Abstract
There is compelling evidence demonstrating a key role for autophagy in host defense against microbial infections. Induction and regulation of autophagy involves complex pathways including signaling molecules that have widespread roles in cell biological functions. For example, inhibiting mTOR by rapamycin, the most widely used chemical approach to induce autophagy, can also result in immunosupression. Nevertheless, advances in our understanding of autophagy provide a new opportunity to modulate host cellular responses as a potential therapeutic strategy to combat microbial infections in humans.
Introduction
As part of the innate immune response, microbial pathogens are phagocytozed by macrophages and dendritic cells (DCs), where they traffic via the endolysosomal pathway. Subsequently, the macrophage or DC mounts a direct antimicrobial activity to eliminate the pathogen and may also process and present microbial antigens to instruct the acquired immune response. However, microbes have evolved evasion strategies to escape or inhibit lysosomal processing and destruction. For example, Toxoplasma gondii and Mycobacterium tuberculosis are intracellular pathogens that inhibit phagosome maturation and fusion with lysosomes [1,2]. In contrast, Listeria monocytogenes or Shigella flexneri escape from the endolysosomal pathway to reside in the cytoplasm of infected cells [3,4]. In addition, many pathogens live in the extracellular space and must be opsonized to be taken up by cells of the immune system, where they can effectively be killed.
Autophagy is a conserved biological process, in which cytoplasmic material is enclosed in a double-membrane structure, called the autophagosome. Through subsequent fusion with lysosomes, resulting in the formation of an autophagolysosome, the cytoplasmatic material is subjected to lysosomal degradation. In the last decade, collective evidence has established a role for autophagy as a host defense mechanism to counteract immune evasion strategies of numerous pathogens, including extracellular, phagosomal and cytoplasmic infection (reviewed in [5]). Autophagy impacts the host response on several levels, including antimicrobial activity, regulation of thymic selection [6], and modulation of MHC class I- and MHC class II-dependent antigen-presentation (reviewed in [7]). However, it has also become evident that several pathogens have evolved strategies to escape autophagy mediated killing (reviewed in Ogama et al [8]). Autophagy has even been described as a bacterial escape mechanism resulting in enhanced pathogen survival [9]. Furthermore, there is conflicting information regarding autophagy related genes and their ability to negatively and positively regulate type I interferon production in the antiviral response [10,11]. Here we review recent advances in understanding the role of autophagy in combating microbial pathogens towards the potential regulation of the autophagic process as a novel therapeutic strategy against human infectious disease.
Induction of autophagy during microbial infection
Several studies have demonstrated the ability of microbial ligands to trigger autophagy and autophagy-related pathways through activation of pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs) [12–19]. In addition, the human inhibitory complement receptor CD46 has been reported to be a direct inducer of autophagy [20]. CD46 is a type I glycoprotein expressed by all nucleated human cells and binds multiple pathogens, including measles virus, human herpes virus 6 (HHV6), Neisseria bacteria, and several serotypes of group A streptococcus.
Although in many instances the innate immune system is sufficient to protect against infection, some conditions, in particular when bacterial immune evasion strategies are efficient, require the effector functions of the acquired immune system. Thus, several studies have investigated the role of the acquired immune system, in particular T cells to activate innate immune cells and induce autophagy. Andrade et al showed that activation of macrophages by CD40L expressing T cells was sufficient to restrict intracellular growth of toxoplasma in macrophages and was dependent on CD40 ligation [21]. As opposed to Th2 cytokines which inhibit autophagy [22], the key Th1 cell derived cytokine IFN-γ, was also found to be sufficient to trigger autophagy and control intracellular infection in macrophages [22–24]. IFN-γ induced autophagy in mouse macrophages was mediated via the function of immunity-related GTPases (IRGs) [24,25]. In contrast, human IRGs are not inducible by IFN-γ [26], although this does not preclude its involvement. Nevertheless, it is reasonable to infer that IFN-γ-induced autophagy in human and mouse macrophages involve distinct mechanisms.
