Abstract
Gait dysfunctions are highly prevalent in individuals post-stroke and affect multiple lower extremity joints. Recent evidence suggests that treadmill walking at faster than self-selected speeds can help improve post-stroke gait impairments. Also, the combination of functional electrical stimulation (FES) and treadmill training has emerged as a promising post-stroke gait rehabilitation intervention. However, the differential effects of combining FES with treadmill walking at the fast versus a slower, self-selected speed have not been compared previously. In this study, we compared the immediate effects on gait while post-stroke individuals walked on a treadmill at their self-selected speed without FES (SS), at the SS speed with FES (SS-FES), at the fastest speed they are capable of attaining (FAST), and at the FAST speed with FES (FAST-FES). During SS-FES and FAST-FES, FES was delivered to paretic ankle plantarflexors during terminal stance and to paretic dorsiflexors during swing phase. Our results showed improvements in peak anterior ground reaction force (AGRF) and trailing limb angle during walking at FAST versus SS. FAST-FES versus SS-FES resulted in greater peak AGRF, trailing limb angle, and swing phase knee flexion. FAST-FES resulted in further increases in peak AGRF compared to FAST. We posit that the enhancement of multiple aspects of post-stroke gait during FAST-FES suggest that FAST-FES may have potential as a post-stroke gait rehabilitation intervention.
Keywords: Stroke, Gait, Functional Electrical Stimulation, Treadmill, Fast walking
INTRODUCTION
Gait dysfunctions are prevalent in post-stroke individuals and contribute to slowed walking speeds 1. Recently, decreased propulsive force generation during paretic terminal stance was recognized as a critical post-stroke gait deficit that is correlated with hemiparetic severity and walking speed 2. Although gait impairments in individuals post-stroke span multiple joints, typically functional electrical stimulation (FES) is only delivered to ankle dorsiflexors to correct footdrop 3, 4. Recent literature suggests that delivering FES to multiple muscles 5, especially the ankle plantarflexors 6, 7, may help to maximize improvements in post-stroke gait with FES.
Recently, treadmill training has emerged as an intervention for improving walking performance post-stroke, providing the advantage of safely training at faster speeds 5, 8. Fast treadmill walking results in improved temporal symmetry and hip extension compared to treadmill walking at slower, self-selected speeds, and may improve energy efficiency of walking 9, 10. During treadmill training, FES applied at appropriate time in conjunction with volitional activation can facilitate motor learning and further enhance the therapeutic benefits on gait 5.
The current study tested the hypothesis that delivering plantar- and dorsi-flexor FES while post-stroke subjects walked at their fastest sustainable speeds would provide biomechanical advantages compared to delivering FES at the slower, self-selected walking speeds. We posited that during fast walking, a better paretic trailing limb position would enable greater contributions of ankle plantarflexor FES to increasing forward propulsion, which would thereby result in greater swing phase knee flexion 11, 12. Therefore, we anticipated that combined effects of FES and fast walking on post-stroke gait would be greater than the effects of fast walking without FES or walking at a slower, self-selected speed with or without FES.
METHODS
Subjects
Thirteen post-stroke subjects (Age =49 – 72 years; 9 males) who were >6 months following a single stroke, able to walk continuously for 5 minutes at their self-selected speed, and had sufficient passive ankle dorsiflexion range of motion to enable their paretic ankle joint to reach at least 5° plantarflexion (5° short of neutral) with the knee flexed were recruited (Table 1). Exclusion criteria included an inability to communicate and co-morbidities, orthopedic, or other neurologic conditions affecting walking. The over ground self-selected speed (SS) and the fastest speed the subjects could sustain for ~ 40-seconds during treadmill walking (FAST) were determined for each subject. All subjects signed consent forms approved by the Human Subjects Review Board of the University of Delaware.
Table 1.
