Abstract
The neural correlates of emotion processing have been shown to vary with age: older adults (OAs) exhibit increased frontal activations and, under some circumstances, decreased amygdala activations relative to young adults (YAs) during emotion processing. Some of these differences are additionally modulated by valence, with age-related biases toward positive versus negative stimuli, and are thought to depend on OAs’ capacity for controlled elaboration. However, the role of semantic elaboration in mediating valence effects in the aging brain has not yet been explicitly tested. In the present study, YAs and OAs were scanned while they viewed negative, neutral, and positive pictures during either a deep, elaborative task or a shallow, perceptual task. FMRI results reveal that emotion-related activity in the amygdala is preserved in aging and insensitive to elaboration demands. This study provides novel evidence that differences in valence processing are modulated by elaboration: relative to YAs, OAs show enhanced activity in the medial prefrontal cortex (PFC) and ventrolateral PFC in response to positive versus negative stimuli, but only during elaborative processing. These positive valence effects are predicted by individual differences in executive function in OAs for the deep but not shallow task. Finally, psychophysiological interaction analyses reveal age effects on valence-dependent functional connectivity between medial PFC and ventral striatum, as well as age and task effects on medial PFC-retrosplenial cortex interactions. Altogether, these findings provide support for the hypothesis that valence shifts in the aging brain are mediated by controlled processes such as semantic elaboration, self-referential processing, and emotion regulation.
Keywords: affective, valence, functional MRI, neuroimaging, elderly, levels of processing
Emotion processing is well-preserved relative to other cognitive functions in aging, with little structural decline in the amygdala (Grieve, Clark, Williams, Peduto, & Gordon, 2005; Soininen, et al., 1994) as well as few changes in the ability to detect emotional information (LaBar et al., 2000; Mather & Knight, 2006). However, there may be alterations in how older adults (OAs) prioritize emotional information relative to young adults (YAs), in that OAs tend to focus more on positively-valenced information and less on negatively-valenced information (e.g., Isaacowitz, Wadlinger, Goren, & Wilson, 2006; Kennedy, Mather, & Carstensen, 2004; Mather & Carstensen, 2003; Mather & Knight, 2005; but see Leclerc & Kensinger, 2008b; Mickley Steinmetz, Muscatell, & Kensinger, 2010). Complementing these behavioral findings, OAs tend to show different patterns of brain activity in response to emotional material when compared to YAs. These differences have been characterized as under-recruitment of the amygdala (e.g., Gunning-Dixon, et al., 2003; Iidaka, et al., 2002; Tessitore, et al., 2005) and over-recruitment of frontal control regions (e.g., Leclerc & Kensinger, 2008a; St. Jacques, Dolcos, & Cabeza, 2010), a pattern that has been referred to as Fronto-amygdalar Age-related Differences in Emotion (FADE; St. Jacques, Bessette-Symons, & Cabeza, 2009).
Despite this gross characterization, amygdala findings have been mixed—although some studies report amygdala under-recruitment in aging (Fischer, et al., 2005; Gunning-Dixon, et al., 2003; Iidaka, et al., 2002; Murty, et al., 2009; Tessitore, et al., 2005), others report no change (Leclerc & Kensinger, 2008a; Mather, et al., 2004; St. Jacques, et al., 2010; Wright, Wedig, Williams, Rauch, & Albert, 2006). This discrepancy has been linked to differences in emotion ratings between age groups: those studies that report rating differences tend to also report amygdala differences, whereas the others do not (St. Jacques, et al., 2009). There may also be differences in responses to negatively- versus positively-valenced stimuli, with the latter eliciting enhanced activity in OAs relative to YAs (Mather, et al., 2004). Frontal findings, however, have been fairly consistent: OAs tend to recruit greater activity in frontal regions such as the medial prefrontal cortex (PFC) (St. Jacques, et al., 2010; Tessitore, et al., 2005) and lateral PFC (Gunning-Dixon, et al., 2003; Murty, et al., 2009; Tessitore, et al., 2005) during emotion processing relative to YAs. Taken together, these findings are consistent with the assumptions that amygdala responses map onto OAs’ reported emotional experience, and that this experience may be affected by age-related increases in emotion control, mediated by the frontal lobes. At the neural level, these findings are also compatible with a general pattern of neural activity in aging, referred to as the Posterior- to-Anterior Shift in Aging (PASA). This shift has been hypothesized to reflect heightened recruitment of frontal control regions as compensation for reductions in processing in posterior brain regions, such as perceptual processing in visual cortex, that typically accompany aging (Grady, et al., 1994). PASA has been identified in numerous functional neuroimaging studies of aging across multiple cognitive domains (e.g., Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Dennis & Cabeza, 2008).
Within the domain of emotion processing, increased recruitment of frontal regions may be indicative of enhanced semantic elaboration of positively-valenced stimuli or down-regulation of responses to negatively-valenced stimuli. Behavioral studies of emotion processing in OAs have been marked by shifts in valence processing, characterized both as positivity shifts (Mather & Carstenson, 2005; Mather & Knight, 2005) and negativity reductions (Charles, Mather, & Carstenson, 2003; St. Jacques, et al., 2010). OAs exhibit attentional biases toward positive and away from negative information relative to YAs (Isaacowitz, et al., 2006; but see Leclerc & Kensinger, 2008b; Mather & Carstensen, 2003) and retrieve more positive memories than negative whereas YAs tend to show the reverse pattern (Kennedy, et al., 2004; Mather & Knight, 2005). Theories espousing positivity shifts have tended to emphasize enhanced elaboration and up-regulation of positively-valenced stimuli as OAs attempt to maximize positive affect as they get older (socioemotional selectivity theory; reviewed by Mather & Carstenson, 2005).
In keeping with these theories, behavioral positivity shifts are correlated with individual differences in executive function (Mather & Knight, 2005), which are presumably supported by the frontal lobes (Miller & Cohen, 2001), and are eliminated under divided attention conditions (Mather & Knight, 2005). Within the frontal lobes, the medial PFC is a likely candidate for mediating age-related positivity shifts. Positive versus negative or neutral stimuli tend to elicit greater activations in medial PFC in young populations (Dolcos, LaBar, & Cabeza, 2004; E.A. Kensinger & Schacter, 2006), though medial PFC regions also may be more broadly involved in emotional experience (Phan, Wager, Taylor, & Liberzon, 2002) and control (Ochsner & Gross, 2005). OAs have been shown to over-recruit the medial PFC in response to positive versus negative stimuli relative to YAs (Gutchess, Kensinger, & Schacter, 2007; Leclerc & Kensinger, 2008a). A similar pattern has also been observed in lateral PFC (Gutchess, et al., 2007). Enhanced medial PFC activation in response to positive stimuli may be attributable to self-referential processing. The medial PFC shows heightened activity when participants attend to stimuli that are self-relevant versus non-relevant (Gutchess, et al., 2007; Kelley, et al., 2002) and retrieve autobiographical memories (Cabeza, et al., 2004; Levine, et al., 2004). The capacity for self-referential processing appears to be intact in aging (Glisky & Marquine, 2009), and its association with the medial PFC has been replicated in OAs, in that both young and older adults show heightened activity in this region when evaluating whether adjectives describe one’s self or another person (Gutchess, et al., 2007). Altogether, these findings engender the interpretation that age-related over-recruitment of the frontal lobes during positive valence processing may reflect controlled, elaborative processes instantiated in the medial PFC (Kensinger & Leclerc, 2009).
In contrast to these positive valence effects, there is additional evidence that OAs over-recruit the PFC in response to negatively-valenced stimuli. Reductions in attention to and memory for negative stimuli in OAs have been interpreted as the results of emotion regulation processes designed to mitigate negative emotion (as reviewed by Mather & Carstenson, 2005; St. Jacques, et al., 2009). In keeping with this idea, heightened frontal responses to negative stimuli have been interpreted as indexing emotion regulation processes in OAs, evidenced by concomitant increases in emotional stability (Williams, et al., 2006) and decreases in amygdala response to negative stimuli (Gunning-Dixon, et al., 2003; Tessitore, et al., 2005), as well as negative correlations between frontal and amygdala regions (St. Jacques, et al., 2010; Urry, et al., 2006). One of these studies also identified decreases in amygdala response to stimuli that normative ratings classify as negative but OAs’ individual ratings classify as neutral, suggesting reductions in perceived negativity and corresponding amygdala response (St. Jacques, et al., 2010). A recent study explicitly tested the neural consequences of reappraisal, an emotion regulation strategy, in both YAs and OAs, and showed that reappraisal was associated with increased frontal and decreased amygdalar activations (Winecoff, Labar, Madden, Cabeza, & Huettel, 2010). Finally, there is evidence that in addition to predicting behavioral valence shifts (Mather & Knight, 2005), individual differences in executive function modulate the degree to which OAs engage ventrolateral PFC while inhibiting negative responses to stigmatized individuals (Krendl, Heatherton, & Kensinger, 2009), as well as the degree to which OAs down-regulate amygdala activation during reappraisal (Winecoff, et al., 2010).
Although OAs’ dependency on medial and lateral PFC regions during emotional processing has been hypothesized to reflect controlled processes, direct evidence supporting this hypothesis is scarce. Divided attention studies have provided behavioral evidence for a relationship between controlled processes and age effects on emotional processing (Mather & Knight, 2005); however, there is no direct evidence tying these effects to age differences in medial and lateral PFC. In addition, divided attention impacts multiple aspects of controlled processing, leaving open the question of which core mechanisms drive age effects on emotional processing. One way to address these questions is to modulate the task demands on semantic elaboration, thus targeting a specific candidate mechanism for the described age effects. Semantic elaboration is likely to be a subcomponent of emotion regulation and appraisal (Ochsner, et al., 2009). By definition, the emotion regulation strategy of reappraisal involves re-interpreting the affective meaning of an event or stimulus, and not surprisingly, compared with distraction, reappraisal involves increased recruitment of regions associated with semantic processing such as left lateral prefrontal and temporal regions (McRae, et al., 2010). Furthermore, reappraisal of emotional pictures boosts subsequent memory for those pictures, thought to be driven by this regulation strategy’s reliance on semantic processing (Dillon, Ritchey, Johnson, & LaBar, 2007). Self-referential processing also shares some features and substrates with semantic processing, in that both promote enhanced subsequent memory and left lateral PFC recruitment (Craik, et al., 1999; Kelley, et al., 2002). Although medial PFC involvement in self-referential processing may reflect a more specialized mechanism that is not exclusively driven by semantic functions (Glisky & Marquine, 2009; Kelley, et al., 2002), enhancements in semantic processing may increase the likelihood that a stimulus may be interpreted as self-relevant and thus facilitate the recruitment of these other self-referential processes. Thus, because of these links to other higher-order functions, semantic elaboration may provide an access point by which one can test the influence of aging on emotional control. The goal of the present study was to specifically assess the role of semantic elaboration in mediating the effects of aging on emotion processing. To this end, older and younger adults were scanned while viewing emotionally negative, neutral, and positive stimuli during two separate tasks that varied in semantic elaboration demands: a deep, semantically-focused task and a shallow, perceptually-focused task. Arousal ratings were matched between groups to control for confounds in perceived arousal or responsivity. Sex differences in emotion processing were additionally assessed, since these differences have been characterized within YAs (Hamann & Canli, 2004) yet have been rarely considered in neuroimaging studies with OAs. Within this experimental design, the present study further aimed to link elaboration effects on age-related valence shifts with individual differences in executive function, as well as with changes in functional connectivity with frontal structures.
The present study employed 3 main analysis strategies to subserve these experimental goals. First, this study examined the main effects and interactions of age group, task, and valence on emotion effects in the brain. It is expected that, because arousal ratings are matched, there will be no age differences in emotion-related activity in the amygdala. Age differences in PFC activations associated with positive valence are anticipated, with the additional expectation that these differences will be augmented in the semantic elaboration task. Second, this study explored the link between neural differences associated with valence effects in aging and individual differences on a battery of tests indexing executive function. It is predicted that valence effects in the PFC will be associated with executive function in OAs. Finally, as an exploratory measure, functional connectivity analyses queried the relationship between the medial PFC and the rest of the brain as a function of stimulus valence.
Methods
Participants
Twenty-one YAs and 19 OAs participated in the study. Participants were healthy, right-handed, native English speakers, with no disclosed history of neurological or psychiatric episodes. Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board. Due to excessive head movement, 1 young adult participant was excluded from all analyses; an additional 3 OA participants were unable to complete the experiment due to technical problems. All behavioral analyses were conducted on the remaining 20 young adult participants (10 female; mean Age = 23.2, SD = 3.1) and 16 older adult participants (7 female; mean Age = 66.7, SD = 5.7). After excluding trials on the basis of arousal ratings (see below), 2 participants (1 YA and 1 OA) had fewer than 8 trials for one of the trial types of interest, and were additionally excluded from the fMRI analysis.
Materials
Stimuli
Stimuli consisted of 630 pictures. These were selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2001) as well as from an in-house, standardized database that allowed us to better equate the pictures for visual complexity and content (e.g., human presence). Pictures were assigned on the basis of a 9-point normative valence scale to emotionally negative (valence: 1–3.99), neutral (valence: 4–5.99), and positive (valence: 6–9) conditions. Negative and positive pictures were selected to be high in arousal (arousal > 5), as indexed by a 9-point normative arousal scale (1 = calm, 9 = excited), whereas neutral pictures were chosen to be low in arousal (arousal < 5). Normative arousal ratings for negative (M = 5.72, SD = 0.49) and positive (M = 5.68, SD = .59) did not significantly differ (t (418) = .62, p = .54).
Neuropsychological Testing
In a separate session, a subset of our OA sample (N = 12; 4 females; mean Age = 67.5 ± 6.3) was additionally tested on a battery of neuropsychological tests designed to assess executive functions (Glisky et al. 1995; 2001). These tasks included: the Wisconsin Card Sorting Task, Controlled Oral Word Association Test (FAS), Mental Arithmetic (Wechsler Adult Intelligence Scale-III), Mental Control and Backward Digit Span (Wechsler Memory Scale-III). Several tasks indexing memory function were also administered, but will not be considered here. This testing session lasted approximately 2.5 hours, including breaks, and was conducted an average of 9 months before the scan session.
Results of neuropsychological testing were used to generate composite measures of executive function among OAs. The executive function composite score represented the mean of the z-scores (calculated within the present sample) for each neuropsychological test listed above (for more detail, see Glisky, Polster, & Routhieaux, 1995; Glisky, Rubin, & Davidson, 2001). After calculating the composite executive function score, 1 participant was identified as an outlier (> 2 standard deviations from the mean) and was excluded from analyses using the composite scores.
Procedure
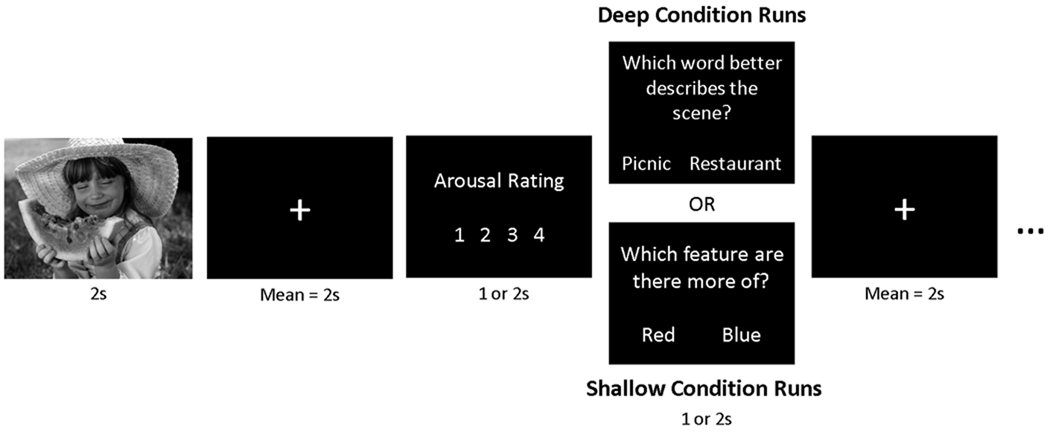
Participants viewed 140 negative, 140 positive, and 140 neutral pictures while functional MR images were recorded. The scan session consisted of 10 functional runs, across which negative, positive, and neutral pictures were evenly divided. Runs alternated between two distinct tasks, deep and shallow, described below. To avoid the induction of long-lasting mood states, the pictures within each block where pseudo-randomized so that no more than three pictures of the same valence were consecutively presented. The assignment of stimulus lists to the deep versus shallow task was counterbalanced across participants.
