Abstract
Stress and obesity are highly prevalent conditions, and the mechanisms through which stress affects food intake are complex. In the present study, stress-induced activation in neuropeptide systems controlling ingestive behavior was determined. Adult male rats were exposed to acute (30 min/d × 1 d) or repeated (30 min/d × 14 d) restraint stress, followed by transcardial perfusion 2h after the termination of the stress exposure. Brain tissues were harvested, and 30 µm sections through the hypothalamus were immunohistochemically stained for Fos protein, which was then co-localized within neurons staining positively for the type 4 melanocortin receptor (MC4R), the glucagon-like peptide-1 receptor (GLP1R), or agouti-related peptide (AgRP). Cell counts were performed in the paraventricular (PVH), arcuate (ARC) and ventromedial (VMH) hypothalamic nuclei and in the lateral hypothalamic area (LHA). Fos was significantly increased in all regions except the VMH in acutely stressed rats, and habituated with repeated stress exposure, consistent with previous studies. In the ARC, repeated stress reduced MC4R cell activation while acute restraint decreased activation in GLP1R neurons. Both patterns of stress exposure reduced the number of AgRP-expressing cells that also expressed Fos in the ARC. Acute stress decreased Fos-GLP1R expression in the LHA, while repeated restraint increased the number of Fos-AgRP neurons in this region. The overall profile of orexigenic signaling in the brain is thus enhanced by acute and repeated restraint stress, with repeated stress leading to further increases in signaling, in a region-specific manner. Stress-induced modifications to feeding behavior appear to depend on both the duration of stress exposure and regional activation in the brain. These results suggest that food intake may be increased as a consequence of stress, and may play a role in obesity and other stress-associated metabolic disorders.
Keywords: HPA Axis, PVH, ARC, LHA, Fos, MC4R, AgRP, GLP1R
Introduction
Stress is a major factor that contributes to the incidence and severity of various disease states. The generation of systemic stress responses occurs following disruptions to homeostasis, and serves an adaptive function to promote the survival of an organism under conditions of real or perceived threat. Physiological compensations to short-term crises, however, can become increasingly harmful if these changes are maintained for an extensive period of time. Chronic stress has thus been implicated in the etiology of affective, immune and cardiovascular disorders ranging from obesity to depression and cognitive dysfunction [1, 2]. Stress has also been shown to exert powerful effects on feeding and appetite, with differential responses seen based on stressor intensity and duration. Acute stress typically suppresses appetite, but feeding behaviors fluctuate less predictably across species during prolonged exposure to stress and complex relationships between ingestive motivation and chronic stress have been suggested through numerous studies (e.g., [3]). In rats, acute immobilization stress leads to a decrease in food intake and body weight one day post-stress [4], intensely painful or psychologically threatening stressors have been shown to inhibit feeding [5], and restraint stress has been used as a model for anorexia nervosa [6]. In contrast, others have shown that mild stressors, such as a gentle tail-pinch, can induce spontaneous feeding [7]. In humans, chronic stress can either enhance food intake and stimulate weight gain, or lead to weight loss through diminished food intake [1]. Given that both obesity rates and chronic stress are significant and growing concerns in Western societies, the interplay between these two conditions has become an increasingly important area of study. We therefore investigated how acute and repeated emotional stress exposure affects the central control of feeding, by evaluating the stress-induced activation of hypothalamic neurons expressing peptides or receptors that are known to constitute orexigenic or anorexigenic signals. We hypothesized that cells participating in anorexigenic pathways would be active under conditions of acute stress, while chronic stress would shift the balance of activated neurons toward those involved in orexigenic pathways. Understanding the influence of stress on the expression of orexigenic and anorexigenic signals that stimulate and inhibit feeding, respectively, would advance our knowledge regarding the pathophysiology of feeding and appetite during chronic stress, and provide a link between brain mechanisms involved in processing stressful stimuli and metabolic consequences that may result from chronic stress exposure.
Previous studies have identified several neuropeptides as feeding-related peptides, and many of these have further been implicated in the regulation of stress responses [8]. Appetite is controlled by a neuronal circuitry that consists of an interconnected network of pathways that contain both orexigenic and anorexigenic signals. Discrete hypothalamic regions that have been identified as contributing to the neural control of ingestive behavior include the PVH, the ARC, the VMH and the LHA, all of which are considered in the present work. The PVH is principally responsible for initiating the stress response, plays a role in the coordination of multiple systems, and is thought to be the main site for the integration of various signals related to energy balance [9]. Many neuropeptides, neuromodulators and receptors that contribute to the stress- and/or feeding-related neural circuitry are expressed in the PVH, which also receives projections from peptide-expressing neurons in other regions [10]. Indeed, previous studies have shown the PVH to be a site of convergence of fibers containing AgRP, the MC4R, and the GLP1R, markers on which this study was focused [11–13]. The ARC has recently gained prominence among sites associated with the hypothalamic integration of energy balance. A major subpopulation of neurons in the ARC expresses the orexigenic AgRP peptide along with neuropeptide Y (NPY); both of these signals decrease energy expenditure, promote an increase in fat deposition, and cause a redistribution of fat stores [14]. Microinjections of these neuromodulators into the ARC have been shown to affect feeding behavior in rats [15, 16]. Overall, the integration of signals from ARC neurons is thought to occur in the PVH [11]. The LHA is another important region involved in the control of ingestive behavior, as it houses cells containing various orexigenic neuropeptides. Lesions to the LHA have resulted in a total inhibition of feeding behavior, whereas stimulation of this region has been shown to increase food intake [17], supporting the notion that the LHA may serve as a primary feeding-stimulatory center in the brain.
Within the hypothalamic regions identified above, the activation of neurons expressing feeding-related markers was assessed. Neuronal activation was determined by the expression of Fos, the protein product of the immediate-early gene c-fos, as consistently used throughout the literature to identify stress-sensitive cells and circuitries (e.g., [10]). Fos was then co-localized within neurons expressing AgRP, the MC4R and the GLP1R.
The MC4R is one of two melanocortin system receptor subtypes expressed in the brain, and is involved in the control of food intake and energy expenditure [18]. Defects in the MC4R gene lead to a deterioration of health status [19], and MC4R knockout mice display an obese and hyperphagic phenotype with increased linear growth and metabolic defects [20]. The endogenous ligand for the MC4R is α–melanocyte stimulating hormone (α-MSH), an anorexigenic peptide derived from the pro-opiomelanocortin gene [21]. Conversely, the orexigenic AgRP acts as both an antagonist and inverse agonist of the MC4R [22], preventing α-MSH binding and suppressing the basal activity of this receptor. Thus, the central control of feeding and energy balance is largely dependent on the combined actions of α-MSH and AgRP on the MC4R [20].
