Abstract
In this study, we report a novel treatment strategy that could potentially be used to improve efficacy of adoptive cell therapy for patients with prostate cancer. We show that female C57BL/6 mice are able to effectively reject two syngeneic prostate tumors (TRAMP-C2 and RM1) in a T cell-dependent manner. The protective antitumor immunity appears to primarily involve T cell responses reactive against general prostate tumor/tissue antigens, rather than simply to male-specific H-Y antigen. For the first time we show that adoptive transfer of lymphocytes from TRAMP-C2-primed or naïve female mice effectively control prostate tumor growth in male mice, when combined with host pre-conditioning (i.e., non-myeloablative lymphodepletion) and IL-2 administration. No pathological autoimmune response was observed in the treated tumor-bearing male mice. Our studies provide new insights regarding the immune-mediated recognition of male-specific tissue, such as the prostate, and may offer new immunotherapy treatment strategies for advanced prostate cancer.
Keywords: Prostate cancer, Female lymphocyte, Adoptive cell therapy, Immunotherapy
Introduction
Early diagnosis and treatment cure most clinically localized prostate cancers (CaP). However, treatment options are limited in patients who suffer recurrences due to micrometastatic disease at the time of diagnosis or minimal residual disease from non-curative local therapy. Application of specific immunotherapies has shown significant promise due to low toxicity and exquisite specificity [1]. Indeed, adoptive cell therapy (ACT) using autologous tumor-infiltrating lymphocyte (TILs) has emerged as an effective treatment for some patients with certain cancers (e.g., metastatic melanoma) [2, 3]. However, only a small fraction of patients benefit and new strategies are needed to improve the success rate of ACT.
High affinity of the T cell receptor (TCR) for antigen is a key feature for efficient elimination of tumors by adoptively transferred T cells or active immunization. Unfortunately, most tumor-associated antigens are unaltered self-proteins. In the case of CaP, antigen targets identified so far are prostate-associated self-antigens or tissue-differentiation antigens that are overexpressed in advanced prostate cancer, such as prostate-specific antigen (PSA), prostate stem cell antigen (PSCA), prostate-specific membrane antigen (PSMA), prostatic acid phosphatase (PAP) and six-transmembrane epithelial antigen of the prostate (STEAP) [4–8]. Given the self nature of these antigenic targets, it is conceivable that central and peripheral tolerance as well as anergy, the host mechanisms essential for prevention of autoimmune diseases, represent significant obstacles to the induction of effective antitumor immune responses [9]. Central tolerance leads to deletion of T cells that express high-avidity TCR specific for self-antigens. In addition, low numbers of autoreactive T cells in the periphery, which are more likely to bear a low-affinity TCR for self-antigens [10, 11], may be responsible for the limited clinical efficacy of cancer immunotherapy trials [12]. This inherent limited T cell recognition of self-antigens also restricts the wide application of tumor-specific T cells for adoptive therapy of solid tumors, including CaP.
Given that the prostate exists only in the male reproductive system, the immune system of a female host is unlikely to develop central tolerance to PSA. In principle then, T cell clones with high-avidity TCR specific for CaP/tissue antigens are more likely to be present or generated in a female T cell repertoire. Given the recent successes in T cell-mediated adoptive immunotherapy of solid tumors and advantageous features of cellular therapy in certain disease settings such as bulky established malignancy [13], a high frequency of prostate tissue antigen-specific T cells in the female T cell repertoire or generated through vaccination may be harnessed for development of a novel adoptive T cell therapy for the treatment of metastatic CaP. Indeed, female C3H/HeJ mice have previously been seen to reject syngeneic PMC-1 prostate tumors, implicating the potential involvement of immune mechanisms [14].
In the current study using C57BL/6 mice, we show that female mice develop a robust T cell-dependent immune response against syngeneic prostate tumor. Immune-mediated recognition of prostate tumor or tissue-associated antigens (e.g., STEAP), not minor HY antigen, appears to be primarily involved in the observed antitumor activities. Furthermore, adoptive transfer of primed or naïve female lymphocytes results in effective control of established prostate tumors in male mice. Our studies suggest that donor immune cells from a female host, which potentially recognize prostate cancer/tissue antigens, may be manipulated and optimized for eradiation of CaP.
Materials and methods
Mice and cells
C57BL/6 mice (wild-type) were purchased from National Cancer Institute (Bethesda, MD, USA). Age-matched mice were used in all the experiments. All animal procedures used in these studies complied with guideline by Institutional Animal Care and Use Committee of Virginia Commonwealth University. Prostate cell lines, RM1 [15], kindly provided by Dr. TC Thompson (MD Anderson Cancer Center), TRAMP-C2 [16], and Lewis lung carcinoma cell D121 were maintained in Dulbecco modified Eagle medium, supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. BM-DCs were generated by culture of mouse bone-marrow cells in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) as previously described [17].
Reagents
CellTrace 5-(and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE) cell proliferation kit was purchased from Molecular Probes (Eugene, OR, USA). Recombinant human interleukin (IL)-2 was obtained from NCI Preclinical Repository (Frederick, MD, USA). H-Y antigen peptides (H-2Db-restricted Smcy738–746, Uty246–254 and I-Ab-restricted Dby608–622) and mouse prostate antigen peptide (mSTEAP326–335) were obtained from AnaSpec Inc. (San Jose, CA, USA). UTY antibody (ab95113) was purchased from Abcam (Cambridge, MA, USA). FITC-conjugated anti-mouse CD3, PE/Cy5-CD8 and PE-IFN-γ mAbs were purchased from BioLegend Inc. (San Diego, CA, USA). ELISA kit for mouse IL-2 and IFN-γ were from eBioscience (San Diego, CA, USA). 7-Amino-actinomycin D staining solution (7-AAD) and BD Cytofix/Cytoperm™ Plus Fixation/permeabilization kit from BD Pharmingen (San Jose, CA, USA).
Tumor challenge
Male or female mice were challenged with 2 × 106 TRAMP-C2 (slow growing) or 1 × 105 RM1 (fast growing) prostate cancer cells. Tumor growth was monitored by measuring tumor sizes. For some experiments, mice were immunized s.c with 1 × 105 irradiated (IR) RM1 cells, followed by tumor challenge with 5 × 105 RM1 cells 2 weeks later.
T cell proliferation assays
Male or female mice were challenged subcutaneously (s.c) with TRAMP-C2 cells. Some female mice were injected with prostate cells or male lymphocytes. For T cell proliferation assay, splenocytes were stimulated with different H-Y peptides or co-cultured with dendritic cells (DCs) that had been pulse by cell lysates from TRAMP-C2 tumor, D121 tumor or prostate tissue. Cell proliferation was measured by 3H-TdR incorporation as described [17].
Intracellular cytokine staining
Splenocytes were stimulated with mSTEAP326–335 peptide (1 μg/ml) in the presence of GolgiPlug (brefeldin A; BD PharMingen). After surface staining with FITC-CD8 mAb, cells were permeabilized and stained for intracellular IFN-γ with PE-IFN-γ according to the manufacturer’s instruction (BD PharMingen).
In vitro cytotoxicity assay
Four weeks after TRAMP-C2 inoculation, splenocytes were prepared and treated with bryostatin-1 (5 nM), ionomycin (1 μM, CalbioChem, Gibbstown, NJ, USA) and rhIL-2 (80 IU/ml) for 16 h as previously described [18]. Cells were washed and cultured in the presence of IL-2 (40 IU/ml) for 5 days. In some experiments, splenocytes were stimulated with irradiated TRAMP-C2 cells (100:1) or Smcy738–746 and Uty246–254 peptide in vitro for 5 days. Live cells were isolated with Lymphocyte™ as effector cells. TRAMP-C2 cells were treated with IFN-γ (20 ng/ml) for 24 h and labeled with 0.5 μM CFSE as target cells. Effector cells and target cells were co-cultured at a ratio of 10:1, at 37°C for 24 or 48 h. Cells were stained with 7-AAD (50 μg/ml) on ice for 20 min, and analyzed by FACS for the percentage of 7-AAD+ cells gating in CFSE+ cells.
Adoptive cell therapy (ACT)
Male C57BL/6 mice (n = 5 for all groups) were injected subcutaneously with 2 × 106 TRAMP-C2 prostate tumor cells. After 5 days, mice were treated with intravenous adoptive transfer of 7 × 107 lymphocytes pooled from spleen and lymph nodes of naïve or TRAMP-C2-challenged female mice. In some cases, IL-2 was given at 2 × 105 international units (IU) intraperitoneally daily for 5 doses. Lymphopenia was induced by non-myeloablative (5 Gy) whole body irradiation (WBI) on the day before the cell transfer as described [19]. Prostates from naïve or treated tumor-bearing mice were collected, fixed with 10% buffered formalin and subjected to H&E staining.
Reverse transcription-polymerase chain reaction
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). For reverse transcription, 1 μg of total RNA and 50 ng of oligo-dT primer were used for 20 μl reaction volumes with RevertAid™ MMLV Reverse Transcriptase (Ferments Inc., Glen Burnie, MA, USA). The sequences of the polymerase chain reaction (PCR) primer pairs used to amplify the respective cDNAs were as follows: mUTY (Forward: 5′-AAATGCAGCTCGGACCAAATC-3′, reverse: CTGAATGATGTGAAGCTGTC); mRPII (RNA polymerase II, Forward: 5′-ccctgcaggaatggatctta-3′, reverse: 5′-ccacggcatgtcatagtgtc-3′). PCR reactions were carried out in BioMix Red PCR buffer (Bioline, Taunton, MA, USA) and PCR products were analyzed on 1% agarose containing ethidium bromide.
Statistical analysis
All experiments were performed independently at least twice with similar results. Differences between groups within experiments were tested for significance with analysis of variance (ANOVA) test or Student t test using GraphPad Prism software (GraphPad, San Diego, CA, USA). p values <0.05 were considered statistically significant.
Results
Immune-mediated rejection of prostate tumors in female mice
To examine the tumorigenesis of mouse prostate tumor cells in male and female mice, TRAMP-C2 tumor line, which was derived from the spontaneous TRAMP prostate tumor model, was used in tumor challenge study. Upon inoculation of tumor cells, male mice developed slowly growing tumors as expected. In contrast, TRAMP-C2 tumors grew initially in female mice but then regressed completely (Fig. 1a). This was expected given the previous data of Labarthe et al. [14], although a different prostate tumor line was used.
Fig. 1.
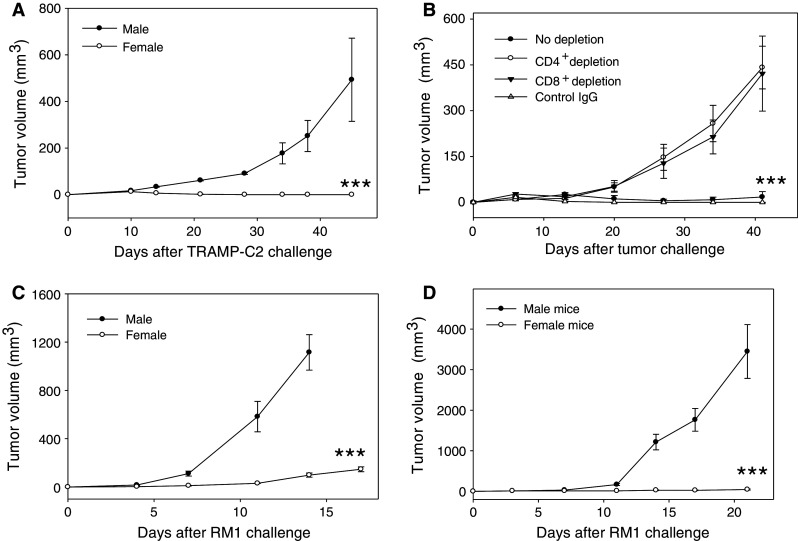
Female mice develop potent antitumor immunity to prostate cancer. a Rejection of TRAMP-C2 tumor in female mice. Male or female mice (n = 5) were challenged with 2 × 106 TRAMP-C2 tumor cells and tumor growth was then followed (***p < 0.001, male vs. female). b Involvement of CD4+ and CD8+ cells in the TRAMP-C2 tumor rejection. Female mice were inoculated with TRAMP-C2 cells. 7 days later, T cell subsets were depleted using GK1.5 (anti-CD4 antibody) and 2.43 (anti-CD8 antibody) (***p < 0.001, no depletion vs. CD8 or CD4 depletion). c Suppression of RM1 prostate tumor growth in female mice. RM1 cells (1 × 105) were injected s.c to male or female mice (***p < 0.001, male vs. female) and tumor growth was followed by measuring the tumor sizes. d Rejection of RM1 tumors by female mice following immunization. Male or female mice (n = 5) were immunized s.c with irradiated RM1 cells (IR-RM1). 2 weeks later, mice were challenged with 5 × 105 live RM1 cells (***p < 0.001 male vs. female). The results are representative of three independent experiments
To dissect the potential involvement of immune cells in the rejection of TRAMP-C2 tumors by female mice, we depleted CD4+ or CD8+ T cells in female mice as previously described by our group [20]. It was seen that tumor rejecting capability of female mice was abolished following depletion of either CD4+ or CD8+ T cells, whereas untreated or control IgG-treated female mice were able to reject the tumors (Fig. 1b), suggesting that both CD4+ and CD8+ T cells participate in tumor rejection. Depletion of NK cells also resulted in partial reduction in the tumor inhibitory effects (data not shown).
Similar observations were made when RM1, a fast growing prostate tumor line, was used in tumor challenge study. RM1 tumors grew significantly slower in female mice than in male counterparts (Fig. 1c). Since naïve female mice did not completely reject RM1 tumors, we further explored the immunogenicity of RM1 tumor cells in male and female mice using a standard vaccination and challenge protocol. To this end, mice were immunized with irradiated RM1 cells, followed by tumor inoculation with viable RM1 cells. It was seen that male mice developed aggressively growing tumors, whereas female mice readily rejected RM1 tumors, even though a large number of RM1 tumor cells (5 × 106) were inoculated into the immunized mice (Fig. 1d). These studies showed that RM1 tumors are weakly immunogenic in male mice, but were readily recognized and rejected by female mice following the cell-based vaccination. The differences in the tumorigenesis of TRAMP-C2 and RM1 cells in female mice may be attributed to the different growth rate. Nonetheless, immune activation was clearly involved in the rejection of prostate tumors in female mice.
Augmentation of a prostate tumor-specific immune response in female mice
Given the immune-mediated rejection of TRAMP-C2 tumors observed in female mice, we next assessed the tumor reactivity of splenocytes derived from TRAMP-C2 tumor-challenged mice. When co-cultured with irradiated TRAMP-C2 cells, naïve female mice-derived lymphocytes produced substantially higher levels of IL-2 (Fig. 2a) and IFN-γ (Fig. 2b) than did male mice-derived lymphocytes, suggesting the presence of immune precursor cells recognizing TRAMP-C2 target cells. Prior tumor challenge or sensitization in vivo strongly enhanced the cytokine production in female mice-derived lymphocytes upon stimulation with TRAMP-C2 cells. In addition, splenocytes of TRAMP-C2-challenged female mice displayed robust cytolytic activity when co-cultured with CFSE-labeled TRAMP-C2 target cells (Fig. 2c).
Fig. 2.
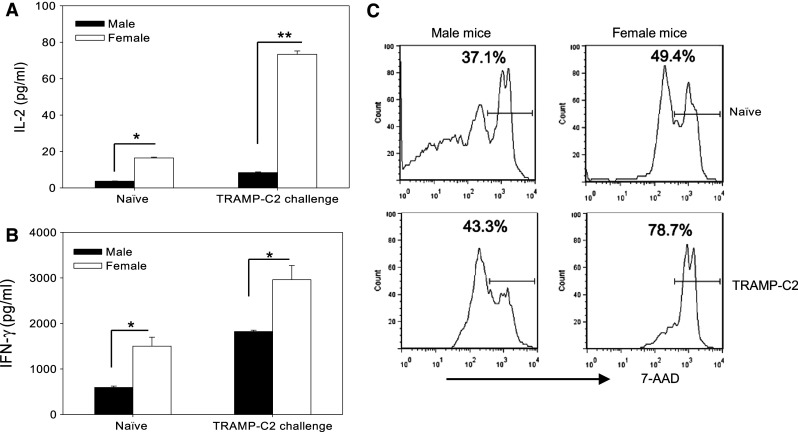
Enhanced antitumor response in female mice is associated with increased recognition of prostate tumor by immune cells. Increased IL-2 (a) and IFN-γ (b) production in primed female lymphocytes. Splenocytes from naïve or TRAMP-C2 challenged mice were co-cultured with irradiated TRAMP-C2 cells at a ratio of 50:1 for 72 h. Supernatant was collected and subjected to ELISA assays for cytokine production (*p < 0.01; **p < 0.001). The results are presented as means + SD and are representative of two independent experiments. c Enhanced cytolytic activity of splenocytes from TRAMP-C2-challenged female mice. Splenocytes from naïve or TRAMP-C2 challenged mice were expanded. Effector cells were co-cultured with CFSE-labeled TRAMP-C2 target cells for 48 h. Tumor death was detected using 7-AAD staining and FACS by gating on CFSE-positive target cells. The representative results of three independent experiments are shown
To determine whether the generated immune response was directed specifically against prostate tumors, female mice were immunized with irradiated TRAMP-C2, RM1 or D121 (a mouse lung carcinoma cell line), followed by tumor challenge. Use of TRAMP-C2 cells in the setting of priming and challenging is not feasible, since female mice without prior exposure to prostate tumor cells are able to reject TRAMP-C2 tumors. Therefore, RM1 prostate tumor cells and D121 lung tumor cells were used in this study. While immunization with D121 cells did not provide appreciable protection against RM1 tumors, immunization with either TRAMP-C2 or RM1 prostate tumor cells induced potent antitumor activities against RM1 tumor (Fig. 3a), suggesting that the antitumor activity involved immune recognition of shared antigens from these two cell lines of the prostate origin. Although, RM1 tumors were not completely eradicated in female mice receiving TRAMP-C2 cells, the tumors grew at a significantly slower rate compared to D121 lung carcinoma in the immunized female mice (Fig. 3a).
Fig. 3.
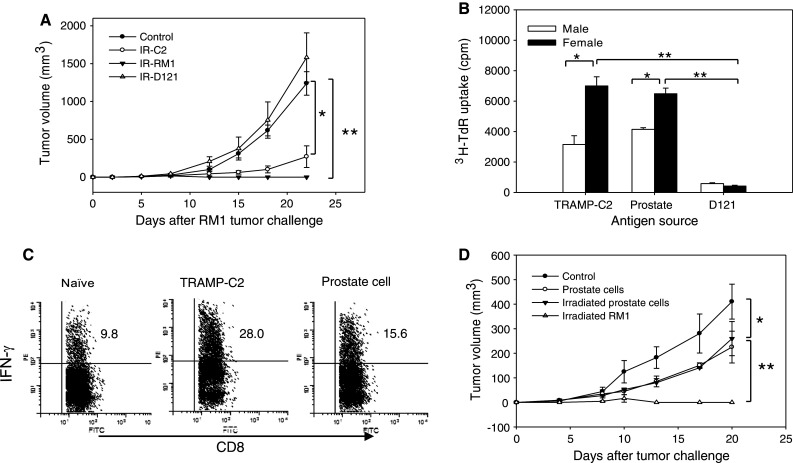
Prostate tumor challenge elicits prostate antigen-specific immune response in female mice. a Cross-protection against TRAMP-C2 and RM1 tumors. Female mice (n = 5) were immunized with irradiated (IR) TRAMP-C2, RM1 prostate tumor cells or D121-lung carcinoma cells, followed by challenge with 5 × 105 live RM1 cells (*, **p < 0.001). b Recognition of prostate tissue antigens by primed female lymphocytes. Lymphocytes from TRAMP-C2 challenged female or male mice were co-cultured with female DCs, which were pulsed with the lysates prepared from TRAMP-C2, D121 or prostate tissue. Cell proliferation were measured by 3H-TdR incorporation (*p < 0.01; **p < 0.001). c Increased STEAP-specific IFN-γ production in TRAMP-C2 challenged female mice. Splenocytes from female mice inoculated with TRAMP-C2 cells, normal prostate cells or left untreated were stimulated with mSTEAP326–335 peptides, followed by intracellular staining for IFN-γ production and FACS analysis gating on CD8+ cells. d Prostate tumor-specific response responsible for subsequent tumor rejection. Female mice (n = 5) were immunized with normal prostate cells, irradiated prostate cells or RM1 tumor cells, followed by challenge with RM1 cells (*p < 0.05; **p < 0.001). The representative results of three independent experiments are shown
The in vitro proliferation of splenocytes derived from TRAMP-C2 tumor-challenged male or female mice was determined in the presence of different antigenic sources using 3H-thymidine incorporation assays. When co-cultured with prostate tissue or TRAMP-C2 tumor lysates, lymphocytes from female mice displayed a robust proliferative response than those from male mice. However, lymphocytes from female or male mice did not respond to in vitro stimulation with D121 lung tumor lysates (Fig. 3b), further supporting that prostate tumor or tissue-specific antigens may serve as the targets of the immune recognition.
Six-transmembrane epithelial antigen of the prostate is highly expressed in advanced human prostate cancer and mouse prostate tumor [8, 21], representing one of CaP antigens for immunotherapeutic targeting. Therefore, we sought to determine whether female mice challenged with TRAMP-C2 tumor cells may develop STEAP-specific immune responses. Splenocytes were stimulated with MHC Class I-restricted mSTAEP326–335 peptide, followed by intracellular staining for IFN-γ. CD8+ T cells from naïve female mice responded to STEAP stimulation, suggesting the existence of the antigen-specific T cell precursors in T cell repertoire of female mice. Compared to naïve female mice, those receiving TRAMP-C2 cells showed a significantly higher frequency of IFN-γ producing CD8+ T cells (Fig. 3c), indicating that the rejection of TRAMP-C2 tumor in female mice may have involved the immune recognition of prostate tumor antigens (e.g., STEAP). Since STEAP, a prostate tissue differentiation antigen, is also expressed in normal prostate, we hypothesized that inoculation of normal prostate cells into female mice should induce the STEAP-specific immune response in a similar manner. Indeed, CD8+ T cells from female mice injected with prostate cells showed increased IFN-γ production compared to control naïve mice, but this production was significantly lower than that seen from CD8+ T cells isolated from TRAMP-C2-challenged mice (Fig. 3c). This might be attributed to the low expression levels of STEAP in normal prostate tissue compared to prostate tumor.
Given the nature of antigens shared between prostate tumor and normal prostate tissue, we next examined the immunogenicity of prostate cells in the context of the induction of anti-prostate tumor response. While immunization with irradiated RM1 cells resulted in the rejection of subsequently inoculated RM1 tumors, female mice receiving normal prostate cells (irradiated or not) only modestly delayed RM1 tumor growth (Fig. 3d), suggesting that there is a different capacity of these two antigenic sources in augmenting an anti-tumor immune response in female mice.
Immune response to H-Y antigen does not play a predominant role in tumor rejection in female mice
In MHC-matched human transplantation and animal models, immune response to the male minor histocompatibility H-Y antigen can be responsible for host versus graft rejection [22]. Therefore, we assessed the immune recognition of the male-specific, Y chromosome-encoded H-Y antigens in female mice following immunization with TRAMP-C2, RM1, normal prostate cells or male lymphocytes. Compared to untreated mice, all immunized mice generated immune responses against the defined H-Y epitopes, including H-2Db-restricted Smcy738–746, Uty246–254 and I-Ab-restricted Dby608–622, as indicated by T cell proliferation assays. Among all the groups, male lymphocytes appeared to induce a slightly higher T cell response against H-Y epitopes (Fig. 4a). PCR analysis indicates that similar mRNA levels of UTY antigen are present in TRAMP-C2 and RM1 tumors, male lymphocytes and prostate tissue (Fig. 4b, left). However, protein levels of UTY are modestly higher in prostate tissue compared to prostate tumors (Fig. 4b, right).