In the deep task, participants were instructed to carefully analyze each picture for its meaning and interpretation. In the shallow task, participants were instructed to carefully analyze each picture for its perceptual features, particularly colors and lines. Critically, participants were cued before each run as to which task was next, and were instructed to tailor their processing of each picture to the current task. Trial structure was similar between tasks (see Figure 1). For each trial a picture was presented for 2 seconds, followed by a jittered fixation interval (mean = 2 s). After this interval the participant was instructed to rate the picture for its emotional arousal or intensity on a 4-point scale (1 = calm, 4 = excited); YAs were given 1 s to make this rating whereas OAs were given 2 s. Finally, a question screen appeared that was designed to test the participant’s semantic or perceptual analysis of the picture, and provided ongoing reminders and motivation to complete the requisite task. In the deep task, the question screen said, “Which word best describes the picture?” Two possible options were presented on-screen, both of which were written for each picture such that both could be related to the picture but only one described the true meaning of the picture. For example, an image depicting people climbing down the stairs from an airplane might be followed by the options “arrival” and “departure.” In the shallow task, the question screen said, “Which feature is there more of?” Two possible options were presented on-screen: either two color names or the words horizontal and vertical. These options were displayed for 1 s in YAs and 2 s in OAs. Trials were separated by an additional jittered fixation interval (mean = 2 s). Either 1 (YAs) or 2 (OAs) days after the scan session, participants completed a recognition task for the pictures. Memory effects for the YAs are presented elsewhere (Ritchey, LaBar, & Cabeza, in press) and will not be discussed further.
Figure 1.
Schematic of the experimental design for a single trial during encoding. Separate lists of 70 negative, 70 neutral, and 70 positive pictures were assigned to deep and shallow conditions. Deep and shallow conditions were blocked across runs.
Behavioral Analysis
Average arousal ratings were calculated separately for each trial type and entered into separate repeated-measures ANOVAs with emotion (negative, neutral, positive), task (deep, shallow), and sex (male, female) as factors. Although no significant group effects were identified (see Results), previous studies have identified some differences between YA and OA ratings of the IAPS pictures (Backs et al., 2005; Gruhn et al., 2008). Therefore, to more carefully match arousal responses between groups as well as reduce variability across individuals within each group, each individual’s arousal ratings were used to exclude from analysis any negative or positive picture that was rated low in arousal (rating 1 or 2) and any neutral picture that was rated high in arousal (rating 3 or 4). For any pictures in which the arousal rating response was missed due to the rapid-paced design (4.8 ± 3.5% of trials in OAs, 10.9 ± 6.6 % in YAs), the normative IAPS arousal ratings were used. Thus, both behavioral and fMRI analyses were restricted to highly-arousing negative (mean N = 47.4 for OA, 46.9 for YA) and positive pictures (mean N = 33.3 for OA, 36.2 for YA) and low-arousing neutral pictures (mean N = 64.7 for OA, 66.9 for YA) within each task. This procedure had the further benefit of minimizing differences in arousal between negatively- and positively-valenced stimuli as well as sex-related differences.
fMRI Methods
Scanning
Images were collected using a 4T GE scanner. Stimuli were presented using liquid crystal display goggles (Resonance Technology, Northridge, CA), and behavioral responses were recorded using a four-button fiber optic response box (Resonance Technology). Scanner noise was reduced with earplugs and head motion was minimized using foam pads and a headband. Anatomical scanning started with a three-plane localizer series. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC-PC plane. High-resolution T1-weighted structural images were collected with a 24-cm field of view (FOV), a 2562 matrix, 68 slices, and a slice thickness of 1.9 mm. Functional images were acquired using an inverse spiral sequence with a 2-sec TR, a 31-msec TE, a 24-cm FOV, a 642 matrix, and a 60° flip angle. Thirty-four contiguous slices were acquired in an interleaved fashion with the same slice prescription as the anatomical images. Slice thickness was 3.8 mm, resulting in 3.75 × 3.75 × 3.8 mm voxels.
fMRI analyses
Preprocessing and data analyses were performed using SPM5 software implemented in Matlab (www.fil.ion.ucl.ac.uk/spm/). After discarding the first 6 volumes, the functional images were slice-timing corrected and motion-corrected, spatially normalized to the Montreal Neurological Institute (MNI) template, spatially smoothed using an 8 mm isotropic Gaussian kernel, and resliced to a resolution of 3.75 × 3.75 × 3.8 mm voxels. Data were high-pass filtered using a cutoff of 128 seconds. For each subject, evoked hemodynamic responses to event types were modeled with a delta (stick) function corresponding to stimulus presentation convolved with a canonical hemodynamic response function within the context of the general linear model, as implemented in SPM5. Six main event types were modeled, representing all possible combinations of emotion (negative, neutral, positive) and task (deep, shallow). As described above, these main event types were restricted to highly-arousing emotional stimuli and low-arousing neutral stimuli. Stimuli falling into the other categories were modeled by separate regressors (i.e., low-arousing negative, low-arousing positive, highly-arousing neutral) but not analyzed further. An additional regressor also modeled the separate effects of the arousal rating and question period, but this regressor was not included in any analyses. Thus, all effects reflect activity during the picture period only. Because participants were instructed to attend to either the semantic or perceptual attributes of the stimulus at this time, depending on prior task instructions, this period captures processing differences due to deep versus shallow tasks that are unconfounded with differences that may arise during the question period. Confounding factors (head motion, session means) were also included in the model.
Estimates for the contrast of negative or positive versus neutral, binned by task, were generated for each participant, and then entered into a mixed ANOVA with factors for age group (YA, OA), valence (negative versus neutral, positive versus neutral), and task (deep, shallow). Thus, the neutral trial types serve as a baseline for all effects reported here. Main effects and interactions were evaluated at p < .001, extent threshold = 10 voxels. All main effects and 2-way interactions were exclusively masked with corresponding higher-order effects at p < .05 (a conservative threshold for exclusive masking), since lower-order effects are interpretable only in the absence of higher-order effects. Because the interaction term tests all possible patterns of interactions, the 3-way group x valence x task interaction also was inclusively masked with the contrast indexing positive > negative x deep > shallow in the OA group (p < .05). This verified the direction of the interaction as well as the presence of a significant effect within the OA group alone. For a subset of the regions identified by the ANOVA, mean contrast values for the negative or positive versus neutral comparisons, for both the deep and shallow tasks, were extracted from each region for each OA participant. This included a cluster within medial PFC that extended into right insula; for all subsequent analyses, this cluster was anatomically-constrained within medial frontal gyrus and anterior cingulate, using Talairach Daemon labels (Lancaster, Summerin, Rainey, Freitas, & Fox, 1997; Lancaster, et al., 2000) implemented in the WFU Pickatlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). These regional means were then correlated across-subjects with composite scores of executive function. Statistical outliers were identified (standardized residual > 2) in SPSS and excluded. It should be noted that the identification of each region and evaluation of its mean were conducted independently of the individual difference measures themselves. Finally, the effects of sex were likewise assessed for a subset of regions (bilateral amygdala, medial PFC, left vlPFC, and striatum) by entering the mean contrast values into a mixed ANOVA with factors for age group, valence, task, and sex.
Psychophysiological interaction analysis
A region in medial PFC identified in the previous ANOVA was used as the seed in an exploratory psychophysiological interaction (PPI) analysis (Friston, et al., 1997). PPI tests the hypothesis that functional coupling between a seed region and the rest of the brain varies as a function of task conditions. In this analysis, deconvolved activity time-courses are extracted from a seed region and then multiplied by the psychological variable of interest—namely, a contrast between conditions. The resulting term denotes the PPI, which is used as a regressor for activity across the rest of the brain. In this analysis, we tested the influence of valence on medial PFC interactions by generating two separate PPIs indexing two psychological variables of interest: negative versus neutral and positive versus neutral, represented by a contrast set to 1 for negative or positive trials and −1 for neutral trials. To simplify calculation of the timecourses, we constructed a new general linear model in which data were concatenated across runs, separately for each task. This concatenation procedure allowed us to evaluate a single VOI and PPI model for each task, valence, and participant, rather than one for each run. The concatenated model was identical the model described above, with the addition of regressors for each run to account for any session effects. For our seed region, we used the anatomically-constrained medial PFC cluster from the ANOVA analysis described above. Within this cluster, we extracted the representative timecourse for voxels that showed any effect across an omnibus F-test at p < .05. The PPI models included regressors for the physiological variable (medial PFC timecourse), psychological variable (emotion contrast), and the PPI, the latter of which was our primary regressor of interest. Contrasts corresponding to the PPI terms were extracted for each task and each participant, and entered into a mixed ANOVA with age group (YA, OA), task (deep, shallow), and valence (negative, positive) as factors. As in the previous ANOVA, we tested the full complement of main effects and interactions (p < .005, extent threshold = 10), with exclusive masking of higher-order effects (p < .05). Because the medial PFC seed region was chosen on the basis of its interesting valence interactions with group and task, we focus on only those effects that are modulated by valence. Each PPI contrast indicates that the degree to which functional coupling between the medial PFC and every other voxel is modulated by emotion; thus, the PPI ANOVA tests how age and task influence valence-dependent interactions between the medial PFC and the rest of the brain.
