Abstract
Neural testosterone (T) metabolism, particularly the synthesis of oestradiol (E2) via the aromatase enzyme, is important for sexual behaviours in many vertebrates. In green anole lizards, E2 metabolized from T facilitates female receptivity and increases sexual motivation in males. T treatment increases aromatase activity in whole brain homogenates of gonadectomized male, but not female, anoles, which is an effect limited to the breeding season (BS). To investigate the potential for local effects of this enzyme in reproductive behaviour, we used in situ hybridization for aromatase mRNA to examine expression during the BS and non-breeding season (NBS) in areas of the brain that control male sexual behaviours (preoptic area and amygdala; POA and AMY), as well as one regulating female reproductive behaviours (ventromedial hypothalamus, VMH). Males had a greater total number of aromatase-expressing cells in the POA than females, and the density of aromatase-expressing cells (number per unit volume) was greater in the VMH and AMY of females. This density was also higher during the BS than NBS in the POA. Expression of aromatase in the AMY appeared lateralised, as trends were detected for the left side to have more total cells and more cells per unit volume than the right. These results suggest that, similar to other vertebrates, regional aromatization of T may be important for control of sex-specific reproductive behaviours.
Keywords: Testosterone metabolism, preoptic area, amygdala, ventromedial hypothalamus
Introduction
Steroid hormones are important for the expression of sexual behaviours in a variety of animals. Specifically, the neural metabolism of testosterone (T) to oestradiol (E2) by aromatase regulates expression of these behaviours in vertebrates including Japanese quail, midshipman fish, musk shrews, songbirds, and mice (1-5). In males, inhibition of aromatase blocks normal copulatory behaviours in Japanese quail, and aromatase knock-out mice do not display complete sexual behaviour unless given oestrogens (5, 6). Aromatase is also important for the expression of female behaviours. Inhibitors of this enzyme decrease female canary sex behaviours and aromatizable androgens facilitate copulatory behaviour in female musk shrews (3, 7).
In most species, aromatase is concentrated in limbic regions of the brain, including those important for the expression of male specific sexual behaviours such as the preoptic area (POA) and amygdala (AMY), as well as the ventromedial hypothalamus (VMH), which is important for female receptivity (6, 8). Aromatase is commonly upregulated by androgens and is found in cells that also express androgen receptor (9-11). Oestrogen receptors are commonly located in brain regions that express aromatase, but cellular co-localization varies substantially, suggesting that in at least some areas (such as the POA) autocrine regulation of the enzyme by E2 is unlikely (12, 13). Males generally express higher levels of neural aromatase than females (14-17). A sex difference in responsiveness to T also exists; the steroid can have a greater effect on aromatase activity and mRNA expression in males than in females (15, 18, 19). Although expression of aromatase activity has been documented in the reptilian brain (20), less is known about its distribution, especially in regions that control sex behaviours.
Green anole lizards are seasonally breeding animals, with the breeding season (BS) lasting typically from April to August. Males have higher levels of circulating T than females, and both sexes have increased plasma T during the BS compared to the non-breeding season (NBS). For example, males during the BS have on average about 20ng/ml T, whereas the mean value in females is less than 1ng/ml. In both sexes, BS levels of T are at least twice those detected in the NBS (21). The same limbic brain regions are critical to the display of reproductive behaviours in reptiles as mammalian and avian systems. Lesions to the POA and AMY in green anoles impair male sexual behaviours (22, 23). Although the experiment has not been conducted in this species, lesions to the VMH inhibit receptivity in other female reptiles, including whiptail lizards (e.g. 24). Also as in other vertebrates, T is critical for the display of male sexual behaviours, and E2 activates receptivity in anoles (25, 26). Unlike rodents and birds, aromatase is not critical for the expression of male sexual behaviours in green anoles (27). Experiments in gonadectomized anoles, however, have documented facilitation of sexual motivation in males by E2 and a role for aromatization of T in female receptivity (27, 28).
Aromatase activity is relatively high in tissue containing the POA and hypothalamus compared to other parts of the anole brain (29). Males have greater whole brain aromatase activity than females, and breeding males have higher aromatase activity than do males during the NBS (30). Exogenous T increases whole brain aromatase activity only in BS males and has no effect in females, which suggests sexually and seasonally dimorphic regulation of this enzyme in the entire brain (31). However, specific distributions and relative patterns of expression of aromatase in areas likely to control reproductive behaviours of males and females are unknown.
This study was designed to begin to test the hypothesis that natural variations in T levels between the sexes and seasons mediate local synthesis of aromatase in the brain. To our knowledge, this is the first study to quantify aromatase expression in three regions controlling sex behaviour of reptiles, examining potential sex and seasonal differences in these regions. We investigated numbers of cells containing aromatase mRNA in areas of the brain that control sexual behaviours (POA, AMY and VMH) under conditions when T normally differs.