Fig. 4.

Immune response to H-Y antigen is unable to control TRAMP-C2 tumor growth in female mice. a Comparable H-Y antigen reactive response in female mice following stimulation. Female mice were challenged with prostate tumor cells (TRAMP-C2, RM1), normal prostate cells or male lymphocytes. 2 weeks later, splenocytes were harvested and stimulated with H-Y antigen peptides. Cell proliferation assay was performed using 3H-TdR assays. b Expression of UTY antigen in different male tissues and cells. mRNA and protein levels of UTY antigen were examined in RM1 tumor cells, TRAMP-C2 tumor cells, normal prostate tissue and male lymphocytes using PCR and immunoblotting analysis, respectively. RNA polymerase II (RPII) and β-actin were used as internal controls. c Lymphocytes from prostate tumor-sensitized female mice exhibit increased cytolytic activity. Splenocytes from TRAMP-C2 or male lymphocyte-immunized female mice were stimulated with irradiated TRAMP-C2 cells, or H-Y peptides, respectively. Effector cells were then co-cultured with CFSE-labeled TRAMP-C2 cells as targets for 24 h, tumor death was detected using 7-AAD staining gated on CFSE-positive target cells. d Failure of controlling aggressive RM1 prostate tumors by priming an H-Y antigen-specific immune response in female mice. Female mice (n = 5) were immunized by injection of TRAMP-C2 cells, male lymphocytes or left untreated, followed by tumor challenge with 5 × 105 RM1 cells and tumor growth was monitored twice weekly (*p < 0.001). The results are representative of three independent experiments
Immunization with male lymphocytes is known to efficiently prime T cells for this minor histocompatibility antigen [23]. We next examined whether immune response against H-Y antigens in female mice resulted in the cytotoxic killing of prostate tumor cells. Splenocytes from male lymphocyte-challenged female mice displayed significantly weaker cytolytic activity than those from TRAMP-C2-challenged mice (Fig. 4c). To further confirm this observation in vivo, female mice were inoculated with male lymphocytes or TRAMP-C2 tumor cells. One week later, mice were challenged with high dosage of RM1 prostate tumor cells (5 × 105). As expected, untreated female mice developed RM1 tumors upon challenge with high dose RM1 cells. It was observed that RM1 tumors grew more aggressively in mice immunized with male lymphocytes than in naïve female mice. In contrast, RM1 tumors were completely rejected by female mice that were challenged with TRAMP-C2 tumor cells, indicating that H-Y antigen-specific immune response does not play a significant role in the antitumor response against RM1 tumors in female mice (Fig. 4d). The frequency of STEAP-stimulated, IFN-γ producing CD8 T cells in male lymphocyte-primed female mice was almost the same as that of naïve female mice (data not shown). These data suggest that the immune response against RM1 cells was prostate tumor-specific rather than merely male antigen-specific.
Adoptive transfer of female lymphocytes suppresses prostate tumor growth in male mice
Lymphodepletion with total body irradiation (TBI) increases the efficacy of adoptively transferred tumor-specific CD8+ T cells by depleting inhibitory lymphocytes and increasing homeostatic cytokine levels [19, 24, 25]. To determine whether the TRAMP-C2 tumor protective immunity in female mice can be transferred to male mice, total lymphocytes from TRAMP-C2-challenged female mice were adoptively transferred to recipient male mice using a modified protocol combining ACT, host pre-conditioning (i.e., 5 Gy WBI), vaccination and IL-2 administration [13, 19]. The growth of TRAMP-C2 tumors was profoundly suppressed in male mice that received ACT. Additional vaccination with irradiated TRAMP-C2 tumor cells did not further enhance ACT-mediated antitumor activity (Fig. 5a).
Fig. 5.
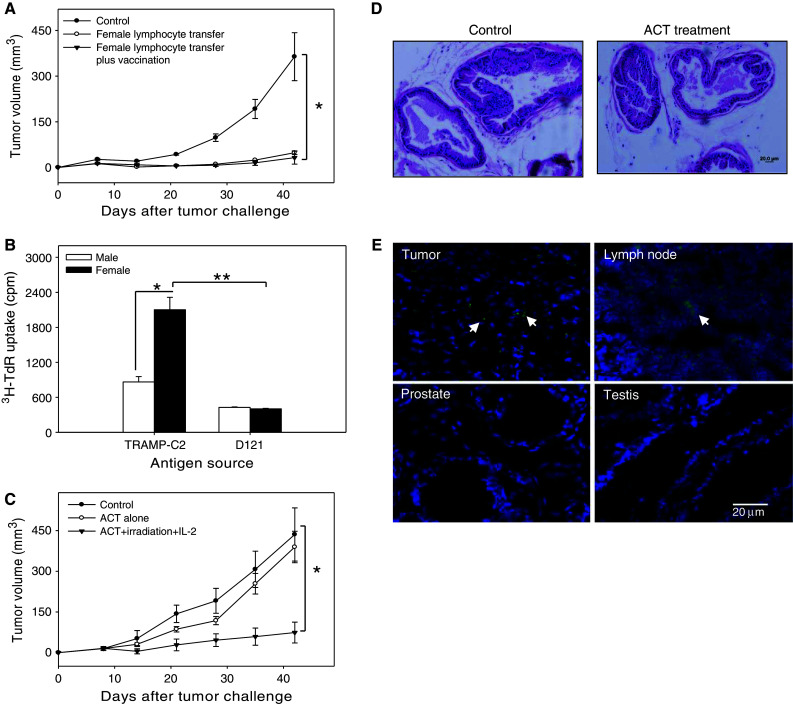
Adoptive transfer of female donor lymphocytes efficiently controls prostate tumor growth in male mice. a Adoptive cell therapy (ACT) using TRAMP-C2 tumor-primed female donor lymphocytes. TRAMP-C2 tumor-bearing male mice (n = 5) were whole body irradiated (5 Gy) and received lymphocytes from TRAMP-C2-challenged female donor mice. IL-2 was administrated daily for the next 3 days. Tumor growth was monitored at indicted time points (*p < 0.001). b Prostate tumor-specific reaction exhibited by naïve female lymphocytes. Lymphocytes from naïve female or male mice were co-cultured with female donor DCs that were pulsed with TRAMP-C2 or D121 tumor lysates. Cell proliferation was measured by 3H-TdR incorporation assays (*p < 0.01; **p < 0.001). c ACT using lymphocytes from naïve female mice. Tumor-bearing male mice (n = 5) were treated with WBI, and received naïve lymphocytes from female donor mice and IL-2. One cohort of male mice without pre-conditioning only received adoptively transferred female lymphocytes (*p < 0.001, ACT plus WBI vs. control or ACT alone). d Absence of inflammation and immune cell infiltrates in the prostates from ACT-treated male mice. The prostate tissues were collected from TRAMP-C2 bearing mice after treatment, and subjected to H&E staining. The representative results of at least two independent experiments are shown. e Preferential trafficking of adoptively transferred T cells from female donors to subcutaneous tumors. TRAMP-C2 tumors, tumor-draining lymph nodes, prostate and testis were collected 3 days after adoptively transfer of CFSE-labeled T cells into tumor-bearing male recipients. Tissue frozen sections were stained with DAPI and analyzed using fluorescence microscope
We predicted that a high frequency of prostate tumor/tissue reactive T cells or their precursors existed in untreated female mice, which was also supported by the observed STEAP-specific T cells in naïve female mice (Fig. 2e). Indeed, naïve female lymphocytes responded more strongly than those from male mice to the stimulation with TRAMP-C2, not D121 lung tumor cells, (Fig. 5b), even though it was not as robust as that seen in lymphocytes from TRAMP-C2-challenged mice.
We next assessed the ability of naïve female lymphocytes to control pre-established TRAMP-C2 tumors in male mice. Tumor-bearing male recipients were subjected to ACT with or without non-myeloablative conditioning. ACT alone failed to result in any significant tumor suppression in male mice (Fig. 5c). However, ACT in conjunction with WBI and IL-2 administration effectively delayed tumor growth (Fig. 5c). In addition, ACT plus WBI, or ACT plus IL-2 failed to defer tumor progression (data not shown).
ACT using female mice-derived lymphocytes does not augment detectable inflammation in prostate
Given that antigens recognized by female donor lymphocytes are likely shared by normal prostate tissue and prostate tumor, adoptive T cell therapy using female donor lymphocytes may carry a potential risk of destruction of the prostate organ. Prostate tissues-derived from untreated and ACT-treated male mice were analyzed by H&E staining. Interestingly, the prostates from ACT-treated TRAMP-C2-bearing mice did not show any obvious signs of an active inflammatory pathology compared to untreated controls (Fig. 5d). Indeed, following adoptive transfer, it was seen that CFSE-labeled T cells from female mice were primarily present in tumor-draining lymph nodes and tumor tissues, but not in prostates, testis (Fig. 5e) and liver (data not shown), suggesting that T cells from female donors preferentially recognize prostate tumors compared to major normal or male organs and that ACT does not appear to induce significant infiltration by inflammatory cells or cause tissue damage in healthy prostate organs. However, the possibility of eventually generating damage in normal prostate is lessened in actual clinical settings, since normal prostate would no longer be present in most patients with advanced or metastatic prostate cancer disease.
Discussion
Cancer patients usually exhibit tumor-associated immune suppression and most cancer-associated antigens, including CaP antigens, are ‘self-proteins’, blocking them from immune recognition. The presence of the prostate organ in male but not female hosts presents a unique opportunity for the development of a novel ACT for CaP treatment. In the current study, we provide the first experimental evidence that T cells from female mice are highly reactive to prostate tumors, and thus may be utilized for more effective adoptive immunotherapy of CaP.
We have shown that male mice were susceptible to prostate tumor growth, whereas, female mice were able to reject or effectively control two different syngeneic prostate tumor lines, TRAMP-C2 and RM1. In the case of RM1 model, while female mice did not completely eliminate the tumors (perhaps due to the rapid growing rate of the RM1 tumors), tumor development was nevertheless profoundly suppressed compared with that seen in male mice. In addition, sensitizing female mice with live TRAMP-C2 cells or irradiated RM1 cells resulted in a complete rejection of the subsequently inoculated RM1 tumors, due to the expansion of tumor-reactive T cells. In this MHC-matched syngeneic system, the immune system of female host may see implanted prostate tumor as “non-self” because of the expression of tumor/tissue-specific antigens or minor histocompatibility antigens, and therefore initiate a strong, coordinated antitumor immune response. The immune-mediated rejection of prostate tumors by the female mice was supported by the observation that depletion of either CD4+ or CD8+ T cells abrogated the tumor control.
Upon stimulation with prostate tumor cells, splenocytes from tumor-sensitized female mice displayed a robust proliferation, high levels of IFN-γ production and cytolytic activity compared to those from male mice. Interestingly, similar results were obtained when splenocytes were stimulated with normal prostate tissue lysates, although the response was much more modest. CaP antigens identified so far are unaltered prostate differentiation antigens, which are overexpressed in primary or metastatic CaP. Based on observations of cross-protection conferred by TRAMP-C2 and RM1 prostate tumors in female mice and the immune responses in female mice against prostate tissue lysates, we speculate that upon prostate tumor challenge, target antigens recognized by the female immune system are mostly prostate-associated proteins highly elevated in CaP. Nonetheless, the immune responses generated for as yet unknown, true CaP-specific antigens may also exist in female mice. Given the higher expression of tumor/prostate tissue-associated antigens in malignant cells, differential cytokine responses stimulated by tumor cells vs. prostate tissue was predictable.
Our results show that mSTEAP-specific CD8+ T cell response was induced in TRAMP-C2-challenged or prostate-immunized female mice. STEAP is constitutively expressed in normal prostate tissue, but highly elevated in malignant prostate tissue, including TRAMP-C2 cells [26]. It has been reported that an mSTEAP-based vaccine significantly prolonged the survival of tumor-bearing mice [8, 27], suggesting that STEAP is a potential candidate antigen for prostate cancer immunotherapy. Therefore, the potent response against mSTEAP observed in our studies could be involved in the immune rejection of prostate tumors in female mice.
It is recognized that minor H antigens encoded by genes on the Y chromosome could contribute to a selective graft versus leukemia (GVL) effect against myeloid and lymphoid leukemias after hematopoietic stem cell transplantation [28]. Therefore, we have also conducted studies to assess whether the protection seen in females may simply be due to H-Y antigens. TRAMP-C2 or RM1-challenged female mice developed a similar H-Y antigen-specific response as did immunization with male lymphocytes, which is known to efficiently prime T cells for this minor histocompatibility antigen [23]. However, female mice immunized with male lymphocytes were still susceptible to RM1 tumor challenge compared to TRAMP-C2 sensitized counterparts, which were able to completely reject subsequent RM1 tumor challenge. In vitro CTL assay also showed that H-Y antigen stimulated lymphocytes were not as effective as CaP-primed lymphocytes in killing RM1 tumor cells. Together, these results suggest that the H-Y antigen-specific immune response did not appear to play a major role in the protection against CaP in primed female mice. However, more studies are needed to further define the contributions of prostate tumor/tissue and H-Y antigens to the observed tumor rejection.
There was a concern that an autoimmune response may be elicited in the prostate organ that shares antigens with prostate tumor. Intriguingly, ACT of tumor-bearing male mice in our studies did not result in visible pathologic autoimmunity. While prostate tumors overexpress certain prostate-associated antigens, making them ready targets for the prostate tumor/tissue-specific T cells from female mice, low levels of self-antigens (e.g., STEAP) in the normal prostate may be insufficient for stimulation of adoptively transferred T cells [29], suggesting that even under experimental conditions with a high frequency of autoreactive T cells, a therapeutic window can be defined, in which ACT can reject tumor cells without causing severe autoimmunity. Additionally, it is expected that this would not be a major problem in the clinical setting, since the normal prostate tissue would likely be already removed through surgery in advanced patients or those with metastatic disease.
Lymphodepletion before ACT has been shown to dramatically improve the antitumor efficacy of transferred T cells by removing endogenous regulatory or suppressive cells and cytokine ‘sinks’ [19]. We have demonstrated that adoptive transfer of TRAMP-C2-sensitized female lymphocytes to male mice treated with non-myeloablative or “low-intensity” conditioning regimens (i.e., low dose WBI) profoundly suppressed tumor growth. However, additional vaccination with irradiated TRAMP-C2 cells failed to further improve the therapeutic efficacy of ACT as reported previously [13]. It is possible that irradiated tumor cells may not be the best form of vaccination for T cell stimulation in male mice. Although WBI is used as an experimental model in the present studies, future studies should test the novel ACT in conjunction with chemotherapies used for allo-transplantation in the clinic, which is expected to have a similar lymphodepleting effect as WBI.
While this study was conducted using syngeneic models, this is not a feasible approach in humans. However, allogeneic cell therapy, as a means to break immunotolerance to hematologic malignancies as well as solid tumors, is increasingly used for successful cancer treatment [30–32]. In fact, it has been observed that T cells from HLA-identical sibling donors also showed a superior tumor-reactive response compared to autologous patients’ TILs [33]. The use of tumor-reactive female donor lymphocytes for CaP therapy may be able to offer combined therapeutic effects derived from both tumor-specific and alloreactive immune responses. Recent progress in allogeneic transfer therapy has highlighted that augmenting a graft versus tumor effect (GVT) by T cell transfer in the absence of measurable graft versus host disease (GVHD) appears to be achievable in the foreseeable future [34, 35]. The improved safety and preliminary success of ACT have justified applying female donor lymphocyte-based immunotherapy to patients with advanced or treatment-refractory CaP.
Surprisingly, naïve female lymphocytes also effectively inhibited TRAMP-C2 growth in male mice as did those from TRAMP-C2-challenged female mice, when combined with pre-conditioning of recipient mice and IL-2 administration. Emerging evidence in mouse models indicates that the differentiation status of transferred cells is also important to the success of T cell-based therapies [36, 37]. Naïve, rather than central memory T cells, were found to be superior in potentiating tumor immunity upon adoptive transfer [38]. Thus, prostate cancer/tissue antigen-specific T cell precursors in female naïve cells may efficiently differentiate and become activated in recipient mice undergoing pre-conditioning. However, it remains to be tested whether undifferentiated female lymphocytes is preferable for augmenting ACT compared to the primed counterparts.
Our studies suggest that rather than using traditional TILs for ACT, high-avidity prostate tumor/tissue reactive T cells, which are present in female T cell repertoire, may be exploited as the source of immunotherapeutic ‘effector’ cells. Compared to the extensive effort needed to apply individualized conventional ACT, the use of female lymphocytes could reduce the time and labor required, and therefore represents a conceptually attractive therapy for advanced CaP with a broad applicability. However, several challenges need to be overcome before this approach is translated in the clinic. Ex vivo ‘programming’ and expansion of naïve female lymphocyte or its subsets and lineage still have to be defined to achieve optimal antitumor activity [39]. Although, the polyvalent nature of antigenic specificity clearly provides additional therapeutic benefits in CaP treatment, knowledge of female donor lymphocyte-mediated antigen recognition for GVT and GVHD will guide choice of right T cell candidate. Selective enrichment of prostate tumor/tissue-specific T-effector cells for ACT would greatly reduce the risk of GVHD in the recipient host. Generation of female T cell lines or clones reactive with prostate tumor will permit to address this issue directly. With the emergence of TCR gene therapy [40], identification of specific TCRs and novel prostate tumor antigens via reactive female T cells will provide new and attractive opportunities for rational design of targeted immunotherapies, including adoptive therapy, against CaP.
Acknowledgments
This work was supported by NIH research grant CA129111, CA154708 American Cancer Society (ACS) RSG-08-187-01-LIB, Harrison endowed scholarship (X-Y W) and NCI Cancer Center Support Grant to Massey Cancer Center.
