Abstract
In this report, stereospecific structural and dynamic features in DNA are studied using the site-directed spin labeling technique. A stable nitroxide radical, 1-oxyl-4-bromo-2,2,5,5-tetramethylpyrroline (R5a), was attached postsynthetically to phosphorothioates that were chemically introduced, one at a time, at five sites of a DNA duplex. The two phosphorothioate diastereomers (Rp or Sp) were separated, and nitroxide rotational motions were monitored using electron paramagnetic resonance spectroscopy. The resulting spectra vary according to diastereomer identity and location of the labeling site, with Rp-R5a spectra effectively reporting on local DNA structural features and Sp-R5a spectra sensing variations in local DNA motions. This establishes Rp- and Sp-R5a as unique probes for investigating nucleic acids in a site- and stereospecific manner, which may aid studies of stereospecific DNA/protein interactions. In addition, weighted averages of individual Rp and Sp spectra match those of R5a attached to mixed diastereomers. This suggests that R5a linked to mixed diastereomers reports on the composite behaviors of Rp- and Sp-R5a and is useful in initial probing of the DNA local environment. This work advances understanding of R5a/DNA coupling, and is a key step forward in developing a nucleotide-independent spectroscopic probe for studying nucleic acids.
Introduction
Site-directed spin labeling (SDSL) is a biophysical tool that utilizes electron paramagnetic resonance (EPR) spectroscopy to monitor a chemically stable nitroxide radical covalently linked to a specific site of a macromolecule (1). One can use SDSL to obtain information about macromolecular structure and dynamics at both the local and global levels. This technique requires a relatively small amount of sample (tens to hundreds of picomoles) and allows one to study high-molecular-weight systems under physiological conditions. It has been proven to be valuable in probing structure and dynamics of proteins, biological membranes, nucleic acids, and their assemblies (see recent reviews (2,3)).
A number of methods for attaching nitroxides to DNA and RNA have been reported (3,4). In particular, a phosphorothioate labeling scheme has been developed (5–7) in which a nitroxide molecule is attached to a phosphorothioate (PS) group introduced at a defined location of the nucleic acid backbone during solid-phase chemical synthesis (Fig. 1 A). This methodology is a simple and efficient means to link a nitroxide label to a target nucleotide within an arbitrary sequence. A number of nitroxide probes, designated as the R5 series, have been attached to DNA and RNA molecules utilizing the PS labeling chemistry, and have been used to monitor RNA/RNA interactions (5), to study motions of an RNA element within a large folded ribozyme (8), to probe local structural and dynamic features in DNA (7), and to measure nanometer distances in nucleic acids (9,10).
Figure 1.
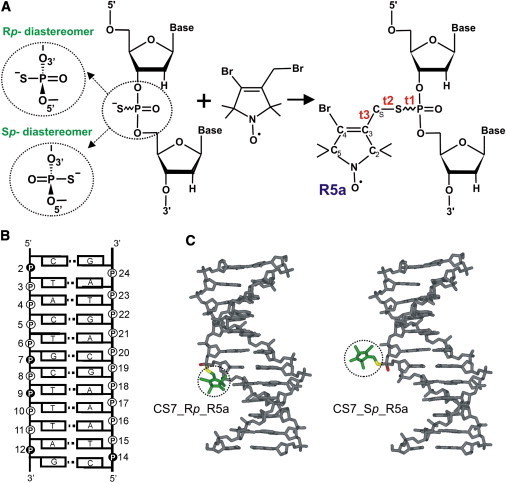
(A) The phosphorothioate labeling scheme. A reaction between a phosphorothioate-modified oligonucleotide and 4-bromo-3-bromomethyl-2,2,5,5-tetramethyl-1-oxylpyrroline yields an R5a-labeled DNA. Chirality of the phosphorothioate linkage results in two oligonucleotide diastereomers known as Rp and Sp. The three torsion angles of the DNA-nitroxide linker are marked as t1(O5′-P-S-CS), t2(P-S-CS-C3), and t3(S-CS-C3-C2). (B) Sequence and secondary structure of the CS DNA duplex, with R5a labeling sites indicated by solid circles. (C) Structural models of R5a (dotted circle) attached to the Rp (left) and Sp (right) diastereomers at CS7.
The PS labeling scheme relies on the PS modification in which sulfur is substituted for one of the two nonbridging oxygens in the naturally occurring phosphodiester, thus introducing a chiral center at the phosphorus atom. Using the current solid-phase synthesis scheme, a modified oligonucleotide is obtained as a mixture of two diastereomers, designated as Rp and Sp (Fig. 1 A), in an ∼50/50 proportion. Both diastereomers have the ability to react with a nitroxide precursor. Nucleic acid systems containing mixtures of Rp- and Sp-nitroxides have been successfully used in many SDSL studies, including monitoring molecular interactions (5), measuring interspin distances (9,10), and exploring structural and dynamic features at the labeled nucleotide (7).
