Abstract
The importance of the extracellular matrix (ECM) in muscle is widely recognized since ECM plays a central role in proper muscle development (Buck and Horwitz 1987), tissue structural support (Purslow 2002), and transmission of mechanical signals between fibers and tendon (Huijing 1999). Since substrate biomechanical properties have been shown to be critical in the biology of tissue development and remodeling (Engler et al. 2006, Gilbert et al. 2010), it is likely that mechanics are critical for ECM to perform its function. Unfortunately, there are almost no data available regarding skeletal muscle ECM viscoelastic properties. This is primarily due to the impossibility of isolating and testing muscle ECM. Therefore, this note presents a new method to quantify viscoelastic ECM modulus by combining tests of single muscle fibers and fiber bundles. Our results demonstrate that ECM is a highly nonlinearly elastic material while muscle fibers are linearly elastic.
Keywords: Muscle, Extracellular matrix, Connective tissue, Mechanical properties
1. Introduction
Extracellular matrix (ECM) is essential for the development, maintenance and regeneration of skeletal muscle (Buck and Horwitz 1987, Purslow 2002). ECM is involved in the macrostructure of muscle, arranging fibers into bundles, bundles into fascicles and integrating muscle structure with aponeurosis and tendon. Additionally, ECM is thought to play a vital role in mechanotransduction, transmitting force laterally from the fiber to the tendon and vice versa (Fomovsky et al. 2010, Huijing 1999, Purslow and Trotter 1994, Street 1983). The mechanical strength and elasticity of the ECM are critical to its functional performance—it must be strong enough to sustain the loads of contraction yet elastic enough to prevent tearing under externally applied strains(Purslow 2002). These properties change both with age and disease, where chronic alterations to the ECM appear to impair muscle function (Lieber et al. 2003, Zhou and Lu 2010). Unfortunately, there are almost no data available regarding skeletal muscle ECM viscoelastic properties. This is primarily due to the impossibility of isolating and testing muscle ECM.
Attempts to remove muscle cells chemically from the ECM to test its properties directly have all met with some degradation or compromised mechanical properties (Borschel et al. 2004, Qing and Qin 2009). Additionally, the geometry of the ECM structure is modified when part of the composite structure (the fiber) is removed, which likely affects the orientation of collagen fibers and thus the modulus of elasticity. This report describes a new technique for indirectly determining the mechanical properties of the ECM without digestion, by combining tests from single muscle fibers and fiber bundles and using the analytical approach of composite theory.
2. Methods
Experiments were performed on single muscle fibers and muscle fiber bundles from the 5th toe of the extensor digitorum longus (EDL) muscle in mice (129/Sv 7–9 weeks old; Taconic Farms, Germantown, NY). Details of the dissection procedure and the solutions used have been described previously (Shah et al. 2004). All procedures were performed in accordance with the NIH Guide for the Use and Care of Laboratory Animals and were approved by the University of California and Department of Veteran’s Affairs Committees on the Use of Animal Subjects in Research.
