disappeared in a concentration-dependent manner (Fig. 4A). The IC50 of PLL50 was ~0.015 µM. We also assessed whether PLL could permanently cure cells of a chronic prion infection. ScN2a cells pre-incubated with 0.05 µM PLL50 for six days were further cultured for additional 30 or 60 days in the absence of PLL50. The undetectable level of PrPSc in pre-treated cells remained the same even after the extended cell culture under PLL-free conditions (Fig. 4B), suggesting a complete extinction of PrPSc in the cells by PLL50.
Figure 4.
Prion inhibitory effect of PLL50. A. The IC50 of PLL50. ScN2a cells were incubated with no (lane 1), 0.02 µM (lane 2), 0.04 µM (lane 3), 0.06 µM (lane 4), 0.08 µM (lane 5), and 0.1 µM (lane 6) PLL50. Two mg of cell lysates were used for the analysis of PrPSc in Western blots. −PK, total PrP; +PK, PK-resistant PrPSc. The IC50 of PLL50 was obtained from densitometry data that present the means of relative PrPSc levels and standard deviations (error bars, n=3). B. Extinction of PrPSc from ScN2a cells treated with PLL50. ScN2a cells incubated with 0.05 µM PLL50 for six days were further cultured in the absence of PLL50 for 30–60 days. Untreated (−) control (lane 1); after six day treatment with (w/) PLL50 (lane 2); 30 day (lane 3) and 60 day (lane 4) culture post-treatment without (w/o) PLL50.
3.4. PrP conversion blocked by PLL50
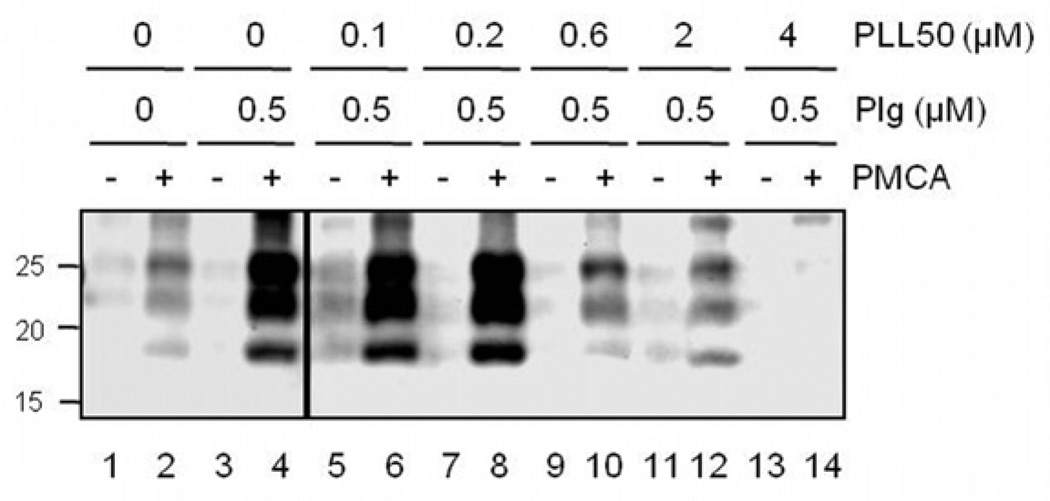
Because LL competes with PrP for kringle domains of plasminogen that include the lysine binding motifs [20] and interferes with PrP conversion in PMCA supplemented with plasminogen [18], we investigated whether PLL inhibits prion propagation by the same mechanism. PLL50 inhibited PrP conversion during plasminogen-supplemented PMCA in a concentration-responsive manner (Fig. 5). This indicates that anti-prion activity of PLL is attributed to the plasminogen-targeted inhibition of PrP conversion as shown by LL.
Figure 5.
Inhibition of PrP conversion by PLL50. PMCA supplemented with (0.5 µM, lanes 3–14) or without (lanes 1–2) plasminogen (Plg) was performed in the presence of 0.1 – 4 µM PLL50 (lanes 5–14). Odd number lanes (−), the PrPSc levels of initial seeds before PMCA; even number lanes (+), the levels of newly generated PrPSc after PMCA.