Autophagy as a therapeutic target
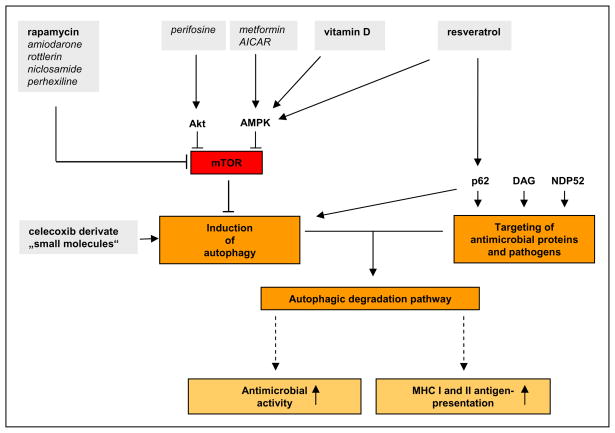
Based on the important role of autophagy in combating microbial pathogens, it is tempting to speculate that it may be possible to target autophagy, i.e. induce autophagy, as a novel therapeutic strategy against human infectious diseases. Insight into this possibility can be derived from the clinical use of agents which induce autophagy. For example, rapamycin is a pharmaceutical agent that induces autophagy, and is approved for use in humans. The mammalian target of rapamycin (mTOR), which is the catalytic subunit of at least two distinct multiprotein complexes (mTORC1 and mTORC2), negatively regulates autophagy. Inhibition of mTOR by rapamcyin and its chemical derivates are the most widely used chemical approaches to induce autophagy. A large screen of chemical inducers of autophagy has identified numerous drugs that promote autophagy by inhibiting the mTOR complex 1 (mTORC1) function, including amiodarone, rottlerin, niclosamide and perhexiline [27].
In addition to inducing autophagy, the pharmacologic inhibition of mTOR function has widespread effects on cell biological function. In the case of T cells, inhibition of mTOR function induces anergy and inhibits cell proliferation [28]. These immunosuppressive effects have been exploited clinically; for instance rapamycin is used in transplantation medicine to prevent organ rejection. Thus, concerns about the strategy of targeting mTOR to treat infections have been raised [29]. Indeed, viral and mycobacterial infections, have been documented as side effects of rapamycin treatment [30]. It has been stated that the rational design of therapeutics will required a better understanding on how autophagy is induced and regulated by the interaction of the host immune system with the invading pathogen [29]. However, our rapidly growing understanding of the role of autophagy and the underlying signaling pathways provide new tools to address those questions [31]. Inhibiting mTOR by rapamycin is not only immunosuppressive, but also has complex immunostimulatory effects [28]. In this regard, a recent study by Araki et al describes an important role of mTOR signaling in CD8 T-cell differentiation [32]. The authors show that rapamycin treatment improves both the quality and quantity of the memory CD8 T-cell responses against viral infection in mice and non-human primates.
Potential approaches to treat microbial infections
There is limited data on the potential utility of pharmaceutical agents that induce autophagy as therapeutic agents for human infectious disease. Sarkar et al performed a two-step screen of small molecules to identify inducers of autophagy [33]. First, small molecules were tested for their ability to modulate growth inhibitory effects of rapamycin in yeast. Subsequently, a second screening step was performed in mammalian cells, and several molecules that induced autophagy downstream or independent of mTOR were found. Within this set of molecules, two were shown to restrict the growth of M. bovis in human macrophages in vitro [34]. In a human monocytic cell line, another small molecule, a celecoxib derivate, induced autophagy and eradicated intracellular Francisella tularensis without cytotoxicity to the host cells [35]. The same drug cleared intracellular Salmonella enterica serovar Typhimurium infection from murine macrophages [36].
The ability of the active form of vitamin D, 1,25-dihydroxivitamin D (1,25D), to induce autophagy has provided a new approach to both understand and potentially treat microbial infections in humans [37,38]. A link between insufficient vitamin D levels and increased risk of infection has been widely reported [39]. In the case of M. tuberculosis infection, the identification of a vitamin D-dependent antimicrobial pathway resulting in the induction of autophagy in combination with the generation of antimicrobial peptides provides an underlying mechanism for the anti-infective effects of vitamin D [15,38,40,41]. Those studies provide evidence that the intracrine conversion of biologically inactive 25-hydroxy vitamin D (25D) to the active form 1,25D is key for the induction of antimicrobial peptides and autophagy. The responsible enzyme, CYP27b1, was induced by TLR2/1 ligation, and its ability to convert 25D to 1,25D to sufficient intracellular levels to activate the vitamin D receptor was dependent on the bioavailability of 25D. Additionally, vitamin D has been linked to naïve T cell signaling, in which CYP27b1 and vitamin D receptor function were both required for inducing PLC-γ1 [42].
In vivo systemic 1,25D levels are controlled by parathyroid feed-back on the activity of renal CYP27b1. However, elevated levels of 1,25D can cause live threatening hypercalcaemia. Thus, 1,25D might not be an optimal candidate to induce autophagy therapeutically. However, vitamin D analogues and nonsecosteroidal vitamin D receptor ligands with reduced calcemic effects are being developed and will potentially be of therapeutic use in this regard [43]. Since the intracellular conversion of 25D to 1,25D in T cells and macrophages controls the intracellular level of 1,25D, it may be important to maintain serum 25D levels in a sufficient range for the natural host response and in immunomodulatory strategies that aim to trigger autophagy. However, studies investigating the effect of vitamin D supplementation alone have not provided satisfying answers about the therapeutic effectiveness of vitamin D in tuberculosis [44] or any other infectious disease. It remains to be determined whether the form, dose and duration of vitamin D treatment are relevant determinants of therapeutic efficacy.
A key question in autophagy research is how cytoplasmic material can be selected for autophagy mediated delivery to lysosomal compartments. Recent findings have shown an important role of ubiquitination in targeting cytosolic antimicrobial peptides to autophagolysosomes as well as cytosolic bacteria to autophagosomes. In starvation and IFN-γ induced mouse macrophages, the p62/SQSTM1 dependent autophagy pathway resulted in the delivery of ubiquitinated cytosolic precursor proteins of antimicrobial peptides to autolysosomes [45]. The induction of autophagy and the key adaptor molecule p62, which targets the ubiquitinated proteins to autolysosomes, provided a protective mechanism against M. tuberculosis infection in this model. Another ubiquitin-binding adaptor molecule, NDP52, was found to bind ubiquitinated Salmonella enterica serotype Typhimurium and Streptococcus pyogenes in the cytosol and deliver these bacteria to autophagosomes [46]. In summary, these ubiquitin-dependent mechanisms provide potential targets to selectively promote autophagy as a host defense mechanism against microbial infection. An additional target might be a recently described diacylglycerol-dependent signaling pathway that contributes to autophagy independently of p62, yet results in an antibacterial response [47].
Approaches in neurodegenerative disease and cancer research and potential use in infections
Insight into mechanisms of autophagy has been applied to the development of therapies in human diseases in the fields of cancer and neurodegenerative diseases research (reviewed in [31,48,49]). The underlying concept is that the induction of autophagy would result in a reduction in cell growth or inflammation, respectively, by promoting apoptosis or autophagic cell death. Thus, these strategies need careful evaluation regarding their usefulness in infectious conditions, in which reduction of inflammation or cell death might be harmful to the host. Nevertheless, based on these studies, numerous drugs have been identified that target AMPK and Akt signaling pathways, including metformin and AICAR, a performance enhancing drug listed on the World Anti-Doping prohibited list [50–52]. AMPK is a critical component of TLR induced and vitamin D mediated autophagy leading to antimicrobial activity against M. tuberculosis [15,38]. However, the broad roles of AMPK and Akt signaling pathways in cell biology, including regulation of mTOR, could make them less attractive direct targets for the treatment of infectious diseases.
Resveratrol, a natural phytoalexin found in grapes, induces autophagy independent of beclin-1 via the p62/SQSTM1 dependent pathway [53]. In addition, resveratrol was also found to inhibit mTOR and activate AMPK. Interestingly, resveratrol also has antiviral activity in vitro, for example against influenza a virus infection [54]. However, more intensive studies will be necessary to determine whether resveratrol as an autophagy activator can be used therapeutically. It is intriguing that red wine is a major source of resveratrol and has been implemented in the health promoting effect of the French diet.
Enhancing acquired immune response
The induction of autophagy has been associated with enhanced antigen presentation of cytosolic and nuclear peptides, some of which originate from viral or bacterial antigens. Thus targeting autophagy therapeutically could not only enhance direct antimicrobial functions, but also augment the acquired immune response. In this regard, it was shown that mice with DC-conditional deletion in Atg5, a key autophagy gene, have impaired CD4+ T cell priming by DCs as a result of inefficient MHC class II antigen presentation [55]. In accordance with these studies, induction of autophagy through NOD2 signalling facilitated the intracellular trafficking of antigen-MHC class II complexes to augment antigen presentation to T cells required for anti-bacterial host defence [16,17].
Early HIV infection of DCs was recently shown to inhibit initiation of autophagy by HIV envelope-mediated mTOR activation [56]. Inhibition of autophagy resulted in increased viral content in DCs, enhanced viral transfer from DCs to CD4+ T cells, and diminished MHC class II-dependent presentation of HIV antigens. In contrast, enhancement of autophagy by rapamycin-mediated mTOR inhibition lead to a reduction of HIV content in DCs and decreased viral cell-to-cell transfer. These studies suggest that inhibiting mTOR pharmacologically could be a therapeutic strategy to restrict early HIV infection and at the same time increase MHC class II-dependent immune responses against the virus.
Furthermore, HSV-1 infection in macrophages involves an alternative MHC class I antigen presentation pathway linked to autophagy. A vacuolar pathway with autophagic components was identified in late phase HSV-1 infection, a process which increased presentation of HSV-1 antigen via MHC class I as well as CD8+ T cell stimulation in accordance with the induction of autophagy in the infected cell [57]. In addition, autophagy was shown to enhance cross-priming in pre-apoptotic viral infected DCs by prolonging MHC I/peptide complex presentation, resulting in increased virus specific IFN-γ producing CD8+ T cells [58].
Evidence that demonstrates the potential of inducing autophagy as a therapeutic approach has been shown in mouse model experiments, which involved vaccination with the attenuated mycobacterial strain Bacille Calmette Guerin (BCG) [59]. In this study, mice were vaccinated with BCG-infected DC that were treated with rapamycin or left untreated, then challenged via the aerosol route with virulent M. tuberculosis. Mice vaccinated with rapamycin treated DCs showed greater expansion of mycobacterial antigen specific T cells as well as a greater reduction in lung bacterial counts compared to mice vaccinated with untreated DCs. Thus, the induction of autophagy in conjunction with attenuated vaccines could help facilitate antigen presentation in order to elicit a more effective T cell response.
Conclusions
Recent advances in our understanding of the mechanisms that regulate autophagy and the biologic function of autophagy in disease provide an opportunity to target autophagy as a therapeutic strategy to combat microbial infections. It is not known whether the in vitro or animal trials of autophagy activators can be translated into useful therapeutics against microbial infections in humans. Nevertheless, such approaches would not only augment direct antimicrobial activity against the invading pathogen, but could also enhance natural acquired immune responses and vaccination strategies. Therefore, there is an urgent need to assess whether autophagic enhancers can be applied to human clinical trials.
Figure 1.
Potential strategies to induce autophagy as an antimicrobial therapy (Bold: Drugs with known antimicrobial activity; Italic: Drugs shown to induce autophagy, but antimicrobial effect unknown).
Acknowledgments
This work was supported by the NIH grants AI073539, AI047868 and AI022553, by the Deutsche Forschungsgemeinschaft FA849/1-1 and FA849/2-1, and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R13-2007-020-01000-0).
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. The Journal of Experimental Medicine. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 5.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 7.Munz C. Antigen processing via autophagy--not only for MHC class II presentation anymore? Curr Opin Immunol. 2010;22:89–93. doi: 10.1016/j.coi.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa M, Sasakawa C. Bacterial evasion of the autophagic defense system. Curr Opin Microbiol. 2006;9:62–68. doi: 10.1016/j.mib.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Schnaith A, Kashkar H, Leggio SA, Addicks K, Kronke M, Krut O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem. 2007;282:2695–2706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- 10.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 11.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumpter R, Jr, Levine B. Autophagy and innate immunity: Triggering, targeting and tuning. Semin Cell Dev Biol. 2010;21:699–711. doi: 10.1016/j.semcdb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. Embo Journal. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial Lipoprotein Activates Autophagy via TLR2/1/CD14 and a Functional Vitamin D Receptor Signaling. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •16.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- •17.Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, Yuan L, Soares F, Chea E, Le Bourhis L, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. These two studies link bacterial sensing by NOD receptors to the induction of autophagy and establish a link between two Crohn’s disease associated genes, NOD2 and Atg16L1, in one functional pathway. [DOI] [PubMed] [Google Scholar]
- 18.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 19.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 20.Meiffren G, Joubert PE, Gregoire IP, Codogno P, Rabourdin-Combe C, Faure M. Pathogen recognition by the cell surface receptor CD46 induces autophagy. Autophagy. 2010;6:299–300. doi: 10.4161/auto.6.2.11132. [DOI] [PubMed] [Google Scholar]
- 21.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- 25.Tiwari S, Choi HP, Matsuzawa T, Pypaert M, MacMicking JD. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat Immunol. 2009;10:907–917. doi: 10.1038/ni.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bekpen C, Hunn JP, Rohde C, Parvanova I, Guethlein L, Dunn DM, Glowalla E, Leptin M, Howard JC. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6:R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, Nabi IR, Roberge M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS ONE. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev. 2010;235:234–243. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subauste CS. Autophagy as an antimicrobial strategy. Expert Rev Anti Infect Ther. 2009;7:743–752. doi: 10.1586/eri.09.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021083s033,021110s043lbl.pdf