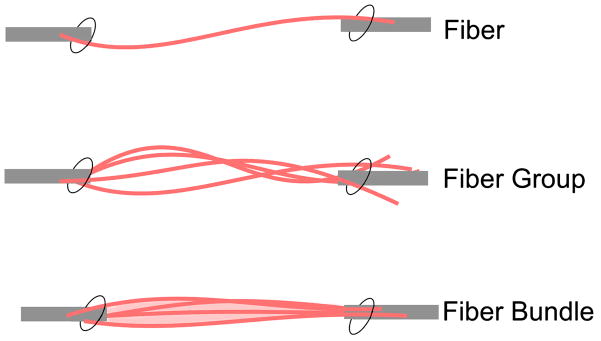
Briefly, muscles were skinned overnight in a glycerinated relaxation solution and single fibers and bundles (composed of 10–20 muscle fibers) were dissected in chilled relaxation solution composed of composed of (mM): EGTA (7.5), potassium propionate (170), magnesium acetate (2), imidazole (5), creatine phosphate (10), ATP (4), leupeptin (17 μg/ml), and E64 (4 μg/ml), to prevent protein degradation. One end of the specimen (either fiber, fiber group or fiber bundle) was attached via 10-0 suture to a motor arm (Newport MT-RS; Irvine, CA) that controlled specimen length and the other to a force transducer (Aurora Scientific 405A; Aurora, Ontario, Canada) that recorded force. The experimental paradigm (Fig. 1) illustrates the three types of specimens tested: (1) single fibers (2) bundles of 10–20 fibers or (3) 10–20 single fibers dissected individually that were then secured together to approximate the size of a bundle, but these “fiber groups” contained no interfibrillar ECM. Sarcomere lengths provided objective assessments of muscle strain and myofibrillar array quality control and were measured by transilluminating the specimen with a low power laser diode.
Figure 1.
Schematic illustration of the arrangement of the three specimen types. Single fibers (pink lines) were isolated from the muscle and either tested individually or secured in groups. Bundles of a similar number of fibers embedded in ECM (light pink) were isolated and similarly secured.
Elastic properties were derived from an incremental stress relaxation protocol. Specimens were stretched in ~10% strain increments at 2000%/sec to impose a 0.25 μm sarcomere length change per stretch. Length was then maintained for 3 minutes while the specimen was allowed to stress-relax. Elastic properties were determined from quadratic fits to fully stress-relaxed data. Elastic properties of the ECM were derived using the rule of mixtures for composites (Equation 1) where Em is the modulus of the ECM, Ef is the fiber modulus, Ec is the composite or bundle modulus and Am is the cross-sectional area fraction occupied by ECM in the bundle.
| (Equation 1) |
Data were compared across sample type by one-way ANOVA and considered significant (α) at p<0.05. Statistical power (1-β) was calculated for differences that were not significant. Individual experimental groups were compared using Tukey’s multiple comparison test. Data are presented as mean±SEM.
3. Results
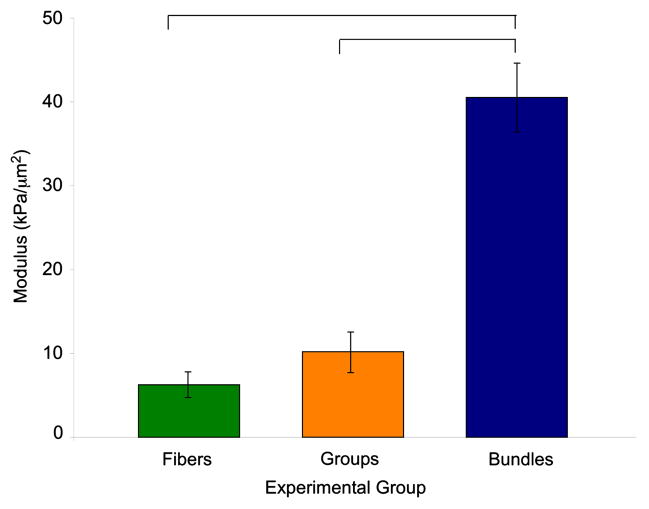
The relaxed quadratic modulus, representing the amount of nonlinearity present in the stress-sarcomere length relationship of the three experimental groups revealed that fiber bundle modulus was six fold greater than the modulus of individual fibers where elasticity is essentially linear (Fig. 2). This indicates that a source of nonlinearity present in the fiber bundles is not present in isolated fibers.
Figure 2.
Quadratic moduli for fibers, fiber groups and fiber bundles. Fiber bundles have a significantly higher modulus than either individual fibers or fiber groups. Fiber and fiber group moduli were not significantly different from each other (p>0.1). Fibers: n=17, Fiber Groups: n=6, Fiber Bundles: n=10. Bars indicate p<0.05.