3.5. Efficacy of PLL50 in a mouse model of prion disease
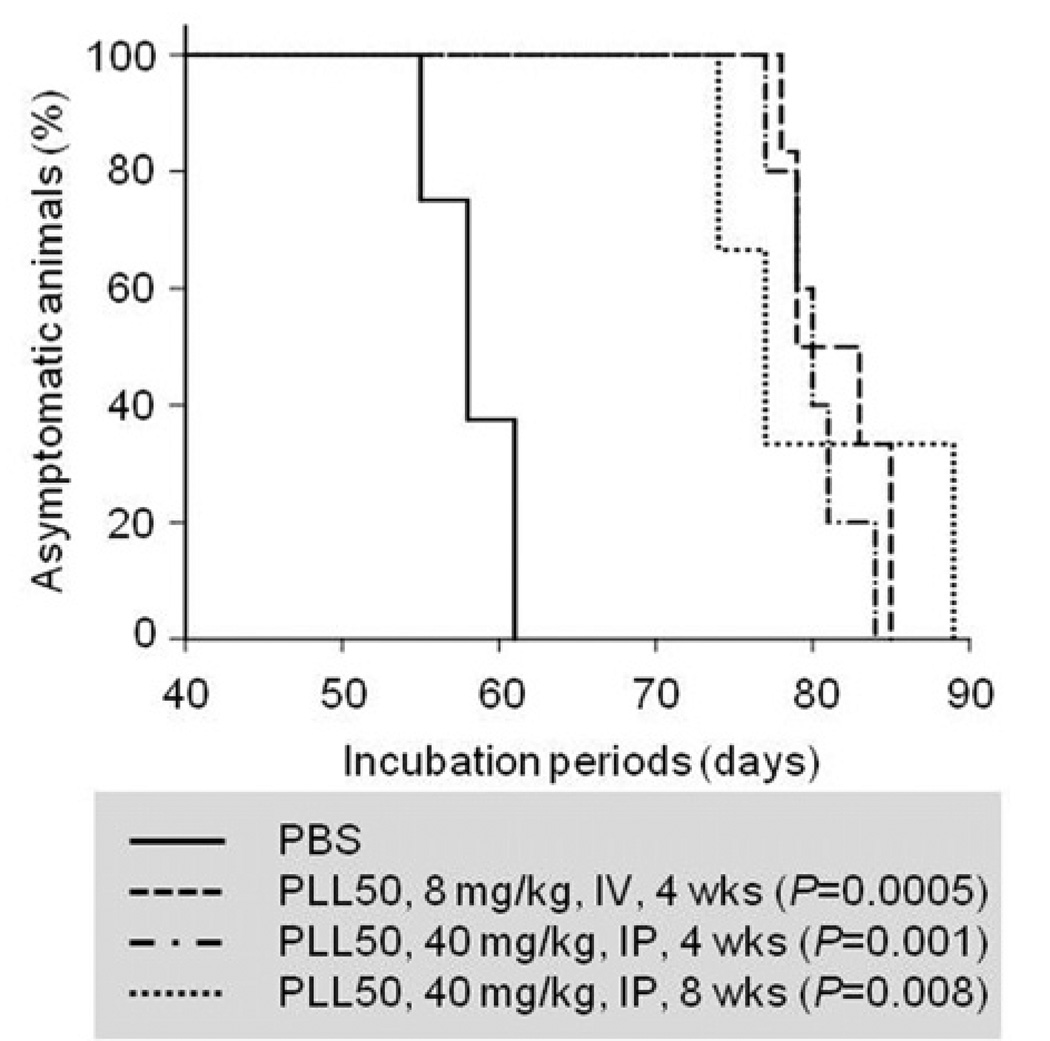
We then assessed in vivo anti-prion efficacy of PLL50. We administered PLL50 for four to eight weeks either intraperitoneally (40 mg/kg) or intravenously (8 mg/kg) to Tg4112 mice inoculated with prion. These PLL50 doses are below the sub-half-maximal lethal doses (LD50) [5, 28] and was tolerated in Tg4112 mice. The mice received PLL50 showed temporary hypoactivity immediately following injection, but recovered within 30 min and behaved as usual. Compared to that of control group, the mean incubation times were prolonged ~40% in three separate PLL-treated groups of mice (Table 2 and Fig. 6). Mice treated with PLL50 succumbed to disease at ~80–82 days, while the control group developed clinical signs at ~58 days post-inoculation. The log-rank test demonstrated that the prolongation of incubation periods in the PLL-treated groups were statistically significant (P = 0.0005 – 0.008). These results demonstrate that PLL is effective in delaying the onset of disease in prion-infected mice.
