Abstract
Intercellular communication via GAP Junctions plays an important role in tissue homeostasis, apoptosis, carcinogenesis, cell proliferation and differentiation. Hepatocyte connexins (Cx) 26 and 32 levels are decreased during the de-differentiation process of primary hepatocytes in culture, a situation that is also characterised by a decrease in S-Adenosylmethionine (SAMe) levels. In this current study, we show that SAMe supplementation in cultured hepatocytes every 12h, leads to an up-regulation of Cx26 and 32 mRNA and protein levels and blocks culture-induced Cx43 expression, although it failed to increase Cx 26 and 32 membrane localization and GAP junction intracellular communication. SAMe reduced nuclear β-catenin accumulation, which is known to stimulate the TCF/LEF-dependent gene transcription of Cx43. Moreover SAMe-induced reduction in Cx43 and β-catenin- was prevented by the proteasome inhibitor MG132, and was not mediated by GSK3 activity. SAMe, and its metabolite 5′-Methyltioadenosine (MTA) increased Cx26 mRNA in a process partially mediated by Adenosine A2A receptors but independent of PKA. Finally livers from MAT1A knockout mice, characterized by low hepatic SAMe levels, express higher Cx43 and lower Cx26 and 32 protein levels than control mice. These results suggest that SAMe maintains a characteristic expression pattern of the different Cxs in hepatocytes by differentially regulating their levels.
Keywords: SAMe, MTA, Adenosine A2A receptor, Hepatocytes, Connexin, cAMP
1. Introduction
Intercellular communication via Gap Junctions (GJIC) plays an important role in liver homeostasis, as well as in liver cell growth, cell differentiation, and cell death. (Warner, 1988; Bruzzone et al., 1996; Trosko and Ruch, 1998, Vinken et al., 2008, Decrock et al. 2009). A functional Gap Junction is formed by two hemi channels, each composed of 6 protein subunits termed connexins (Cx), in the membranes of neighbouring cells (Makowski et al., 1977). About 20 members of the connexin gene family have been identified and individual tissues show characteristic expression patterns of individual connexins (Sohl and Willecke, 2003). In the liver, expression of Cx32, 26, 43, 40, 37, 39, 31.9 and 30.2 have been identified (Nicholson et al., 1987, Shiojiri et al., 2006, Cicirata et al., 2004, Belluardo et al., 2001, Nielsen et al., 2003). Most of the non-parenchyma cells express connexin 43 (Zhang et al, 1989); Cx40 and 37 are expressed by liver vascular cells (Field et al, 2003); Cx32 is present in hepatocytes, biliary epithelial cells and sinusoidal endothelial cells (Nicholson et al, 1987, Bode et al, 2002), whereas Cx26 is expressed in hepatocytes, Kuppfer cells, hepatic stellate cells and sinusoidal endothelial cells (Nicholson et al., 1987, Zhang et al, 1989, Fischer et al 2005, Kumar and Gilula, 1986). The adult pattern of hepatocyte Cx expression (26 and 32) has been associated with a differentiated and non-proliferating hepatocyte phenotype (Zhang and Thorgeirsson, 1994; Kojima et al., 1996).
In the liver, S-Adenosylmethionine (SAMe) is a precursor of glutathione and polyamine synthesis, and it serves as a methyl donor in cellular transmethylation reactions (Eloranta and Kajander, 1984). Methionine Adenosyltransferase (MAT) catalyzes the formation of SAMe from methionine and ATP (Finkelstein, 1990; Mato, et al., 1997). In mammals, there are liver-specific and non-liver-specific MAT enzymes, which are the products of two genes, MAT1A and MAT2A respectively (Kotb et al., 1997). MAT1A-expressing hepatocytes produce higher amounts of SAMe and grow more slowly than cells expressing MAT2A (Mato et al., 2002). MAT2A is predominantly expressed in the fetal liver and is progressively replaced by MAT1A during development (Gil et al., 1996). The opposite switch between MAT1A and MAT2A, correlated with decreases in SAMe levels, has been observed during the de-differentiation process of hepatocytes in culture (Garcia-Trevijano et al., 2000). SAMe is involved in the control of hepatocyte development and de-differentiation status (Gil B. et al, 1996; Garcia-Trevijano et al., 2000) and a tight regulation of SAMe levels is essential for the maintenance of a functional liver (Martinez-Lopez et al, 2008).
During liver development, Cx43 is first expressed, and as Cx32 and Cx26 abundance increases between 19th and 21st gestational days, Cx43 expression is suppressed (Berthoud et al., 1992; Iwai et al., 2000). Conversely, during the de-differentiation process of primary hepatocytes in culture, a decrease in Cx26 and 32 accompanied by a rise in Cx43 have been observed (Kojima et al., 1995; Stutenkemper et al., 1992). This regulation of the differential expression of connexins in the liver is poorly understood. Since both SAMe levels and Cxs have a similar pattern of expression in hepatocytes during liver development and in culture, we decided to investigate whether SAMe regulated Cxs 26, 32 and 43 expression during the culture-induced hepatocyte de-differentiation process and also examined some of the molecular mechanisms.
3. Materials and Methods
Reagents
S-Adenosylmethionine, in the stable form of sulfate-p-toluensulfonate salt produced by Knoll (Milan, Italy) was obtained from Europharma (Madrid, Spain). ZM241385 from Tocris Bioscience (UK). H-89 Dihydrochloride, PHZ1123, Kenpaullone and MG132 was purchased from Calbiochem (USA and Canada). Cell culture reagents were from Gibco BRL (UK). Rat-tail collagen type I, from BD Biosciences (Bedford). All other reagents were of analytical grade, and unless otherwise stated, were purchased from Sigma.
MAT1A-KO and WT mice experiments
MAT1A deficient and wild type (WT) mice (Lu, S.C et al, 2001) were maintained in the CICbioGUNE animal facility and all procedures conducted in accordance with the Spanish guide for the care and use of laboratory animals, and protocols were approved by the CICbioGUNE ethical review committee. Eight-month-old male homozygous MAT1A-KO and WT were killed and liver specimens were snap-frozen for subsequent analysis.
Isolation and Culture of Rat Hepatocytes
Hepatocytes were isolated from male Sprague-Dawley rats (200 g) by collagenase perfusion (Gibco-BRL) as described previously (Leffert et al., 1979). Animals were treated humanely, and all procedures complied with our institutions' guidelines and UK legislation for the use of laboratory animals. Viability of the cells, as judged by the trypan blue exclusion test, was more than 90%. Isolated hepatocytes were seeded over collagen-coated tissue culture dishes or cover slips at a density 16.600 cells/mm and cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum in a 5% CO2-95 % air incubator at 37 °C. After two hours of attachment, the culture medium was removed and replaced by serum-free MEM with or without SAMe (4 mM) or MTA (500 μM). Subsequently the medium was replaced every 12 hours with fresh medium in both control and supplemented cells. The appropriate concentrations of SAMe (4 mM) or MTA (500 μM) treatments were established previously (Garcia-Trevijano et al., 2000; Hevia, H et al., 2004). In some experiments, prior to SAMe or MTA addition, cells were pre-treated every 24h for 2 hours with the inhibitors of PKA (H-89, 1 μM), adenosine A2A receptors (ZM241385, 30μM), proteasome (MG132, 5μM), GSK3β (Kenpaullone, 5μM and PHZ1123, 25μM), and MAT (Cycloleucine, 20mM).