Conflict of interest
All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2(4):227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117(5):1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109(3):409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Luz Garcia-Hernandez M, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68(3):861–869. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 6.Kuratsukuri K, Sone T, Wang CY, Nishisaka N, Jones RF, Haas GP. Inhibition of prostate-specific membrane antigen (PSMA)-positive tumor growth by vaccination with either full-length or the c-terminal end of PSMA. Int J Cancer. 2002;102(3):244–249. doi: 10.1002/ijc.10700. [DOI] [PubMed] [Google Scholar]
- 7.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18(23):3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Hernandez MdlL, Gray A, Hubby B, Kast WM. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: a candidate antigen for treating prostate cancer. Cancer Res. 2007;67(3):1344–1351. doi: 10.1158/0008-5472.CAN-06-2996. [DOI] [PubMed] [Google Scholar]
- 9.Mathis D, Benoist C. Levees of immunological tolerance. Nat Immunol. 2010;11(1):3–6. doi: 10.1038/ni.1833. [DOI] [PubMed] [Google Scholar]
- 10.Walker LSK, Abbas AK. The enemy within: keeping self-reactive t cells at bay in the periphery. Nat Rev Immunol. 2002;2(1):11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 11.Rizzuto GA, Merghoub T, Hirschhorn-Cymerman D, Liu C, Lesokhin AM, Sahawneh D, Zhong H, Panageas KS, Perales M-A, Altan-Bonnet G, Wolchok JD, Houghton AN. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med. 2009;206(4):849–866. doi: 10.1084/jem.20081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarassoff CP, Arlen PM, Gulley JL. Therapeutic vaccines for prostate cancer. Oncologist. 2006;11(5):451–462. doi: 10.1634/theoncologist.11-5-451. [DOI] [PubMed] [Google Scholar]
- 13.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labarthe MC, Theocharous P, Russell N, Todryk S, Bangma C, Thraves P, Dalgleish AG, Whelan MA. A novel murine model of allogeneic vaccination against prostate cancer. Cancer Immunol Immunother. 2008;57(4):453–465. doi: 10.1007/s00262-007-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baley PA, Yoshida K, Qian W, Sehgal I, Thompson TC. Progression to androgen insensitivity in a novel in vitro mouse model for prostate cancer. J Steroid Biochem Mol Biol. 1995;52(5):403–413. doi: 10.1016/0960-0760(95)00001-G. [DOI] [PubMed] [Google Scholar]
- 16.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57(16):3325–3330. [PubMed] [Google Scholar]
- 17.Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, Kim HL, Subjeck JR, Wang XY. Pattern recognition scavenger receptor SRA/CD204 down-regulates toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113(23):5819–5828. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of her2/neu-specific t cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58(6):941–953. doi: 10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, Fisher PB, Wang XY. Secretable chaperone grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/il-24. Cancer Res. 2008;68(10):3890–3898. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, Madraswala R, Zhou Y, Kuo J, Raitano AB, Jakobovits A, Saffran DC, Afar DEH. Steap: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96(25):14523–14528. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson E, Scott D, James E, Lombardi G, Cwynarski K, Dazzi F, Millrain M, Dyson PJ. Minor h antigens: genes and peptides. Eur J Immunogenet. 2001;28(5):505–513. doi: 10.1046/j.0960-7420.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 23.Millrain M, Chandler P, Dazzi F, Scott D, Simpson E, Dyson PJ. Examination of HY response: T cell expansion, immunodominance, and cross-priming revealed by HY tetramer analysis. J Immunol. 2001;167(7):3756–3764. doi: 10.4049/jimmunol.167.7.3756. [DOI] [PubMed] [Google Scholar]
- 24.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17(2):195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Holt GE, Velders MP, Kwon ED, Kast WM. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001;61(15):5857–5860. [PubMed] [Google Scholar]
- 27.Rodeberg DA, Nuss RA, Elsawa SF, Celis E. Recognition of six-transmembrane epithelial antigen of the prostate expressing tumor cells by peptide antigen induced cytotoxic T lymphocytes. Clin Cancer Res. 2005;11(12):4545–4552. doi: 10.1158/1078-0432.CCR-04-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347–352. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 29.Weinhold M, Sommermeyer D, Uckert W, Blankenstein T. Dual T cell receptor expressing CD8+ T cells with tumor- and self-specificity can inhibit tumor growth without causing severe autoimmunity. J Immunol. 2007;179(8):5534–5542. doi: 10.4049/jimmunol.179.8.5534. [DOI] [PubMed] [Google Scholar]
- 30.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 31.Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, Read EJ, Tisdale J, Dunbar C, Linehan WM, Young NS, Barrett AJ. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343(11):750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 32.Childs RW, Barrett J. Nonmyeloablative allogeneic immunotherapy for solid tumors. Annu Rev Med. 2004;55(1):459–475. doi: 10.1146/annurev.med.55.091902.104511. [DOI] [PubMed] [Google Scholar]
- 33.Kausche S, Wehler T, Schnurer E, Lennerz V, Brenner W, Melchior S, Grone M, Nonn M, Strand S, Meyer R, Ranieri E, Huber C, Falk CS, Herr W. Superior antitumor in vitro responses of allogeneic matched sibling compared with autologous patient CD8+ T cells. Cancer Res. 2006;66(23):11447–11454. doi: 10.1158/0008-5472.CAN-06-0998. [DOI] [PubMed] [Google Scholar]
- 34.Boni A, Muranski P, Cassard L, Wrzesinski C, Paulos CM, Palmer DC, Gattinoni L, Hinrichs CS, Chan CC, Rosenberg SA, Restifo NP. Adoptive transfer of allogeneic tumor-specific t cells mediates effective regression of large tumors across major histocompatibility barriers. Blood. 2008;112(12):4746–4754. doi: 10.1182/blood-2008-07-169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valenzuela JO, Iclozan C, Hossain MS, Prlic M, Hopewell E, Bronk CC, Wang J, Celis E, Engelman RW, Blazar BR, Bevan MJ, Waller EK, Yu X-Z, Beg AA. PkCtheta¸ is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119(12):3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulos CM, Suhoski MM, Plesa G, Jiang T, Basu S, Golovina TN, Jiang S, Aqui NA, Powell DJ, Jr, Levine BL, Carroll RG, Riley JL, June CH. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol Res. 2008;42(1–3):182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]