Table 2.
Incubation time of prion-infected Tg 4112 mice that received treatment with PLL50.
| Group | Inoculated prion strain (route) |
Treatment | Mean incubation time (days ± S.E.M.) |
n/n0 | P-value | ||
|---|---|---|---|---|---|---|---|
| Dose | Frequency (duration) |
route | |||||
| control | RML (IC) | 58.4 ± 0.9a | 8/8 | ||||
| I | RML (IC) | 8 mg/kg | Once/week (4 weeks) | IV | 81.5 ± 1.3 | 6/6 | 0.0005 |
| II | RML (IC) | 40 mg/kg | 3 times/week (4 weeks) | IP | 80.2 ± 1.2 | 5/5 | 0.001 |
| III | RML (IC) | 40 mg/kg | 3 times/week (8 weeks) | IP | 80.6 ± 4.6 | 3/3 | 0.008 |
S.E.M., standard error of the mean; n, number of mice diagnosed ill with prion diseases; n0, number of mice inoculated; IC, intracranial; IV, intravenous; IP, intraperitoneal.
Adopted from [26]. The P-values were calculated using the log-rank test.
Figure 6.
Clinical onset of prion disease in Tg 4112 mice treated with PLL50. The percent of mice remained asymptomatic was plotted over the course of disease. PBS, vehicle; IV, intravenous delivery once a week for 4 weeks (wks); IP, intraperitoneal delivery three times a week for 4 and 8 wks. The P-values were obtained by the statistical analysis using the log-rank test.
3.6. Reduction of PrPSc accumulation in the brains of PLL50-treated mice
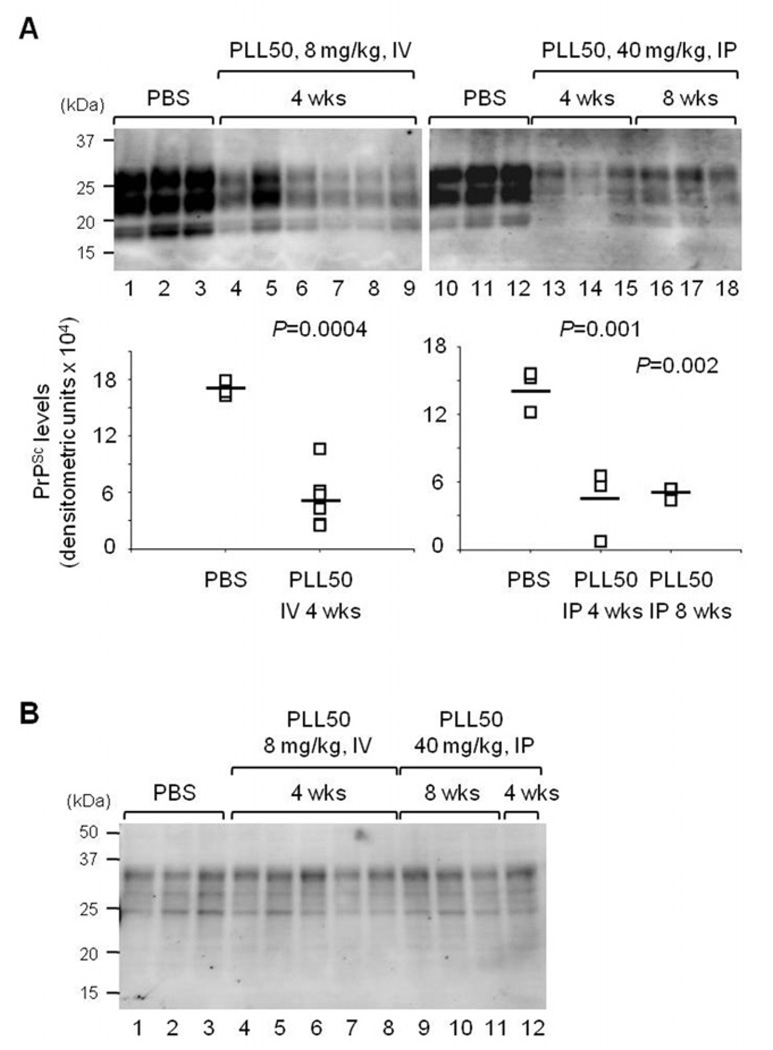
We assessed whether administration of PLL reduces the PrPSc level in vivo. The brains of mice from each group were collected at the terminal stage of disease when multiple clinical signs were presented. Western blotting to measure the PrPSc level in brain homogenate showed that the accumulation of PrPSc was reduced in the brains of mice treated with PLL50 compared to controls (Fig. 7A, P = 0.0004 – 0.002), while the level of PrPC remained the same (Fig. 7B). These results suggest that the PrPSc levels decreased in the brains of PLL-treated mice were the result of abrogated PrPSc generation, but not altered PrPC expression, by PLL.