RNA isolation, RT-PCR and real-time PCR
Total RNA was isolated using PureLink™ Purification System (Invitrogen, UK). 1 μg of total RNA was treated with DNAse (Promega, UK) and reverse transcribed into cDNA using SuperscriptIII (Invitrogen, UK). To compare the transcript level of connexin genes, real-time PCR was performed using Platinum® SYBR® Green qPCR SuperMix-UDG (Invitrogen, UK) and the LightCycler ® 2.0 Real-Time PCR System (Roche, UK). PCR was executed with the following primers: Cx26F 5′-AGAGGTTCGGTTTGTGATGTG-3′, Cx26R 5′-ACGAGAAGCTTCCAGTTTGTC-3′; Cx32F 5′-ATCTGCTCTACCCGGGCTATG-3′, Cx32R 5′-AGACGGTTTTCTCAGTGGG-3′, Cx43F 5′-ACCTACATCATCAGCATCC-3′, Cx43R 5′-AGCGAGAGACACCAAGGAC-3′, 5′-G6PaseR 5′-AGTATCCCAACCACAAGACG-3′, G6PaseF 5′ACCTGTGAGACTGGACCAG-3′. qPCR conditions were as follows: 50 °C for 2 minutes and 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 5 seconds, 60 °C for 10 seconds, and 72 °C for 10 seconds. Albumin, α-Fetoprotein (α-FP), and Hepatocyte nuclear factor 1-alpha (HNF-1α) primers and PCR conditions were described previously (Schwartz, R.E. et al., 2002). No-template controls were run for each primer set as a negative control. All reactions were performed in triplicate, and expression levels were normalised to the average level of 18s rRNA in each sample, since we observed that 18S rRNA, unlike Actin and GAPDH, was not significantly affected either during the de-differentiation process of hepatocytes in culture or by SAMe treatment.
Total protein isolation
Cultured hepatocytes were washed twice with PBS buffer and resuspended in 1 ml lysis buffer (10 mM Tris/HCl pH 7.6. 5 mM EDTA, 50 mM NaCl, 1% Triton X–100, complete protease inhibitor cocktail, and 50 mM NaF). Frozen liver tissue samples were homogenized in lysis buffer and were centrifuged (15.000 g, 1h, 4 °C) and supernatants were collected. Samples were sonicated 3 times for 20 second cycles, at intensity 10. After sonication samples were mixed for 1h with DTT 0.1 M and urea 8 M, at 4 °C.
Nuclear protein isolation
Cytoplasmic and nuclear lysates for β-catenin analysis were prepared from rat hepatocytes as described in the subcellular proteome extraction kit from Calbiochem.
Western blot analysis
The protein concentration of the samples was determined by Bradford assay. For immunoblotting analysis, 30 μg of protein were electrophoresed on SDS-polyacrylamide gels and transferred onto membranes. After blocking, membranes were incubated o/n at 4 °C with specific antibody (Table I). This was followed by 1h of incubation with goat anti-mouse or goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase; immunoreactive proteins were detected by an enhanced chemiluminiscent system (ECL, Amersham International plc, Little Chalfont, UK).
Table I. Specifications and conditions of the antibodies used.
| Antibody | Supplier | Catalog Number | Western blot | Immunocytochemistry |
|---|---|---|---|---|
| Cx26 | Zymed (Invitrogen, UK) | 710500 | 1/500 | 1/100 |
| Cx32 | Zymed (Invitrogen, UK) | 358900 | 1/250 | 1/100 |
| Cx43 | Zymed (Invitrogen, UK) | 710700 | 1/250 | 1/100 |
| Z0-1 | Zymed (Invitrogen, UK) | 40-2300 | 1/1000 | 1/100 |
| β-catenin | Cell Signalling Technology | 9562 | 1/750 | - |
| phospho-β-catenin (Ser33/37/Thr41) | Cell Signalling Technology | 9561 | 1/1000 | - |
| GSK3 | Cell Signalling Technology | 9332 | 1/1000 | - |
| phospho-Ser9-GSK3 | Cell Signalling Technology | 9331 | 1/1000 | - |
| Actin | Sigma | A5316 | 1/1000 | - |
| HPR-conjugated secondary goat antibody to mouse | (Promega Ltd, UK) | W4021 | 1/1000 | - |
| HPR-conjugated secondary goat antibody to rabbit | (Biorad, UK) | 172-1019 | 1/1000 | - |
| (FITC)-conjugated secondary goat antibody to Rabbit IgG | Jackson Immunoresearch | 111-095-003 | - | 1/600 |
| (FITC)-conjugated secondary goat antibody to Mouse IgG | Jackson Immunoresearch | 115-095-003 | - | 1/200 |
| Cy3-conjugated secondary goat antibody to Rabbit IgG | Jackson Immunoresearch | 115-165-003 | - | 1/1000 |
| Cy3-conjugated secondary goat antibody to Mouse IgG | Jackson Immunoresearch | 111-165-003 | - | 1/500 |
Immunocytochemistry and Fluorescent F-actin staining
Hepatocytes grown on collagen-coated glass cover slips were treated as described above. Hepatocytes were fixed for 10 minutes with ethanol at RT. After fixation cells, were washed 3 times with phosphate-buffered saline (PBS), blocked for 30 minutes with PBS containing 0.1% BSA and 10% horse serum, and then incubated o/n at 4°C with primary antibodies diluted in blocking solution (Table I). After washing with 0.1% BSA/PBS, the cells were incubated for 45 min with fluoresceine isothiocyanate (FITC)-conjugated secondary antibodies (Table I), and the DNA-binding fluorochrome Hoechst 33342 (0.5 Ag/ml) for cell nuclear staining. Finally, cells were washed 3 times in PBS, before being mounted in glycerol/PBS (1:1) CitiFluor Glycerol/PBS solution AF1 (Citifluor Ltd. UK) and examined under Leica TCS-SP (UV) confocal laser microscope. In control experiments, cells were incubated only with appropriate secondary antibodies. No unspecific cross reactivity was seen.
For F-actin staining, the cells were incubated with Rhodamine-Phalloidin diluted in blocking solution for 1 hour, and washed 3 times with PBS before being examined.
Measurement of intracellular cAMP
Hepatocytes were lysed in 200 μl of lysis buffer, and 100 μl of cell extracts used to measure the cAMP intracellular levels as described in the cAMP Biotrak enzymeimmunoassay (EIA) system, from Amersham Biosciences (UK).
Glycogenolysis (breakdown of glycogen)
Basal and glucagon induced-glycogenolysis were measured as glucose released into the buffer, using the glucose oxidase kit (Sigma) as described previously (Loven et al., 2005).
Ammonia clearance
Hepatocytes were cultured in MEM and SAMe-supplemented MEM in the presence of 2.5 mM of NH4Cl. 24h and 36h later, the medium was collected and NH4Cl concentration measured using the ammonia assay (Sigma) (Banas et al., 2007).
Gap Junction Intracellular Communication (GJIC) assay
GJIC was studied using the Scrape-loading/dye transfer method as described previously (El-Fouly et al., 1987) with some modifications. Briefly, hepatocytes were plated in 12-well plates at a density of 0.5 × 106 cells/well in minimum essential medium (MEM) with or without SAMe supplementation as described above. After 24h, hepatocytes were rinsed three times with PBS, and a scrape line made by gently passing a yellow tip across the cultures. Cells were then incubated with a 10 mM solution of Lucifer Yellow CH (Sigma) in PBS during 5 minutes at 37 °C. Cells were washed three times with PBS, fixed with 4% paraformaldehyde and then examined under a Zeis Axiover 200 fluorescence microscope.
Statistical analysis
Data were analyzed using the Kruskal-Wallis test to determine differences between all independent groups. When significant differences were obtained (p < 0.05), differences between two groups were tested using the Mann-Witney U test.
4. Results
Maintenance of hepatocyte differentiation in culture
Primary hepatocytes supplemented with SAMe showed different morphological characteristics from control cells cultured in serum-free medium. SAMe-cultured hepatocytes were square-shaped and highly refringent as compared to untreated cells (Figure 1A). Fluorescent staining of F-actin protein showed that non-supplemented cells have stress fibres, while SAMe supplemented hepatocytes showed F-actin mostly localised in membrane areas. In addition, SAMe supplementation increased Zonula occludens 1 (ZO-1) membrane localization, indicating hepatocyte polarization.
Figure 1. Changes in morphological characteristics, glucose secretion from glycogen and Albumin, α–FP, and HNF-1α PEPCK and G6Pase mRNA expression in hepatocytes culture with SAMe supplementation.

(A) Morphology by light microscopy (Original magnification ×100), fluorescent staining of F-actin protein and immunofluorescence of ZO-1 protein in serum-free cultured hepatocytes (Control) and SAMe-supplemented hepatocytes. Original magnification ×200. Real-time PCR analysis of (B) Albumin, (C) α–FP and (D) HNF-1α mRNA levels in hepatocytes cultured with or without SAMe supplementation. Real-time PCR analysis of mRNA levels of (E) PEPCK at 12, 24 and 48h and (F) G6Pase at 12h in hepatocytes cultured with or without SAMe supplementation, and with and without glucagon (10 nM). Values were normalized with 18S ribosomal RNA expression. (G) Glucose release into the culture medium was assayed in control and SAMe-supplemented hepatocytes during 12h. Release of glucose was stimulated with glucagon (10 nM) during 90 minutes. Each bar represents the mean ± SD of at least quadruplicate experiments (*p<0.05; vs. control).
Markers of mature hepatocytes, including mRNA of albumin (Figure 1B), HNF-1α (Figure 1D) and phosphoenolpyruvate carboxykinase (PEPCK) (Figure 1E), were significantly increased after 36 and 48h of SAMe supplementation when compared with control cells. In addition, SAMe increased glucagon-induced PEPCK and glucose-6-phosphatase (G6Pase) mRNA expression (Figures 1E and 1F), without affecting glucagon receptor mRNA expression (not shown). Finally, culture-induced expression of α-FP mRNA was prevented by SAMe supplementation (Figure 1C).
Glucose production from glucogenolysis was measured in hepatocytes after culture in basal medium supplemented with 15 mM glucose and 9 nM insulin during 16 hours, with or without SAMe supplementation. Hepatocytes were stimulated with glucagon (10 nM) during 90 minutes. We observed that SAMe increased basal and glucagon-induced glucose production (Figure 1G).
Finally, we did not observe any difference in the capacity to clear ammonia from culture medium in hepatocytes cultured in the presence or absence of SAMe, 24 and 36 hours after the addition of NH4Cl (data non shown).
Taken together, our data suggest that our culture conditions with SAMe supplementation every 12h, partially reverses the de-differentiation process of hepatocytes in culture.
Connexin 26, 32 and 43 protein and mRNA expression
Supplementation of SAMe in cultured hepatocytes every 12h for 48h induced a significant increase in Cx26 mRNA at 12, 24, 36 and 48h (Figure 2A), and in Cx32 mRNA after 24, 36, and 48h (Figure 2B) compared to controls. In cells treated with only an initial dose of SAMe at time 0, there was an initial increase in Cx26 and 32 mRNA expression, which declined to basal levels with time (Figure 2A, B). On the other hand, SAMe was able to prevent culture-induced increase in Cx43 mRNA expression observed at 48h both after sustained SAMe supplementation or after a single initial treatment (Figure 2C). Thus, the observed changes in Cx26 and 32 mRNA expression are dependent on a sustained supplementation of SAMe, whereas only a single dose of SAMe is required for the observed changes in Cx43 mRNA expression.
Figure 2. Connexin 26, 32 and 43 mRNA and protein expression in hepatocytes cultured with SAMe supplementation.
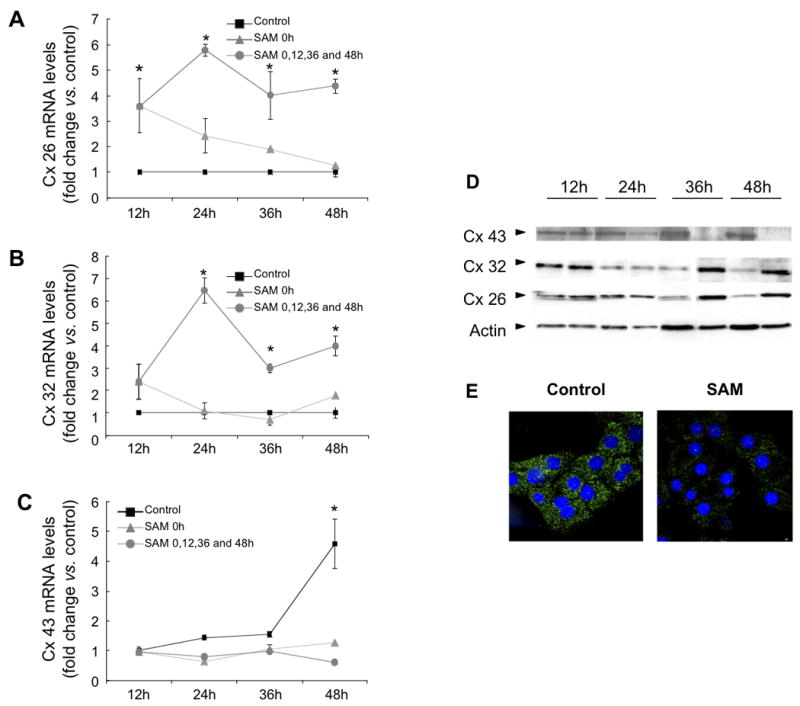
Real-time PCR analysis of (A) Cx26, (B) Cx32 and (C) Cx43 mRNA expression of hepatocytes cultured in serum-free medium (control), with a single SAMe supplementation at time 0h (SAMe 0h), or with SAMe supplementation every 12h (SAMe 0h+12h+24h). Each bar represents the mean ± SD of at least quadruplicate experiments (*p<0.05; vs. control). Values were normalized with 18S ribosomal RNA expression. (D) Western blot analysis was performed with total proteins extracted at 12, 24, 36 and 48 h from cultured hepatocytes in control medium or SAMe supplemented MEM. Blots were incubated with anti-Cx32, Cx26, Cx43 and actin antibodies. Results are representative of at least three independent experiments. (E) Immunofluorescent staining of Cx43 protein in control and SAMe supplemented hepatocytes during 48h. Original magnification ×200.
SAMe supplementation also increased Cx26 and 32 protein expression and completely abrogated culture-induced Cx43 protein expression after 36 and 48h (Figure 2D). It has been suggested that increases in Cx43 hepatocytes in culture could arise from contaminating non-parenchyma cells such as oval cells (Stutenkemper et al., 1992). Immunocytochemistry analysis showed a decrease of Cx43 positive spots in the cytosol of hepatocytes supplemented with SAMe when compared with control cells at 48h (Figure 2E), which is in keeping with the suggestion that the appearance of Cx43 could be considered as a part of the de-differentiation process (Zhang et al., 1994; Rosenberg et al., 1996; Vinken et al., 2006), which is partially reversed by SAMe.
The increase Cx26 mRNA expression induced by SAMe supplementation is partially mediated by Adenosine A2A receptors
Dibutyl cyclic AMP (cAMP) has been shown to increase the stability of Cx32 mRNA and increase Cx26 mRNA expression in hepatocytes in primary culture (Saez et al., 1989, Kojima et al., 1995b). It has also been shown that SAMe increases cAMP levels by activating Adenosine A2A receptors in the RAW 267 cell line (Zanotti et al., 1998; Song et al., 2005). Intracellular levels of cAMP in cultured hepatocytes were 2.4 times higher 12h after the first SAMe supplementation, and 1h after the second SAMe addition (Table II). SAMe pre-treatment of hepatocytes with the Adenosine Receptor A2A inhibitor ZM241385 (30 μM) prevented the SAMe induced Cx26 mRNA expression (Figure 3A), but had no significant effect on the SAMe-induced Cx32 mRNA (Figure 3B).