Systems containing pure Rp- or Sp-nitroxides have been explored to a much smaller extent, although we have previously demonstrated the feasibility of obtaining diastereopure-nitroxide-labeled samples with certain DNA and RNA molecules (11). Modeling studies have shown that at a given site of a parent macromolecule, the Rp- and Sp-nitroxides are spatially distinct (9,12) (Fig. 1 C) and may experience a different local environment, defined as the structural and dynamic features of a macromolecule at the labeling site. This may give rise to detectable differences in EPR observables. For example, in a recent study of nanosecond dynamics of an RNA element in a large ribozyme (8), it was found that Rp- and Sp-nitroxides gave different spectra in either the docked or the undocked state of the ribozyme but reported similar spectral changes upon transition between the ribozyme states. The availability of both Rp and Sp data provided an important support in assigning the observed EPR spectral change to RNA motions. Overall, SDSL studies using pure Rp- and/or Sp-nitroxides may become a unique methodology for examining a given nucleic acid site in a stereospecific manner, thus increasing the amount of information one can obtain. It may aid studies of stereospecific interactions with the two nonbridging oxygens, particularly those related to enzymatic cleavage and ion coordination.
This study explores the feasibility of probing DNA local environment with Rp- and Sp-nitroxides by attaching an R5a probe (Fig. 1 A) to a DNA duplex in a stereospecific fashion. The model DNA, designated CS (Fig. 1 B), is a dodecamer with a near-canonical B-form structure in solution (PDB ID 1CS2 (13)) and has been used in a number of previous SDSL studies (7,9,12). In particular, X-band continuous-wave (cw) EPR spectra have been reported for R5a attached to mixtures of PS diastereomers at eight CS sites (7). These mixed-diastereomer studies have established that local DNA environments differentially influence rotational motions of R5a in the regime of 0.1–30 ns, giving rise to observable spectral variations along the DNA duplex.
In this work, X-band cw-EPR spectra were obtained for pure Rp- and Sp-R5a labeled at five different positions of the CS duplex (Fig. 1 B). At each site studied, the Rp-R5a spectrum was found to differ from that of Sp-R5a, with rotational diffusion of Rp-R5a being more restricted. This is attributed to the fact that in a B-form DNA duplex, Rp diastereomers position the nitroxide toward the groove of the DNA, whereas Sp diastereomers position the nitroxide toward the solvent. Linear additions of Rp- and Sp-R5a spectra satisfactorily recover the respective measured mixed-diastereomer spectra, supporting the notion that studies using mixtures of PS diastereomers report the combined behaviors of Rp- and Sp-nitroxides. Furthermore, variations between different DNA sites are observed when comparing spectra within the Rp or the Sp series. Two of the five Rp spectra show observable low-mobility components, which are likely due to site-specific nitroxide-DNA interactions. The Sp spectra, on the other hand, seem to be less sensitive to variations in local structural features, and they report primarily on DNA backbone flexibility. These results together indicate that Rp- and Sp-R5a provide information on the DNA local environment in a stereo- and site-specific manner.
Materials and Methods
DNA oligonucleotides and nomenclature
In the text, CSx designates site x within the CS duplex, whereas CSx_Rp and CSx_Sp refer to the Rp and Sp PS configurations, respectively, at site x. CSx_R5a indicates the CS duplex with R5a nitroxide attached to a diastereomeric mixture at the specific site x or the corresponding EPR spectrum, and CSx_Rp_R5a and CSx_Sp_R5a refer to stereoregular R5a-labeled DNA duplexes or their EPR spectra. For example, CS7_Rp represents site 7 with an Rp configuration and thus the local environment at the Rp PS of nucleotide 7; CS7_Rp_R5a designates a duplex with an R5a attached to the Rp diastereomer or the corresponding EPR spectrum of that duplex.
All deoxyoligonucleotides used in this work were obtained commercially from Integrated DNA Technologies (Coralville, IA). Oligonucleotide concentrations were determined by UV absorption at 260 nm, as previously described (7).
Preparation of Rp-R5a and Sp-R5a oligonucleotides
To attach R5a in a stereospecific manner requires two general steps: nitroxide coupling and diastereomer separation. Nitroxide coupling was carried out by incubating a PS-modified oligonucleotide with a reactive derivative, 4-bromo-3-bromomethyl-2,2,5,5-tetramethyl-1-oxylpyrroline (R5a precursor, kindly provided by Kálmán Hideg, University of Pécs, Hungary), according to a published protocol (7). For CS2, CS9, CS12, and CS14, diastereomer separation was carried out on the unlabeled DNA, and R5a was then attached to the diastereopure oligonucleotides. For CS7, diastereomer separation was feasible after R5a coupling, which significantly shortened the preparatory procedure.