Figure 7.
The PrPC and PrPSc levels in the brains of PLL50-treated mice. A. The level of PrPSc accumulated in the brains of mice treated with PLL50. Individual mouse brains from spate groups: the PBS-treated control group (Lanes 1–3 and 10–12, n=3 each); the group received IV 8 mg/kg weekly for 4 wks (lanes 4–9, n=6); the group received IP 40 mg/kg three times a week for 4 wks (lane 13–15, n=3); and the group received IP 40 mg/kg three times a week for 8 wks (lanes 16–18, n=3). The PrPSc levels of individual brains were plotted with the averages (bars). The P-values were obtained by the statistical analysis using ANOVA. B. The levels of PrPC in the brains of mice treated with PLL50. Individual mouse brains from the PBS-treated control group (lanes 1–3); the group received IV 8 mg/kg weekly for 4 wks (lanes 4–8); and the group received IP 40 mg/kg three times a week for 8 wks (lane 9–11) and 4 wks (lane 12).
3.7. Distribution of PLL50 to the brain
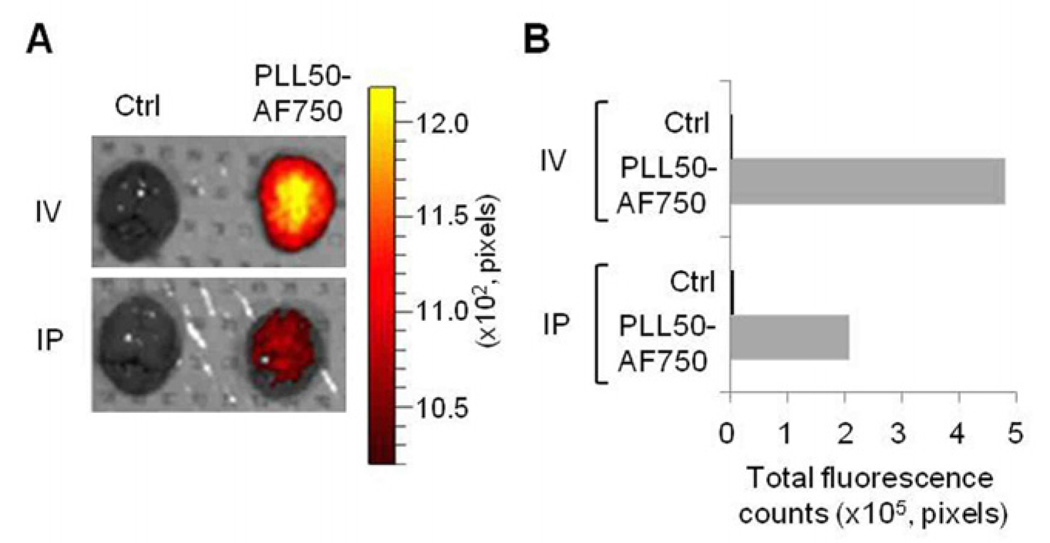
We examined whether injected PLL50 was distributed to the brain where prion replication takes place and the PrPSc level was decreased by PLL. W assessed the accumulation of fluorephore AF750-conjugated PLL50 (PLL50-AF750) in the brain by ex vivo biofluorescence imaging. The brain extracted at 24 h post-injection from mice that intravenously or intraperitoneally received PLL50-AF750 showed that PLL was available to the brain (Fig. 8A). The photon count analysis revealed that the intravenous injection resulted in greater accumulation of PLL50-AF750 in brain (Fig. 8B). These results suggest that PLL targets the brain.