Methods
Animals and tissue processing
Adult male and female green anole lizards were purchased from Charles Sullivan (Nashville, TN) during the BS and NBS (April and November, respectively). They were wild-caught and shipped within a few days to our animal facilities, where they were housed individually in 10-gallon aquaria that contained peat moss for substrate, rocks, sticks for climbing, and water dishes. During the BS, animals were kept on a 14:10 light/dark cycle and ambient temperatures ranged from 28 °C during the day to 19 °C at night. During the NBS, animals were kept at a 10:14 light/dark cycle and ambient temperatures ranged from 24 °C during the day to 15 °C at night. Under these conditions, previous studies have determined that plasma T levels are high during the BS and low during the NBS (See above; 21). Full spectrum and heat lamps were provided above each cage to allow lizards to bask in temperatures 10 °C above ambient. Relative humidity was maintained at approximately 70% throughout both seasons. Cages were misted daily with water and the animals were fed crickets or mealworms three (BS) or two times a week (NBS).
After being in the lab for 15 to 24 days, animals were rapidly decapitated, and the brains were frozen in methylbutane and kept at −80 °C until processing. At this time, breeding state was confirmed by visual inspection of the reproductive system of each lizard. During the BS, females had thick, convoluted oviducts and at least one yolking follicle, both of which are indicative of high E2 and reproductive activity. All males had large, well-vascularized testes and milky, thick vasa deferentia, consistent with high T and sperm production. During the NBS, females had small oviducts with no or one tiny (< 1 mm in diameter) yolking follicle, and all males had small, non-vascularised testes and thin, clear vasa deferentia.
Brains were sectioned coronally at 20 μm into four alternate series of sections and thaw-mounted onto SuperFrost Plus (Fisher Scientific; Hampton, NH) slides. Slides were stored at −80 °C with dessicant until further processing. All procedures adhered to NIH guidelines and were approved by the Michigan State University IACUC.
Cloning of aromatase
Whole brain RNA was extracted from two breeding males. Tissue was homogenised in Trizol (Invitrogen Corporation; Carlsbad, CA), and RNA was separated using chloroform. Then, the RNA was isolated using RNeasy minicolumns (Qiagen Sciences; Valencia, CA) and concentrated using ethanol precipitation. It was reconstituted in DEPC-treated water and stored at −80 °C. RNA was converted into cDNA with the SuperScript III Reverse Transcriptase kit (Invitrogen) per manufacturer's instructions, and stored at −20 °C until use.
Searches of available databases using a variety of relevant key words did not provide a sequence for aromatase in the green anole genome, which is publicly available but not fully annotated. Therefore, we identified an appropriate sequence by blasting the aromatase cDNA sequence from a whiptail lizard, Cnemidophorus uniparens (NCBI: EU310875) from Dias et. al. (32), to the anole genome using the Ensembl Genome Broswer. Primers were designed using the Oligo Analysis Tool program (Eurofins MWG Operon; Huntsvile AL; Table 1) and purchased from Invitrogen. PCR reactions included 1 unit of Platinum TAQ High Fidelity DNA polymerase (Invitrogen), 0.2 mM dNTP mixture, 0.2 μM primer mix, 2 mM MgSO4, and template cDNA. The PCR reaction went through 40 cycles of 94 °C for 15 seconds, 50 °C for 30 seconds, and 68 °C for 1 minute. Aromatase was amplified twice using the same primers, first by using cDNA from brain, and then using the PCR product from the first reaction as the template for the second reaction.
Table 1.
Primers used to clone aromatase from the green anole brain. Melting temperatures (TM) are indicated.
| Primer Name | Sequence (5′ to 3′) | TM (°C) |
|---|---|---|
| Forward | GACATGCCGAAGCTGAA | 56.72 |
| Reverse | TTGGGAAGAACTCAAGCCGA | 63.12 |
The amplicon was cloned following Zhou and Gomez-Sanchez (33). Briefly, glycerol stocks of E. coli cells containing pBluescript II Exo/Mung DNA (Stratagene; La Jolla, CA) were cultured, and plasmids were isolated using Wizard Plus Miniprep kits (Promega Corporation, Madison, WI) per manufacturer's instructions. Vectors were digested overnight at 4 °C with a blunt-end restriction enzyme (EcoRV, New England BioLabs; Ipswich, MA) and purified using the QIAquick PCR Purification Kit (Qiagen Sciences). T-overhangs were added to the blunt-ended plasmid vector using a terminal transferase reaction (Roche Diagnostics; Indianapolis, IN), incubated at 37 °C for 1.5 hours. The T-tailed vector was repurified, and the A-tailed aromatase PCR product was ligated to the vector using T4 DNA ligase as per manufacturer's instructions (Promega).