Table II. Intracellular cAMP levels after SAMe or MTA supplementation.
cAMP intracellular levels were measured as described in Material and Methods from primary hepatocytes in culture, 1h and 12h after the first MTA supplementation, and 1h after the second MTA supplementation. Each data represents the mean ± SD of at least quadruplicate experiments (*p<0.05; vs. control).
| cAMP (fmol/1.5 million cells) | 1h | 12h | 13h |
|---|---|---|---|
| CONTROL | 498 ± 22.5 | 523.9 ± 26.1 | 481.0 ± 16 |
| MTA 500 μM | 1253.9 ± 59.7* | 1162.5 ± 95.9* | 1767.7 ± 132* |
| SAMe 4mM | 589.2 ± 32.5 | 1287.3 ± 58.4* | 1112.4 ± 74.8* |
Figure 3. Effect of PKA inhibitor (H-89) or Adenosine A2A receptors inhibitor (ZM241385) on Cx26 and 32 mRNA expression in response to SAMe supplementation.
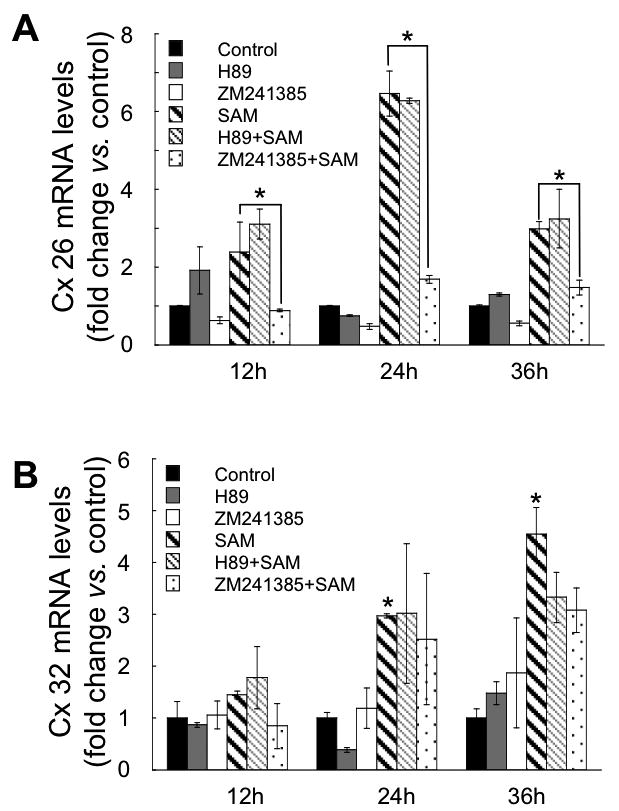
Real-time PCR analysis of Cx26 (A), and Cx32 mRNA levels (B), at 12, 24, and 36h of control and SAMe supplemented hepatocyte culture, and pre-treated during 2 hours, with or without ZM211385 30 μM, or H-89 1 μM, every 24h, before each SAMe supplementation. Each bar represents the mean ± SD of at least quadruplicate experiments (*p<0.05; vs. control). Values were normalized with 18S ribosomal RNA expression.
Recent studies have shown that repeated SAMe treatment (12 days) induced an increase in the binding of cAMP to the RII PKA regulatory unit in cerebrocortical microtubules (Zanotti et al., 1998). We observed that pre-incubation with the PKA inhibitor, H-89 (1μM) did not prevent the increases in the Cx26 (Figure 3A) and Cx32 (Figure 3B) mRNA levels induced by SAMe.
MTA supplementation increased cAMP levels and Cx 26 mRNA expression
SAMe can be converted to MTA, which is itself converted into methionine and subsequently into SAMe (methionine salvage pathway) (Figure 4A). Several of the SAMe effects in hepatocytes (e.g. inflammatory response) have been attributed to its conversion into MTA (Simile et al., 2001; Ansorena et al., 2002). To test whether the SAMe effects on connexin expression were due to its conversion into MTA, we examined their mRNA and protein expression in response to MTA treatment. MTA-supplemented hepatocytes showed an increase in Cx26 mRNA levels at 12, 24, and 36h of treatment (Figure 4B) and in Cx26 protein expression after 36h (Figure 4D). There was no significant effect on Cx32 or 43 mRNA and protein expression at any time (data not shown).
Figure 4. Cx26 mRNA and protein expression in response to MTA supplementation. Effect of PKA inhibitor (H-89), MAT inhibitor (Cycloleucine) or Adenosine A2A receptors inhibitor (ZM241385).
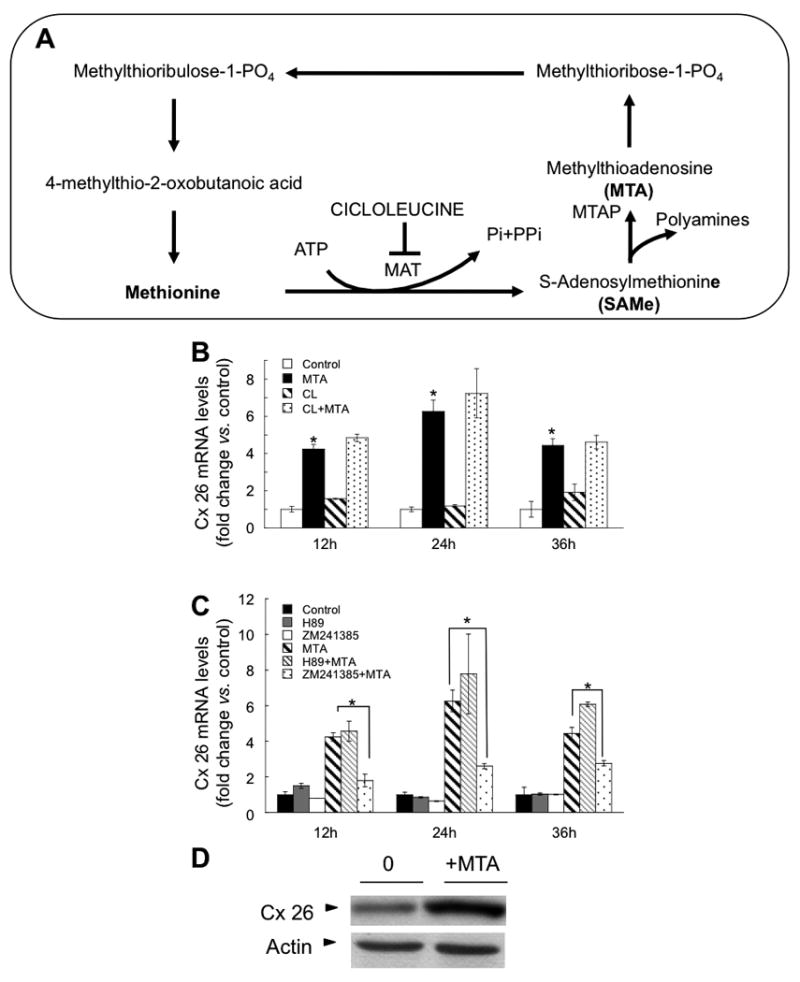
(A) Schematic representation of the methionine salvage pathway. SAMe can be converted into MTA, which is converted into methionine. SAMe can be synthesized from methionine and ATP in a reaction catalyzed by Methionine Adenosyltransferase (MAT) enzyme. Cycloleucine is a specific inhibitor of MAT activity. Methylthioadenosine phosphorylase (MTAP). (B) Real-time PCR analysis of Cx26 mRNA levels, at 12, 24, and 36h of cultured hepatocytes control and with MTA supplementation, and pre-treated during 2h, with or without cycloleucine 20 mM (A), ZM211385 30 μM or H89 1 μM (B), every 24h, before each MTA supplementation. Each bar represents the mean ± SD of at least quadruplicate experiments (*p<0.05; vs. control). Values were normalized with 18S ribosomal RNA expression. (C) Westernblot analysis was preformed with total proteins extracted at 36h from hepatocytes control or MTA-supplemented MEM. Blots were incubated with anti-Cx26 and actin antibodies. Results are representative of at least three independent experiments.
To confirm that the effect of MTA on Cx26 expression was not due to its conversion into methionine and then into SAMe through MAT activity, we pre-treated hepatocytes with the MAT inhibitor cycloleucine (20 mM), before MTA supplementation. Cycloleucine did not block the MTA effect on Cx26 mRNA expression (Figure 4B), indicating that the MTA effect was independent of its conversion into SAMe.
MTA, like SAMe, has been described as a partial agonist at Adenosine A2A receptors (Munshi et al., 1988). It is also able to increase intracellular cAMP levels either through the inhibition of cAMP phosphodiesterase (Riscoe and Ferro, 1984; Maher, 1993), or by its conversion into adenosine. Thus, we measured cAMP production 1h and 12h after the first MTA supplementation, and 1h after the second MTA supplementation. MTA induced a rapid increase in cAMP 1h after each MTA supplementation; these increases were sustained for 12h (Table II). Pre-incubation with the Adenosine A2A receptors inhibitor ZM241385 (30 μM) prevented the MTA-induced Cx26 mRNA expression (Figure 4C), suggesting that its effect is mediated by Adenosine A2A receptors. Finally, as observed in SAMe-treated hepatocytes, the PKA inhibitor, H-89 (1 μM) did not prevent MTA-induced increases in Cx 26 mRNA (Figure 4C).
SAMe supplementation decreases phospho-Ser9-GSK3β, Cx43 and β-catenin protein levels
Several studies have demonstrated that β-catenin interacts with Cx43 promoter regulating its mRNA expression (Xia et al., 2010; Du W et al., 2008; Zhai Y et al., 2002; Ai Z. et al., 2000; Van der Heyden et al., 1998). Analysis by western blot of nuclear protein extracts from hepatocytes supplemented with SAMe 1h after the second supplementation, showed decreased nuclear β-catenin levels (Figure 5A). The canonical mechanism of β-catenin regulation involves its phosphorylation by glycogen synthase kinase 3β (GSK3β) at the Thr-41, Ser-37, and Ser-33 sites (Rubinfeld et al., 1996). This phosphorylation targets β-catenin to ubiquitination and degradation by the proteasome system (Aberle et al. 1997; Liu et al. 2002). Phosphorylation of GSK3β is correlated with a decrease in GSK3β activity. SAMe supplementation blocked GSK-3β phosphorylation and decreased β-catenin protein levels at 24, 36 and 48h (Figure 5B).
Figure 5. GSK-3β phosphorylation and β-catenin protein expression in hepatocytes cultured with SAMe. Effect of proteasome inhibitor (MG132) and GSK-3β inhibitor (Kenpaullone).

(A) Western blot analysis was performed with nuclear proteins extracted from hepatocytes 1 hour after the second SAMe supplementation at 12h, and from control cells. Blots were incubated with anti β-catenin and HDAC1 antibodies. (B) Westernblot analysis was performed with total proteins extracted at 24, 36 and 48h from hepatocytes cultured in control or SAMe-supplemented MEM. Blots were incubated with anti-β-catenin, GSK3β and phospho-ser9-GSK3β and actin (C) Westernblot analysis was preformed with total proteins extracted at 48h from hepatocytes control and with SAMe supplementation, and pre-treated during 2h with or without kenpaullone (5μM), every 24h, before each SAMe supplementation. Blots were incubated with anti β-catenin, phospho-β-catenin, Cx43 and actin antibodies. (D) Western blot analysis was performed with total proteins extracted at 36h from cultured hepatocytes with SAMe or without supplementation, and pre-treated with or without MG132 (5 μM) every 24h, before each SAMe supplementation. Blots were incubated with anti-β-catenin, Cx43 and actin antibodies. Results are representative of at least three independent experiments. (E) Real-time PCR analysis of Cx43 mRNA was preformed with total mRNA extracted at 48h from control or SAMe-supplemented cultured hepatocytes, and pre-treated with or without MG132 5μM every 24h, before each SAMe supplementation. Each bar represents the mean ± SD of at least quadruplicate experiments (*p<0.05; vs. control). Values were normalized with Ribosomal 18S expression.
SAMe treatment did not increase GSK3β-mediated β-catenin phosphorylation at 48h (Figure 5C), and moreover the GSK-3β inhibitors, Kenpaullone (5 μM) or PHZ1123 (25 μM), did not prevent the SAMe-induced reduction in β-catenin and Cx43 protein (data not shown). The proteasomal inhibitor, MG132 (5 μM), prevented the SAMe-mediated Cx43 and β-catenin protein down-regulation at 36h (Figure 5D), and reduction in Cx43 mRNA expression at 48h (Figure 5E).
SAMe supplementation does not lead to functional GJIC between hepatocytes
We next determined whether the effects of SAMe on connexin expression could lead to functional GJIC between hepatocytes. To examine this, we performed the scrape-loading/dye transfer method (el-Fouly et al., 1987). SAMe supplementation did not improve the spread of the dye Lucifer Yellow CH, indicating that SAMe supplementation does not improve GAPJ intracellular communication between hepatocytes (Figure 6). Also, SAMe supplementation did not increase Cx32 or Cx26 membrane localization as analysed by immunocytochemistry (data not shown). Altogether, these results suggest that, although SAMe plays a role in regulating the expression of connexin mRNA and protein, it is not involved in membrane localization and formation of functional GJIC, and that other factors are necessary to form functional GAPJ.
Figure 6. Effect of SAMe supplementation on GJIC in hepatocytes.

Hepatocytes cultured during 24h with and without SAMe supplementation were subjected to scrape-loading/dye transfer analysis as described in material and methods. Results are representative of at least three independent experiments.
Connexin 32, 26 and 43 mRNA and protein expression in MAT1A-KO livers
To test if SAMe could be regulating Cx expression in vivo, we examined MAT1A knockout (MAT1A-KO) mice. These mice have markedly lower hepatic SAMe levels, hepatic hyperplasia and develop spontaneous steatohepatitis at 8 months and hepatocellular carcinoma (HCC) at 15 months (Lu S.C. et al., 2001). Livers from 8 months old MAT1A-KO mice showed decreased expression of Cx26, Cx32 and ZO-1 proteins and increased expression of Cx43 and β-catenin proteins, when compared with WT mice (Figure 7A). Analysis by real time PCR also showed that MAT1A-KO livers have less Cx32 mRNA expression and more Cx43 mRNA expression than WT liver, with no change in Cx26 mRNA expression between MAT1A-KO livers and WT (Table III). These results suggest that SAMe could be regulating Cx expression in hepatocytes in vivo.
Figure 7. Connexin 26, 32 and 43, β-catenin and ZO-1 protein expression in liver tissue extracted from MAT1A-KO and WT.