Results
Behavioral
Arousal Ratings
Average arousal rating scores (Table 1) were entered into a mixed ANOVA with age group (YA, OA), emotion (negative, neutral, positive), task (deep, shallow), and sex (male, female) as factors. There was a significant main effect of emotion, F(2, 64) = 268.95, p < .001. Follow-up tests revealed that negative pictures were rated as more arousing than neutral, F(1, 32) = 494.92, p < .001, or positive, F(1, 32) = 31.66, p < .001 pictures. Positive pictures were also rated as more arousing than neutral pictures, F(1, 32) = 350.68, p < .001. There was no main effect of task, F(1, 32) = .18, p = .68, or interaction of task and emotion, F(2, 64) = 1.84, p = .17, indicating that our task manipulation did not alter the participants’ perceived emotional responses to the stimuli. There was no main effect of age group or interactions of age group with task or emotion, all Fs < 1, p > .6, suggesting that older and younger adults had comparable arousal responses to the stimuli. Finally, there was a significant sex by emotion interaction, F(2, 64) = 7.21, p = .002, and a marginally-significant age group by sex by emotion interaction, F(2, 64) = 2.53, p = .09. The latter effects reflect mainly that females rated the negative items as higher in arousal than males, F(1, 32) = 7.55, p = .01, a pattern that tended to be accentuated in OAs. All other effects involving sex were null, all Fs < 1.2, p > .2. Because the included trials were subsequently tailored to each individual’s arousal ratings, the influences of valence and sex on arousal was mitigated with respect to the fMRI analyses.
Table 1.
Arousal ratings: Mean (SD)
| Deep Task | Shallow Task | |||||
|---|---|---|---|---|---|---|
| Group | Negative | Positive | Neutral | Negative | Positive | Neutral |
| YA male | 2.71 (.35) | 2.47 (.40) | 1.43 (.21) | 2.68 (.43) | 2.41 (.47) | 1.43 (.22) |
| YA female | 2.92 (.41) | 2.46 (.46) | 1.35 (.21) | 2.97 (.44) | 2.44 (.44) | 1.35 (.18) |
| OA male | 2.59 (.37) | 2.58 (.45) | 1.38 (.26) | 2.6 (.36) | 2.5 (.45) | 1.39 (.28) |
| OA female | 3.04 (.35) | 2.3 (.45) | 1.56 (.26) | 3.06 (.36) | 2.31 (.45) | 1.58 (.32) |
Note: Arousal ratings range from 1 (calm) to 4 (excited), independent of valence.
fMRI
Emotion-related activity: Main effects and interactions
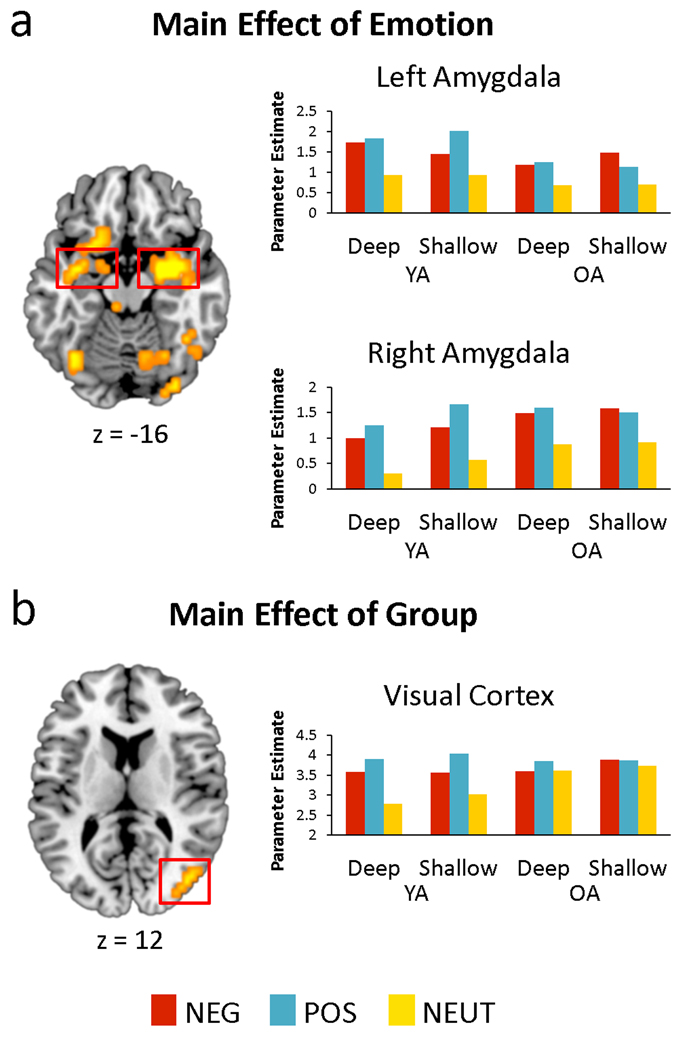
Contrasts of negative or positive versus neutral for each task and participant were entered into a mixed ANOVA with age group, valence, and task as factors. The full complement of main effects and interactions were tested, and are reported in Table 2. Of particular interest, the main effect of condition (effects spanning all contrasts, with no other main effects or interactions) yielded significant effects in bilateral amygdala together with a widespread network across the brain (Figure 2a), suggesting that the influence of arousal on the amygdala is independent of age, valence and task. Region-of-interest analyses revealed a task by valence by sex interaction in the right amygdala cluster, F(1, 30) = 6.54, p = .016, reflecting enhanced activity for negative stimuli during deep processing in females relative to males, but this effect was independent of age. A main effect of age group, regardless of valence or task, was also identified in the middle occipital gyrus (BA 19/37), indicating greater emotion-related activity in the YAs than OAs (Figure 2b). Age-independent main effects of task were identified in bilateral dorsolateral PFC (BA 6/9), indicating enhanced emotion effects in these frontal regions during deep relative to shallow processing. Finally, age-independent main effects of valence (positive > negative) were identified in a widespread network including several midline regions, such as ventromedial PFC, anterior cingulate, posterior cingulate, and precuneus. Additional findings are reported in Table 2.
Table 2.
Main Effects on Emotion-Related Activity
| BA | Hem | x | y | z | F | voxels | |
|---|---|---|---|---|---|---|---|
| Main Effect of Condition: Negative and Positive > Neutral | |||||||
| Postcentral Gyrus | 3 | L | −41 | −19 | 57 | 73.46 | 369 |
| -- | 2 | L | −45 | −27 | 40 | 68.71 | -- |
| -- | 3 | L | −55 | −17 | 22 | 53.83 | -- |
| Superior Frontal Gyrus | 6 | L | −19 | −8 | 53 | 49.35 | -- |
| Cingulate Gyrus | 24 | L | −8 | 6 | 35 | 36.58 | -- |
| Middle Temporal Gyrus | 39 | L | −52 | −61 | 10 | 63.77 | 85 |
| Fusiform Gyrus | 37 | L | −38 | −63 | −13 | 25.94 | -- |
| Inferior Frontal Gyrus | 47 | L | −41 | 29 | −8 | 57.99 | 172 |
| Putamen/Amygdala | L | −30 | −8 | −6 | 40.87 | -- | |
| Globus Pallidus | L | −8 | 0 | −7 | 32.92 | -- | |
| Insula | 13 | L | −41 | 1 | 10 | 31.76 | -- |
| Inferior Frontal Gyrus | 47 | L | −26 | 10 | −13 | 25.88 | -- |
| Postcentral Gyrus | 40 | R | 59 | −21 | 22 | 50.47 | 42 |
| Precentral Gyrus | 6 | R | 55 | 2 | 31 | 30.88 | -- |
| Fusiform Gyrus | 37 | R | 41 | −59 | −7 | 47.55 | 11 |
| Amygdala | R | 22 | −4 | −13 | 44.38 | 136 | |
| Insula | 13 | R | 38 | −19 | −6 | 20.4 | -- |
| -- | 13 | R | 38 | 4 | 0 | 14.12 | -- |
| Middle Temporal Gyrus | 21 | R | 49 | −12 | −19 | 12.99 | -- |
| Thalamus | L | −7 | −29 | 1 | 42.16 | 30 | |
| Inferior Frontal Gyrus | 47 | R | 41 | 33 | 2 | 39.01 | 32 |
| Fusiform Gyrus | 37 | R | 45 | −59 | −13 | 38.06 | 10 |
| Inferior Occipital Gyrus | 18 | R | 33 | −84 | −5 | 35.54 | 22 |
| Inferior Frontal Gyrus | 44 | R | 52 | 12 | 20 | 27.89 | 36 |
| Precentral Gyrus | 6 | R | 30 | 4 | 23 | 14.67 | -- |
| Medial Frontal Gyrus | 8 | L | −11 | 38 | 40 | 26.95 | 12 |
| Fusiform Gyrus | 19 | R | 22 | −63 | −13 | 23.41 | 13 |
| Main Effect of Group: YA > OA | |||||||
| Middle Occipital Gyrus | 19 | R | 41 | −76 | 14 | 22.71 | 13 |
| Middle Occipital Gyrus | 37 | R | 48 | −62 | −3 | 20.09 | 13 |
| Main Effect of Task: Deep > Shallow | |||||||
| Middle Frontal Gyrus | 6 | R | 22 | 2 | 42 | 20.24 | 22 |
| Middle Frontal Gyrus | 6 | L | −22 | −2 | 39 | 16.82 | 23 |
| Inferior Frontal Gyrus | 9 | L | −48 | 8 | 24 | 13.2 | 10 |
| Main Effect of Valence: Positive > Negative | |||||||
| Precuneus | 31 | L | −4 | −68 | 28 | 55.11 | 1278 |
| -- | 19 | R | 15 | −79 | 39 | 41.08 | -- |
| Inferior Parietal Lobule | 39 | R | 45 | −64 | 38 | 48.08 | -- |
| -- | 39 | L | −45 | −64 | 38 | 40.97 | -- |
| Posterior Cingulate Gyrus | 31 | R | 8 | −38 | 41 | 34.754 | -- |
| Ventral Anterior Cingulate | 32 | 0 | 40 | −8 | 32.12 | 84 | |
| Anterior Cingulate | 24 | 0 | 30 | 12 | 18.81 | -- | |
| Middle Temporal Gyrus | 37 | R | 45 | −69 | 7 | 29.51 | 128 |
| Middle Frontal Gyrus | 10 | L | −30 | 45 | 22 | 26.15 | 37 |
| Medial Frontal Gyrus | 32 | L | −15 | 30 | 26 | 12.67 | -- |
| Cerebellum | L | −37 | −45 | −27 | 25.88 | 92 | |
| -- | L | −34 | −64 | −32 | 20.55 | -- | |
| Inferior Parietal Lobule | 40 | L | −45 | −45 | 44 | 25.63 | 66 |
| Hippocampus | R | 30 | −26 | −8 | 20.46 | 12 | |
| Middle Frontal Gyrus | 10 | R | 37 | 44 | 15 | 20.44 | 12 |
| Medial Frontal Gyrus | 9 | R | 19 | 30 | 26 | 19.64 | 26 |
| Middle Occipital Gyrus | 19 | L | −48 | −69 | 7 | 18.95 | 23 |
| Cerebellum | R | 26 | −60 | −26 | 18.54 | 11 | |
| Inferior Parietal Lobule | 40 | L | −56 | −28 | 22 | 16.92 | 15 |
| Caudate Head | R | 7 | 11 | 3 | 15.89 | 11 | |
| Caudate Tail | R | 19 | −25 | 19 | 15.45 | 12 | |
Up to 5 local maxima set 20 mm apart are reported for each cluster. BA = Brodmann Area, Hem = Hemisphere. Coordinates are in Talairach space.
Figure 2.
a) Main effect of emotion-related activity across all trial types in left and right amygdala, indicating emotion enhancements regardless of age and task. b) Main effect of age group on emotion-related activity in the middle occipital gyrus (BA 19), indicating a reduction in emotion enhancements with aging. Activation effects are overlaid on a T1 MNI template, and mean contrast estimates within each region are plotted for each condition.
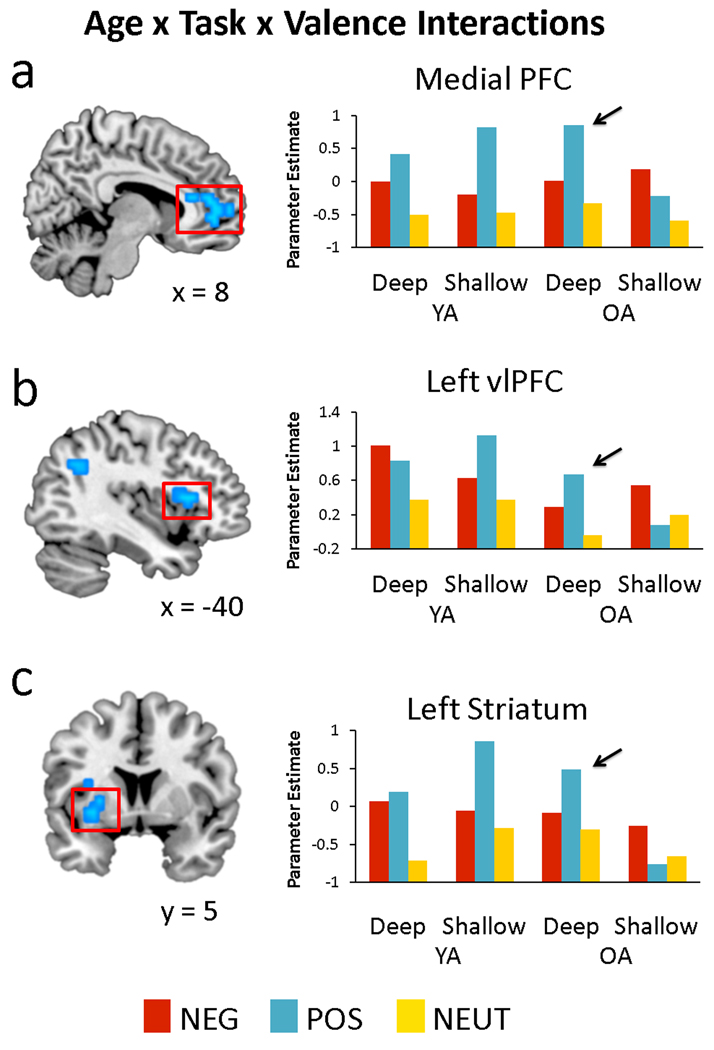
Although there were not any regions that displayed a significant 2-way interaction, activity in many regions were characterized by a 3-way interaction of age group, task, and valence. This interaction was driven predominately by greater positive versus negative valence effects in the deep condition in OAs (and the reverse in YAs), verified by inclusive masking with the contrast for the corresponding task by valence interaction in OAs alone. The results of this analysis are listed in Table 3, and include significant interactions in the bilateral vlPFC, medial PFC, and striatum. As can be seen in Figure 3, these regions were often accompanied by a significant interaction in YAs. To isolate those regions that show significant task by valence interactions only in the OAs, we further masked the interaction results by excluding all voxels showing a significant task by valence interaction in YAs. Only 2 regions remained: the medial PFC and the right insula, extending into vlPFC. Finally, region-of-interest analyses on the results of the 3-way interaction revealed a main effect of sex in the striatum, F(1, 30) = 6.61, p = .015, with greater emotion-related activity in females than males. Again, this effect was independent of age, providing evidence that sex effects may be stable across the lifespan. Altogether, these results indicate that the vlPFC, medial PFC, and ventral striatum show preferential activity for positive than negative valence in OAs, but only when OAs engage in deep, semantic processing. Furthermore, valence effects in the medial PFC are not affected by task in the YAs.
Table 3.
Interaction Effects on Emotion-Related Activity
| BA | Hem | x | y | z | F | voxels | |
|---|---|---|---|---|---|---|---|
| Group x Task x Valence Interaction, with mask for Task x Valence in OAs | |||||||
| Anterior Cingulate | 32 | R | 19 | 37 | 12 | 19.43 | 251 |
| Medial Frontal Gyrus | 10 | L | −8 | 44 | 12 | 18.49 | -- |
| Superior Frontal Gyrus | 10 | L | −23 | 54 | 1 | 18.05 | -- |
| -- | 10 | R | 23 | 54 | −6 | 16.77 | -- |
| Insula | 13 | R | 34 | 23 | 6 | 16.55 | -- |
| Inferior Parietal Lobule | 40 | L | −45 | −53 | 34 | 16.95 | 17 |
| Ventral Striatum | L | −30 | 11 | −1 | 16.37 | 22 | |
| Inferior Frontal Gyrus | 45 | L | −41 | 19 | 10 | 14.96 | 17 |
| Thalamus | R | 4 | −10 | 11 | 14.28 | 14 | |
| Putamen | L | −19 | −7 | 11 | 13.22 | 12 | |
| Group x Task x Valence Interaction, with mask for Task x Valence in OAs but not YAs | |||||||
| Medial Frontal Gyrus | 10 | R | 0 | 51 | −6 | 17.23 | 12 |
| Superior Frontal Gyrus | 10 | R | 22 | 54 | −6 | 16.77 | 45 |
| Medial Frontal Gyrus | 10 | R | 4 | 44 | 12 | 16.61 | -- |
| Insula/Inferior Frontal Gyrus | 13 | R | 33 | 22 | 6 | 16.55 | 24 |
Up to 5 local maxima set 20 mm apart are reported for each cluster. BA = Brodmann Area, Hem = Hemisphere. Coordinates are in Talairach space.
Figure 3.
The interaction of age, task, and valence on emotion-related activity in a) medial PFC, b) left vlPFC (BA 45), and c) striatum, all indicating a greater difference between positive than negative trials during deep versus shallow processing in OAs. Mean contrast estimates within each region are plotted for each condition.
Correlations with neuropsychological scores
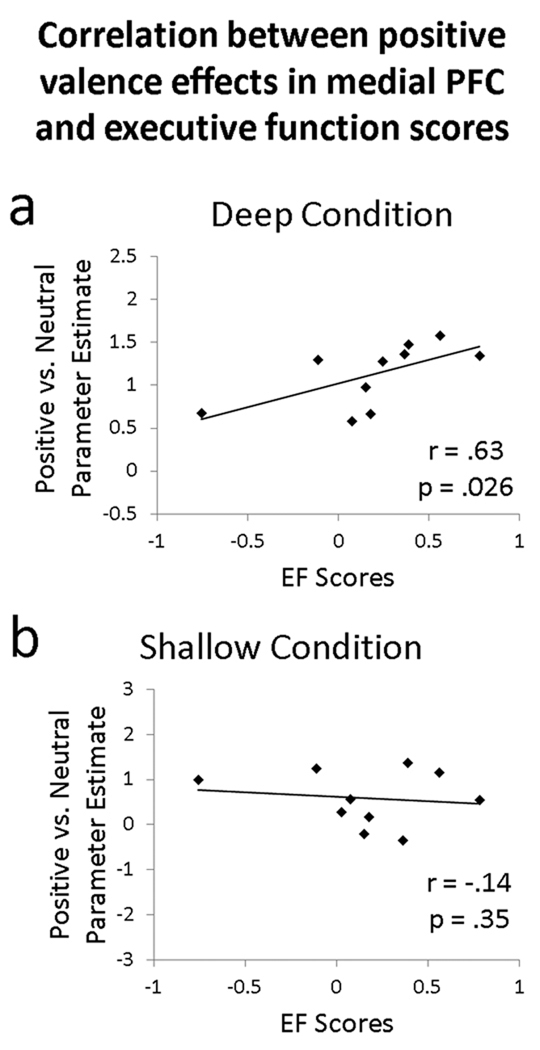
Based on the results of the previous ANOVA, we extracted mean contrast estimates indexing negative or positive versus neutral for each task from the left vlPFC and medial PFC ROIs described above. For the OA participants who completed our neuropsychological battery, we correlated the mean contrast estimates from each ROI with individual differences in executive function (Figure 4). The correlation between executive function scores and positive valence effects were significant for medial PFC (r = .63, p = .026, one-tailed) and marginally significant for left vlPFC (r = .52, p = .052, one-tailed). Effects were specific to the deep condition; equivalent correlations for the shallow condition were non-significant, p > .15. These correlations indicate that, within regions previously linked to enhanced processing of positive emotion in aging, OAs with better executive function showed greater activity for positive versus neutral stimuli during elaborative processing. The correlation between executive function scores and negative valence effects in the deep condition was significant for left vlPFC (r = .58, p = .041, one-tailed) but not medial PFC (r = .38, p = .123, one-tailed). Again, equivalent correlations for the shallow condition were non-significant, p > .15, suggesting a specific link between executive function and elaborative processing of emotional stimuli.
Figure 4.
Across OA participants, composite scores of executive function (EF) correlate with positive valence-related activity (positive vs. neutral contrast) in the medial PFC. Correlations are plotted separately for the a) deep, semantically-focused condition and b) shallow, perceptually-focused condition.
Psychophysiological interaction
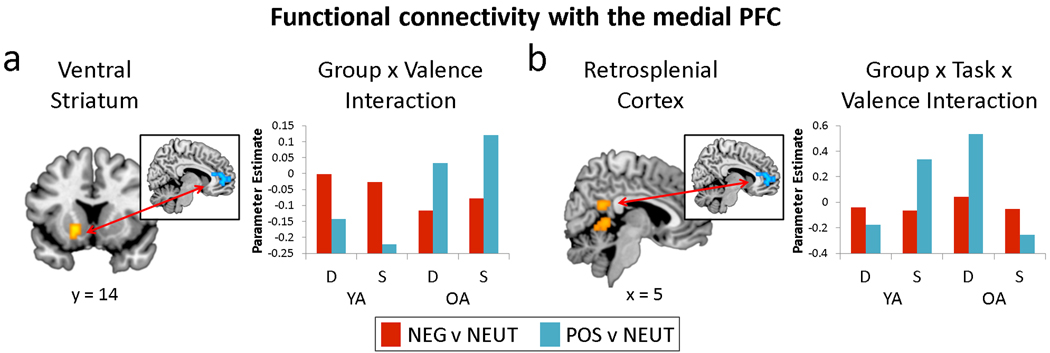
Motivated by this pattern of findings in medial PFC, we conducted an exploratory psychophysiological interaction (PPI) analysis that measured the effect of valence on the relationship between the medial PFC and the rest of the brain. PPI contrasts were entered into a mixed ANOVA with age group, task, and valence as factors. The list of regions showing valence-dependent interactions with the medial PFC is presented in Table 4 (for valence-independent effects, see Supplementary Table 1). Although the main effect of valence was null, there was an interaction of age group and valence in the ventral striatum, representing stronger links with the medial PFC for positively- than negatively-valenced trials in OAs, but the reverse in YAs (Figure 5a). Age effects on this relationship may reflect the ventral striatum’s role in processing reward (Delgado, 2007) or saliency (Zink, Pagnoni, Martin, Dhamala, & Berns, 2003), providing a possible candidate mechanism for age-related differences in emotional processing. Finally, there was an age group by task by valence interaction in the retrosplenial cortex, demonstrating that this region is functionally linked to the medial PFC during positive valence processing in the deep task in OAs and the shallow task in YAs (Figure 5b).
Table 4.
Regions showing valence-dependent differences in functional connectivity with medial PFC as a function of group and/or task
| BA | Hem | x | y | z | F | voxels | direction | |
|---|---|---|---|---|---|---|---|---|
| Group x Valence Interaction | ||||||||
| Ventral Striatum | L | −19 | 11 | −7 | 19.08 | 18 | OA: Pos > Neg | |
| Supramarginal Gyrus | 40 | R | 45 | −46 | 34 | 12.17 | 16 | -- |
| Parahippocampal Gyrus | 35 | R | 15 | −29 | −5 | 14.65 | 11 | OA: Neg > Pos |
| Group x Task x Valence Interaction | ||||||||
| Middle Frontal Gyrus | 6 | R | 30 | −4 | 56 | 14.58 | 38 | OA D: Pos > Neg |
| Retrosplenial Cortex | 23, 30 | R | 4 | −46 | 23 | 11.76 | 12 | -- |
| Cerebellum | L | −7 | −47 | −1 | 11.54 | 16 | OA D: Neg > Pos | |
BA = Brodmann Area, Hem = Hemisphere, Neg = Negative, Pos = Positive, Neut = Neutral, D = Deep, S = Shallow. Coordinates are in Talairach space. The main effect of Valence and Task x Valence interaction were null.
Figure 5.
Psychophysiological interaction results, reflecting a) the interaction of age and valence on the relationship between the medial PFC and the ventral striatum, and b) the interaction of age, task, and valence on the relationship between the medial PFC and retrosplenial cortex. Mean parameter estimates within each region are plotted for each condition. S = Shallow, D = Deep.
Discussion
The present study resulted in 4 main findings. First, overall emotion effects (both positive and negative) were intact across age groups in the amygdala, but reduced by aging in visual cortex. Second, preferential activity for positive versus negative stimuli in the medial PFC and vlPFC within OAs emerged only during semantic elaboration. Third, emotion effects in the medial PFC correlated with individual differences in executive function among OAs, but again only under semantic elaboration. Finally, aging altered the interactions of medial PFC with the ventral striatum and the retrosplenial cortex during emotional processing.
Intact amygdala and reduced posterior emotion-related activity in aging
Bilateral amygdala responded more for negative and positive stimuli than neutral, regardless of age group and task (see Figure 2a), suggesting that amygdala responsivity in OAs is comparable to that in YAs. This finding is inconsistent with studies that found age-related reductions in amygdala activity (Fischer, et al., 2005; Gunning-Dixon, et al., 2003; Iidaka, et al., 2002; Murty, et al., 2009; Tessitore, et al., 2005), although there have been other reports of comparable amygdala activity between young and older adults (Leclerc & Kensinger, 2008a; Mather, et al., 2004; St. Jacques, et al., 2010; Wright, et al., 2006). One possible explanation for this discrepancy, suggested by St. Jacques et al. (2009), is that OAs may have reduced arousal responses and corresponding ratings compared to YAs, and that these rating differences may engender differences in amygdala activity. This explanation could account for preserved amygdala activity in OAs in the present study, given that individual differences in arousal responses were mitigated by excluding emotional trials with low arousal ratings and neutral trials with high arousal ratings. However, age effects in the amygdala remained absent even when all trials were included (data not shown), consistent with our behavioral analysis indicating that there were no overall group differences in arousal ratings. Alternatively, this discrepancy may emerge from differences in stimuli or task design. Of the above studies, all but one (Murty, et al., 2009) of the studies reporting age differences in amygdala activity used face stimuli, and all used blocked designs. By comparison, studies reporting equivalent amygdala effects across groups tend to use object or scene stimuli and event-related designs (but see Wright, et al., 2006). The use of face stimuli, which typically comprise young faces, may introduce differences with respect to the relevancy of the stimuli to each age group. This is further complicated by the differential sensitivity of blocked versus event-related designs to sustained and transient forms of processing, respectively, which may impacted by aging in different ways (Dennis, Daselaar, & Cabeza, 2007). Thus, it remains an open question for future research to clarify which factors determine whether or not OAs show reduced amygdala activity during emotional processing. The present study furthers our previous knowledge by showing that, in addition to equivalent amygdala activity in YA and OA, emotion-related activity in the amygdala is insensitive to task-related elaboration, as evidenced by equivalent effects in the two tasks. This finding is consistent with the proposal that the amygdala tends to be engaged automatically in response to emotionally-salient stimuli (Dolan & Vuilleumier, 2003). It has alternatively been proposed that amygdala recruitment is subject to attentional control (Pessoa, McKenna, Gutierrez, & Ungerleider, 2002), in which case it is likely that both of our tasks permitted sufficient attentional allocation to the emotional stimuli to elicit comparable amygdala responses.
In contrast with the amygdala, there was a group difference in emotion-related activity in visual cortex, indicating that in YAs, this region showed a greater difference between emotional and neutral stimuli than in OAs. This group effect echoes previous results demonstrating a reduction in activity related to perceptual processing in posterior regions of the brain, the aforementioned Posterior-Anterior Shift with Aging (PASA) pattern (Davis, et al., 2008; Dennis & Cabeza, 2008). However, it should be noted that in the present study, the pattern of activation seems to suggest that the OAs tend to activate visual cortex as much for neutral stimuli as for emotional stimuli (see Figure 2b). This pattern suggests that OAs tend to exhibit reduced differentiation of stimulus processing relative to YAs, rather than an overall reduction in perceptual-related activity. This pattern has likewise been identified in the context of emotion processing, marked by reduced emotion-related activity in visual processing regions in OAs relative to YAs (Tessitore, et al., 2005; Wright, et al., 2006), as well as reduced functional connectivity between the amygdala and visual cortices during negative versus neutral picture viewing (St. Jacques, et al., 2010). The present study further demonstrates that these activity reductions are insensitive to task-related elaboration.
Age-related valence shifts in medial PFC and vlPFC during semantic elaboration
Our second main finding consisted of regions where age effects on brain activity were modulated by valence and elaborative processing (Figure 3). These regions included medial PFC and vlPFC, which were differentially engaged by OAs for processing positive emotions, but only during the semantic elaboration task. Previous studies have linked posterior reductions with frontal increases in aging (Davis, et al., 2008; Grady, et al., 1994; St. Jacques, et al., 2010). In the present study, however, over-recruitment of frontal regions, particularly for positive valence, was contingent on controlled, elaborative processing. This pattern is reminiscent of the behavioral finding that positivity shifts in aging, as indexed by memory for positive versus negative stimuli, are eliminated under divided attention conditions (Mather & Knight, 2005). Similar to that study, the present study had two conditions under which the ability to engage in controlled elaboration was modulated. In the deep task, participants were able to devote full resources toward processing the semantic content of the pictures, whereas in the shallow task, they were attending primarily to the perceptual features. Thus, whereas behavioral effects of positive valence in aging have been observed in full relative to divided attention conditions (Mather & Knight, 2005), here we observe neural effects of positive valence during semantic elaboration relative to shallow processing conditions. Although we did not identify any behavioral effects of valence that differed by age, it may be that neural measures may provide a more sensitive metric for identifying these shifts: whereas behavioral measures collapse over many simultaneous brain processes, neural measures may index subtle effects on one of these processes.
The localization of effects in the medial PFC is consistent with previous reports of valence shifts in this region in aging (Gutchess, et al., 2007; Leclerc & Kensinger, 2008a) and again, extends this literature to include the finding that these effects are restricted to tasks involving deep processing. Medial PFC has been linked to self-referential processing in both younger (Gutchess, et al., 2007; Kelley, et al., 2002) and older adults (Gutchess, et al., 2007). Furthermore, its differential deployment with respect to encoding condition in the present study is predicted by a self-referential processing account. Self-referential processing depends in part on controlled semantic elaboration processes, as they share some common substrates (Craik, et al., 1999; Kelley, et al., 2002) and their effects on memory are similarly affected by semantic processing deficits (Glisky & Marquine, 2009). However, semantic elaboration does not fully account for self-referential processing: there is evidence that medial PFC involvement in self-referential processing dissociates from semantic processing (Kelley, et al., 2002) and that age-related semantic processing impairments do not completely eliminate the additive memory benefits of self-referential processing (Glisky & Marquine, 2009). Nevertheless, the availability of semantic resources should affect the recruitment of self-referential processing. It is furthermore likely that attending to the meaning of a stimulus increases the likelihood that it will be interpreted as self-relevant, facilitating medial PFC involvement during deep relative to shallow processing. Further research into the relationship between semantic elaboration and self-referential processing and their contributions to medial PFC activity is warranted. It should be additionally noted that YAs show greater activity for positive than negative stimuli in medial PFC for both the deep and shallow tasks. One possible explanation for this pattern is that compared to OAs, YAs were more likely to engage in semantic elaboration even when attention was diverted to the shallow perceptual features of the stimulus—consistent with stronger effects of divided attention in OAs than YAs (McDowd & Craik, 1988). This interpretation, as well as the task by valence interaction in OAs, underscores the idea that over-recruitment of frontal regions with aging does not occur automatically: frontal over-recruitment is more likely to occur during effortful processing.
Interestingly, whereas YAs showed a main effect of valence within medial PFC, they showed a valence by task interaction within left vlPFC. Although the interpretation of this finding is not straightforward, it is possible that the left vlPFC activation corresponds to valence responses that OAs up-regulate and YAs down-regulate when each group engages in deep, semantic processing, perhaps due to differences in stimulus prioritization. Despite the admittedly tentative nature of this interpretation, it may be consistent with the idea that attending more to positive than negative valence is adaptive for OAs but not for YAs, due to possible differences in the relevance of positive versus negative stimuli to immediate emotional goals and knowledge-related goals, respectively (Carstensen, Isaacowitz, & Charles, 1999).