Figure 8.
Distribution of PLL to brain. Wild type FVB mice received PLL50-AF750 either IV or IP. The brain extracted at 24 h post-injection was subjected to detect biofluorescence using the IVIS Spectrum imaging system. A. Ex vivo fluorescence image of brains. B. Total photon counts of fluorescence. Ctrl, vehicle (PBS) injected.
4. Discussion
The current study demonstrates that PLL is effective in inhibiting prions in the cell culture and animal models of prion diseases. Since therapy for prion disease does not exist, development of effective agents and/or methodologies to prevent or cure prion diseases has been a high priority in prion research. In particular, an approach to an unexplored target to hinder the progression of prion disease is of great importance. Unlike a number of previous strategies that target the isoforms of PrP [17], we targeted a cellular protein cofactor for PrP conversion in this study. Such approach has not been possible presumably due to the lack of information on the identity of the cofactor. We previously showed that plasminogen interacts with PrP and stimulates PrP conversion, while LL prevents their interaction and inhibits PrPSc generation [18, 20]. In the current study, as a logical extension from our recent understanding, we performed translational investigations using PLL to target the interaction between PrP and plasminogen, a functional cofactor for PrP conversion. As a result, we validated our hypothesis that PLL interfering with plasminogen-enhanced PrP conversion eliminates PrPSc from prion-infected cultured cells, delays the onset of disease in prion-inoculated mice, and reduces the level of PrPSc in the mouse brains. Our study not only confirms that a cofactor (plasminogen) for PrP conversion is an excellent target to inhibit prion propagation but also provides the evidence that PLL interfering with the function of plasminogen could be the potential lead compounds for future development of anti-prion agents.
Comparison of the IC50 values of LL (~7.36 mM) and PLL50 (~0.015 µM) revealed that anti-prion efficacy was tremendously improved by multimerization of LL. Also, the swtich from LL to PLL improved the ratio of toxicity to efficacy (LC50/IC50) from 2.3 (LL) to 13.3 (PLL50). These results suggest that PLL is more preferable to inhibit prions than LL, although an immediate clinical application of PLL does not appear to be feasible. Furthermore, compared to LL, PLL50 improved anti-prion activity ~ 1.6×103 and 5×105 folds in PMCA and ScN2a cells, respectively (Table 3). These results suggest that application of the mutimerization strategy is effective in enhancing anti-prion activity of LL as previously observed in other biologically or pharmacologically active peptides and chemicals [29, 30]. We postulate that the increased anti-prion potency of PLL is due to the enhanced interaction between the linear chain structure of PLL and kringle domains of plasminogen. The polymer chain might not only bind to the kringle domains but also wrap entire plasminogen more tightly than small molecule LL, preventing plasminogen from interacting with PrP more effectively to inhibit PrP conversion. This hypothesis is supported by the observation that the anti-prion activity is dependent on the molecular weight of PLL rather than the charge density available under the treatment conditions. Therefore, the interaction between PLL and plasminogen may not be attributed to simple neutralization of charges.