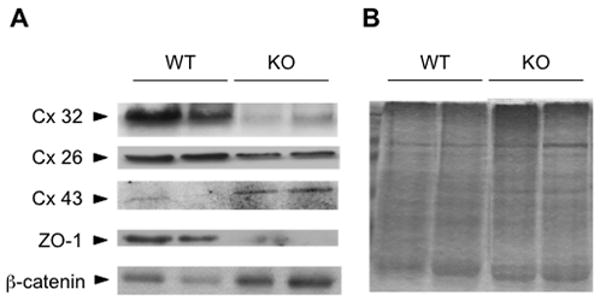
(A) Western blot analysis was preformed with total proteins extracted from MAT1A-KO (KO) and WT mice. Blots were incubated with anti-Cx32, Cx26, Cx43, ZO-1 and β-catenin antibodies. Results are representative of at least 10 independent samples. (B) Ponceau S staining of the nitrocellulose membranes was performed to ensure equal loading.
Table III. Connexin 26, 32 and 43 mRNA expression in liver tissue extracted from MAT1A-KO and WT.
Real-time PCR analysis of Cx26, Cx32 and Cx43 mRNA expression in liver samples from MAT1A-KO and WT mice. Values represent the mean ± SD of at least quadruplicate experiments (*p<0.05; vs. control). Values were normalized with respect to 18S ribosomal RNA expression.
| Connexin 26 | Connexin 32 | Connexin 43 | |
|---|---|---|---|
| Wild Type | 1 ± 0.02 | 1 ± 0.01 | 1 ± 0.46 |
| MAT1A-KO | 0.99 ± 0.02 | 0.46 ± 0.06 | 4.72 ± 0.67* |
5. Dicussion
In this study, we show that the regulation of connexin 26, 32 and 43 expression in primary hepatocytes in culture is determined by the availability of S-Adenosylmethionine (SAMe). SAMe supplementation blocked the culture-induced expression of Cx43 mRNA and protein, and prevented the decline of Cx26 and 32 mRNA and proteins. Removal of SAMe supplementation reversed the effects seen on Cx26 and 32 mRNA expression, suggesting that maintenance of SAMe intracellular levels are critical for the differentiated expression pattern of connexin mRNAs. These observations, together with the finding that livers from MAT1A-KO mice, characterized by chronic SAMe deficiency (Lu S.C. et al, 2001) express higher Cx43 and lower Cx26 and 32 protein levels than WT mice, suggest that SAMe could play a role in regulating connexin expression in liver.
Primary hepatocytes lose their hepatic polarity upon isolation and rapidly decline in liver-specific functions over culture time (Allen and Bhatia, 2002). SAMe has been identified as a key molecule that helps to maintain the differentiated phenotype of the hepatocytes in culture (Gil B. et al, 1996; Garcia-Trevijano et al., 2000; Martinez-Lopez et al, 2008). Here we observed that SAMe supplementation every 12h, helped maintain hepatocytes square-shaped morphology for at least 48h in culture and prevented formation of F-actin fibres. Moreover, SAMe restored ZO-1 expression, indicating hepatocyte polarization. Also, SAMe-supplemented hepatocytes showed increased albumin, HNF-1α and PEPCK mRNA expression, reduced αFP mRNA expression and improved glucagon-induced PEPCK and G6Pase mRNA expression. Basal and glucagon-induced glucose production from glucogenolysis were also increased in hepatocytes supplemented with SAMe, whereas ammonia clearance, a characteristic feature of the detoxification process in adult hepatocytes, was unaffected by SAMe supplementation.
In addition, even though we found that SAMe supplementation maintained the adult pattern of Cx expression in hepatocytes, it did not induce localization of Cx32 and Cx26 to the membrane nor improved GJIC. Altogether, our data suggest that SAMe supplementation contributes to Cx26 and 32 mRNA and protein expression, but other factors are responsible for membrane localization of Cx 32 and 26, connexon formation and GAP junction functionality. Thus, even though SAMe improves some of the characteristics of the differentiated phenotype, such as the Cx expression, these effects cannot be attributed to SAMe-mediated increase in GAPJ intracellular communication.
HNF-1α has been suggested to determine the cell-specific expression of Cx32 (Piechocki et al. 2000; Koffler et al. 2002). SAMe supplementation increased HNF-1α mRNA expression suggesting that SAMe-induced expression in HNF-1α could contribute to Cx32 increased expression.
β-catenin nuclear localization has been associated with TCF/LEF-dependent gene transcription of Cx43 (Van der Heyden et al., 1998, Zhai Y et al., 2002). Recently, it was shown that β-catenin associates with the Cx43 promoter, and that silencing of β-catenin inhibits Cx43 mRNA expression (Xia et al 2010). In hepatocytes, SAMe induced a reduction in total and nuclear β-catenin protein. The SAMe-induced decrease in Cx43 mRNA and protein, and β-catenin protein was significantly attenuated by the proteasome inhibitor MG132. This suggests that SAMe reduces the total and nuclear pool of β-catenin through proteasomal degradation, thus blocking Cx43 gene transcription. As shown by others (Bachar-Dahan L., et al, 2006), we found that SAMe-induced β-catenin destabilization is mediated via GSK3β-independent proteasomal degradation. We found that SAMe decreased GSK3β phosphorylation but did not affect β-catenin phosphorylation at the GSK3β phosphorylation sites Thr-41, Ser-37, and Ser-33. Moreover the GSK3β inhibitors, Kenphalloudin and PDH1123, did not prevent SAMe-induced decrease in Cx43 and β-catenin protein.
Several studies suggest an important role for cAMP in the maintenance of Cx26 and 32 expression in hepatocytes (Saez et al., 1989; Kojima et al., 1995b). SAMe and its metabolite MTA are both structurally related to adenosine and are able to interact with adenosine purinergic receptors, increasing intracellular cAMP levels (Maher, 1993; Munshi et al., 1988, Riscoe and Ferro, 1984; Song et al., 2005). However, several of the SAMe effects have been attributed to its conversion into MTA by the methionine salvage pathway (Simile et al., 2001; Ansorena et al., 2002). The present results show that supplementation of hepatocytes with SAMe and its metabolite MTA increased intracellular cAMP levels, and that MTA supplementation increased Cx26 mRNA and protein expression, without having any effect on Cx43 or 32. Moreover, the results indicate that activation of Adenosine A2A receptors was involved in Cx26 mRNA regulation by SAMe and MTA. However, this regulation was not related to PKA activity, since neither SAMe- nor MTA-induced Cx26 mRNA expression was prevented by the PKA inhibitor H-89, nor mimicked by its activator Forskolin (data not shown).
There was no difference in Cx26 mRNA expression between MAT1A-KO and WT mice. However in the knockouts, hepatic SAMe levels are decreased by 74%, whereas MTA levels are unchanged (Lu S.C. et al., 2001). In addition, MTA was able to increase cAMP levels faster than SAMe, and the MAT I/III inhibitor cycloleucine showed that MTA-induced Cx26 mRNA expression was independent of its conversion into SAMe. Taken together these results suggest that the observed SAMe action on Cx26 mRNA expression in cultured hepatocytes could be mediated by its conversion into MTA and subsequent activation of Adenosine receptors A2A. These results provide the first evidence indicating a potential role of Adenosine A2A receptors in Cx26 mRNA regulation. However further studies are needed to establish the molecular mechanism by which Adenosine A2A receptors, and more specifically cAMP, regulates Cx26 mRNA expression in primary hepatocytes.
In summary, our study suggests that the pattern of Cx expression in differentiated hepatocytes both in vitro and in vivo could be controlled by intracellular levels of SAMe. However, although SAMe plays a role in regulation of connexin expression, it is not involved in membrane localisation and formation of functional GJIC.