Rp and Sp diastereomers of a given DNA oligonucleotide were separated on a DNApac PA-100 anion-exchange (AE) column (4 × 250 mm, Dionex, Sunnyvale, CA) using a procedure modified from a published protocol (11). In each round of separation, ∼5 nmol of an oligonucleotide was loaded onto the column and eluted at room temperature using a two-component gradient with a flow rate of 1 ml/min. The elution buffers consisted of 25 mM Tris-HCl, pH 6.8 (buffer A) and 25 mM Tris-HCl, pH 6.8, and 500 mM NaCl (buffer B). In a typical round of high-performance liquid chromatography (HPLC) separation, a DNA sample was subjected to a 15-min segment of 0% Buffer B, followed by a 50- to 60-min segment in which buffer B was increased at a rate of 1.5%/min. Eluted samples were collected in 0.2-ml fractions using an automatic fraction collector. After HPLC runs, respective Rp and Sp fractions were combined and desalted using a homemade G-25 Sephadex column. The desalted samples were subjected to an additional round(s) of AE HPLC separation depending on their purity. To obtain a sufficient amount (4–10 nmol) of Rp and Sp oligonucleotides with >98% purity required 20–30 rounds of separation. Finally, purified and desalted Rp and Sp oligonucleotides were lyophilized, then resuspended in water and stored at −20°C.
EPR sample preparation and measurements
To form a DNA duplex, ∼1 nmol of an R5a-labeled diastereopure oligonucleotide was annealed with a 10% molar excess of an unlabeled complementary strand in buffer 1 (100 mM NaCl and 50 mM Tris-HCl, pH 7.5). After overnight incubation at room temperature, the annealing reaction mixture was diluted to 600 μL with buffer 1 and then concentrated to 5–7 μL using a membrane centrifugal filter (MWCO 5 kD, Millipore, Billerica, MA), which removed unannealed single-stranded DNA and a trace amount of free spin labels. Concentrated DNA was used to prepare an EPR sample containing ∼20–40 μM of an R5a-labeled DNA duplex suspended in buffer 1 and 34% (w/w) sucrose. Sucrose was added to reduce global tumbling of the duplex and to enhance sensitivity of an X-band EPR spectrum to site-specific features of nitroxide rotational motions. A previous study showed that sucrose does not alter key features observed in EPR spectra between different CS sites (7).
EPR spectra were obtained according to procedures previously described (4). Specifically, 13–15 μL of samples were placed in glass capillaries (1.0 × 1.2 mm, Vitrocom, Mountain Lakes, NJ) sealed at one end. X-band EPR spectra were acquired on a Bruker EMX Spectrometer with a high sensitivity cavity (ER 4119HS, Bruker Biospin, Billerica, MA) and acquisition parameters were as follows: incident microwave power, 2 mW; modulation amplitude, 1–2 G; and modulation frequency, 100 kHz. A liquid nitrogen variable temperature setup was used to maintain sample temperature. All EPR spectra were baseline-corrected and normalized to the same number of spins using software provided by the Hubbell group at University of California, Los Angeles.
EPR spectra fitting
Spectral fitting was carried out with the MATLAB-based EPRLL program suite (14) using the microscopic-order-macroscopic-disorder model. Dimensions of the basis set were reduced via the pruning subroutine to Lemx = 6, Lomx = 5, Kmx = 5, Mmx = 4, Kmn = 0, Mmn = 0, and pImx = 2. All spectral calculations used the same set of g and A tensor values (gxx = 2.0083, gyy = 2.0051, gzz = 2.0022, Axx = 6.9, Ayy = 5.7, and Azz = 35), which were determined from fitting near-rigid-limit X-band spectra obtained at 0°C in 65% sucrose (w/w). The diffusion tilt angle (βD = 35°) and number of director orientation (nort = 20) were fixed. Variable fitting parameters included 1), and N, spherical components of the rotational diffusion rate tensor (and N = R‖/R⊥, where R‖ and R⊥ are the respective rate constants for rotations parallel and perpendicular to the nitroxide principal diffusion axis); 2), c20, the coefficient of an ordering potential (, where θ is an instantaneous angle relating the director to the nitroxide diffusion frame); and 3), Δ(0), the Gaussian inhomogeneous broadening factor. From the ordering potential, an order parameter, S20, is computed as
For each measured spectrum, the SIMPLEX minimization method was utilized to find a best-fit spectrum with the lowest root-mean-square deviation (rmsd). An ensemble of acceptable spectra, defined as those with rmsd values within 110% of that of the best-fit spectrum, was then generated using a Monte Carlo restart subroutine. