Abstract
Biliverdin reductase-A is a pleiotropic enzyme involved not only in the reduction of biliverdin-IX -alpha into bilirubin-IX-alpha, but also in the regulation of glucose metabolism and cell growth secondary to its serine/threonine/tyrosine kinase activity. Together with heme oxygenase, whose metabolic role is to degrade heme into biliverdin-IX-alpha, it forms a powerful system involved in the cell stress response during neurodegenerative disorders. In this paper, an up-regulation of the biliverdin reductase-A protein levels was found in the hippocampus of the subjects with Alzheimer disease and arguably its earliest form, mild cognitive impairment. Moreover a significant reduction in the phosphorylation of serine, threonine and tyrosine residues of biliverdin reductase-A was found, and this was paralleled by a marked reduction in its reductase activity. Interestingly, the levels of both total and phosphorylated biliverdin reductase-A was unchanged as well as its enzymatic activity in the cerebella. These results demonstrated a dichotomy between biliverdin reductase-A protein levels and activity in the hippocampus of subjects affected by Alzheimer disease and mild cognitive impairment, and this effect likely is attributable to a reduction in the phosphorylation of serine, threonine and tyrosine residues of biliverdin reductase-A. Consequently, not just the increased levels of biliverdin reductase-A, but also its changed activity and phosphorylation state, should be taken into account when considering potential biomarkers for Alzheimer disease and mild cognitive impairment.
Keywords: Alzheimer Disease, Biliverdin Reductase, Cerebellum, Hippocampus, Mild Cognitive Impairment, Oxidative Stress
1. Introduction
The role of oxidative and nitrosative stress in the pathogenesis of neurodegenerative disorders is no longer matter of question [1]. In the case of prolonged oxidative stress and neuroinflammation, which characterize neurodegenerative disorders, brain tissue is under free radical attack, and the brain reacts to this pro-oxidant condition by up-regulating some genes involved in the cell stress response [2, 3]. Among these, a main role is played by the heme oxygenase/biliverdin reductase (HO/BVR) system, whose up-regulation is one of the earlier events in the adaptive response to stress [4]. Heme oxygenase exists in two main isoforms, HO-1 and HO-2. Heme oxygenase-1 (HO-1), also known as heat shock protein (Hsp)-32, is induced by various stimuli, including oxidative and nitrosative stress, ischemia, heat shock, bacterial lipopolysaccharide (LPS), hemin, and the neuroprotective agent leteprinim potassium (Neotrofin) [5-7]. Heme oxygenase-2, the constitutive isoform, is responsive to developmental factors and adrenal glucocorticoids [5, 7]. Although HO-1 and HO-2 catalyze the same reaction, namely the transformation of iron-protoporphyrin-IX-alpha (heme) into ferrous iron (FeII), carbon monoxide (CO), and biliverdin-IX-alpha (thereafter BV) [5,7], they play different roles in protecting tissues against injuries [8,9]. The most convincing hypothesis suggests that controlled HO-1 induction plays a pivotal role in the earlier stages of cellular responses to tissue damage, whereas HO-2 is constitutively expressed and is primarily involved in maintaining cell heme homeostasis and in sensing the intracellular levels of gaseous compounds including oxygen, nitric oxide (NO), and CO [5,7,9]. Similarly, two isoforms of BVR were described and named BVR-A and BVR-B [10-12]. Both these enzymes generate bilirubin (BR), but only BVR-A reduces BV into the powerful antioxidant molecule BR-IX-alpha (thereafter BR), whereas BVR-B prefers the other BV isoforms, such as BV-β, BV-γ and BV-δ [10-12]. In the central nervous system (CNS), HO-2 is expressed in neuronal populations in almost all brain areas [5], whereas the inducible isoform is present at low levels in sparse groups of neurons, including the ventromedial and paraventricular nuclei of the hypothalamus [5, 7]. Heme oxygenase-1 is also found in cells of glial lineage, where its expression can be induced by oxidative stress [13]. Biliverdin reductase-A is co-expressed with HO-1 and/or HO-2 in cells of the rat brain that express these enzymes under normal conditions. It is also found in regions and cell types that can express heat shock-inducible HO-1 [14].
It is noteworthy to mention that BVR-A not only transforms BV into BR (by reducing the former's C10 [γ bridge]), but it is also a serine/threonine/tyrosine kinase involved in various cellular functions [10,11]. Interestingly, through the autophosphorylation of specific serine residues BVR-A controls its own reductase activity [15]. In addition, phosphorylated BVR-A interacts with members of the mitogen activated protein kinase family, in particular the extracellular signal-regulated kinases 1/2 (ERK1/2), and regulates the expression of oxidative-stress-responsive genes such as HO-1 or inducible nitric oxide synthase (iNOS) [10, 16-18].
With regard to neurodegenerative disorders, the activation of the HO/BVR-A system was demonstrated in the inferior parietal lobule, plasma and lymphocytes of Alzheimer disease (AD) patients [19,20]. In addition, Kimpara et al. shown that AD patients have an increased concentration of BR in the cerebrospinal fluid with respect to control [21]. Recently Mueller et al. showed that also BVR-B is up-regulated in plasma samples of AD patients [20]. Although these lines of evidence corroborate the activation of the HO/BVR-A axis in AD, its pathophysiological and clinical significance is under debate. In fact, although they are well recognized for cytoprotective activity, HO/BVR-A by-products may become toxic for neurons if produced in excess as during condition of prolonged neuroinflammation [22].
The aim of the current study was to investigate the levels, activity and regulation of BVR-A in both hippocampal and cerebellum samples of subjects affected by AD or arguably its earliest form, mild cognitive impairment (MCI), the latter being the transitional phase between normal aging and early AD [23]. Mild cognitive impairment shares with AD both pathological features, such as Aβ accumulation in the neocortex and numerous neurofibrillary tangles (NFTs) in the medial temporal lobe [23, 24], which generate pro-oxidant status, and clinical aspects of both disorders including memory loss. Amnestic MCI is characterized by memory loss but no dementia and normal activities of daily living; AD is characterized by both memory loss and dementia. For this reason, MCI and AD are often compared in terms of brain damage and cellular stress response in order to study progression of AD.