- 31.Turcotte S, Giaccia AJ. Targeting cancer cells through autophagy for anticancer therapy. Curr Opin Cell Biol. 2010;22:246–251. doi: 10.1016/j.ceb.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 32.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. A key publication in demonstrating immune-stimulatory aspects of mTOR inhibition by rapamycin. The study provides striking evidence, that inhibtion of mTOR by rapamycin improves quantity and quality of memory CD8 T cell responses in mice, and for its potential implications in vaccince development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA, Lewis TA, O’Kane CJ, Schreiber SL, et al. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol. 2007;3:331–338. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, Rubinsztein DC. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy. 2007;3:620–622. doi: 10.4161/auto.4898. [DOI] [PubMed] [Google Scholar]
- 35.Chiu HC, Soni S, Kulp SK, Curry H, Wang D, Gunn JS, Schlesinger LS, Chen CS. Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J Biomed Sci. 2009;16:110. doi: 10.1186/1423-0127-16-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu HC, Kulp SK, Soni S, Wang D, Gunn JS, Schlesinger LS, Chen CS. Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob Agents Chemother. 2009;53:5236–5244. doi: 10.1128/AAC.00555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- •38.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell HostMicrobe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. This report shows that autophagy induction by vitamin D leads to in vitro antimicrobial activity against virulent M. tuberculosis infection in human macrophages. [DOI] [PubMed] [Google Scholar]
- 39.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 41.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 43.Sato M, Lu J, Iturria S, Stayrook KR, Burris LL, Zeng QQ, Schmidt A, Barr RJ, Montrose-Rafizadeh C, Bryant HU, et al. A nonsecosteroidal vitamin D receptor ligand with improved therapeutic window of bone efficacy over hypercalcemia. J Bone Miner Res. 2010;25:1326–1336. doi: 10.1002/jbmr.15. [DOI] [PubMed] [Google Scholar]
- 44.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •45.Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, Virgin HWt, Kyei GB, Johansen T, Vergne I, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. This study provides evidence that p62 is a key adaptor molecule that delivers antimicrobial peptides to the mycobacterial autophagosome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •46.Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol. 2009;10:1215–1221. doi: 10.1038/ni.1800. This study shows that the receptor NPD52 binds and recruits ubiquitinated bacteria to autophagosomes resulting in antimicrobial activity. [DOI] [PubMed] [Google Scholar]
- •47.Shahnazari S, Yen WL, Birmingham CL, Shiu J, Namolovan A, Zheng YT, Nakayama K, Klionsky DJ, Brumell JH. A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy. Cell Host Microbe. 2010;8:137–146. doi: 10.1016/j.chom.2010.07.002. In this report the authors describe a distinct diacylglycerol-dependent signaling pahtway of antibacterial autophagy induction that is independent of ubiquitin, NDP52 and p62 adaptor molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puissant A, Robert G, Auberger P. Targeting autophagy to fight hematopoietic malignancies. Cell Cycle. 2010:9. doi: 10.4161/cc.9.17.13048. [DOI] [PubMed] [Google Scholar]
- 50.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 51.Robert G, Ben Sahra I, Puissant A, Colosetti P, Belhacene N, Gounon P, Hofman P, Bost F, Cassuto JP, Auberger P. Acadesine kills chronic myelogenous leukemia (CML) cells through PKC-dependent induction of autophagic cell death. PLoS ONE. 2009;4:e7889. doi: 10.1371/journal.pone.0007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu L, Kim YA, Wang X, Wu X, Yue P, Lonial S, Khuri FR, Sun SY. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–8976. doi: 10.1158/0008-5472.CAN-09-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •53.Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, Auberger P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. This report establishes that the plant phytoalexin resveratrol promotes autophagy via p62/SQSTM1 and AMPK signaling. Resveratrol induced autophagy was different from the canonical pathway of autophagy induction in that it was independent of beclin-1 (atg6). Beclin 1 is a major component of the classical autophagy initiation complex. [DOI] [PubMed] [Google Scholar]
- 54.Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, Ciriolo MR, Garaci E. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- •55.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. This study provides in vivo evidence that autophagy is crucial for optimal MCH class II antigen-presentation by murine DCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •56.Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R, Garcia E, Dinkins C, Leuba F, Wu L, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. The authors describe autophagosomal structures that promote pathogen degradation, amplify TLR signaling and antigen presentation in HIV-1 infected DCs. HIV-1 evades these structures early in infection by inhibiting autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippe R, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uhl M, Kepp O, Jusforgues-Saklani H, Vicencio JM, Kroemer G, Albert ML. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- ••59.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. This report shows that autopagy induction by rapamycin improved BCG vaccine induced protective Th1 reponses in mice challenged with virulent M. tuberculosis. The authors provide a proof of principle that vaccination efficacy can be enhanced by targeting autophagy in DCs. [DOI] [PubMed] [Google Scholar]