Acknowledgments
This work was made possible by a Beacon Project grant from the UK Department of Trade and Industry ref: QCBB/C/012/00005, with additional support from: NIH grant AT1576 (MLM, SCL) and CIBERehd funded by the Instituto de Salud Carlos III (MLM, MVR). We are grateful for this support. We thank our colleagues on the Beacon Project for many useful discussions.
List of abbreviations
- α-FP
Alpha-fetoprotein
- Cx
Connexins
- CL
Cycloleucine
- cAMP
Dibutyl cyclic AMP
- GJIC
Gap Junctions Intercellular communication
- G6Pase
Glucose-6-phosphatase
- GSK3β
Glycogen synthase kinase 3βeta
- HNF-1α
Hepatocyte nuclear factor 1-alpha
- MAT
Methionine Adenosyltransferase
- MTA
5′-Methyltioadenosine
- PEPCK
phosphoenolpyruvate carboxykinase
- PKA
Protein Kinase A
- SAMe
S-Adenosylmethionine
- TCF/LEF
T cell factor/lymphocyte enhancer binding factor
- ZO-1
Zonula occludens-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105(2):161–71. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JW, Bhatia SN. Engineering liver therapies for the future. Tissue Eng. 2002;8:725. doi: 10.1089/10763270260424097. [DOI] [PubMed] [Google Scholar]
- 4.Ansorena E, Garcia-Trevijano ER, Martinez-Chantar ML, Huang ZZ, Chen L, Mato JM, Iraburu M, Lu SC, Avila MA. S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatol. 2002;35:274–80. doi: 10.1053/jhep.2002.30419. [DOI] [PubMed] [Google Scholar]
- 5.Bachar-Dahan L, Goltzmann J, Yaniv A, Gazit A. Engrailed-1 negatively regulates beta-catenin transcriptional activity by desestabilizing beta-catenin via a glycogen synthase kinase-3beta-independent pathway. Mol Biol Cell. 2006;17(6):2572–80. doi: 10.1091/mbc.E06-01-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219–28. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 7.Belluardo N, White TW, Srinivas M, Trovato-Salinaro A, Ripps H, Mudò G, Bruzzone R, Condorelli DF. Identification and functional expression of HCx31.9, a novel gap junction gene. Cell Commun Adhes. 2001;8(4-6):173–8. doi: 10.3109/15419060109080719. [DOI] [PubMed] [Google Scholar]
- 8.Berthoud VM, Iwanij V, Garcia AM, Saez JC. Connexins and glucagon receptors during development of rat hepatic acinus. Am Physiol Soc. 1992:G650–8. doi: 10.1152/ajpgi.1992.263.5.G650. [DOI] [PubMed] [Google Scholar]
- 9.Bode HP, Wang L, Cassio D, Leite MF, St-Pierre MV, Hirata K, Okazaki K, Sears ML, Meda P, Nathanson MH, Dufour JF. Expression and regulation of gap junctions in rat cholangiocytes. Hepatol. 2002;36(3):631–40. doi: 10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- 10.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signalling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 11.Cicirata F, Nicotra A, Cicero D, Parenti R, Zappalà A. Cloning and expression pattern of connexin39, a new member of the gap junction gene family isolated from the neural tube of chicken embryos. Gene. 2004;17;328:121–6. doi: 10.1016/j.gene.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Decrock E, Vinken M, De Vuyst E, Krysko DV, D'Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;(4):524–36. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 13.Du WJ, Li JK, Wang QY, Hou JB, Yu B. Lithium chloride regulates connexin43 in skeletal myoblasts in vitro: possible involvement in Wnt/beta-catenin signalling. Cell Commun Adhes. 2008;15(3):261–71. doi: 10.1080/15419060802198587. [DOI] [PubMed] [Google Scholar]
- 14.el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168(2):422–30. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 15.Eloranta TO, Kajander EO. Catabolism and lability of S-adenosylL-methionine in rat liver extracts. Biochem J. 1984;224:137–144. doi: 10.1042/bj2240137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez L, Perez V, Munoz M, Corpa JM, Abad M, Carbajo MT. Effects of S-adenosylmethionine on hepatic regeneration after partial hepatectomy in the rat. J Physiol Biochem. 2003;59:63–4. doi: 10.1007/BF03179869. [DOI] [PubMed] [Google Scholar]
- 17.Field JM, Tate LA, Chipman JK, Minchin SD. Identification of functional regulatory regions of the connexin32 gene promoter. Biochim Biophys Acta. 2003;1628(1):22–9. doi: 10.1016/s0167-4781(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 18.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–236. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 19.Fischer R, Reinehr R, Lu TP, Schönicke A, Warskulat U, Dienes HP, Häussinger D. Intercellular communication via gap junctions in activated rat hepatic stellate cells. Gastroenterol. 2005;128(2):433–48. doi: 10.1053/j.gastro.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Trevijano ER, Latasa MU, Carretero MV, Berasain C, Mato JM, Avila MA. S-Adenosylmethionine regulates MAT1Aand MAT2A gene expression in cultured rat hepatocytes: a new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000;14:2511–2518. doi: 10.1096/fj.00-0121com. [DOI] [PubMed] [Google Scholar]
- 21.Gil B, Casado M, Pajares MA, Bosca L, Mato JM, Martin-Sanz P, Alvarez L. Differential Expression Patter of S-Adenosylmethionine Synthetase Isoenzimes During Rat Liver Development. Hepatolol. 1996;24:876–881. doi: 10.1002/hep.510240420. [DOI] [PubMed] [Google Scholar]
- 22.Hevia H, Varela-Rey M, Corrales FJ, Berasain C, Martinez-Chantar ML, Latasa MU, Lu SC, Mato JM, Garcia-Trevijano ER, Avila MA. 5′-methylthioadenosine modulates the inflammatory response to endotoxin in mice and in rat hepatocytes. Hepatology. 2004;39:1088–98. doi: 10.1002/hep.20154. [DOI] [PubMed] [Google Scholar]
- 23.Iwai M, Harada Y, Muramatsu A, Tanaka S, Mori T, Okanoue T, Katoh F, 0hkusa T, Kashima K. Development of gap junctional channels and intercellular communication in rat liver during ontogenesis. J of Hepatol. 2000;32:11–18. doi: 10.1016/s0168-8278(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 24.Koffler LD, Fernstrom MJ, Akiyama TE, Gonzalez FJ, Ruch RJ. Positive regulation of connexin 32 transcription by hepatocyte nuclear factor-1alpha. Arch Of Biochem And Biophys. 2002;407:160–167. doi: 10.1016/s0003-9861(02)00488-5. [DOI] [PubMed] [Google Scholar]
- 25.Kojima T, Mitaka T, Paul DL, Mori M, Mochizuki Y. Reappearance and long-term maintenance of connexin32 in proliferated adult rat hepatocytes: use of serum-free L-15 medium supplemented with EGF and DMSO. J Cell Sci. 1995;108:1347–5. doi: 10.1242/jcs.108.4.1347. [DOI] [PubMed] [Google Scholar]
- 26.Kojima T, Mitaka T, Shibata Y, Mochizuki Y. Induction and regulation of connexin26 by glucagon in primary cultures of adult rat hepatocytes. J Cell Sci. 1995b;108:2771–80. doi: 10.1242/jcs.108.8.2771. [DOI] [PubMed] [Google Scholar]
- 27.Kojima T, Yamamoto M, Tobioka H, Mizuguchi T, Mitaka T, Mochizuki Y. Changes in cellular distribution of connexins 32 and 26 during formation of gap junctions in primary cultures of rat hepatocytes. Exp Cell Res. 1996;223:314–326. doi: 10.1006/excr.1996.0087. [DOI] [PubMed] [Google Scholar]
- 28.Kotb M, Mudd HS, Mato JM, Geller MA, Kredich NM, Chou JY, Cantoni GL. Consensus nomenclature for the mammalian methionine adenosyltransferase genes and gene products. Trends Genet. 1997;13:51–52. doi: 10.1016/s0168-9525(97)01013-5. [DOI] [PubMed] [Google Scholar]
- 29.Kumar NM, Gilula NB. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986;103:767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leffert HL, Koch KS, Moran T, Williams M. Liver cells. Methods Enzymol. 1979;58:536–544. doi: 10.1016/s0076-6879(79)58168-3. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Li Y, Semenov M, Han C, Baeg G, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 32.Loven AD, Olsen AK, Friis C, Andersen B. Phase I and II metabolism and carbohydrate metabolism in cultured cryopreserved porcine hepatocytes. Chem Biol Interact. 2005;155(1-2):21–30. doi: 10.1016/j.cbi.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 33.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Nalt Acad Sci USA. 2001;98(10):5560–5. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maher PA. Inhibition of the tyrosine kinase activity of the fibroblast growth factor receptor by the methyltransferase inhibitor 5-methylthioadenosine. J Biol Chem. 1993;268:4244–49. [PubMed] [Google Scholar]
- 35.Makowski L, Caspar DLD, Phillips WC, Goodenough DA. Gap junction structures. II Analysis of the x-ray diffraction data. J Cell Biol. 1977;74:629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin PE, Blundell G, Ahmad S, Errington RJ, Evans WH. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J Cell Sci. 2001;114:3845–55. doi: 10.1242/jcs.114.21.3845. 2001 Nov. [DOI] [PubMed] [Google Scholar]
- 37.Mato JM, Alvarez L, Ortiz P, Pajares MA. S-adenosylmethionine synthesis: molecular mechanisms and clinical implications. Pharmacol Ther. 1997;73:265–280. doi: 10.1016/s0163-7258(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 38.Mato JM, Camara J, Fernandez de Paz J, Caballeria L, Coll S, Caballero A, Garcia-Buey L, Beltran J, Benita V, Caballeria J, Sola R, Moreno-Otero R, Barrao E, Martin-Duce A, Correa JA, Pares A, Barrao E, Garcia-Magaz I, Puerta JL, Moreno J, Boissard G, Ortiz P, Rodes J. S-adenosylmethioinine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter trial. J Hepatol. 1999;30(6):1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 39.Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- 40.Martínez-López N, Varela-Rey M, Ariz U, Embade N, Vazquez-Chantada M, Fernandez-Ramos D, Gomez-Santos L, Lu SC, Mato JM, Martinez-Chantar ML. S-adenosylmethionine and proliferation: new pathways, new targets. Biochem Soc Trans. 2008;36(Pt 5):848–52. doi: 10.1042/BST0360848. [DOI] [PubMed] [Google Scholar]
- 41.Munshi R, Clanachan AS, Baer HP. 5-deoxy-5-methylthioadenosine: a nucleoside which differentiates between adenosine receptor types. Biochem Pharmacol. 1988;37:2085–2089. doi: 10.1016/0006-2952(88)90560-6. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson BJ, Dermietzel R, Teplow D, Traub O, Willecke K, Revel JP. Two homologous protein components of hepatic gap junctions. Nature. 1987;329:732–34. doi: 10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen PA, Kumar NM. Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett. 2003;540(1-3):151–6. doi: 10.1016/s0014-5793(03)00252-7. [DOI] [PubMed] [Google Scholar]
- 44.Piechocki MP, Toti RM, Fernstrom MJ, Burk RD, Ruch RJ. Liver cell-specific transcriptional regulation of connexin 32. Biochim Biophys Acta. 2000;1491:107–22. doi: 10.1016/s0167-4781(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 45.Riscoe MK, Ferro AJ. 5-Methylthioribose: Its effects and function in mammalian cells. J Biol Chem. 1984;259:5465–71. [PubMed] [Google Scholar]
- 46.Rosenberg E, Faris RA, Spray DC, Monfils B, abreu S, Danishefsky I, Reid LM. Correlation of expression of connexin mRNA isoforms with degree of cellular differentiation. Cell Adhes Commun. 1996;4:223–35. doi: 10.3109/15419069609010768. [DOI] [PubMed] [Google Scholar]
- 47.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272(5264):1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 48.Saez JC, Gregory WA, Watanabe T, Dermietzel R, Hertzberg EL, Reid L, Bennett MV, Spray DC. cAMP delays disappearance of gap junctions between pairs of rat hepatocytes in primary culture. Am J Physiol. 1989;257:C1–11. doi: 10.1152/ajpcell.1989.257.1.1-a. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu W, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiojiri N, Niwa T, Sugiyama Y, Koike T. Preferential expression of connexin37 and connexin40 in the endothelium of the portal veins during mouse liver development. Cell Tissue Res. 2006;324(3):547–52. doi: 10.1007/s00441-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 51.Simile MM, Banni S, Angioni E, Carta G, De Miglio MR, Muroni MR, Calvisi DF, Carru A, Pascale RM, Feo F. 5-methyltioadenosine administration prevents lipid peroxidation and fibrogenesis induced in rat liver by carbon-tetrachloride intoxication. J Hepatol. 2001;34:386–394. doi: 10.1016/s0168-8278(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 52.Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–80. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- 53.Song Z, Urinate S, Shoo R, Chen T, Brave S, Hill D, McClain C. S-adenosylmethionine (SAMe) modulates interleukin-10 and interleukin-6, but not TNF, production via the adenosine (A2) receptor. Biochimica et Biophysica Acta. 2005;1743:205–13. doi: 10.1016/j.bbamcr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Stutenkemper R, Geisse S, Schwarz HJ, Look J, Traub O, Nicholson BJ, Willecke K. The hepatocyte-specific phenotype of murine liver cells correlates with high expression of connexin32 and connexin26 but very low expression of connexin43. Exp Cell Res. 1992;201:43–54. doi: 10.1016/0014-4827(92)90346-a. [DOI] [PubMed] [Google Scholar]
- 55.Trosko JE, Ruch RJ. Cell-Cell communication in carcinogenesis. Front Biosci. 1998;3:d208–36. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- 56.Van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. 1998;111:1741–9. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- 57.Vinken M, Henkens T, Vanhaecke T, Papeleu P, Geerts A, Van Rossen E, Chipman JK, Meda P, Rogiers V. Trichostatin A enhances gap junctional intercellular communication in primary cultures of adult rat hepatocytes. Toxicol Sci. 2006;91:484–92. doi: 10.1093/toxsci/kfj152. [DOI] [PubMed] [Google Scholar]
- 58.Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and photobiology of Gap Junctional Channels in hepatocytes. Hepatol. 2008;47:1077–88. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- 59.Warner A. The gap junction. J Cell Sci. 1988;89:1–7. doi: 10.1242/jcs.89.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol Cell Biol. 2010;30(1):206–19. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanotti S, Mori S, Radaelli R, Perez J, Racagni G, Popoli M. Modifications in brain cAMP- and calcium/calmodulin-dependent protein kinases induced by treatment with S-adenosylmethionine. Neuropharmacology. 1998;37:1081–9. doi: 10.1016/s0028-3908(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 62.Zhai Y, Wu R, Schwartz DR, Darrah D, Reed H, Kolligs FT, Nieman MT, Fearon ER, Cho KR. Role of beta-catenin/T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas. Am J Pathol. 2002;160(4):1229–38. doi: 10.1016/s0002-9440(10)62550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang JT, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989;109(6 Pt 2):3391–401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, Thorgeirsson SS. Modulation of connexins during differentiation of oval cells into hepatocytes. Exp Cell Res. 1994;213:37–42. doi: 10.1006/excr.1994.1170. [DOI] [PubMed] [Google Scholar]