2. Materials and Methods
2.1 Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Nitrocellulose membranes and electrophoresis transfer system Trans-blot semi-dry Transfer Cell were obtained from Bio-Rad (Hercules, CA, USA). Anti-mouse and anti-rabbit IgG horseradish peroxidase conjugate secondary antibody and ECL plus Western blot detection reagents were obtained from GE Healthcare Bio-Sciences corp. (Piscataway, NJ, USA).
2.2 Subjects
Frozen hippocampal and cerebellar samples (n=6 each) from well-characterized subjects with AD and MCI and respective age-matched controls (Table 1) were obtained from the University of Kentucky Rapid Autopsy Program of the Alzheimer's Disease Clinical Center (UK ADC) with a post-mortem interval within the range 1.75-5.75 h for AD and MCI patients and relative control subjects. All the subjects were longitudinally followed and underwent annual neuropsychological testing, and neurological and physical examinations. Control subjects were without history of dementia or other neurological disorders and with intact activities of daily living (ADLs), and they underwent annual mental status testing and semi-annual physical and neurological exams as part of the UK ADC normal volunteer longitudinal aging study (Table 2). The control subjects showed no significant histopathological alterations and the Braak score was within the range 1-2 for the MCI age-matched controls, and 1-3 for the AD age-matched controls. Patients suffering from MCI met the criteria described by Petersen [23], which include: a memory complaint supported by an informant, objective memory test impairment (age- and education-adjusted), general normal global intellectual function, intact ADLs, Clinical Dementia Rating score of 0.0 to 0.5, no dementia, and a clinical evaluation that revealed no other cause for memory decline. AD patients diagnosis was made according to criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's disease and Related Disorders Association (ADRDA) [25]. All AD patients displayed progressive intellectual decline. The Braak scores were within the range 3-5 and 5-6 for MCI and AD patients, respectively.
Table 1.
Demographic information of AD and MCI subjects and age-matched controls. The value reported are the average of 6 samples.
| Age (years) |
Sex | Brain Weight (grams) |
PMI (hours) |
Braak stage |
|
|---|---|---|---|---|---|
| Control (AD) | 81 (72–87) |
5M,1F | 1219 (1020–1410) |
2.00–3.75 | 1–3 |
| AD | 85 (80–92) |
2M,4F | 1104 (835–1260) |
2.00–5.75 | 5–6 |
| Control (MCI) | 82 (74–93) |
4F,2M | 1204 (1080–1315) |
1.75–4.00 | 1–2 |
| MCI | 89 (82–99) |
4F,2M | 1102 (930–1200) |
2.00–5.00 | 3–5 |
AD, Alzheimer disease; MCI, mild cognitive impairment; PMI, post-mortem interval
Table 2.
Summary of the causes of death, concomitant neuropathologies and known co-morbidities in AD and MCI subjects and age-matched controls.
| Subjects | Cause of Death | Other Neuropathologies | Co-morbidities | |||
|---|---|---|---|---|---|---|
| Amyloid Angiopathy |
PD | Brain Tumor |
Head Injury |
|||
| Control AD 1 | No | No | No | No | MI, CABG | |
| Control AD 2 | MI | No | ||||
| Control AD 3 | Embolism | No | ||||
| Control AD 4 | Mild | |||||
| Control AD 5 | CHF | No | No | No | Yes | CHF |
| Control AD 6 | No | |||||
| AD 1 | No | No | No | No | Hypertension | |
| AD 2 | No | No | No | No | ||
| AD 3 | Mild | No | No | No | Hypertension | |
| AD 4 | Severe | No | No | No | MI, peptic ulcer | |
| AD 5 | Moderate | No | No | No | ||
| AD 6 | Moderate | No | No | No | Cancer | |
| Control MCI 1 | CD | No | ||||
| Control MCI 2 | No | No | No | No | Arrhythmias | |
| Control MCI 3 | SRD | No | ||||
| Control MCI 4 | Bladder cancer | No | ||||
| Control MCI 5 | Pancreatic cancer | No | No | No | No | Anxiety |
| Control MCI 6 | Cardiac arrest | No | No | No | No | CHF |
| MCI 1 | Heart disease | Mild | Yes | CHF | ||
| MCI 2 | CHF | No | ||||
| MCI 3 | MI-CAD | Mild | ||||
| MCI 4 | Moderate | Yes | Yes | Hypertension | ||
| MCI 5 | CHF | No | ||||
| MCI 6 | CHF | Moderate | No | No | No | MI |
CABG, coronary artery bypass graft; CAD, coronary artery disease; CD, unspecified cardiac disease; CHF, congestive heart failure; MI, myocardial infarction; PD, Parkinson disease; SRD, severe respiratory disease; TIA, transient ischemic attack.
2.3 Sample preparation
Brain tissues samples (hippocampus and cerebellum) from control, MCI and AD subjects were sonicated in Media 1 lysis buffer (pH 7.4) containing 320 mM Sucrose, 1% of 990 mM Tris-HCl (pH=8.8), 0.098 mM MgCl2, 0.076 mM EDTA, proteinase inhibitors leupeptin (0.5mg/mL), pepstatin (0.7 μg/mL), aprotinin (0.5 mg/mL) and PMSF (40 μg/mL) and phosphatase inhibitor cocktail (Sigma-Aldrich). Since the phosphorylation of specific Ser/Thr/Tyr residues by BVR-A itself or other kinases (e.g. the insulin receptor kinase) are involved in both the reductase and metabolic activities of biliverdin reductase [10,15], kinase inhibitors could interfere with such activities. For this reason, kinase inhibitors where not included in Media 1 lysis buffer. Homogenates were centrifuged at 14,000g for 10 min to remove debris. Protein concentration in the supernatant was determined by the Pierce BCA method (Pierce, Rockford, IL, USA).
2.4 Western blot analysis
For Western blot analyses, 50 μg of protein were denaturated in sample buffer for 5 min at 100 °C, and proteins separated on 12% precast Criterion gels (Bio-Rad) by electrophoresis at 100 mA for 2 h in MOPS buffer (Bio-Rad) into Bio-Rad apparatus. The proteins from the gels were then transferred to nitrocellulose membrane using the Transblot-Blot SD Semi-Dry Transfer Cell at 20 mA for 2 h. Subsequently, the membranes were blocked at 4 °C for 1 h with fresh blocking buffer made of 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) containing 0.01% (w/v) sodium azide and 0.2% (v/v) Tween 20 (PBST). The membranes were incubated at room temperature in PBST for 2 h with the following primary antibodies, as separate experiments: polyclonal anti-rabbit BVR-A (Sigma-Aldrich, dilution 1:1000); polyclonal anti-rabbit phosphoserine and anti-phosphothreonine (Zymed, Invitrogen, Camarillo, CA, USA, dilution 1:250); polyclonal anti-rabbit phosphotyrosine, (Zymed, Invitrogen, Camarillo, CA, USA, dilution 1:1000); polyclonal anti-rabbit phosphoERK1/2 (phospho-Thr202/Tyr204; Stressgen, Ann Arbor, MI, USA, dilution 1:1000) and polyclonal anti-rabbit β-actin (Sigma-Aldrich, dilution 1:2000). The membranes were then washed three times for 5 min with PBST followed by incubation with anti-mouse alkaline phosphatase or horseradish peroxidase conjugate secondary antibody (1:3000) in PBST for 2 h at room temperature. Membranes were then washed three times in PBST for 5 min and developed using or 5-bromo-4-chloro-3- indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT) color developing reagent for alkaline phosphatase secondary antibody or ECL plus WB detection reagents for horseradish peroxidase conjugate secondary antibody. Blots were dried, scanned in TIF format using Adobe Photoshop on a Canoscan 8800F (Canon) or STORM UV transilluminator (λex = 470 nm, λem = 618 nm, Molecular Dynamics, Sunnyvale, CA, USA) for chemiluminescence. The images were quantified with Image Quant TL 1D version 7.0 software (GE Healthcare). The optical density of bands was calculated as volume (optical density × area) adjusted for the background.
2.5 Immunoprecipitation
The immunoprecipitation procedure was performed as previously described [26] with modifications. Briefly, 150 μg of protein extracts were dissolved in 500 μl of RIPA buffer (10 mM Tris, pH 7.6; 140 mM NaCl; 0.5% NP40 including protease inhibitors) and then incubated with 1 μg of anti-BVR-A antibody at 4°C overnight. Immunocom plexes were collected by using protein A/G suspension for 2 h at 4°C and washed five times with immunoprecipitation buffer. Immunoprecipitated biliverdin reductase was recovered by resuspending the pellets in reducing SDS buffers and subjected to electrophoresis on 12% gels followed by Western blot analysis. Total BVR-A was used as loading control according to [16, 27].
2.6 Pro-Q Diamond Gel Staining
For detection of BVR-A specific phosphorylation, BVR-A was immunoprecipitated and subjected to electrophoresis as described above. Then, the gel was stained using a Pro-Q Diamond phosphoprotein gel stain kit (Molecular Probes) according to the manufacturer's protocol. Briefly, 1-D gels were fixed with 50% methanol and 10% acetic acid (100 mL per gel) for 30 min two times. Gels were then washed three times with ultrapure water (100 mL per gel) for 30 min. Gels were incubated in Pro-Q Diamond phosphoprotein stain solution (100 mL per gel) for 90 min, and destained by washing three times in 50 mM sodium acetate, pH 4.0, 20% ACN (100 mL per gel) for 30 min. Gels were then washed again three times with ultrapure water (100 mL per gel) for 30 minuts each washing. Pro-Q Diamond images were acquired using a Typhoon transilluminator with excitation/emission wavelength of 555/580 nm. SYPRO ruby-stained gel images were obtained using a STORM phosphoimager. All the images were saved in TIFF format. After the fluorescence scanning was completed for each gels, the gels were incubated in fixing solution (7% acetic acid, 10% methanol) for 20 min and stained overnight at room temperature with 50ml SYPRO Ruby gel stain (Bio-Rad). The SYPRO ruby gel stain was then removed and gels stored in deionized water.
2.7 BVR-A Phosphoserine/threonine and phosphotyrosine detection
For the detection of BVR-A specific phosphorylation on serine/threonine or tyrosine residues, BVR-A immunoprecipitates were recovered by resuspending the pellets in loading buffer, and protein was detected by Western blotting as previously described [16] using a mix of anti-phosphoserine and anti-phosphothreonine primary antibodies as described in section 2.4. Membranes were then stripped and re-probed by using and anti-BVR-A antibody as in section 2.4. Total BVR-A was used as loading control according to [27].
2.8 Biliverdin reductase activity assay
To test BVR activity in hippocampus and cerebellum samples from AD and MCI patients the BVR assay kit (Sigma-Aldrich) was used, following the standard protocol with minor changes. Briefly, 150 μg of protein samples were prepared for the assay and loaded in the 96 well plate. Furthermore, BVR positive control solution (2.5, 5, 10, 15 20 μl) was included in the assay for generation of a standard curve. Then, 50 μl of assay buffer and 150 μl of working solution (containing NAPDH, substrate solution and assay buffer) were added to standard and sample on the plate. This latter was placed on the UV-VIS plate reader at 37° C and read every minute for ten minutes. The reading after 5 min, that presented a linear reaction rate, was chosen for BVR activity calculation.
2.9 Statistical analysis
All statistical analysis was performed using a two-tailed Student's t-test. P < 0.05 was considered significantly different from control.
3. Results
3.1 BVR-A protein levels in AD and MCI
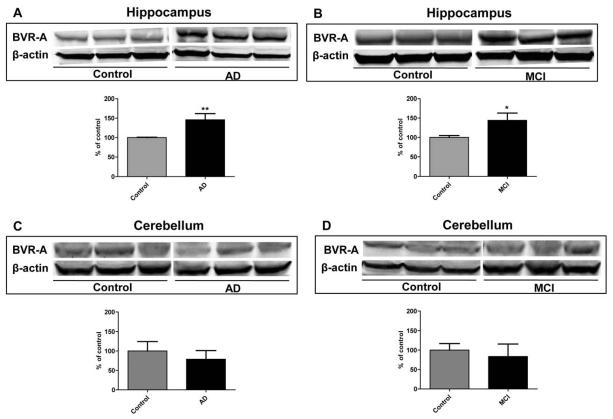
Biliverdin reductase-A protein levels in hippocampus and cerebellum from AD and MCI subjects and age-matched controls was measured by WB analysis. The BVR-A band intensity from control samples normalized to β-actin was considered as 100% and the AD and MCI values were expressed as percentage of controls. As shown in Figure 1, BVR-A levels were significantly increased in hippocampal samples from AD (Figure 1, Panel A) and MCI (Figure 1, Panel B) subjects. Conversely, no significant differences in BVR-A protein levels were found in the cerebellum of AD and MCI subjects (Figure 1, Panels C-D).
Figure 1.
Biliverdin reductase-A (BVR-A) protein levels in hippocampus and cerebellum of Alzheimer diseases (AD) and mild cognitive impairment (MCI) subjects. Brain samples of hippocampus (panels A and B) and cerebellum (panels C and D) of subjects with AD or MCI were assayed for BVR-A by Western Blot as described under Materials and Methods (section 2.4). Densitometric values shown in the histograms are given as percentage of Control, set as 100%, and are the product of the band value of the levels of BVR-A normalized per β-actin as loading control. In panels A-D representative gels are shown. Data are expressed as mean ± SD of six individual samples per group. *P < 0.05 and **P < 0.01 versus Control (Student's t-test).
3.2 BVR-A phosphotyrosine and phoshoserine/threonine detection.
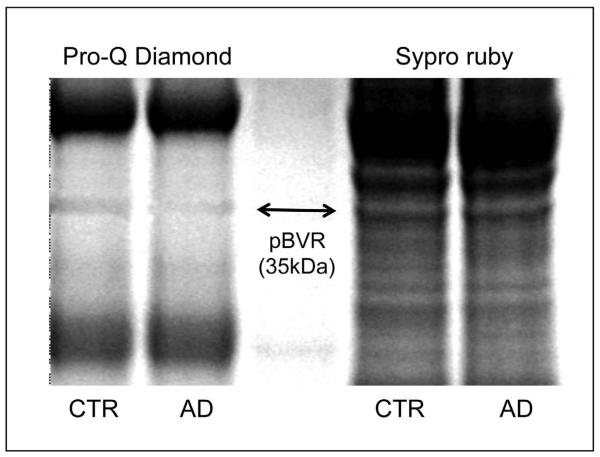
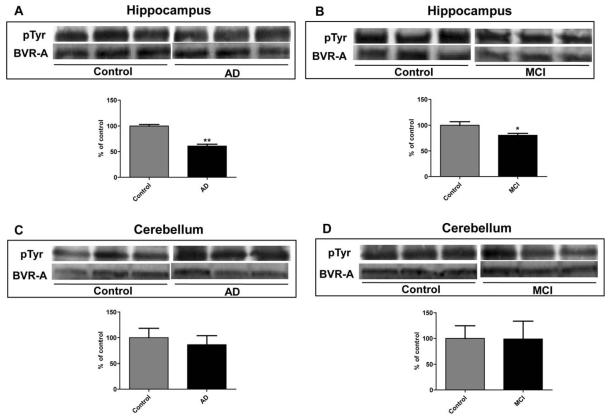
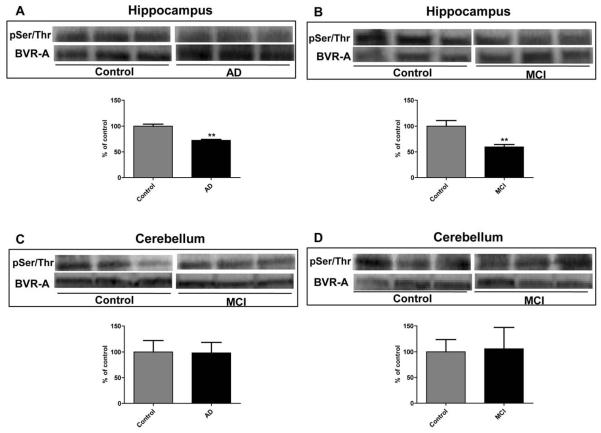
We initially approached the study of BVR-A phosphorylation using Pro-Q Diamond fluorescent staining. ProQ Diamond is capable of sensitive detection of phosphoserine-, phosphothreonine-, and phosphotyrosine-containing proteins directly in sodium dodecyl sulfate (SDS)-polyacrylamide gels. Fluorescence signal intensity of the band correlates with the number of phosphorylated residues on the protein. As shown in Figure 2 the total phosphorylation signal of BVR-A is significantly (p < 0.05) decreased by about 55% in AD compared to control hippocampus. However, to evaluate the extent of phosphorylation for Ser, Thr or Tyr residues of BVR-A, specific antibodies for pTyr, pSer, and pThr were used. pTyr-BVR-A was significantly decreased by 40 and 20% in AD (Figure 3, Panel A) and MCI (Figure 3, Panel B) hippocampal samples, respectively, whereas no significant change in cerebellar tissue was found. Similarly, pSer/pThr-BVR-A was decreased in AD (Figure 4, Panel A) and MCI (Figure 4, Panel B) hippocampus (28 and 40%, respectively) and did not change in cerebellar samples (Figure 4, Panels C-D).
Figure 2.
Biliverdin reductase-A total phosphorylation levels in hippocampus of Alzheimer disease (AD) subjects. Representative 1D gel image of immunoprecipitated BVR-A stained for total phosphorylation (Pro-Q Diamond) and for protein levels level (Sypro Ruby) in control and AD hippocampus.
Figure 3.
Biliverdin reductase-A (BVR-A) phosphorylation on Tyrosine (Tyr) residues in hippocampus and cerebellum of Alzheimer diseases (AD) and mild cognitive impairment (MCI) subjects. Brain samples of hippocampus (panels A and B) and cerebellum (panels C and D) of subjects with AD or MCI were immunoprecipitated by using an anti-BVR-A antibody and assayed for phospho-Tyr (pTyr) by Western Blot as described under Materials and Methods (sections 2.4, 2.5 and 2.7). Densitometric values shown in the histograms are given as percentage of Control, set as 100%, and are the product of the band value of the levels of pTyr-BVR-A normalized per total BVR-A as loading control. In panels A-D representative gels are shown. Data are expressed as mean ± SD of six individual samples per group. *P < 0.05 and **P < 0.01 versus Control (Student's t-test).
Figure 4.
Biliverdin reductase-A (BVR-A) phosphorylation on Serine/Threonine (Ser/Thr) residues in hippocampus and cerebellum of Alzheimer diseases (AD) and mild cognitive impairment (MCI) subjects. Brain samples of hippocampus (panels A and B) and cerebellum (panels C and D) of subjects with AD or MCI were immunoprecipitated by using an anti-BVR-A antibody and assayed for phospho-Ser/Thr (pSer/Thr) by Western Blot as described under Materials and Methods (sections 2.4, 2.5 and 2.7). Densitometric values shown in the histograms are given as percentage of Control, set as 100%, and are the product of the band value of the levels of pSer/Thr-BVR-A normalized per total BVR-A as loading control. In panels A-D representative gels are shown. Data are expressed as mean ± SD of six individual samples per group. **P < 0.01 versus Control (Student's t-test).
3.3 BVR activity assay
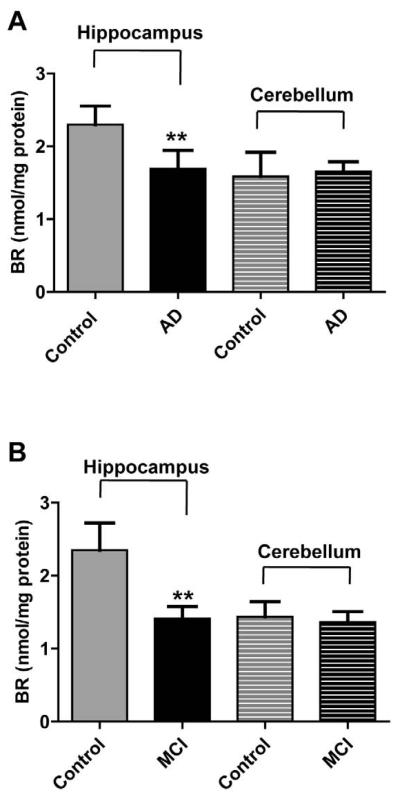
Since BVR autophosphorylation on serine residues affects its enzymatic activity [15], the reductase activity was measured. As shown in Figure 5, BVR activity was significantly decreased in hippocampal samples from AD (Figure 5, Panel A) and MCI (Figure 5, Panel B) subjects (27 and 40% reduction compared to their own controls, respectively). No differences in BVR activity in the cerebellum was found (Figure 5).
Figure 5.
Biliverdin reductase activity in hippocampus and cerebellum of Alzheimer diseases (AD) and mild cognitive impairment (MCI) subjects. Brain samples of hippocampus (panels A and B) and cerebellum (panels C and D) of subjects with AD or MCI were assayed for BVR activity as described under Materials and Methods (section 2.8). Values are given as nmol of bilirubin (BR) formed per mg of protein. Data are expressed as mean ± SD of six individual samples per group. ** P < 0.01 versus Control (Student's t-test).
3.4 BVR-A and ERK 1/2 interaction
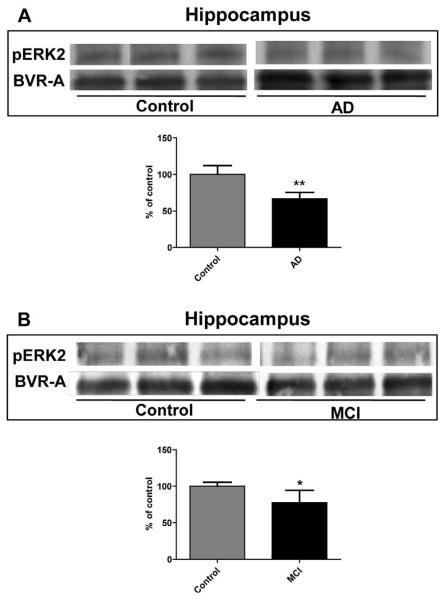
As BVR-A is functionally coupled to ERK1/2 and through this pathway contributes to the regulation of oxidative stress-responsive genes, the interaction between these two system was investigated in the hippocampus of AD and MCI subjects. As shown in Figure 6, phosphorylated ERK 2 co-immunoprecipitates with BVR-A, and its protein levels is significantly reduced in both AD and MCI samples. This result lends support to the evidence that ERK-2 is linked to BVR-A and this is in good agreement with previous data by Maines and co-workers [16].
Figure 6.
Extracellular signal-regulated kinases 1/2 (ERK 1/2) phosphorylation in hippocampus of Alzheimer diseases (AD) and mild cognitive impairment (MCI) subjects. Brain samples of hippocampus (panels A and B) of subjects with AD or MCI were immunoprecipitated with anti-BVR-A antibody and assayed for phospho-ERK1/2 by Western Blot as described under Materials and Methods (sections 2.4 and 2.5). Densitometric values shown in the histograms are given as percentage of Control, set as 100%, and are the product of the band value of the levels of pERK 2 normalized per total BVR-A as loading control. In panels A-B representative gels are shown. Data are expressed as mean ± SD of six individual samples per group. *P<0.05 and **P < 0.01 versus Control (Student's t-test).
4. Discussion
In this paper we report on the first evidence about the regulation of BVR-A protein levels and activity in the brain of AD and MCI subjects. The reason why only the BVR-A isoform was selected is related to the ultimate scope of this paper to dissect the contribution of the HO/BVR-A axis in the adaptive stress response in AD and MCI brains. Indeed, the fetal isoform BVR-B [28] was recently proposed as a serum biomarker in AD individuals [20], but the presence and pathophysiological role of this isoform in brain tissue needs further elucidation.
We chose to investigate the two selected brain areas for this study because of their differential selectivity against free radical-induced injury and pathology. Indeed, hippocampus is broadly recognized as a main target of neurodegenerative damage during AD progression, presenting increased levels of oxidative stress, neuronal loss and marked atrophy in respect to whole brain [29-31]. Conversely cerebellum is largely devoid of pathology and oxidative stress [32], consistent with lack of AD pathology on BVR-A levels, activity and phosphorylation state.
The first novel finding provided by this study is the relationship between BVR-A protein levels and activity. Total BVR-A protein levels was increased in the hippocampus of both AD and MCI subjects, but its activity was reduced. This result opens new avenues in the field of the cell stress response in AD. In fact, previous works led to the hypothesis that an up-regulation of the HO/BVR-A axis could be considered as a potential biomarker of AD. In this light, several groups, including ours, demonstrated increased protein levels and activity of HO-1/ BVR in brain, serum and lymphocytes of AD patients [4, 19, 20]. Furthermore, Kimpara et al. [21] demonstrated an increased concentration of BR in the cerebrospinal fluid of AD patients. The results shown in our study provide a second level of comprehension for this phenomenon and underline the importance of phosphorylation on BVR-A activity. Maines and her group identified Gly148-Ser-Leu-Arg-Phe-Thr-Ala-Ser-Pro as an important motif in the serine/threonine kinase domain and demonstrated that the phosphorylation of Ser149 is essential for BVR activity [27]. The significant reduction of both pSer/Thr-BVR-A (Figure 4) and BVR activity in AD (Figure 5) agree with this paradigm and confirmed the importance of BVR kinase activity even in the modulation of cell stress response. Keeping this in mind, it is no longer correct to measure total BVR-A protein level as an index to evaluate the involvement of this enzyme in the cell stress response since post-translational modifications, such as the phosphorylation of critical Ser/Thr/Tyr residues, play a main role in the regulation of the neuroprotective and/or metabolic activities of this enzyme [22,33,34]. Actually, a strong increase in total BVR-A protein levels could blunt the reduction in the Ser/Thr/Tyr phosphorylation and the consequent decrease in the reductase activity. However, this seems not to be the case in AD hippocampus since, despite a marked increase in total BVR-A protein levels and a significant reduction in both pSer/Thr e pTyr-BVR-A, the production of BR in this tissue remains decreased. In order to complete this intricate puzzle, the role of BVR-B has to be also carefully considered. Whether or not in future studies the presence of BVR-B is confirmed in AD brain, the differential contribution of both BVR isoforms to the cell stress response should be further explored. Indeed, it is not possible to single out the differential contribution of BVR-A and BVR-B to the generation of BR and the improvement of the cell adaptive response. Although it is possible to specifically measure BVR-A activity by using the alpha isomer of BV, it is not possible to specifically measure BVR-B activity because BV-β, BV-δ and BV-γ are also substrates for BVR-A [12].
As mentioned above, through its Ser/Thr/Tyr kinase activities BVR-A is implicated in cell growth and apoptosis as well as glucose metabolism. Specific Tyr residues of BVR-A, particularly Tyr198, Tyr228 and Tyr291, are substrates for insulin receptor kinase (IRK)-1, and phosphorylated BVR serves as a Ser/Thr kinase for insulin receptor substrate (IRS)-1, inhibiting the latter's phosphorylation by the insulin receptor. These processes represent a physiologic mechanism for increasing glucose uptake [10, 15]. The reduced phosphorylation of Ser/Thr/Tyr residues in BVR-A in AD and MCI hippocampus shown in this study (Figures 3 and 4) could also contribute to a further explanation to the impaired glucose metabolism seen in both disorders. A limitation of this study is the impossibility to identify the single Ser/Thr/Tyr residues in BVR-A which are not phosphorylated in the AD hippocampus. However, all the results related to BVR-A phosphorylation and BR formation are in good agreement with previous literature data, and therefore, it is plausible to hypothesize that these functional changes reflected the molecular modifications on specific Ser/Thr/Tyr residues.
Biliverdin reductase-A is also a crucial component of MEK1-ERK-Elk1 signaling. Biliverdin reductase-A functions as a scaffold protein for the activation of ERK by MEK1/2 and of Elk1 by ERK. The first step of this process is the formation of a ternary complex constituted by BVR-A/MEK/ERK, which places ERK in a position that permits its activation by MEK. In addition, the formation of this complex allows BVR-A to be phosphorylated by ERK. Once activated, the complex BVR-A-ERK is separated from MEK and translocates into the nucleus where it binds and activates Elk1, a transcription factor for the expression of oxidative-stress-responsive genes such as HO-1 or inducible nitric oxide synthase (iNOS) [10,16-18]. The reduced phosphorylation of BVR-A coupled with the decreased interaction with ERK-2 found in AD hippocampus (Figure 6) lend support to the hypothesis that BVR could be responsible, at least in part, for the ERK1/2 dysregulation detected in this brain area in AD subjects [35]. That only ERK-2 co-immunoprecipitated with BVR-A in AD hippocampus was quite surprising. A detailed dissection of the relative contribution of ERK-1 and ERK-2 to BVR-induced redox modifications in AD brain requires further analysis and is out of the scope of this paper. However, our result is corroborated by morphological findings which demonstrated that ERK-2 is the main isoform coupled to both senile plaques and neurofibrillary tangles and that its levels are markedly reduced in AD hippocampus and cortex [35,36].