Individual differences in executive function and PFC effects in aging
Turning to our third finding, positive valence effects in the medial PFC were predicted by individual differences in independently-measured composite scores of executive function in the OAs (Figure 4). The left vlPFC also showed a trend toward this correlation. Critically, this relationship was identified for valence effects during the semantic elaboration task only. Whereas previous evidence has linked behavioral valence shifts with executive function in aging (Mather & Knight, 2005), the present study extends these results to the neural domain and highlights the specific link between executive function and elaborative processing of emotional stimuli. Individual differences in executive function in OAs have been linked to lateral PFC activity during the inhibition of negative emotional responses (Krendl, et al., 2009), suggesting a relationship between executive function and emotion regulation processes instantiated in PFC. In the present study, the specificity of this correlation to the elaboration task provides evidence for the assumption that these PFC regions are involved in controlled processes such as emotion regulation, semantic elaboration, and self-referential processing. Furthermore, because the present correlations incorporate the contrast of positively-valenced trials versus neutral, the results indicate that executive function predicts PFC changes that are specific to emotion processing. In sum, the neural valence shifts described above may be attributed to changes in the controlled evaluation and/or regulation of positively-valenced stimuli in OAs, supported by individual differences in executive function.
Medial PFC interactions with ventral striatum and retrosplenial cortex
Finally, the fourth main finding of the study was that aging affected the interactions of medial PFC with the ventral striatum and the retrosplenial cortex during emotional processing (Figure 5). As illustrated by Figure 5a, whereas YAs show a stronger relationship between medial PFC and the ventral striatum for negative than positive trials, OAs show a stronger relationship for positive than negative trials. This finding is interesting due to the ventral striatum’s role in reward processing (Delgado, 2007) or salience detection (Zink, et al., 2003), and recent evidence that striatal activation remains intact in OAs relative to YAs during gain anticipation, but not loss anticipation (Samanez-Larkin, et al., 2007). Altogether, the present finding may speak to differences in the way young and older adults convey information about incentive salience to the medial PFC. Aging also altered medial PFC interactions with the retrosplenial cortex but in this region age and valence effects varied by task: OAs showed a stronger medial PFC-retrosplenial relationship for positive than negative trials during deep processing, whereas YAs showed reverse pattern. Both medial PFC and retrosplenial cortex are cortical midline structures thought to support with self-referential processing (Northoff & Bermpohl, 2004; Northoff, et al., 2006), with the medial PFC being generally linked to representing self-relevancy and the retrosplenial cortex being more specifically tied to autobiographical memory processes (Northoff, et al., 2006). Because of their mutual association with self-referential processing, it may be that fluctuations in the relationship between these regions likewise reflect changes in this form of elaboration. In keeping with this idea, that finding that the medial PFC-retrosplenial relationship is enhanced by positive valence during semantic elaboration in OAs provides further support for a self-referential processing account of neural valence effects in aging.
Conclusions
In conclusion, whereas age-invariant amygdala responses and age-related reductions in the visual cortices are insensitive to task demands, age-related frontal differences associated with valence processing are modulated by level of controlled elaboration. That is, the medial PFC and vlPFC preferentially respond to positive valence in OAs, but only when OAs engage in deep, semantic processing. This suggests that these frontal effects are mediated by emotion control processes, such as self-referential processing or emotion regulation. This hypothesis is further supported by the link between these emotion effects and individual differences in executive function. Finally, functional connectivity analyses demonstrate that the relationships between the medial PFC and other emotion control-related regions are modulated by valence, age, or both.
Supplementary Material
Acknowledgments
The authors would like to thank James Kragel for technical assistance and Elsa Baena for assistance with collection of neuropsychological data.
Funding
This work was supported by the National Institute on Aging [grant numbers R01 AG019731, R01 AG23770, and R01 AG34580 awarded to RC and F32 AG029738 awarded to SMH], the National Institute of Mental Health [F31 MH085384 awarded to MR], and the National Institute of Neurological Disorders and Stroke [R01 NS41328]. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH, NIA, NINDS, or NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backs RW, da Silva SP, Han K. A comparison of younger and older adults' self-assessment manikin ratings of affective pictures. Experimental Aging Research. 2005;31(4):421–440. doi: 10.1080/03610730500206808. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain Activity during Episodic Retrieval of Autobiographical and Laboratory Events: An fMRI Study using a Novel Photo Paradigm. Journal of Cognitive Neuroscience. 2004;16(9):1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54(3):165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstenson LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132(2):310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In Search of the Self: A Positron Emission Tomography Study. Psychological Science. 1999;10(1):26–34. [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The Posterior-Anterior Shift in Aging. Cereb. Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-Related Responses in the Human Striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FI, Salthouse TA, editors. Handbook of Aging and Cognition - III. Mahweh, NJ: Erlbaum; 2008. [Google Scholar]
- Dennis NA, Daselaar SM, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiology of Aging. 2007;28(11):1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Ritchey M, Johnson BD, LaBar KS. Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion. 2007;7(2):354–365. doi: 10.1037/1528-3542.7.2.354. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Annals of the New York Academy of Sciences. 2003;985(1):348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaulation and subsequent memory: An event-related fMRI study. NeuroImage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett. 2005;386(2):99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and Modulatory Interactions in Neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Marquine M. Semantic and self-referential processing of positive and negative trait adjectives in older adults. Memory. 2009;17:144–157. doi: 10.1080/09658210802077405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double Dissociation between Item and Source Memory. Neuropsychology. 1995;9(2):229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology-Learning Memory and Cognition. 2001;27(5):1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Grady C, Maisog J, Horwitz B, Ungerleider L, Mentis M, Salerno J, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J. Neurosci. 1994;14(3):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhn D, Scheibe S. Age-related differences in valence and arousal ratings of pictures from the International Affective Picture System (IAPS): do ratings become more extreme with age? Behavior Research Methods. 2008;40(2):512–521. doi: 10.3758/brm.40.2.512. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience. 2007;2(2):117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14(2):233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging. 2006;21(1):40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the Self? An Event-Related fMRI Study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The Role of Motivation in the Age-Related Positivity Effect in Autobiographical Memory. Psychological Science. 2004;15(3):208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Leclerc CM. Age-related changes in the neural mechanisms supporting emotion processing and emotional memory. European Journal of Cognitive Psychology. 2009;21(2):192–215. [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: Effects of valence and arousal. Cognitive, Affective, & Behavioral Neuroscience. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Heatherton TF, Kensinger EA. Aging minds and twisting attitudes: an fMRI investigation of age differences in inhibiting prejudice. Psychol Aging. 2009;24(3):530–541. doi: 10.1037/a0016065. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Mesulam MM, Gitelman DR, Weintraub S. Emotional curiosity: modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer's disease. Neuropsychologia. 2000;38(13):1734–1740. doi: 10.1016/s0028-3932(00)00077-4. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerin JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach atlas labels. NeuroImage. 1997;5:5633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cogn Affect Behav Neurosci. 2008a;8(2):153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008b;23(1):209–215. doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The Functional Neuroanatomy of Episodic and Semantic Autobiographical Remembering: A Prospective Functional MRI Study. Journal of Cognitive Neuroscience. 2004;16(9):1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [doi: DOI: 10.1016/S1053-8119(03)00169-1] [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner KN, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14(5):409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstenson LL. Aging and motivated cognition: The positivity effect in attention and memory. [Review] Trends in Cognitive Sciences. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20(4):554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Angry faces get noticed quickly: Threat detection is not impaired among older adults. Journal of Gerontology: Psychological Sciences. 2006;61B(1):P54–P57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- McDowd JM, Craik FI. Effects of aging and task difficulty on divided attention performance. Journal of Experimental Psychology: Human Perception and Performance. 1988;14(2):267–280. doi: 10.1037/0096-1523.14.2.267. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The Neural Bases of Distraction and Reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley Steinmetz KR, Muscatell KA, Kensinger EA. The effect of valence on young and older adults' attention in a rapid serial visual presentation task. Psychology and Aging. 2010;25(1):239–245. doi: 10.1037/a0018297. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cogn Neurosci. 2009;21(10):1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [doi: DOI: 10.1016/j.tics.2004.01.004] [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--A meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, et al. Bottom-Up and Top-Down Processes in Emotion Generation. Psychological Science. 2009;20(11):1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI. NeuroImage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Ritchey M, LaBar KS, Cabeza R. Level of processing modulates the neural correlates of emotional memory formation. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21487. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10(6):787–791. doi: 10.1038/nn1894. [10.1038/nn1894] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: Correlation to visual and verbal memory. Neurology. 1994;44(9):1660. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- St. Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: Fronto-amygdalar differences during emotional perception and episodic memory. Journal of the International Neuropsychological Society. 2009;15(06):819–825. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2010;31:315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139(1):9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: neural basis of improving emotional stability over age. J Neurosci. 2006;26(24):6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiology of Aging. 2006;27(2):361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human Striatal Response to Salient Nonrewarding Stimuli. J. Neurosci. 2003;23(22):8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.