Table 3.
Improved anti-prion efficacy of PLL.
| Anti-prion activity | ||
|---|---|---|
| ScN2a cellsa | PMCAb | |
| LL | 7.36 mM | 1 mMc |
| PLL50 | 15 nM | 0.6 µM |
| Efficacy improved | 5 × 105 fold | 1.6 × 103 fold |
The IC50 of LL and PLL50 represent the anti-prion activity in ScN2a cells.
The concentrations of LL and PLL50 that abrogated plasminogen-mediated PrP conversion represent the anti-prion activity in PMCA.
Adopted from [18].
Our data provide evidence that the molecular basis of anti-prion activity of PLL relies on the blockade of the conversion process. The underlying mechanism of anti-prion activity exhibited by both LL and PLL appears to be identical. LL and PLL are considered to be bound to the lysine-binding sites of the kringle domains of plasminogen, which prevents plasminogen from interacting with PrP and results in inhibition of PrP conversion. Alternatively, PLL may directly interact with and destroy PrPSc as previously proposed by a group of synthetic cationic dendrimers and their derivatives that degrade PrPSc at acidic pH [31, 32]. Since PLLs are also cationic polymers, degradation of PrPSc by PLLs at acidic pH may be feasible, although this possibility remains to be explored. Interestingly, it is worthwhile to note that cationic branched polyamines and their derivatives with permanent positive charges variably degrade PrPSc at acidic pH, but uniformly block PrP conversion at neutral pH [33]. Thus, additional investigations are necessary to advance our comprehension on the specific action mechanism of structurally different individual cationic polymers.
The results of our in vivo efficacy and ex vivo distribution studies suggest that intravenous administration was more efficient than intraperitoneal administration in treating the prion diseases and delivering PLL to the brain. Toxicity of PLL, however, still needs to be carefully considered for further development of our PLL-mediated anti-prion therapy because intravenous administration of PLL can cause severe side-effects [28], which limited us to apply regimens with a higher dose and more frequent administration for our current studies. In this respect, further development of the PLL-based anti-prion strategy that can avoid PLL toxicity would be necessary. Although our study demonstrated that PLL exhibits potent anti-prion activity, it is not clear whether PLL is universally effective against different prion strains and in various animal species. Additional evaluations are required to determine whether PLL will be useful in treating various types of prion diseases.
Our study offers a unique opportunity to develop PLL-based multifunctional therapeutics for prion disease. The advantage of PLL is on its versatile applications unlimited to the inhibition of PrP conversion. PLLs can be used for non-viral delivery of protein and nucleotide drugs. Therefore, we envision a PLL-based combination therapy that can, for instance, concurrently inhibit PrP conversion, suppress PrPC expression [34], and destabilize PrPSc [35].
5. Conclusions
We demonstrate here that PLLs are effective inhibitors of prion propagation by targeting plasminogen that stimulates PrP conversion. The study represents an example of successful translation of the knowledge on the cofactor-assisted PrP misfolding mechanism into the development of a potential therapeutic intervention for prion diseases, providing a proof-of-concept that interference with plasminogen using PLL is effective to inhibit PrP conversion. We also explore a new therapeutic target that has hitherto not been recognized to hinder the progression of prion disease, and suggest that PLL can both inhibit prion propagation and deliver other anti-prion agents.