In order to complete the analysis of the potential role of BVR-A in AD, it is necessary to comment on BVR-A as nuclear transporter for heme. Biliverdin reductase-A binds heme, and translocates it from cytosol to the nucleus, where several antioxidant genes including HO-1are up-regulated [17]. The result shown in Figure 1, namely the overexpression of BVR-A in AD hippocampal samples, agrees with previous data showing a significant HO-1 induction in several brain areas and illuminates a complete spectrum of interactions between HO-1 and BVR-A, whose final results are due to the sum of effects related to the total versus phosphorylated fraction of BVRA.
Interestingly, increased BVR-A levels and reduced phosphorylation on Ser/Thr/Tyr residues occurred also in the hippocampus of MCI subjects, thus implying that any modification in terms of cell stress response is an early event in the pathogenesis and progression of AD.
Another consequence of this study is the differential pattern of protein levels and activity of BVR in hippocampus and cerebellum. One of the possible explanations for the lack of oxidative damage in cerebellum characteristic of AD subjects [32] is the abundance of BVR-A and BR in this brain area. Although the results in this paper demonstrated that there is no evidence for an increased protein levels and activity of BVR-A in the cerebellum of AD or MCI subjects with respect to controls, our data are not in conflict with the previous hypothesis, since no difference between control and AD or MCI subjects were found. In fact, phosphorylation of BVR-A is not impaired in the cerebellum of AD or MCI subjects with respect to controls, and consequently, the levels of bilirubin-IX-alpha formed is still high, and this antioxidant may contribute to the lack of oxidative stress in cerebellum in AD and MCI.
However, it is important to recall that the HO-1/BVR system is not the only one involved in cellular stress response. Two other members of the heat shock protein family, such as Hsp60 and Hsp70, were shown to be involved in the adaptive stress response in AD. However, data from the literature failed to show any significant up-regulation of both Hsp60 and Hsp70 as well as HO-1 in the cerebellum of AD patients [37-39].
A potential caveat to the present studies is that some of the AD and MCI cases had evidence of mild to moderate amyloid aniopathay, which, being associated with a potential bleeding-prone condition, conceivably could activate the HO-1/BVR system. That the results reported were observed in hippocampus of all AD and MCI subjects regardless of the presence or absence of amyloid angiiopathy suggests that this condition did not materially contribute to the results observed. Nevertheless, further research will be necessary to completely rule out a contribution of this pathology to the present results.
In conclusion, this paper represents the first report of phosphorylation differences along with the level and activity of BVR in brain of subjects with AD or MCI. The implication of these results for insulin signaling and AD are profound, and research on these subjects is ongoing in our laboratory.
Acknowledgments
This work was supported in part by a NIH grant to D.A.B. [AG-05119]. E.B. is a Ph.D. student of the Catholic University of the Sacred Heart in Rome and is recipient of fellowships from the Society for Free Radical Biology and Medicine and the Italian Society of Pharmacology. F.D.D. was supported by a fellowship from Istituto Pasteur – Fondazione Cenci Bolognetti. We are grateful to the Neuropathology Cores of the University of Kentucky Alzheimer's Disease Clinical Center for providing well characterized specimens for this research.
Abbreviation
- AD
Alzheimer disease
- BVR
biliverdin reductase
- HO
heme oxygenase
- MCI
mild cognitive impairment
- pSer/Thre
phospho-serine/threonine
- pTyr
phospho-tyrosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors state that they have no conflict of interests correlated with this study.
References
- 1.Butterfield DA, Stadtman ER. Paula ST, Bittar EE, editors. Protein Oxidation Processes in Aging Brain. Advances in Cell Aging and Gerontology. 1997:161–191. [Google Scholar]
- 2.Calabrese V, Cornelius C, Mancuso C, Barone E, Calafato S, Bates T, Rizzarelli E, Kostova AT. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front. Biosci. 2009;14:376–397. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso C, Scapagini G, Curro D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front. Biosci. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- 4.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:478–493. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- 5.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 6.Maines MD. The heme oxygenase system and its functions in the brain. Cell. Mol. Biol. (Noisy-le-grand) 2000;46:573–585. [PubMed] [Google Scholar]
- 7.Mancuso C. Heme oxygenase and its products in the nervous system. Antioxid. Redox. Signal. 2004;6:878–887. doi: 10.1089/ars.2004.6.878. [DOI] [PubMed] [Google Scholar]
- 8.Maines MD, Panahian N. The heme oxygenase system and cellular defense mechanisms. Do HO-1 and HO-2 have different functions? Adv. Exp. Med. Biol. 2001;502:249–272. doi: 10.1007/978-1-4757-3401-0_17. [DOI] [PubMed] [Google Scholar]
- 9.Maines MD. The heme oxygenase system: update 2005. Antioxid. Redox. Signal. 2005;7:1761–1766. doi: 10.1089/ars.2005.7.1761. [DOI] [PubMed] [Google Scholar]