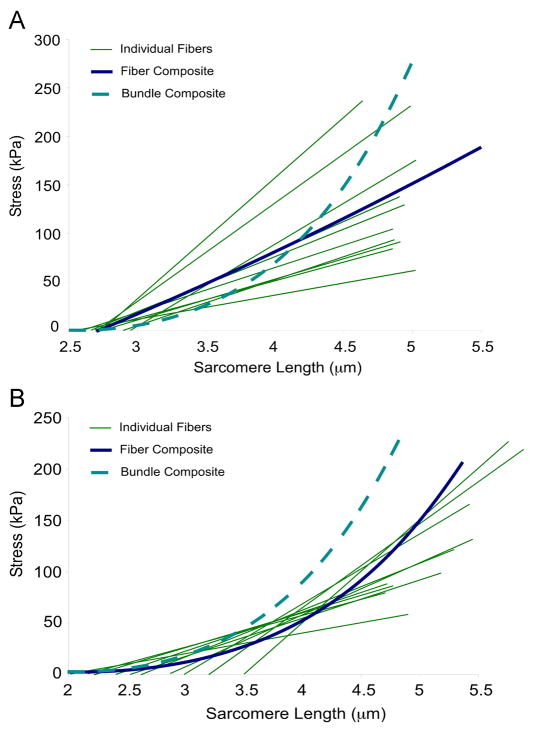
Because fiber bundles are composites of fibers and ECM, nonlinearity could arise from natural variations in the passive tension of fibers or from an intrinsic nonlinearly elastic ECM. This concept is illustrated graphically (Fig. 3) where the relaxed stress-sarcomere length relation is plotted for 10 experimentally tested fibers for two different cases. In the first case, fibers all develop passive tension at approximately the same sarcomere length and the fiber composite stress-strain relationship is linear (Fig. 3A). If this were the case for fibers in the bundle, the observed nonlinearity would have to arise from the ECM surrounding the fibers. In the second case, fiber stress-strain curves are artificially shifted such that each fiber develops passive tension at slightly different sarcomere lengths (Fig. 3B). This results in a nonlinear fiber composite stress-sarcomere length relationship even though the single fibers are linearly elastic. Thus, if fibers in a bundle were at different sarcomere lengths at a given bundle length, bundle stress-strain behavior could appear to be nonlinear whereas each of its components was actually linear. In this case, the contribution of the ECM to the nonlinearity of bundle elasticity could not be determined by subtraction from the contribution of fiber variability and composite theory could not be used to determine ECM elasticity.
Figure 3.
Illustration of the potential sources of nonlinearity in fiber bundles. (A) Experimental data from 10 fibers is plotted in green (each line represents an individual fiber) with composite fiber behavior shown in blue. In this case, all fibers develop tension at the same sarcomere length and the composite behavior is linear. Since nonlinear bundle behavior is observed, ECM elasticity must be nonlinear. (B), Same experimental data as in (A) are plotted but now each fiber has been shifted to develop tension at different sarcomere lengths, which yields a nonlinear composite behavior. In this case, nonlinear bundle elasticity could be obtained with a linearly or nonlinearly elastic ECM.
To differentiate between these possibilities, we combined and biomechanically tested individual isolated fibers into “groups” without the lateral connection of ECM. In this case, the contribution of fiber-to-fiber variability to the nonlinearity of the stress-strain relationship was isolated. Our results demonstrated that “fiber groups” have a quadratic modulus that is significantly lower than fiber bundles indicating that interfibrillar variability is not the primary contributing factor to fiber bundle nonlinearity (Fig. 2). In fact, fiber group modulus was not significantly different from single fiber modulus suggesting that a group of fibers behaves like an individual fiber (p > 0.1, 1-β=0.9). Based on these data, we can calculate bundle ECM modulus using Equation 1, knowing only fiber and bundle mechanics and fiber area fraction.