The GLP1R binds glucagon-like peptide-1 (GLP-1), a brain-gut peptide produced in both the intestine and the brainstem [23]. This amide peptide is structurally related to glucagon and plays a role in controlling blood glucose levels as well as glucose metabolism in the brain [24]. GLP-1 has been implicated in the neuroendocrine control of hypothalamic-pituitary function, feeding behavior, and mediation of the stress response [12, 25]. Although the role of central GLP-1 has not been fully determined, the anorexigenic effects of this neuropeptide are becoming better understood [23], and its physiological importance is highlighted by the fact that it is conserved across all studied mammalian species [26]. Detection of GLP-1-immunoreactive terminals in the PVH has revealed this as an important area for GLP-1 action, and GLP-1 has also been detected in forebrain regions and other hypothalamic nuclei that send projections to the ARC and PVH where GLP-1 binding sites are found [24, 26]. Experiments utilizing a GLP1R antagonist, exendin, showed a stimulation of feeding in satiated rats, while daily administration of exendin increased food intake and body weight [25, 26]. Recent data have additionally suggested a role for GLP-1 in the initiation of stress responses [27].
Taken together, these data suggest that multiple overlapping central pathways may be involved in both the generation of stress responses and the control of feeding. The identification of these pathways will lead to a greater understanding of the relationships between stress, food intake, and energy balance, and may serve to inform future searches into the mechanisms involved in the development or exacerbation of stress-related disorders of metabolism.
Materials and Methods
Experimental animals
Young adult male Sprague/Dawley rats (Harlan, Houston, TX) of similar age (~4 months) and weight (338 ± 3 g) were used in the present experiments. All rats were individually housed in standard cages in a temperature-controlled animal facility maintained on a 12:12 hour light:dark cycle, with food and water provided ad libitum. Following shipment, the rats were allowed at least one week of acclimatization to the facility before experimentation was initiated. Rats were randomly assigned to Control, Acute restraint or Repeated restraint groups (n=5/group). Animal care and use were in accordance with the Guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
Restraint stress
Physical restraint was used as an emotional stressor, and consisted of placing the rats in a plastic restrainer (Kent Scientific, Torrington, CT) allowing only limited movement for 30 min. Repeatedly stressed rats were restrained in their home cages for 30 min daily for 14 consecutive days. Acutely stressed rats were exposed to the open restraining device, placed within their home cages for 30 min/d over the first 13 days, then restrained for 30 min on the 14th day. Control rats were exposed to the open restraint device on each of 14 consecutive days, but never restrained. All restraint and exposure occurred between 9:00 and 11:00 a.m.
Perfusion
Rats were transcardially perfused 2 hours after the termination of their restraint or exposure period on the final (14th) day. Rats were weighed and deeply anesthetized with chloral hydrate (400 mg/kg, i.p.; Sigma, St. Louis, MO). Perfusion through the ascending aorta was done using ~100 mL of ice-cold 0.9% saline, followed by 400–500 mL of ice-cold 4% paraformaldehyde (JTBaker, Inc., Pittsburg, NJ) at pH 9.5 in 0.1 M borate buffer. Brain tissues were then collected and post-fixed for 5 hours at 4°C, followed by cryoprotection overnight at 4°C in 10% sucrose in 0.1M potassium phosphate-buffered saline (KPBS).
Tissue Processing
The following day, each brain was removed from the cryoprotectant solution, mounted on a tabletop freezing microtome (Model SM 2000R, Leica Microsystems, Bannockburn, IL), and serial frozen sections (30 µm in thickness) taken in the coronal plane from a block of brain tissue between the septum and the caudal thalamus. The sections were collected into antifreeze solution (30% ethylene glycol, 20% glycerol) and stored at −20°C until used for immunohistochemical analyses.
Immunohistochemistry
Brain tissue sections were immunohistochemically stained for peptides and receptors thought to play key roles in the stress response and in the regulation of feeding during stress. Staining was performed such that each marker was localized within every fifth tissue section, allowing for: 1) multiple analyses to be conducted on each brain; 2) analysis of multiple sections through the rostrocaudal extent of each region of interest; and 3) the reduction of double-counting errors. First, Fos immunoreactivity was measured using a nickel-intensified avidin-biotin-immunoperoxidase technique. Sections were washed in KPBS, then placed in 0.3% hydrogen peroxide to quench endogenous peroxidases and 1% sodium borohydride to reduce free aldehydes. After being washed thoroughly, the tissue was placed in primary antiserum (rabbit anti-Fos; Oncogene Science, Cambridge, MA) diluted 1:50,000 in KPBS containing 0.3% Triton X-100 and 2% normal goat serum and incubated at 4°C overnight with gentle agitation. On the following day, sections were incubated in secondary antibody (biotinylated goat anti-rabbit IgG, 1:200 dilution; Vector Laboratories, Burlingame, CA) for 1 h. An avidin-biotin-complex solution (Vectastain Elite kit; Vector Laboratories, Burlingame, CA) was then applied for 1 h, and a nickel-enhanced glucose oxidase method using diaminobenzidine (DAB) as a chromogen was utilized to visualize specific binding [28].
Subsequently, Fos was co-localized with other markers using the same immunoperoxidase method as described above but with sequential staining for the second marker done without nickel enhancement. This method allows cells expressing both markers to be observed with black nuclei (Fos) and a brown cytoplasm (second marker). Due to the exothermic nature of the DAB reaction, we ran the reactions on ice in order to prevent the denaturing of tissue proteins and to optimize our double staining. In addition, we changed the concentration of the Fos primary in these analyses to a 1:10,000 dilution and It should be noted that as a result of this modification, increased numbers of Fos-positive cells are occasionally seen in the dual staining experiments compared to those in which staining was done for Fos alone. Fos was co-localized with: 1) GLP1R (1:5000; Abcam, Inc., Cambridge, MA); 2) MC4R (1:8000; Abcam, Inc., Cambridge, MA); and 3) AgRP (1:20,000; Phoenix Pharmaceuticals, Inc., Belmont, CA), to determine the number of stress-activated cells that also expressed these feeding-related markers. In all cases, stained sections were mounted on gelatin-coated slides and allowed to dry overnight before being defatted through a graded ethanol series and xylene and coverslipped. Lastly, one tissue series from each rat was stained with 0.25% thionin for Nissl material. These slides were used to evaluate the basic architecture of the brain regions to be examined, and served as a reference during analysis of staining for other markers.
Quantification of Staining
Light microscopy (AxioScop; Carl Zeiss, Inc., Thornwood, NY) and simple cell counting methods were used to measure immunoreactivity in both single- and dual-staining experiments. Initial counts were made at 20× magnification for Fos-positive and doubly-labeled cells, with a higher magnification (40×) and different focal planes used for verification of double labeling. Cell counts were performed both manually and with the assistance of a digital microimaging system coupled to computer software (AxioCam with AxioVision software; Carl Zeiss, Inc., Thornwood, NY), and quantified in multiple sections through the PVH (−1.30 – −2.12), ARC (−2.12 – −3.30), VMH (−2.12 – −3.30) and LHA (−1.60 – −2.30); coordinates given are approximate distances (in mm) from Bregma. Proper identification of the anatomical regions of interest was assured through use of adjacent Nissl series as well as the atlas of Paxinos and Watson [29]. Specifically, immunopositive cells were counted unilaterally from six sections through each region; the number of cells expressing each marker was then summed, and group means, standard deviations and standard errors were calculated (Table 1).
Table 1.
Quantification of immunostaining.
| Marker | Region | Control | Acute | Repeated |
|---|---|---|---|---|
| Fos | PVH | 106.0 ± 18.5 | 528.6 ± 91.9* | 187.2 ± 48.6† |
| ARC | 20.6 ± 1.6 | 303.2 ± 62.4* | 37.4 ± 2.5† | |
| LHA | 65.8 ± 12.3 | 140.2 ± 14.6* | 110.0 ± 7.8 | |
| Fos+MC4R | PVH | 116.0 ± 4.8 | 127.6 ± 6.4 | 106.6 ± 25.4 |
| ARC | 117.6 ± 3.6 | 105.4 ± 6.4 | 78.2 ± 6.4*† | |
| LHA | 16.6 ± 1.0 | 46.2 ± 2.9* | 32.4 ± 3.3*† | |
| Fos+AgRP | PVH | 47.4 ± 5.4 | 63.0 ± 5.6 | 47.8 ± 4.1 |
| ARC | 93.0 ± 10.4 | 49.0 ± 5.4* | 37.2 ± 8.9* | |
| LHA | 17.4 ± 1.8 | 12.2 ± 1.9 | 41.6 ± 2.28*† | |
| Fos+GLP1R | PVH | 64.2 ± 5.2 | 45.2 ± 6.2 | 45.8 ± 6.6 |
| ARC | 77.8 ± 8.1 | 41.4 ± 5.6* | 70.4 ± 8.2† | |
| LHA | 41.8 ± 0.9 | 25.4 ± 1.9* | 35.2 ± 5.7 | |
Total cell counts were made for all neurons expressing Fos, or Fos in conjunction with the type 4 melanocortin receptor (MC4R), agouti-related peptide (AgRP) or the glucagon-like peptide-1 receptor (GLP1R). Counts were made in 6 sections through the paraventricular hypothalamic nucleus (PVH), the arcuate nucleus (ARC) or the lateral hypothalamic area (LHA), and summed for each animal (n=5/group). Values presented are group means ± SEM.
significantly different (p<0.05) from control;
significantly different (p<0.05) from acute.
Statistical Analysis
The mean number of immunopositive cells in each region was compared and evaluated with a one-way analysis of variance (ANOVA) to determine overall effects, followed by Tukey-Kramer post hoc tests to determine individual differences (JMP program, version 7; SAS Institute, Inc., Cary, NC). Significance was determined at the p<0.05 level.
Results
Neuroanatomy and Fos expression
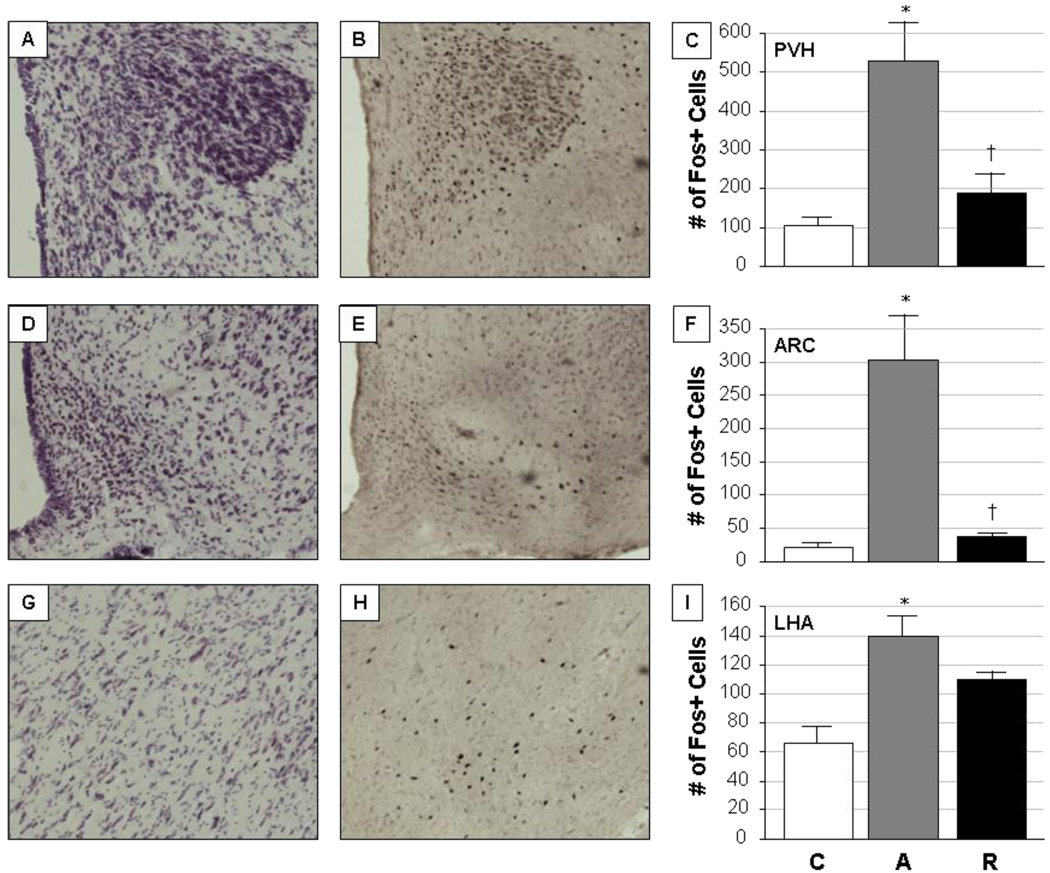
Nissl staining of adjacent brain sections allowed for the proper identification of anatomical regions from which immunostained cells were subsequently quantified (Fig. 1). Sections through the PVH, ARC and LHA showed characteristic appearance and staining patterns (Fig.1A, 1D, and 1G, respectively). Similarly, Fos-immunoreactive cells were easily identified by individual black-stained nuclei in the same regions (Fig. 1B, 1E, and 1H). Differences in Fos expression could be seen in the PVH, ARC and LHA in groups of rats exposed to acute or repeated restraint, compared to unstressed control animals, with variable responses in each region. Significant overall stress treatment effects on Fos expression were seen in the PVH (F=13.53, p=0.0008), the ARC (F=19.34, p=0.0002) and the LHA (F=9.84, p=0.003). Group comparisons revealed that acute stress induced a marked elevation of Fos expression in all three brain regions evaluated (Fig 1C, 1F and 1I), an effect which then habituated with repeated stress exposure in the PVH and ARC but not the LHA, although the LHA did show a trend toward habituation. The PVH showed the most Fos immunoreactivity overall, while the greatest difference in Fos expression between control and acute stress conditions occurred in the ARC. The number of Fos-expressing cells in the VMH was very low in all groups (data not shown), which precluded further analysis of this region with respect to dual labeling of cells for Fos and our secondary markers.
Figure 1. Identification of hypothalamic regions and quantification of Fos expression.
Nissl-stained sections through the adult male rat hypothalamus allowed for the identification of specific brain regions to be analyzed, including the paraventricular hypothalamic nucleus (PVH; panel A), the arcuate nucleus (ARC; panel D) and the lateral hypothalamic area (LHA; panel G). Adjacent sections were then immunohistochemically stained for Fos expression, as a generic marker of neuronal activation under control (C; unstressed), acute restraint stress (A), and repeated restraint stress (R) conditions. Representative images of Fos immunoreactivity are shown for the PVH (panel B), ARC (panel E) and LHA (panel H) of acutely stressed animals. Quantification of the number of Fos-expressing cells in each region is given in panels C (PVH), F (ARC), and I (LHA); bars represent the mean ± SEM for each group of animals (n=5/group).
*, significantly different (p<0.05) from control; †, significantly different (p<0.05) from acute. Photomicrographs were taken at 20× magnification.
Activation of MC4R-expressing neurons
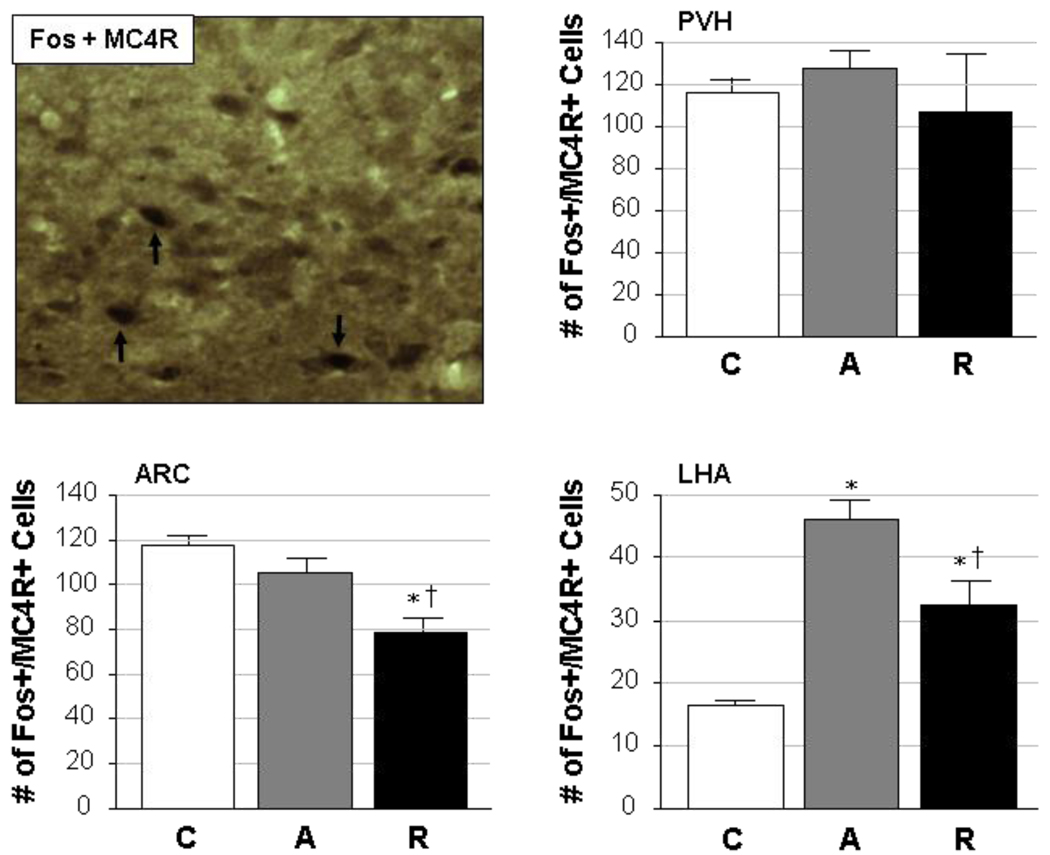
Localization of MC4R expression within stress-sensitive (Fos-positive) neurons showed different patterns across brain regions in response to acute and repeated stress (Fig. 2). Dual labeling for both markers within individual cells (see Fig. 2, upper left panel) was observed in all three regions, and quantification of the data further illustrated the differential responses seen in these hypothalamic areas. In the PVH, Fos/MC4R co-expression did not change as a function of stress exposure; control, acutely restrained and repeatedly restrained rats all showed similar numbers of dually-stained cells (F=0.47, p=0.6). The LHA, in contrast, showed significant changes in the level of activation of MC4R-positive cells following acute or repeated restraint stress (F=31.70, p<0.0001). Changes in the amount of dual staining for Fos and MC4R in this region, however, likely result from stress-induced changes in Fos expression rather than a specific recruitment of MC4R neurons in particular. Interestingly, both control and acutely stressed rats had similar numbers of Fos- and MC4Rpositive cells in the ARC, but repeatedly restrained rats showed a significant reduction in the number of activated MC4R-expressing cells in this nucleus. The overall effects of stress on Fos and MC4R co-expression in the ARC were significant (F=13.03, p=0.001).
Figure 2. Cellular co-localization of Fos with MC4R.
A representative photomicrograph is given in the upper left panel, in which dual staining for activated cells (Fos-positive; black nuclei) and the type 4 melanocortin receptor (MC4R; brown cytoplasm) was performed in the ARC of a repeatedly restrained adult male rat. Neurons expressing both markers can be seen (black arrows) in this image, which was captured at 20× magnification. The remaining panels illustrate quantified data from the PVH, ARC and LHA, in which the number of cells (mean ± SEM) co-expressing Fos and MC4R are given from rats exposed to control (C), acute restraint stress (A), or repeated restraint stress (R) conditions (n=5/group).
*, significantly different (p<0.05) from control; †, significantly different (p<0.05) from acute.
Activation of AgRP-expressing neurons
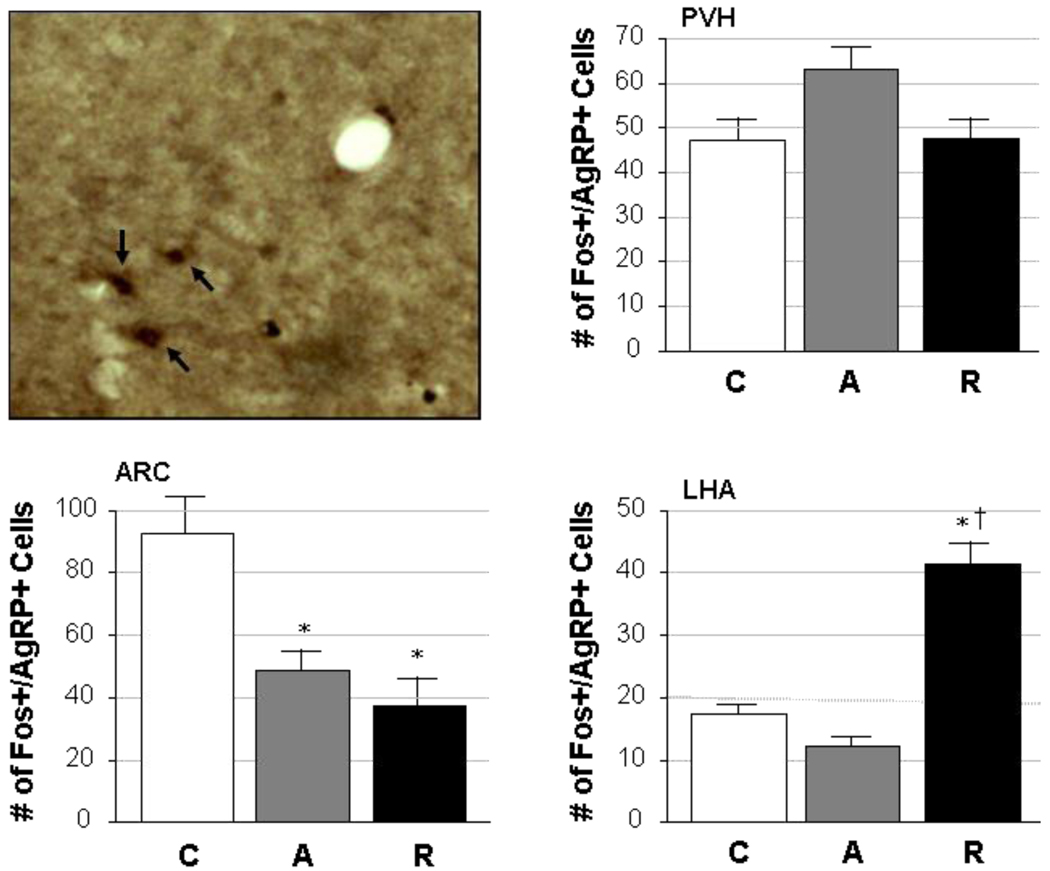
Figure 3 shows the degree of co-localization of Fos and AgRP in hypothalamic areas associated with stress and feeding. As with MC4R, AgRP was shown to co-localize with Fos in stress-sensitive neurons (Fig. 3, upper left panel) in the PVH, ARC and LHA. Quantification of the number of dually-stained neurons, however, also showed differences across each of the brain regions analyzed. No effect of stress was seen in the PVH in terms of Fos and AgRP co-expression (F=3.08, p=0.08), but largely opposite effects were observed in the ARC compared to the LHA. The ARC had the highest number of Fos- and AgRP-positive cells under basal (control) conditions, but these numbers were significantly reduced after stress exposure regardless of whether the stress was acute or repeated (F=12.03, p=0.001). In contrast, cells expressing both Fos and AgRP in the LHA were few in control and acute stress conditions but significantly elevated in response to repeated restraint stress (overall effect: F=62.27, p<0.0001).
Figure 3. Cellular co-localization of Fos and AgRP.
A representative photomicrograph is given in the upper left panel, in which dual staining for activated cells (Fos-positive; black nuclei) and agouti-related peptide (AgRP; brown cytoplasm) was performed in the ARC of a repeatedly restrained adult male rat. Neurons expressing both markers can be seen (black arrows) in this image, which was captured at 20× magnification. The remaining panels illustrate quantified data from the PVH, ARC and LHA, in which the number of cells (mean ± SEM) co-expressing Fos and AgRP are given from rats exposed to control (C), acute restraint stress (A), or repeated restraint stress (R) conditions (n=5/group).
*, significantly different (p<0.05) from control; †, significantly different (p<0.05) from acute.
Activation of GLP1R-expressing neurons
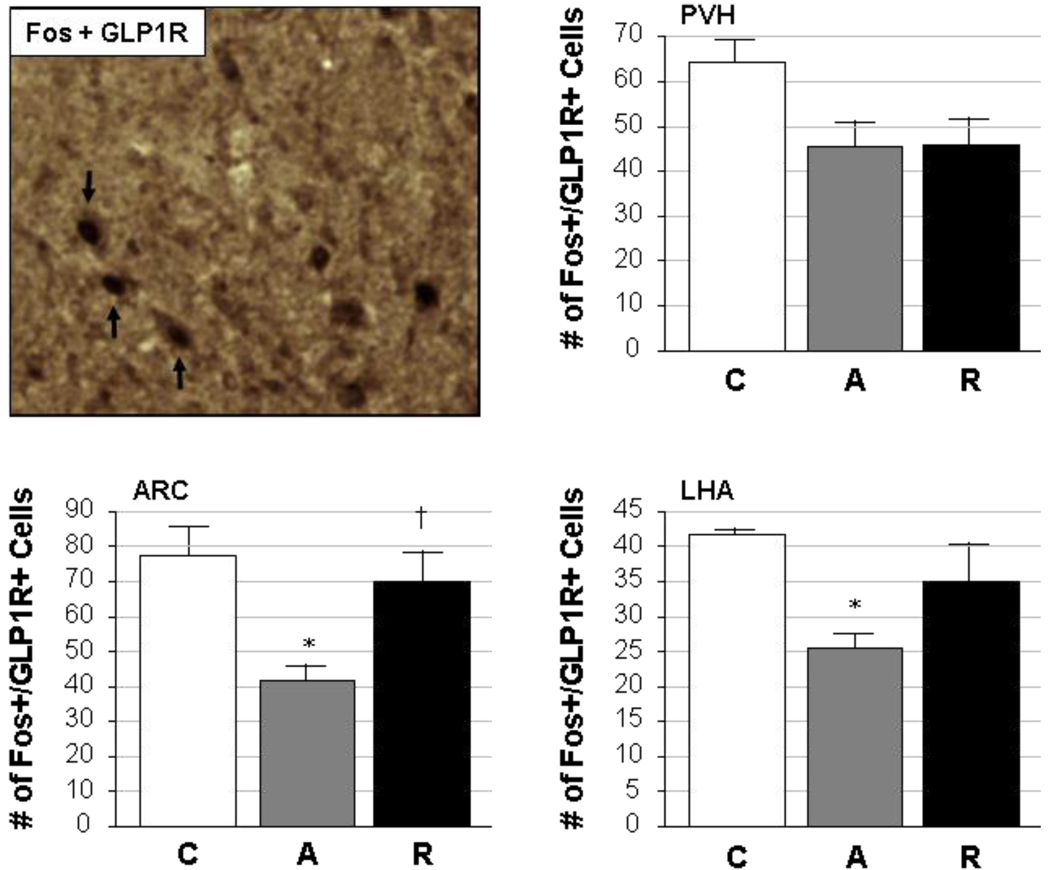
While the co-expression of Fos with GLP1R was also demonstrated (Fig. 4, upper left panel) in PVH, ARC and LHA neurons, variable patterns were again seen in the three hypothalamic regions when quantitative cell counts were made (Fig 4). No significant changes were seen in the number of cells in which Fos and GLP1R were co-localized in the PVH of control, acutely stressed and repeatedly stressed rats, although stress tended to decrease the level of staining for both markers regardless of whether it was acutely or repeatedly applied (p=0.05 by Student’s t-test in control compared to acute restraint, and p=0.06 in control compared to repeated restraint). The overall effect of stress on Fos and GLP1R co-expression in PVH neurons was a nearly-significant trend (F=3.21, p=0.076) toward decreased numbers of cells staining positively for both markers. Acute restraint led to a significant reduction in Fos- and GLP1R-expressing neurons in both the ARC and the LHA. Repeated exposure to restraint stress, however, restored GLP1R neuron activation in the ARC to levels seen in unstressed controls. In the LHA, a similar trend was seen, although the number of Fos- and GLP1R-positive LHA cells following repeated restraint was neither different from the control nor the acute stress condition. The overall effects of stress treatment on Fos and GLP1R coexpression were significant for both the ARC (F=6.73, p=0.01) and the LHA (F=5.56, p=0.02).
Figure 4. Cellular co-localization of Fos and GLP1R.
A representative photomicrograph is given in the upper left panel, in which dual staining for activated cells (Fos-positive; black nuclei) and the glucagon-like peptide-1 receptor (GLP1R; brown cytoplasm) was performed in the ARC of a repeatedly restrained adult male rat. Neurons expressing both markers can be seen (black arrows) in this image, which was captured at 20× magnification. The remaining panels illustrate quantified data from the PVH, ARC and LHA, in which the number of cells (mean ± SEM) co-expressing Fos and GLP1R are given from rats exposed to control (C), acute restraint stress (A), or repeated restraint stress (R) conditions (n=5/group).
*, significantly different (p<0.05) from control; †, significantly different (p<0.05) from acute.
Discussion
Neuronal activation in hypothalamic regions implicated in stress and feeding
Neurons are capable of simultaneously expressing multiple peptides, and determining the co-expression of neuromodulators within brain regions and individual cells improves our ability to explain the regulatory controls involved in both the generation of stress responses and the control of feeding behavior. In this study, our goal was to assess the levels of stress-induced activation in distinct neuroanatomical regions by localizing and quantifying Fos, the protein product of the immediate early gene c-fos. Subsequently, co-localization of the Fos signal with feeding-related neuropeptides or receptors was performed in order to examine potential interactions between these peptides and to determine how the activation of cells expressing these proteins differs in acutely and repeatedly stressed rats.
Acute stress has been shown to induce transient increases in Fos levels in brain regions involved in the processing of stressful stimuli and the generation of appropriate responses [30]. Results from this study showed enhanced Fos induction in the PVH, primarily in the medial parvocellular subdivision of this nucleus, in response to acute restraint stress. The number of Fos positive cells was also significantly increased in the ARC and LHA of acutely stressed rats, indicating roles for these regions in the stress response. These results are in line with previously published findings, where consistent increases in Fos expression have been seen in the PVH and other areas in response to stressors of various types, intensities and durations. Immobilization studies in mice have shown c-fos mRNA induction the PVH, ARC, and LHA, with the PVH being the most highly activated region [31]. Increased expression of c-fos mRNA in response to 90-minutes of immobilization has been demonstrated in both acutely and repeatedly stressed rats compared to unstressed controls [32]. In this study, habituated responses were seen in repeatedly immobilized rats; this pattern was also observed in the ARC. Finally, c-fos induction occurred in the LHA to a similar degree in both acutely and repeatedly immobilized rats, with no evidence of habituation in the repeated group.
Numerous studies have provided insight into the plasticity of stress responses following repeated homotypic stress exposure. The majority of brain regions that are highly activated by an acute stressor show diminished Fos expression after repeated exposure to the same stressor, implying that adaptation to that stressor has occurred. The anticipated habituation of Fos in the repeated restraint model used in this study was observed in both the PVH and the ARC. In the LHA, conversely, a significant increase in the number of Fos-expressing cells was detected in both the acutely and repeatedly restrained groups compared to the controls. This suggests that LHA activation is enhanced by stress regardless of whether that stress is acute or chronic, and may provide a link between stress and feeding behavior. Repeated restraint has been used in previous studies as a model of anorexia nervosa [6], demonstrating that reduced feeding can also result from this type of stress. However, since the majority of LHA neurons are orexigenic in nature [33], and an overall higher activation of these cells was seen in the present study, future studies will seek to more clearly define the mechanisms through which the LHA may modulate feeding behavior during stress.
Role of the melanocortin system and MC4R
Although we have not examined the corticotropin-releasing factor (CRF) system in the present work, the importance of CRF as an anorexigenic peptide which is extensively involved in the control of the stress system must be at least briefly mentioned. Previous work has shown a high degree of co-localization between Fos and CRF in the PVH of stressed rats [34], and co-expression of MC4R and CRF has also been seen in PVH neurons [35, 36], indicating a potential interaction between systems regulating stress and feeding in this nucleus. Enhanced secretion of CRF is thought to be one mechanism through which melanocortin signaling can effect downstream changes in neuroendocrine and feeding responses [37]. The activation of CRF neurons and the expression and secretion of CRF during both acute and repeated stress have been extensively documented in the literature, and several recent papers have postulated that this system is one of the most prominent mediators of the inhibitory effects of stress on feeding (e.g., [19]). Therefore, the contribution of the CRF system to the central control of energy homeostasis is of significance.
A novel aspect of the current study included determining the extent to which co-localization of Fos and MC4R occurs in the PVH in order to further define cells that may play a dual role in the control of stress and feeding. While Fos expression was increased in the PVH of acutely restrained rats, and habituated following repeated stress exposure, the number of cells in which MC4R co-localized with Fos did not significantly change. One possible explanation for this finding may be that different subsets of PVH neurons contribute to the regulation of feeding and the response to stress. Further experiments are needed to clarify this interaction. Similarly, increased Fos expression in the ARC after acute restraint was not accompanied by an increase in the number of cells in which MC4R co-localized with Fos. The MC4R itself, therefore, cannot be the key regulator in mediating feeding effects in the ARC of acutely stressed animals. This does not, however, preclude the possibility that changes in MC4R binding or signaling in response to changing agonist or antagonist levels may contribute to alterations in the control of feeding. The LHA, conversely, showed an upregulation in the number of Fos- and MC4R-expressing cells in acutely stressed rats. While this appears mainly to be a consequence of the increased expression of Fos instead of changes in MC4R expression per se, the fact that many activated cells contain MC4R implies a greater role for MC4R in the LHA during the generation of responses to stress.
Although repeated exposure to stress did not change the number of Fos-positive cells in the PVH that also express MC4R, a significant decrease in the number of Fos- and MC4R-expressing neurons was observed in the ARC compared to both control and acutely stressed groups. This reduction in MC4R cell activation may signify a desensitization of feeding regulatory pathways in the ARC after repeated stress exposure. Moreover, this result may be indicative of a shift toward more orexigenic behaviors, as signals promoting feeding become more prominent. In the LHA, while the number of Fos- and MC4R-expressing cells increased after acute stress, a habituation of this response was observed in repeatedly restrained rats. Again, this appears to be a consequence of changing Fos expression and not specifically of MC4R, but still implies a greater role for the LHA in the control of stress responses. Studies in stressed and adrenalectomized rats did not show altered MC4R expression [38], but the effects of prolonged exposure to elevated levels of circulating glucocorticoids on the melanocortin system and, specifically, on MC4R expression, are still relatively unknown. Therefore, determining repeated stress effects are modulated at the level of the MC4R or at the level of the neuropeptides which bind to this receptor must be investigated further.
AgRP expression in stress-sensitive neurons
AgRP expression in response to metabolic stressors such as fasting has been investigated, but the effects of emotional stressors on this orexigenic peptide are not entirely known [38]. Previous studies have shown that although NPY and AgRP are present in the same cells in the ARC and have similar responses to fasting, they become differentially regulated following acute stress, where NPY mRNA is upregulated and AgRP mRNA is concurrently downregulated [39]. In our hands, the number of Fos-positive cells expressing AgRP in the PVH was not significantly altered by acute stress. The same was true in the LHA, where acute restraint had no effect on the number of neurons in which Fos and AgRP were co-localized. In contrast, a significant decrease in cells staining for both Fos and AgRP after acute restraint was observed in the ARC. This is consistent with the idea that orexigenic signaling is decreased in response to acute stress.
Although the activation of neurons expressing AgRP did not change significantly in the PVH of either acutely or repeatedly stressed rats, differences were observed in both the ARC and LHA following repeated restraint exposure. Specifically, a significant decrease in Fos- and AgRP-positive neurons was seen in the ARC of repeatedly stressed animals, compared to controls. This response was equivalent to that seen after acute stress, suggesting that AgRP neurons in the ARC are de-recruited under conditions of emotional stress regardless of whether that stress is acute or repeated. The regulation of energy balance in the ARC in response to emotional stress may therefore involve suppression of feeding, at least as far as AgRP is concerned. In the LHA, however, while both control and acutely stressed rats showed a relatively low level of Fos and AgRP co-localization, repeatedly stressed rats showed a marked increase in the number of doubly-labeled cells. Increased orexigenic signaling in this region following repeated exposure to emotional stress is directly in line with the hypothesis of this study.
Stress and the GLP1R
The GLP1R is an anorexigenic signal-transducing receptor, and an increased activation of GLP1R-expressing cells was anticipated following acute restraint. In contrast to our expectations, however, significant decreases in were seen in the number of neurons co-expressing Fos and GLP1R in the ARC and LHA of acutely stressed rats compared to unstressed controls. In the PVH, no significant differences in the numbers of Fos- and GLP1R-expressing neurons were seen following either acute or repeated restraint, although a trend was seen toward decreased dual labeling. One possible explanation for these results might simply be that GLP1R neurons are not involved in modifying appetite and energy balance during episodes of acute stress. Alternatively, increased systemic levels of GLP-1, the agonist for this receptor, could reduce the expression of the receptor and decrease our ability to localize it in our tissue. Circulating or tissue GLP-1 concentrations were not measured in this study, and to our knowledge it is unclear whether emotional stress can modulate GLP-1 expression. Additional experiments are underway to address these questions. A final explanation could be that GLP-1 exerts its effects through a more complex neurocircuitry involving the CRF system, as GLP-1 terminals have been shown to innervate CRF neurons in the PVH, and GLP1R is present on ~65% of CRF neurons in the rat PVH [13]. The anorexic effects of GLP-1 may therefore be achieved through mechanisms that would not be identified in this study.
The only significant difference in the activation of GLP1R-expressing cells in repeatedly stressed rats occurred in the ARC, where the number of Fos- and GLP1R-expressing cells was equivalent to control levels. This was again contrary to our original hypothesis, and the implications of these results cannot be fully ascertained until GLP-1 levels are assessed in the system. It is possible that GLP-1 plays more of a role in the stress response itself, rather than in the regulation of feeding and appetite post-stress.
Summary and Conclusions
The aim of this study was to identify hypothalamic mechanisms involved in the regulation of feeding and energy balance following acute or repeated activation of the stress response. As both stress and obesity become increasingly prevalent problems in our society, learning about the neuronal mechanisms underlying these conditions becomes increasingly important. Despite individual differences in the activation patterns of neurons in brain regions implicated in the control of food intake, energy balance and stress, the overall suggestion is that acute restraint stress shifts these mechanisms in an orexigenic or feeding-stimulatory direction. In general, a trend toward increasing orexigenic signals in the brains of stressed animals appears to be evident. The relative balance between orexigenic and anorexigenic pathway activation, however, appears to be dependent on whether the stress is acute or repeated. Prolonged increases in feeding-stimulatory signals, in conjunction with repeated stress, would eventually lead to both overeating and a redistribution of energy stores due to the actions of glucocorticoids. Together, these stimuli could contribute substantially to the epidemics of obesity and overall poor health. A final point of interest is that the ARC seems to play a larger role in acute restraint, whereas greater effects are seen in the LHA in repeatedly stressed animals. This implies that the goal of activated neuronal networks during acute stress is to satisfy a short-term need for increased energy resources, while in repeated stress the entire drive of the response is shifted in favor of a long-term increase in feeding.
Research Highlights
Acute and repeated emotional stress increase hypothalamic orexigenic signaling
Acute stress appears to primarily engage the arcuate hypothalamic nucleus
The lateral hypothalamic area gains importance during repeated stress exposure
We have identified pathways through which stress may contribute to obesity
Acknowledgements
The authors would like to acknowledge the technical support of the NIH/RCMI-funded core facilities, and the Analytical Cytology Core in particular, of the Border Biomedical Research Center at UTEP. We would also like to express our appreciation for UTEP’s Veterinary Services Department which provided excellent support for our vivarium work. This project was supported by NIH Grant RR008124 (RCMI, to UTEP), and NSF Grant No. 0245071 (GRA, to SLC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg N, Carr JA, Summers CH. Ethological causes and consequences of the stress response. Int Comp Biol. 2002;42(3):508–516. doi: 10.1093/icb/42.3.508. [DOI] [PubMed] [Google Scholar]
- 3.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinol. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 4.Valles A, Marti O, Garcia A, Armario A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol Reg Int Comp Physiol. 2000;279:R1138–R1144. doi: 10.1152/ajpregu.2000.279.3.R1138. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu N, Oomura Y, Kai Y. Stress-induced anorexia in rats mediated by serotonergic mechanisms in the hypothalamus. Physiol Behav. 1989;46(5):835–841. doi: 10.1016/0031-9384(89)90045-0. [DOI] [PubMed] [Google Scholar]
- 6.Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol Reg Int Comp Physiol. 1997;273:R1612–R1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- 7.Morley JE, Levine AS, Rowland NE. Stress induced eating. Life Sci. 1983;32:2169–2182. doi: 10.1016/0024-3205(83)90415-0. [DOI] [PubMed] [Google Scholar]
- 8.Ueta Y, Ozaki Y, Saito J, Onaka T. Involvement of novel feeding-related peptides in neuroendocrine response to stress. Exp Biol Med. 2003;228:1168–1174. doi: 10.1177/153537020322801011. [DOI] [PubMed] [Google Scholar]
- 9.Powley TL, Opsahl CH, Cox JE, Weingarten HP. The role of the hypothalamus in energy homeostasis. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus. Part A: Behavioral Studies of the Hypothalamus. vol 3. New York: Marcel Dekker, Inc.; 1980. pp. 211–298. [Google Scholar]
- 10.Swanson LW, Sawchenko PE. Hypothalamic Integration: organization of the paraventricular and supraoptic nuclei. Ann Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 11.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: Evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 12.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinol. 1997;138(10):4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Fekete C, Légrádi G, Lechan RM. Glucagon like peptide-1 (7–36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985(2):163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- 14.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 15.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20(1):68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 16.Beck B. Neuropeptides and Obesity. Nutrition. 2000;16:916–923. doi: 10.1016/s0899-9007(00)00410-x. [DOI] [PubMed] [Google Scholar]
- 17.Cho YK, Li SC, Smith DV. Taste responses of neurons of the hamster solitary nucleus are enhanced by lateral hypothalamic stimulation. J Neurophysiol. 2002;87(4):1981–1992. doi: 10.1152/jn.00765.2001. [DOI] [PubMed] [Google Scholar]
- 18.Butler A, Cone R. The melanocortin receptors: Lessons from knockout models. Neuropeptides. 2003;36(2–3):77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 19.Cone RD. The corticotropin-releasing hormone system and feeding behavior—a complex web begins to unravel. Endocrinol. 2000;141:2713–2714. doi: 10.1210/endo.141.8.7700. [DOI] [PubMed] [Google Scholar]
- 20.Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- 21.Car JA. Stress, neuropeptides, and feeding behavior: A comparative perspective. Int Comp Biol. 2002;42(3):582–590. doi: 10.1093/icb/42.3.582. [DOI] [PubMed] [Google Scholar]
- 22.Boston BA. Pro-opiomelanocortin and weight regulation: from mice to men. J Pediatr Endocrinol Metab. 2001;14 Suppl 6:1409–1416. [PubMed] [Google Scholar]
- 23.Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, Sanz C, Vazquez P, Maldonado A, de Caceres J, Desco M, Pozo MA, Blazquez E. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92(4):798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 24.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessio DA, Sandoval DA, Seeley RJ. New ways in which GLP-1 can regulate glucose homeostasis. Clin Invest. 2005;115(12):3406–3408. doi: 10.1172/JCI27207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 27.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 28.Shu S, Ju G, Fan L. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. 4th Ed. San Diego: Elsevier (Academic Press); 1998. [Google Scholar]
- 30.Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: A review. Stress. 2002;5(1):3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- 31.Pirnik Z, Kiss A. Fos expression variances in mouse hypothalamus upon physical and osmotic stimuli: Co-staining with vasopressin, oxytocin, and tyrosine hydroxylase. Brain Res Bull. 2005;65(5):423–431. doi: 10.1016/j.brainresbull.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol Regul Integr Comp Physiol. 1998;275(5):R1438–R1449. doi: 10.1152/ajpregu.1998.275.5.R1438. [DOI] [PubMed] [Google Scholar]
- 33.Hillebrand JJ, de Wied D, Adan RA. Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides. 2002;23(12):2283–2306. doi: 10.1016/s0196-9781(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 34.Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress. J Comp Neurol. 2002;445:229–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- 35.Lu X, Barsh GS, Akil H, Watson SJ. Interaction between α-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci. 2003;23(21):7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinol. 1998;139(10):4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 37.Wachira SJ, Hughes-Darden CA, Nicholas HB, Jr, Taylor CV, Robinson TJ. Neural melanocortin receptors are differentially expressed and regulated by stress in rat hypothalamic-pituitary-adrenal axis. Cell Mol Biol. 2004;50(6):703–713. [PubMed] [Google Scholar]
- 38.Sebag JA, Hinkle PM. Regulation of endogenous melanocortin-4 receptor expression and signaling by glucocorticoids. Endocrinol. 2006;147(12):5948–5955. doi: 10.1210/en.2006-0984. [DOI] [PubMed] [Google Scholar]
- 39.Kas MJH, Bruijnzeel AW, Haanstra JR, Wiegant VM, Adan RAH. Differential regulation of agouti-related protein and neuropeptide Y in hypothalamic neurons following a stressful event. J Mol Endocrinol. 2005;35:159–164. doi: 10.1677/jme.1.01819. [DOI] [PubMed] [Google Scholar]