One Shot TOP10 Chemically Competent E. coli cells (Invitrogen) were transformed with the ligated vector. The cells were grown on LB agar plates containing 100 μg/ml of ampicillin and spread with 5-bromo-4-chloro-3-indolyl-β-D-galactosidase (X-Gal). White colonies were selected and grown overnight in LB broth containing 100 μg/ml of ampicillin. Vector DNA was isolated using Wizard Plus Miniprep kits (Promega), and the sequence of the insert was confirmed in both directions on a Perkin Elmer/Applied Biosystems 3100 capillary sequencer. The 181bp green anole insert (GenBank HM585359) shared 86.7% identity with the whiptail sequence. The insert was also 88% identical to the aromatase sequences of both leopard geckos and Italian wall lizards, and ranged from 78-82% identical to aromatase in a variety of avian and mammalian species. Vector DNA was then isolated using Wizard Plus Maxiprep kits (Promega), linearized using restriction enzymes (Xho1 and BamH1; New England BioLabs) and stored at −20 °C.
In situ hybridization
Sense (T7) and antisense (T3) probes were transcribed using the Digoxigenin RNA Labeling Kit per manufacturer's instructions (Roche), which labels RNA with digoxigenin-UTPs. Probes were cleaned using a G50 sephadex bead column prior to use.
All slides were processed at the same time. One set of slides from each animal was used for the antisense reaction. As a control, another set of slides from one animal from each group was used for the sense reaction. No labeling was detected in tissue exposed to the sense probes. Slides were allowed to thaw and then fixed for 10 mins in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS; pH 7.4). They were treated with 0.25% acetic anhydrase in triethanolamine-HCl with 0.9% NaCl buffer (pH 8.0). Next, slides were incubated overnight at 55 °C in hybridization buffer (50% formamide, 4× SSC, 1× Denhardt's solution, 200μg/ml fish sperm DNA, 10% dextran sulfate, 20 mM dithiothreitol, 250 μg/ml tRNA, 2 mM EDTA, and 0.1% Tween-20) with 200 ng/ml probe. The following day, slides were rinsed in 2× SSC and 0.2× SSC and then treated with 0.9% H2O2 in maleic acid buffer (pH 7.5) with 0.1% Tween-20 (MABT) for 30 mins. They were incubated in a blocking solution of 5% normal sheep serum (Jackson Immuno Research; West Grove, PA) in MABT for 30 mins, and treated with 0.5 μl/ml Anti-Digoxigenin-AP Fab fragments (Roche Diagnostics; Mannheim, Germany) in MABT. After two hours, slides were treated with 4.5μl/ml NBT and 3.5 μl/ml BCIP (Roche) in 0.1M Tris-Hcl and 0.1M NaCl (pH 9.5) for colour detection. The colour reaction was stopped after 10 mins with 1M Tris and 0.5M EDTA (pH 8.0).
Aromatase analysis
The slides were examined under brightfield illumination using Stereo Investigator software (MicroBrightfield, Inc.; Williston, VT) following Beck et al. (34) by a user blind to experimental group. Estimates of aromatase expressing cells were determined using the Optical Fractionator function within the POA, VMH, and AMY. Both the left and right sides of each region were analyzed. After tracing the outline of one side of a brain region in each tissue section in which it existed, the software placed a user-defined grid over each area (POA: 100×100 μm2, AMY: 40×40 μm2, VMH: 80×80 μm2) and sampling sites (30×30 μm2) were placed randomly within the defined region. The software calculated a volume for the brain region, and computed the number of aromatase-positive cells based on it and the samples in which manual counts were taken. To further characterise the patterns of expression, densities of aromatase-positive cells were determined by dividing the number of cells by the calculated volumes of each brain region. Final sample sizes are indicated in the figure captions.
Statistical Analysis
Analysis was conducted separately for each brain region. The left and right sides of the POA and VMH did not differ in volume, estimated number of aromatase positive cells, or density of these cells (all F < 2.13, p > 0.155). Thus, the average of both sides for these two regions was used for statistical analyses reported for these brain regions. Within the POA and VMH, two-way ANOVAs were used to compare main effects of and interactions between sex and season. Interactions were further broken down by one-way ANOVAs and pairwise comparisons as appropriate.
Unlike the POA and VMH, paired t-tests indicated that the left and right sides of the AMY differed in the number of aromatase expressing cells (F = 5.45, p = 0.03). Thus, we accounted for side in all subsequent analysis by using 3-way mixed model ANOVAs.
Results
We detected aromatase mRNA in a variety of diencephalic and telencephalic areas in the green anole lizard brain, a pattern very similar to the distribution found in whiptail lizards (32) and red-sided garter snakes (35).
POA
Similar to previous work using Nissl-stained tissue from other individuals (34), the POA defined by aromatase positive cells was larger in males than females (F = 6.848 p = 0.014, data not shown). There was a trend for males to have more aromatase-expressing cells than females (F = 4.04, p = 0.054; Fig. 1). Effects of season and interactions between sex and season were not detected on the number of aromatase-expressing cells (all F < 0.95, p > 0.339). The density of aromatase cells was higher during the BS than the NBS (F = 5.77, p = 0.023; Fig. 1). There was no effect of sex or an interaction for the density of aromatase cells (all F < 1.47, p > 0.236).
Figure 1.
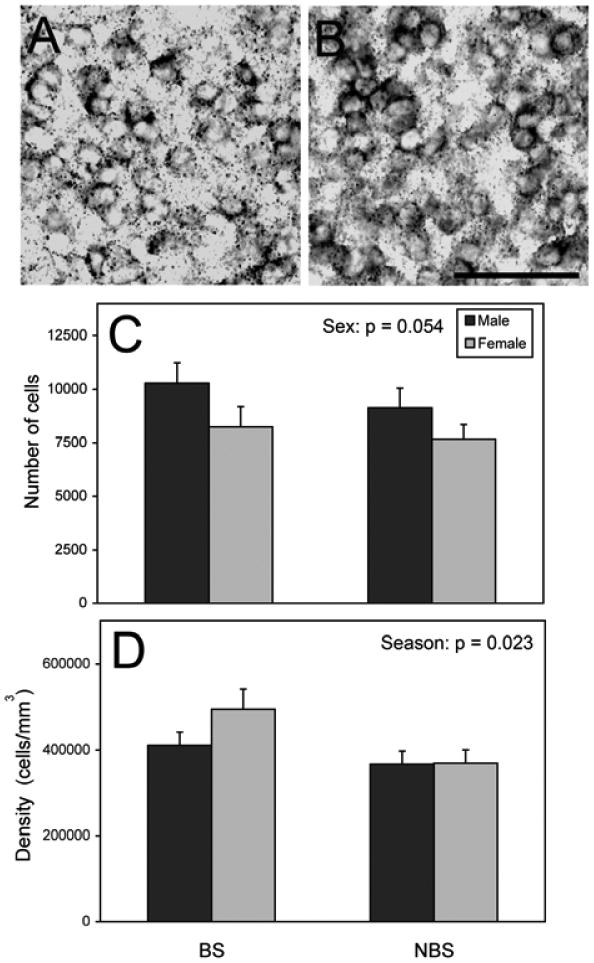
Aromatase expression in the POA (mean + S.E.). The photograph in panel (A) depicts a BS male, and (B) shows a NBS male. Panel (C) indicates the number of aromatase expressing cells; (D) shows the increased density of aromatase expressing cells overall in the BS compared to NBS. Scale bar = 50 μm. = 9 NBS females, 8 for the rest of the groups.
AMY
Parallel to the analysis of Nissl-stained tissue (34), volume of this region did not differ among the groups (all F < 2.73, p > 0.116, data not shown). There also were no effects of sex or season on the number of aromatase-positive cells (all F < 0.14, p > 0.713, Fig. 2). However, the left side of the AMY had a trend for more aromatase expressing cells than the right side (F = 4.18, p = 0.056). Females had a higher density of aromatase expressing cells than males (F = 17.04, p = 0.001, Fig. 2). A trend for greater density of aromatase cells on the left was also detected (F = 4.19, p = 0.056), as well as a trend for an interaction between side of the brain and season (F = 3.79, p = 0.067). There were no other effects detected on the density of aromatase expressing cells (all F < 2.03, p > 0.171).
Figure 2.
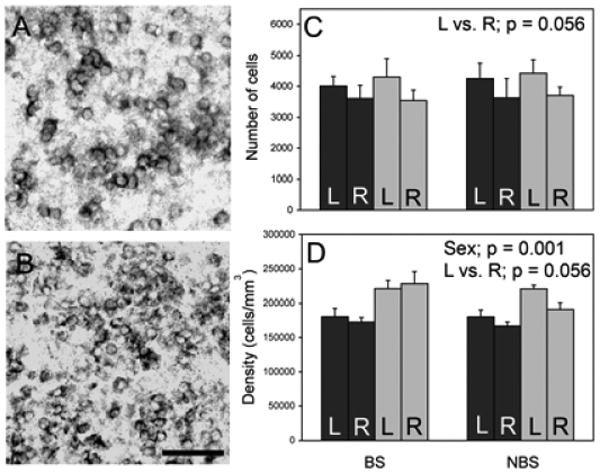
Aromatase mRNA in the AMY. Panel (A) is from the left AMY of a male. Panel (B) shows the left AMY of a female. Breeding animals are represented in all panels. Mean + SE of the number of aromatase expressing cells is indicated in (C). The density of aromatase expressing cells is in (D). Scale bar = 50 μm. n = 5 BS and NBS males, 6 BS females, and 8 NBS females. Black bars = males; grey bars = females.
VMH
Although Nissl stains show cell bodies throughout (34), we only detected aromatase in the lateral portion of the VMH. Lesions to this portion of the VMH abolished female sexual behaviours in a different species of lizard and seems likely to be the portion of this region important in the control of female reproductive behaviors (24). Thus, we confined our analysis of the VMH to this region.
The lateral VMH was larger in volume in males than females (F = 4.76, p =0.041, data not shown). There were no effects detected on number of aromatase cells (all F < 0.07, p > 0.800; Fig. 3). However, the density of aromatase expressing cells was greater in females than males (F = 9.24, p = 0.006; Fig. 3). Neither a main effect of season nor an interaction between sex and season were detected on the density of these cells (all F < 0.35, p > 0.563).
Figure 3.
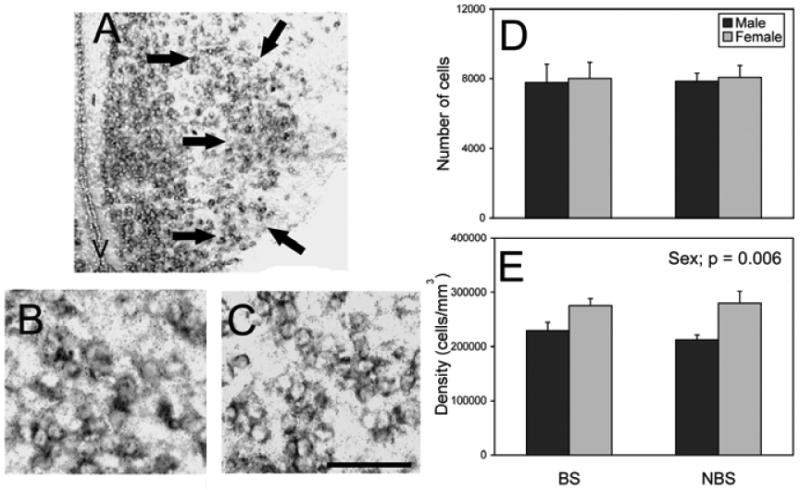
Aromatase mRNA expression in the VMH. Panel (A) is a photomicrograph of the VMH from a BS female with arrows indicating the boundary of the region quantified. The photograph in panel (B) depicts a BS male, and panel (C) depicts a BS female. No differences were detected in the total number (mean + S.E.) of aromatase expressing cells (D). Females have a greater density of aromatase cells than males (E). Scale bar = 150 μm (A) and 50 μm (B and C). V = 3rd ventricle. n = 7 BS males, 5 NBS males, 4 BS females, and 8 NBS females.
Discussion
This is the first study in anoles and one of the few in reptiles to quantify aromatase expression in three brain regions that are important in the control of sexual behaviors. We document a number of effects of sex and season on aromatase expression in these three areas, with specific patterns differing among them. In the POA, the estimated number of aromatase expressing cells was greater in males than females, and their density was increased in the BS compared to NBS. In the AMY, we found that females had a higher density of aromatase expressing cells than males and detected a trend for more cells with aromatase mRNA on the left side of the brain. Females had a higher density of aromatase expressing cells in the VMH. These results allow us to formulate hypotheses about potential roles and regulation of aromatase within these specific brain regions.
Aromatase in the POA
The increase in aromatase-expressing cells in the POA of males compared to females is consistent with a variety of other vertebrates. Males have more aromatase expressing cells, as well as more activity, in the POA in musk shrews, zebra fish, guinea pigs, Japanese quail, cowbirds, and rats (9, 14, 15, 36-38). Additionally, parthenogenic whiptail lizards show increased aromatase mRNA in the POA after ovulation, when male-like sexual behaviours occur in these genetic females (32). Collectively, the data suggest that higher aromatase expression in the POA in males may be important for sex-specific behaviours. In green anoles, this function is likely focused on motivation to display reproductive behaviours, as E2 appears mainly to increase mount attempts (28).
Consistent with a role of the POA in male sexual behavior, the volume of this region is sexually dimorphic, determined both from borders defined with Nissl-staining and labeling of aromatase mRNA (see above). While there were no seasonal differences in the volume of the region or number of cells, we did detect an effect of season on the density of aromatase cells. This increased density of aromatase-expressing cells in the BS compared to NBS is probably due to subtle differences in aromatase expression and volume that were undetected in our previous analysis. This result does support the idea that aromatase in the POA is important for sexual behaviours. Seasonal differences in aromatase expression have been studied (e.g., 39, 40), although relatively few studies have examined both sex and seasonal differences. Male little brown bats have more overall neural aromatase activity than females during the BS, and this activity does not differ between males and females prior to emergence from hibernation (41). This is consistent with activity data from green anoles (see above), as well as the results from the present study.
Aromatase in the AMY
The lack of effects in aromatase expressing cells due to sex and season is consistent with previous studies that have found no differences in aromatase activity between males and females in similar brain areas in Japanese quail, rats, and guinea pigs (18, 36), although male turtles (Chrysemys picta) have higher aromatase activity in this general region than do females (20). Activity does not necessarily parallel mRNA expression such that male rats appear to express more aromatase mRNA (represented by silver grains) per cell even though activity does not differ (8, 42). In the present study, we cannot detect relative quantities of aromatase mRNA in each cell due to the constraints of the labeling procedure, which facilitated counting of individually labeled cells. However, that type of analysis could be undertaken in the future.
We also found a trend for lateralisation, such that a greater number of more densely packed cells appeared to be present on the left than the right. In other species, morphology and neurochemistry of the AMY is lateralised (43, 44). However, straightforward hypotheses have not been suggested regarding the roles of these lateralisations, and one hesitates to speculate based on values that only approach statistical significance.
We found that the density of aromatase cells was higher in females than in males and a trend for lateralisation existed on this measure. Density was calculated from the number of cells and the volume of the region, neither of which differed between the sexes. However, in both the BS and NBS, more cells were present in a smaller volume in females compared to males, on average. The biological relevance of the sexual dimorphism in density is unclear, but could reflect an increased need for sensory integration in females (perhaps of courtship cues from males; reviewed in 45, 46). While it is difficult to speculate on whether the trend for lateralization is associated with a particular function, it is consistent with what we observed for the number of aromatase-expressing cells.
Aromatase in the VMH
The similarity in aromatase expression in the VMH of males and females suggests that it may be important for both sexes. This result differs somewhat from other species, although relatively few studies have examined aromatase distribution in both males and females. For example, more VMH cells express aromatase mRNA and activity is higher in male than in female rats (8, 14, 42). Male turtles also have more aromatase activity in the entire hypothalamus than do females (20). However, no sex difference was detected in activity in the VMH equivalent in Japanese quail (15). Much of the data on aromatase in the VMH represents regional activity rather than quantification of cell number. The former technique does not differentiate between portions of the VMH (lateral vs. medial). Our analysis of only the lateral portion of this region may relate to some of the differences with other species. It also is likely to be the reason we found a novel sex difference in volume compared to earlier work on intact adult green anoles, which quantified the entire VMH (34). Similar to the present study, however, a larger sample size in gonadectomized animals (some E2-treated) did reveal a male-biased sex difference in the volume of the VMH in Nissl-stained tissue (47).
Although the number of aromatase expressing cells does not differ, female anoles may have proportionally more aromatase in the lateral VMH than do males, as the density of aromatase expressing cells is higher in females than in males. This result is consistent with the idea that E2 production via aromatase in the VMH is important for female receptivity. Similar to the current data, female anoles also express more estrogen receptor α in the VMH than do males (48). Thus, locally produced E2 could have more of an effect in the VMH in females.
Conclusion
Aromatase is expressed in the brain of vertebrates, specifically in portions that control sexual behaviours. The present data indicate that this pattern holds for green anole lizards. There were more cells that expressed aromatase in the POA of males than in females, and the density of VMH cells expressing aromatase was greater in females. These data follow the idea that aromatase in the POA is important for male specific behaviours, and aromatase in the VMH is important for female specific behaviours. Collectively, the present data considered in the context of those available from other taxa on aromatase suggest evolutionary conservation. However, at least in terms of male sexual behaviour, oestrogens appear to have a larger role in birds and mammals (5, 49). As these groups both evolved from reptilian ancestors (50), investigations in reptiles such as green anoles can elucidate the evolution of mechanisms regulating reproduction. Increasing our understanding of changes in the role of aromatase will be particularly informative.
Acknowledgments
We thank Camilla Peabody, Yu Ping Tang, and Di Wu for technical assistance, and a variety of members of the Wade lab for help with animal care and cage set-up. This work was supported by NSF grant IOS-0742833 to J. Wade.
References
- 1.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27(3):247–74. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44(4-6):521–40. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 3.Rissman EF. Evidence that neural aromatization of androgen regulates the expression of sexual-behavior in female musk shrews. J Neuroendocrino. 1991;3(4):441–8. doi: 10.1111/j.1365-2826.1991.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 4.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38(4):250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 5.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46(1):1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Balthazart J. Testosterone-metabolism in the avian hypothalamus. J Steroid Biochem Mol Biol. 1991;40(4-6):557–70. doi: 10.1016/0960-0760(91)90277-c. [DOI] [PubMed] [Google Scholar]
- 7.Leboucher G, Beguin N, Mauget R, Kreutzer M. Effects of fadrozole on sexual displays and reproductive activity in the female canary. Physiol Behav. 1998;65(2):233–40. doi: 10.1016/s0031-9384(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 8.Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370(1):71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Veney SL, Rissman EF. Immunolocalization of androgen receptors and aromatase enzyme in the adult musk shrew brain. Neuroendocrinology. 2000;72(1):29–36. doi: 10.1159/000054568. [DOI] [PubMed] [Google Scholar]
- 10.Gelinas D, Callard GV. Immunolocalization of aromatase- and androgen receptor-positive neurons in the goldfish brain. Gen Comp Endocrinol. 1997;106(2):155–68. doi: 10.1006/gcen.1997.6891. [DOI] [PubMed] [Google Scholar]
- 11.Balthazart J, Foidart A, Houbart M, Prins GS, Ball GF. Distribution of androgen receptor-immunoreactive cells in the quail forebrain and their relationship with aromatase immunoreactivity. J Neurobiol. 1998;35(3):323–40. [PubMed] [Google Scholar]
- 12.Balthazart J, Foidart A, Absil P, Harada N. Effects of testosterone and its metabolites on aromatase-immunoreactive cells in the quail brain: relationship with the activation of male reproductive behavior. J Steroid Biochem Mol Biol. 1996;56(1-6):185–200. doi: 10.1016/0960-0760(95)00236-7. [DOI] [PubMed] [Google Scholar]
- 13.Veney SL, Rissman EF. Co-localization of estrogen receptor and aromatase enzyme immunoreactivities in adult musk shrew brain. Horm Behav. 1998;33(3):151–62. doi: 10.1006/hbeh.1998.1446. [DOI] [PubMed] [Google Scholar]
- 14.Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase-activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117(6):2471–7. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 15.Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990;2(5):675–83. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 16.Negri-Cesi P, Poletti A, Celotti F. Metabolism of steroids in the brain: a new insight into the role of 5alpha-reductase and aromatase in brain differentiation and functions. J Steroid Biochem Mol Biol. 1996;58(5-6):455–66. doi: 10.1016/0960-0760(96)00083-0. [DOI] [PubMed] [Google Scholar]
- 17.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67(1):1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- 18.Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol. 1997;61(3-6):365–74. [PubMed] [Google Scholar]
- 19.Roselli CE, Klosterman SA, Fasasi TA. Sex differences in androgen responsiveness in the rat brain: Regional differences in the induction of aromatase activity. Neuroendocrinology. 1996;64(2):139–45. doi: 10.1159/000127111. [DOI] [PubMed] [Google Scholar]
- 20.Callard GV, Petro Z, Ryan K. Identification of Aromatase in the Reptilian Brain. Endocrinology. 1977;100:1214–8. doi: 10.1210/endo-100-4-1214. [DOI] [PubMed] [Google Scholar]
- 21.Lovern MB, McNabb FM, Jenssen TA. Developmental effects of testosterone on behavior in male and female green anoles (Anolis carolinensis) Horm Behav. 2001;39(2):131–43. doi: 10.1006/hbeh.2000.1637. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler JM, Crews D. The role of the anterior hypothalamus-preoptic area in the regulation of male reproductive behavior in the lizard, Anolis carolinensis: lesion studies. Horm Behav. 1978;11(1):42–60. doi: 10.1016/0018-506x(78)90057-0. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg N, Scott M, Crews D. Role of the amygdala in the reproductive and aggressive behavior of the lizard, Anolis carolinensis. Physiol Behav. 1984;32(1):147–51. doi: 10.1016/0031-9384(84)90088-x. [DOI] [PubMed] [Google Scholar]
- 24.Kendrick AM, Rand MS, Crews D. Electrolytic lesions to the ventromedial hypothalamus abolish receptivity in female whiptail lizards, Cnemidophorus uniparens. Brain Res. 1995;680(1-2):226–8. doi: 10.1016/0006-8993(95)00191-r. [DOI] [PubMed] [Google Scholar]
- 25.Mason P, Adkins EK. Hormones and social behavior in the lizard, Anolis carolinensis. Horm Behav. 1976;7(1):75–86. doi: 10.1016/0018-506x(76)90006-4. [DOI] [PubMed] [Google Scholar]