Acknowledgements
The authors thank Glenn Telling for providing Tg4112 mice, Linda Van Eldik for constructive suggestions, and Paula Thomason for editing this manuscript. This work was supported in part by NIH grant (P20 RR 020171) and funds from the College of Medicine, University of Kentucky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meister A. Biochemistry of the amino acids. 2nd ed. New York: Academic Press; 1965. [Google Scholar]
- 2.Langeland N, Moore LJ, Holmsen H, Haarr L. Interaction of polylysine with the cellular receptor for herpes simplex virus type 1. J Gen Virol. 1988;69(6):1137–1145. doi: 10.1099/0022-1317-69-6-1137. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas Delihas LWR, Loo Winnie, Berkowitz Jonathan, Poltoratskaia Natalia. High sensitivity of mycobacterium species to the bactericidal activity by polylysine. FEMS Microbiol Lett. 1995;132(3):233–237. doi: 10.1016/0378-1097(95)00304-n. [DOI] [PubMed] [Google Scholar]
- 4.Shima S, Matsuoka H, Iwamoto T, Sakai H. Antimicrobial action of epsilon-poly-L-lysine. J Antibiot. 1984;37(11):1449–1455. doi: 10.7164/antibiotics.37.1449. [DOI] [PubMed] [Google Scholar]
- 5.Arnold LJJ, Dagan A, Gutheil J, Kaplan NO. Antineoplastic activity of poly(L-lysine) with some ascites tumor cells. Proc Natl Acad Sci U S A. 1979;76:3246–3250. doi: 10.1073/pnas.76.7.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu B, Wiehle S, Roth JA, Cristiano RJ. The contribution of poly-L-lysine, epidermal growth factor and streptavidin to EGF/PLL/DNA polyplex formation. Gene Ther. 1998;5:1235–1243. doi: 10.1038/sj.gt.3300719. [DOI] [PubMed] [Google Scholar]
- 8.Wagner E, Cotten M, Foisner R, Birnstiel ML. Transferrin-polycation-DNA complexes: The effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991;88(10):4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plank C, Tang MX, Wolfe AR, Szoka FC. Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum Gene Ther. 1999;10(2):319–332. doi: 10.1089/10430349950019101. [DOI] [PubMed] [Google Scholar]
- 10.Klink D, Yu Q-C, Glick MC, Scanlin T. Lactosylated poly-L-lysine targets a potential lactose receptor in cystic fibrosis and non-cystic fibrosis airway epithelial cells. Mol Ther. 2003;7(1):73–80. doi: 10.1016/s1525-0016(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 11.Ward CM, Read ML, Seymour LW. Systemic circulation of poly(L-lysine)/DNA vectors is influenced by polycation molecular weight and type of DNA: differential circulation in mice and rats and the implications for human gene therapy. Blood. 2001;97(8):2221–2229. doi: 10.1182/blood.v97.8.2221. [DOI] [PubMed] [Google Scholar]
- 12.Harada-Shiba M, Yamauchi K, Harada A, Takamisawa I, Shimokado K, Kataoka K. Polyion complex micelles as vectors in gene therapy - pharmacokinetics and in vivo gene transfer. Gene Ther. 2002;9:407–414. doi: 10.1038/sj.gt.3301665. [DOI] [PubMed] [Google Scholar]
- 13.Sakharov DV, Jie AFH, Bekkers MEA, Emeis JJ, Rijken DC. Polylysine as a vehicle for extracellular matrix-targeted local drug delivery, providing high accumulation and long-term retention within the vascular wall. Arterioscler Thromb Vasc Biol. 2001;21(6):943–948. doi: 10.1161/01.atv.21.6.943. [DOI] [PubMed] [Google Scholar]
- 14.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 15.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 17.Trevitt CR, Collinge J. A systematic review of prion therapeutics in experimental models. Brain. 2006;129(9):2241–2265. doi: 10.1093/brain/awl150. [DOI] [PubMed] [Google Scholar]
- 18.Mays CE, Ryou C. Plasminogen stimulates propagation of protease-resistant prion protein in vitro. FASEB J. 2010;24(12):5102–5112. doi: 10.1096/fj.10-163600. [DOI] [PubMed] [Google Scholar]
- 19.Fischer MB, Roeckl C, Parizek P, Schwarz HP, Aguzzi A. Binding of disease-associated prion protein to plasminogen. Nature. 2000;408:479–483. doi: 10.1038/35044100. [DOI] [PubMed] [Google Scholar]