Assuming that 5% of the bundle cross sectional area is made up of ECM (Lieber et al. 2003), and using experimentally-derived modulus values (Fig. 2) the quadratic modulus of ECM in fiber bundles from the 5th toe EDL muscle is 692 kPa/μm2. This indicates that the elasticity of ECM in muscle is highly nonlinear.
4. Discussion
Here we describe a new method to quantify passive mechanical properties of the muscle ECM without tissue digestion. Previous studies used methods of subtraction, where the ECM was “preferentially” digested from muscle and its properties inferred from subtracting the digested state from the undigested state (see review by Fomovsky et al. 2010 and Granzier and Irving 1995). However, this method includes the uncertainty of incomplete or non-specific digestion. Additionally, it has not been rigorously demonstrated that the differences observed were due only to the removal of ECM. The current study provides a control by using groups of fibers, which differ from bundles only in that they contain no ECM that could interconnect muscle fibers. Such interconnections have been shown to be significant in defining the mechanical properties of tendon (Haraldsson et al. 2008). The modulus of the fiber groups was not significantly different from the modulus of individual fibers indicating that the observed difference between the elasticity of fibers and bundles is likely due to ECM.
It is possible that our fiber isolation had some effect on mechanical properties. Fibers were dissected from intact muscles after chemical skinning and the shear stress applied during isolation or the skinning procedure itself could cause fibers to behave differently in isolation than in their unperturbed state. For instance, the sarcolemma and associated proteins could be disrupted or collagen fibrils could remain attached during isolation, affecting the elasticity of the fiber. Further studies will be needed to determine the extent to which this affects our conclusions.
Bundle quadratic modulus was almost four fold larger than that of fiber groups. The magnitude of this effect supports the conclusion that the primary contributor to bundle stiffness is the ECM. Small perturbations to proteins on the fiber surface would likely contribute minimally to elasticity, as would the occasional remaining collagen fiber. Although this method was evaluated on bundles of only 10–20 fibers it could be extended to larger scales using fascicles and whole muscles for a more complete picture of the material properties of the ECM.
Acknowledgments
We gratefully acknowledge the National Institutes of Health grant AR40050 and the Department of Veterans Affairs. We also thank Dr. Sam Ward and Lucas Smith for helpful discussions.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borschel GH, Dennis RG, Kuzon WM., Jr Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast Reconstr Surg. 2004;113:595–602. doi: 10.1097/01.PRS.0000101064.62289.2F. discussion 603–594. [DOI] [PubMed] [Google Scholar]
- 2.Buck CA, Horwitz AF. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- 3.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol. 2010;48:490–496. doi: 10.1016/j.yjmcc.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haraldsson BT, Aagaard P, Qvortrup K, Bojsen-Moller J, Krogsgaard M, Koskinen S, Kjaer M, Magnusson SP. Lateral force transmission between human tendon fascicles. Matrix Biol. 2008;27:86–95. doi: 10.1016/j.matbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 1999;32:329–345. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 9.Lieber RL, Runesson E, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28:464–471. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 10.Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:947–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- 11.Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J Muscle Res Cell Motil. 1994;15:299–308. doi: 10.1007/BF00123482. [DOI] [PubMed] [Google Scholar]
- 12.Qing Q, Qin T. [Optimal method for rat skeletal muscle decellularization] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:836–839. [PubMed] [Google Scholar]
- 13.Shah SB, Davis J, Weisleder N, Kostavassili I, McCulloch AD, Ralston E, Capetanaki Y, Lieber RL. Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys J. 2004;86:2993–3008. doi: 10.1016/S0006-3495(04)74349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114:346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Lu H. Targeting fibrosis in duchenne muscular dystrophy. J Neuropathol Exp Neurol. 2010;69:771–776. doi: 10.1097/NEN.0b013e3181e9a34b. [DOI] [PMC free article] [PubMed] [Google Scholar]