Demographic and clinical information about the 13 recruited subjects. Data for Subjects # 3, 4, 12, and 13 were not included in the analysis because these subjects were unable to walk faster than their self-selected speeds without compromising safety during data collection (shown as X for the Fast speed). FES-logic refers to the logic (1 versus 2) used for timing of plantarflexor FES for each subject. Logic 1 delivered FES from the time the paretic hindfoot foot switch was off the ground until the paretic forefoot foot switch was off the ground. Logic 2 delivered FES from the time the non-paretic hindfoot foot switch contacted the ground until the paretic forefoot foot switch was off the ground. Ankle ROM refers to the passive ankle range of motion measured with the knee flexed at ~90°; N/A: ankle ROM data were not obtained from these subjects. The means and standard deviations presented are for the 9 subjects included in the analysis, who were able to walk at both SS and FAST speeds.
| Subject | Gender (M/F) | Age (yrs.) | Stroke onset (yrs.) | Hemiparesis (L/R) | SS Speed (m/s) | Fast Speed (m/s) | FuglMeyer LE Score | FES Logic | Ankle ROM (°) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 66 | 2.4 | L | 0.9 | 1 | 24 | L1 | 5 |
| 2 | M | 52 | 6.3 | L | 0.6 | 1.4 | 20 | L2 | N/A |
| 3 | F | 58 | 21.3 | L | 0.2 | X | 23 | X | N/A |
| 4 | F | 51 | 1.9 | L | 0.3 | X | 20 | X | N/A |
| 5 | M | 49 | 9.3 | R | 0.9 | 1 | 28 | L2 | 0 |
| 6 | M | 72 | 6.1 | R | 0.5 | 0.7 | 18 | L1 | 8 |
| 7 | M | 57 | 2.7 | R | 0.7 | 1 | 22 | L1 | 8 |
| 8 | M | 58 | 9.9 | R | 0.7 | 0.9 | 21 | L1 | 2 |
| 9 | M | 60 | 5.8 | R | 0.8 | 1.3 | 25 | L2 | 0 |
| 10 | M | 74 | 4.7 | R | 0.7 | 1.1 | 31 | L2 | 10 |
| 11 | M | 56 | 9.8 | R | 1.2 | 1.7 | 25 | L1 | 4 |
| 12 | F | 46 | 2.2 | L | 0.9 | X | 23 | X | 0 |
| 13 | F | 66 | 1.4 | R | 0.3 | X | 18 | X | 15 |
| Mean | 60.4 | 6.3 | 0.8 | 1.1 | 23.8 | 4.6 | |||
| StDev | 8.6 | 2.9 | 0.2 | 0.3 | 4.1 | 3.8 | |||
Electrical Stimulation was delivered to paretic ankle dorsiflexor (50.8 × 50.8 mm electrodes, TENS Products, CO) and plantarflexor muscles (76 × 127 mm electrodes, ConMed, NY) using surface electrodes. A Grass S8800 stimulator in combination with an SIU8 stimulus isolation unit was used to deliver electrical stimulation (Grass Instruments, MA). Two foot switches (forefoot and hindfoot; 25-mm MA-153, Motion Lab Systems Inc., LA) were attached bilaterally to the soles of both shoes. A customized FES-system, using input from footswitch signals (CompactRIO, National Instruments, TX) 7, 13, was used to stimulate the ankle dorsiflexors during paretic swing phase and plantarflexors during paretic terminal stance phase using one of two timing logics 7 (Table 1). Novel 30-Hz variable-frequency trains, beginning with a 200-Hz triplet, were used during FES 7, 13, 14.
Gait Analysis was performed as subjects walked on a split-belt treadmill (AMTI, Watertown, MA) instrumented with two force platforms. Subjects used a handrail and wore a harness attached to an overhead support (without bodyweight support) for safety. Marker data were collected using an 8-camera motion analysis system (Vicon 5.2, Oxford, UK) at 100-Hz and synchronized with analog data (2000-Hz). (See Kesar et. al. 2009 for detailed methodology 7).
These data were collected as part of a larger study. The testing session comprised 18 walking trials (40-seconds each) with 5-minute rest between consecutive trials. In this paper, we report data from walking: (1) without FES at the self-selected walking speed (SS), (2) without FES at the FAST walking speed (FAST), (3) at self-selected speed with FES delivered to both the ankle dorsi- and plantar-flexor muscles (SS-FES), and (4) at the FAST speed with FES delivered to both the ankle dorsi- and plantar-flexor muscles (FAST-FES). SS and FAST data were obtained by averaging data collected during the beginning (1st and 2nd trials), middle (8th and 9th trials), and end (17th and 18th trials) of the session. Data for SS-FES and FAST-FES conditions were collected either during the 3rd through 7th walk trials or 10th through 16th walk trials. The order of testing of walking conditions with FES was randomized across subjects.
For SS-FES and FAST-FES, for each subject, data from the timing logic (Logic 1 versus Logic 2) that generated greater peak anterior ground reaction force (AGRF) was included in statistical analyses (Table 1). All variables were computed using Visual 3D (C-Motion, Rockville, MD).
Dependent Variables
Peak AGRF during Paretic Stance: maximum AGRF between the onset of the propulsion (anterior) phase of antero-posterior GRFs and toe-off.
Percent Paretic Propulsion: ratio of AGRF integral from the onset of propulsion through to the end of stance phase for the paretic leg versus total AGRF integral for the paretic and non-paretic legs 2.
Peak Knee Flexion during Swing phase for the paretic leg.
Trailing Limb Angle: sagittal plane angle between the lab’s vertical (Z) axis and a vector joining markers located on the lateral malleolus and the greater trochanter of the paretic lower extremity. Trailing limb angle at paretic toe-off was used for analysis.
Statistical Analysis
A priori comparisons using paired t-tests were performed to compare SS versus FAST, SS-FES versus FAST-FES, and FAST versus FAST-FES for each dependent variable (SPSS 16.0, Chicago, IL). Based on a priori directional hypothesis, one-tailed pair-wise comparisons were performed to compare SS versus FAST (Effect of FAST speed alone), SS-FES versus FAST-FES (effect of FES delivered at the FAST versus the SS speed), and FAST versus FAST-FES for all dependent variables except trailing limb angle. Alpha level was set at 0.05. The Shapiro-Wilk test was used to test for normality (SPSS 16.0, SPSS Inc., Chicago, IL). Descriptive statistics in the text are Mean ± Standard errors.
RESULTS
Four of 12 subjects could not walk faster than their self-selected speeds safely during testing (Table 1). Thus, data for 9 subjects are included in the analyses. For each subject, we used data from 6 gait cycles because 6 was the maximum number of gait cycles with usable data for all 9 subjects.
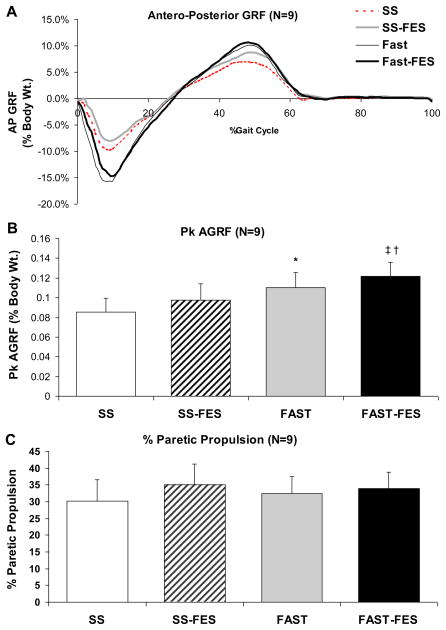
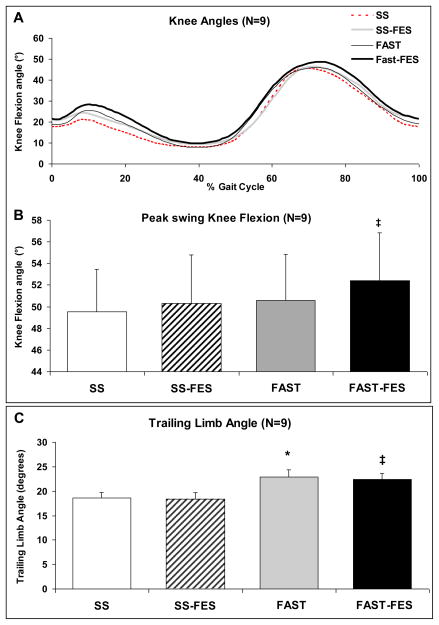
During walking without FES, greater peak AGRFs were observed at the FAST versus the SS speed (p≤0.01) (Figure 1). FAST-FES produced greater peak AGRFs compared to SS-FES (p≤0.05), and FAST-FES produced greater peak AGRF compared to FAST (p≤0.05). All three of these differences exceeded the recently reported within-session minimal detectable change (MDC) for peak AGRF (0.80 % body weight) 15. In contrast, no differences were detected in % propulsion. Significantly greater knee flexion was produced during FAST-FES (52.4±4.4°) compared to SS-FES (50.3±4.5° (p≤0.05) (Figure 2). This significant change was close to the within-session MDC for peak swing phase knee flexion (1.9°)15. There was no difference in knee flexion during walking at the FAST (50.6±4.3°) versus SS speeds (49.5±3.9°) (p=0.11) or during FAST-FES versus FAST (p=0.09). As hypothesized, greater trailing limb angle was observed during FAST (22.9±1.5°) versus SS (18.6±1.3°) (p≤0.01), and during FAST-FES (22.4±1.3°) versus SS-FES (18.4±1.3°) (p≤0.01) (Figure 2). Changes in trailing limb angle were also greater than the within-session MDC (1°) for this variable15. No other pair-wise differences were detected in trailing limb angle.
Figure 1.
(A) Average (N=9 subjects) time normalized antero-posterior GRF data (0%–100% = initial contact to initial contact).
Average values (N=9 subjects) and standard error bars for (B) peak anterior GRF (AGRF) and (C) percent paretic propulsion during paretic stance during SS, SS-FES, FAST, and FAST-FES. * Significant difference from SS (p≤0.05). † Significant difference from FAST (p≤0.05). ‡ Significant difference from SS-FES (p≤0.05).
Figure 2.
(A) Average (N=9 subjects) sagittal plane knee joint angles time-normalized to the gait cycle. Average values (N=9 subjects) and standard error bars for (B) peak knee flexion angles during paretic swing phase and (C) paretic trailing limb angle during SS, SS-FES, FAST, and FAST-FES. ‡ Significant difference from SS-FES (p≤0.05). * Significant difference from SS (p≤0.05).
DISCUSSION
Post-stroke individuals demonstrated immediate speed-related improvements in gait, and these improvements were further enhanced by delivering FES at the FAST walking speed. As hypothesized, FAST-FES produced greater peak AGRFs and swing phase knee flexion compared to SS-FES. Plantarflexor weakness can result in decreased forward propulsion during terminal stance, thereby leading to decreased swing phase knee flexion 11, 12. Our current results, along with our recent findings 7, provide experimental evidence for the relationship between plantarflexor activation, forward propulsion, and swing phase knee flexion in post-stroke individuals. Our current results also showed that FAST walking enabled a greater paretic trailing limb angle at toe-off, indirectly supporting our hypothesis that a greater trailing limb angle during FAST walking would place the paretic limb in a better biomechanical position at terminal stance, enabling plantarflexor FES to better contribute to forward propulsion at the FAST speed.
Our findings that FAST-FES produced greater improvements in peak AGRF, trailing limb position, and knee kinematics compared to SS-FES have clinical implications for the design of future gait rehabilitation studies. Our study is a step towards development of gait rehabilitation interventions that address specific post-stroke gait impairments. By using a hypothesis-based approach, we demonstrated immediate improvements in targeted post-stroke gait impairments across multiple joints (hip, knee, ankle) and multiple phases of the gait cycle (stance and swing phases). The improvements in gait demonstrated in the current study, albeit small, exceeded the MDC values15, were demonstrated despite a relatively small sample size and with very little walking practice with FES. These results support the need for future studies investigating the effectiveness and generalizability of FAST-FES as a gait rehabilitation intervention.
Acknowledgments
The authors would like to thank the following funding sources: National Institutes of Nursing Research R01 grant NR010786 and Bioengineering Research partnership R01 grant HD038582; NIH K01 HD050582; NIH Shared Instrumentation Grant S10 RR022396-01; DOD Grant W911NF-05-1-0097; University of Delaware Dissertation Fellowship.
The authors also thank Ms. Margie Roos, PT, NCS for clinical testing and subject recruitment, and Ms. Leigh Shrewsbury for scheduling and recruitment; thanks to Sarah Flynn for assistance with data-analysis. The authors also acknowledge the helpful reviews of this manuscript provided by Dr. Andrew J. Fuglevand, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olney SJ, Richards C. Hemiparetic gait following stroke. Part i: Characteristics. Gait Posture. 1996;4:136–148. [Google Scholar]
- 2.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37:872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 3.Weber DJ, Stein RB, Chan KM, Loeb G, Richmond F, Rolf R, James K, Chong SL. Bionic walkaide for correcting foot drop. IEEE Trans Neural Syst Rehabil Eng. 2005;13:242–246. doi: 10.1109/TNSRE.2005.847385. [DOI] [PubMed] [Google Scholar]
- 4.Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, MJIJ The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: A systematic review. Artif Organs. 2004;28:577–586. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- 5.Daly JJ, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, McCabe J, Fredrickson E, Marsolais EB, Ruff RL. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37:172–178. doi: 10.1161/01.STR.0000195129.95220.77. [DOI] [PubMed] [Google Scholar]
- 6.Embrey DG, Holtz SL, Alon G, Brandsma BA, McCoy SW. Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Arch Phys Med Rehabil. 91:687–696. doi: 10.1016/j.apmr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Kesar TM, Perumal R, Reisman DS, Jancosko A, Rudolph KS, Higginson JS, Binder-Macleod SA. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: Effects on poststroke gait. Stroke. 2009;40:3821–3827. doi: 10.1161/STROKEAHA.109.560375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: A randomized controlled trial. Stroke. 2002;33:553–558. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 9.Lamontagne A, Fung J. Faster is better: Implications for speed-intensive gait training after stroke. Stroke. 2004;35:2543–2548. doi: 10.1161/01.STR.0000144685.88760.d7. [DOI] [PubMed] [Google Scholar]
- 10.Reisman DS, Rudolph KS, Farquhar WB. Influence of speed on walking economy poststroke. Neurorehabil Neural Repair. 2009 doi: 10.1177/1545968308328732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 12.Anderson FC, Goldberg SR, Pandy MG, Delp SL. Contributions of muscle forces and toe-off kinematics to peak knee flexion during the swing phase of normal gait: An induced position analysis. J Biomech. 2004;37:731–737. doi: 10.1016/j.jbiomech.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Kesar TM, Perumal R, Jancosko A, Reisman DS, Rudolph KS, Higginson JS, Binder-Macleod SA. Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Phys Ther. 90:55–66. doi: 10.2522/ptj.20090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder-Macleod S, Kesar T. Catchlike property of skeletal muscle: Recent findings and clinical implications. Muscle Nerve. 2005;31:681–693. doi: 10.1002/mus.20290. [DOI] [PubMed] [Google Scholar]
- 15.Kesar TM, Binder-Macleod SA, Hicks GE, Reisman DS. Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke. Gait Posture. doi: 10.1016/j.gaitpost.2010.11.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]