- 20.Ryou C, Prusiner SB, Legname G. Cooperative binding of dominant-negative prion protein to kringle domains. J Mol Biol. 2003;329:323–333. doi: 10.1016/s0022-2836(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 21.Mays CE, Ryou C. Plasminogen: A cellular protein cofactor for PrPSc propagation. Prion. 2011;5(1):22–27. doi: 10.4161/pri.5.1.14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott MRD, Butler DA, Bredesen DE, Walchli M, Hsiao KK, Prusiner SB. Prion protein gene expression in cultured cells. Protein Eng. 1988;2:69–76. doi: 10.1093/protein/2.1.69. [DOI] [PubMed] [Google Scholar]
- 23.Mays CE, Kang H-E, Kim Y, Shim SH, Bang J-E, Woo H-J, et al. CRBL cells: Establishment, characterization and susceptibility to prion infection. Brain Res. 2008;1208:170–180. doi: 10.1016/j.brainres.2008.02.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryou C, Legname G, Peretz D, Craig JC, Baldwin MA, Prusiner SB. Differential inhibition of prion propagation by enantiomers of quinacrine. Lab Invest. 2003;83:837–843. doi: 10.1097/01.lab.0000074919.08232.a2. [DOI] [PubMed] [Google Scholar]
- 25.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 26.Mays CE, Titlow W, Seward T, Telling GC, Ryou C. Enhancement of protein misfolding cyclic amplification by using concentrated cellular prion protein source. Biochem Biophys Res Commun. 2009;388(2):306–310. doi: 10.1016/j.bbrc.2009.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Song J, Mays C, Titlow W, Yoon D, Ryou C. Changes in gene expression of kringle domain-containing proteins in murine brains and neuroblastoma cells infected by prions. Mol Cell Biochem. 2009;328:177–182. doi: 10.1007/s11010-009-0087-4. [DOI] [PubMed] [Google Scholar]
- 28.De Vries A, Feldman JD, Stein O, Stein Y, Katchalski E. Effects of intravenously administered poly-DL-lysine in rats. Proc Soc Exptl Biol Med. 1953;76:237–240. doi: 10.3181/00379727-82-20078. [DOI] [PubMed] [Google Scholar]
- 29.Schick BP, Maslow D, Moshinski A, Antonio JDS. Novel concatameric heparin-binding peptides reverse heparin and low-molecular-weight heparin anticoagulant activities in patient plasma in vitro and in rats in vivo. Blood. 2004;103(4):1356–1363. doi: 10.1182/blood-2003-07-2334. [DOI] [PubMed] [Google Scholar]
- 30.May BCH, Fafarman AT, Hong SB, Rogers M, Deady LW, Prusiner SB, et al. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc Natl Acad Sci USA. 2003;100:3416–3421. doi: 10.1073/pnas.2627988100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Supattapone S, Nguyen H-OB, Cohen FE, Prusiner SB, Scott MR. Elimination of prions by branched polyamines and implications for therapeutics. Proc Natl Acad Sci USA. 1999;96(25):14529–14534. doi: 10.1073/pnas.96.25.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supattapone S, Wille H, Uyechi L, Safar J, Tremblay P, Szoka FC, et al. Branched polyamines cure prion-infected neuroblastoma cells. J Virol. 2001;75:3453–3461. doi: 10.1128/JVI.75.7.3453-3461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim Y-b, Mays CE, Kim Y, Titlow WB, Ryou C. The inhibition of prions through blocking prion conversion by permanently charged branched polyamines of low cytotoxicity. Biomaterials. 2010;31(8):2025–2033. doi: 10.1016/j.biomaterials.2009.11.085. [DOI] [PubMed] [Google Scholar]
- 34.White MD, Farmer M, Mirabile I, Brandner S, Collinge J, Mallucci GR. Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc Natl Acad Sci U S A. 2008;105(29):10238–10243. doi: 10.1073/pnas.0802759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soto C, Kascsak RJ, Saborio GP, Aucouturier P, Wisniewski T, Prelli F, et al. Reversion of prion protein conformational changes by synthetic beta-sheet breaker peptides. Lancet. 2000;355:192–197. doi: 10.1016/s0140-6736(99)11419-3. [DOI] [PubMed] [Google Scholar]