- 26.Tokarz RR, Crews D. Induction of sexual receptivity in the female lizard, Anolis carolinensis: effects of estrogen and the antiestrogen CL-628. Horm Behav. 1980;14(1):33–45. doi: 10.1016/0018-506x(80)90013-6. [DOI] [PubMed] [Google Scholar]
- 27.Winkler SM, Wade J. Aromatase activity and regulation of sexual behaviors in the green anole lizard. Physiol Behav. 1998;64(5):723–31. doi: 10.1016/s0031-9384(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 28.Latham S, Wade J. Estradiol facilitates mounting behavior in male green anole lizards. Physiol Behav. 2010;99(1):78–81. doi: 10.1016/j.physbeh.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Wade J. Androgen metabolism in the brain of the green anole lizard (Anolis carolinensis) Gen Comp Endocrinol. 1997;106(1):127–37. doi: 10.1006/gcen.1996.6860. [DOI] [PubMed] [Google Scholar]
- 30.Rosen GJ, Wade J. Androgen metabolism in the brain of the green anole lizard (Anolis carolinensis): effects of sex and season. Gen Comp Endocrinol. 2001;122(1):40–7. doi: 10.1006/gcen.2001.7616. [DOI] [PubMed] [Google Scholar]
- 31.Cohen RE, Wade J. Testosterone selectively affects aromatase and 5alpha-reductase activities in the green anole lizard brain. Gen Comp Endocrinol. 2010;166(1):128–33. doi: 10.1016/j.ygcen.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias BG, Chin SG, Crews D. Steroidogenic enzyme gene expression in the brain of the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Brain Res. 2009;1253:129–38. doi: 10.1016/j.brainres.2008.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou MY, Gomez-Sanchez CE. Universal TA cloning. Curr Issues Mol Biol. 2000;2(1):1–7. [PubMed] [Google Scholar]
- 34.Beck LA, O'Bryant EL, Wade JS. Sex and seasonal differences in morphology of limbic forebrain nuclei in the green anole lizard. Brain Res. 2008;1227:68–75. doi: 10.1016/j.brainres.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Krohmer RW, Bieganski GJ, Baleckaitis DD, Harada N, Balthazart J. Distribution of aromatase immunoreactivity in the forebrain of red-sided garter snakes at the beginning of the winter dormancy. J Chem Neuroanat. 2002;23(1):59–71. doi: 10.1016/s0891-0618(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 36.Connolly PB, Roselli CE, Resko JA. Aromatase activity in adult guinea pig brain is androgen dependent. Biol Reprod. 1990;43(4):698–703. doi: 10.1095/biolreprod43.4.698. [DOI] [PubMed] [Google Scholar]
- 37.Saldanha CJ, Schlinger BA. Estrogen synthesis and secretion in the brown-headed cowbird (Molothrus ater) Gen Comp Endocrinol. 1997;105(3):390–401. doi: 10.1006/gcen.1996.6841. [DOI] [PubMed] [Google Scholar]
- 38.Goto-Kazeto R, Kight KE, Zohar Y, Place AR, Trant JM. Localization and expression of aromatase mRNA in adult zebrafish. Gen Comp Endocrinol. 2004;139(1):72–84. doi: 10.1016/j.ygcen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol. 2003;56(3):209–21. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- 40.Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm Behav. 2004;45(4):225–34. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Callard GV, Kunz TH, Petro Z. Identification of androgen metabolic pathways in the brain of little brown bats (Myotis lucifugus): sex and seasonal differences. Biol Reprod. 1983;28(5):1155–61. doi: 10.1095/biolreprod28.5.1155. [DOI] [PubMed] [Google Scholar]
- 42.Wagner CK, Morrell JI. Neuroanatomical distribution of aromatase MRNA in the rat brain: indications of regional regulation. J Steroid Biochem Mol Biol. 1997;61(3-6):307–14. [PubMed] [Google Scholar]
- 43.Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;501(6):904–15. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- 44.Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008;506(5):851–9. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- 45.Moreno N, Gonzalez A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. J Anat. 2007;211(2):151–63. doi: 10.1111/j.1469-7580.2007.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55(3):257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 47.Beck LA, Wade J. Effects of estradiol, sex, and season on estrogen receptor alpha mRNA expression and forebrain morphology in adult green anole lizards. Neuroscience. 2009;160(3):577–86. doi: 10.1016/j.neuroscience.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 48.Beck LA, Wade J. Sexually dimorphic estrogen receptor alpha mRNA expression in the preoptic area and ventromedial hypothalamus of green anole lizards. Horm Behav. 2009;311(3):162–71. doi: 10.1016/j.yhbeh.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Schlinger BA. Sex steroids and their actions on the birdsong system. J Neurobiol. 1997;33(5):619–31. [PubMed] [Google Scholar]
- 50.Richman JM, Buchtova M, Boughner JC. Comparative ontogeny and phylogeny of the upper jaw skeleton in amniotes. Dev Dyn. 2006;235(5):1230–43. doi: 10.1002/dvdy.20716. [DOI] [PubMed] [Google Scholar]