- 10.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Maines MD. New insights into biliverdin reductase functions: linking heme metabolism to cell signaling. Physiology (Bethesda) 2005;20:382–389. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- 12.Franklin EM, Browne S, Horan AM, Inomata K, Hammam MA, Kinoshita H, Lamparter T, Golfis G, Mantle TJ. The use of synthetic linear tetrapyrroles to probe the verdin sites of human biliverdin-IXalpha reductase and human biliverdin-IXbeta reductase. FEBS J. 2009;276:4405–4413. doi: 10.1111/j.1742-4658.2009.07148.x. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer BE, Nishimura RN, Lu SY. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain Res. Mol. Brain Res. 1995;30:37–47. doi: 10.1016/0169-328x(94)00273-h. [DOI] [PubMed] [Google Scholar]
- 14.Ewing JF, Weber CM, Maines MD. Biliverdin reductase is heat resistant and coexpressed with constitutive and heat shock forms of heme oxygenase in brain. J. Neurochem. 1993;61:1015–1023. doi: 10.1111/j.1471-4159.1993.tb03615.x. [DOI] [PubMed] [Google Scholar]
- 15.Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc. Natl. Acad. Sci. U S A. 2005;102:7109–7114. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerner-Marmarosh N, Miralem T, Gibbs PE, Maines MD. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc. Natl. Acad. Sci. U S A. 2008;105:6870–6875. doi: 10.1073/pnas.0800750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tudor C, Lerner-Marmarosh N, Engelborghs Y, Gibbs PE, Maines MD. Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin. Biochem. J. 2008;413:405–416. doi: 10.1042/BJ20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maines MD. Biliverdin reductase: PKC interaction at the cross-talk of MAPK and PI3K signaling pathways. Antioxid. Redox Signal. 2007;9:2187–2195. doi: 10.1089/ars.2007.1805. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid. Redox Signal. 2006;8:1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- 20.Mueller C, Zhou W, Vanmeter A, Heiby M, Magaki S, Ross MM, Espina V, Schrag M, Dickson C, Liotta LA, Kirsch WM. The heme degradation pathway is a promising serum biomarker source for the early detection of Alzheimer's disease. J. Alzheimers Dis. 2010;19:1081–1091. doi: 10.3233/JAD-2010-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimpara T, Takeda A, Yamaguchi T, Arai H, Okita N, Takase S, Sasaki H, Itoyama Y. Increased bilirubins and their derivatives in cerebrospinal fluid in Alzheimer's disease. Neurobiol. Aging. 2000;21:551–554. doi: 10.1016/s0197-4580(00)00128-7. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr. Drug Metab. 2009;10:579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment clinical trials. Nat. Rev. Drug Discov. 2003;2:646–653. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- 24.Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, Tangalos EG, Boeve BF, Knopman DS, Braak H, Petersen RC. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch. Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Cenini G, Sultana R, Memo M, Butterfield DA. Effects of oxidative and nitrosative stress in brain on p53 proapoptotic protein in amnestic mild cognitive impairment and Alzheimer disease. Free Radic. Biol. Med. 2008;45:81–85. doi: 10.1016/j.freeradbiomed.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salim M, Brown-Kipphut BA, Maines MD. Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J. Biol. Chem. 2001;276:10929–10934. doi: 10.1074/jbc.M010753200. [DOI] [PubMed] [Google Scholar]
- 28.Pereira PJ, Macedo-Ribeiro S, Párraga A, Pérez-Luque R, Cunningham O, Darcy K, Mantle TJ, Coll M. Structure of human biliverdin IXbeta reductase, an early fetal bilirubin IXbeta producing enzyme. Nat. Struct. Biol. 2001;8:215–220. doi: 10.1038/84948. [DOI] [PubMed] [Google Scholar]
- 29.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 30.Markesbery WR. Neuropathologic Alterations in Mild Cognitive Impairment: A. Review. J. Alzheimers Dis. 2010;19:221–228. doi: 10.3233/JAD-2010-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aksenov M, Aksenova MV, Harris ME, Hensley K, Butterfield DA, Carney JM. Enhancement of beta-amyloid peptide A beta(1-40)-mediated neurotoxicity by glutamine synthetase. J. Neurochem. 1995;65:1899–1902. doi: 10.1046/j.1471-4159.1995.65041899.x. [DOI] [PubMed] [Google Scholar]
- 32.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 33.Barone E, Trombino S, Cassano R, Sgambato A, De Paola B, Di Stasio E, Picci N, Preziosi P, Mancuso C. Characterization of the S-denitrosylating activity of bilirubin. J. Cell. Mol. Med. 2009;13:2365–2375. doi: 10.1111/j.1582-4934.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancuso C, Bonsignore A, Capone C, Di Stasio E, Pani G. Albumin-bound bilirubin interacts with nitric oxide by a redox mechanism. Antioxid. Redox Signal. 2006;8:487–494. doi: 10.1089/ars.2006.8.487. [DOI] [PubMed] [Google Scholar]
- 35.Hyman BT, Elvhage TE, Reiter J. Extracellular signal regulated kinases. Localization of protein and mRNA in the human hippocampal formation in Alzheimer's disease. Am. J. Pathol. 1994;144:565–572. [PMC free article] [PubMed] [Google Scholar]
- 36.Trojanowski JQ, Mawal-Dewan M, Schmidt ML, Martin J, Lee V. Localization of the mitogen activated protein kinase ERK2 in Alzheimer's disease neurofibrillary tangles and senile plaque neurites. Brain Res. 1993;618:333–3. doi: 10.1016/0006-8993(93)91286-2. [DOI] [PubMed] [Google Scholar]
- 37.Perez N, Sugar J, Charya S, Johnson G, Merril C, Bierer L, Perl D, Haroutunian V, Wallace W. Increased synthesis and accumulation of heat shock 70 proteins in Alzheimer's disease. Brain Res. Mol. Brain Res. 1991;11:249–254. doi: 10.1016/0169-328x(91)90033-t. [DOI] [PubMed] [Google Scholar]
- 38.Premkumar DR, Smith MA, Richey PL, Petersen RB, Castellani R, Kutty RK, Wiggert B, Perry G, Kalaria RN. Induction of heme oxygenase-1 mRNA and protein in neocortex and cerebral vessels in Alzheimer's disease. J. Neurochem. 1995;65:1399–1402. doi: 10.1046/j.1471-4159.1995.65031399.x. [DOI] [PubMed] [Google Scholar]
- 39.Di Domenico F, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]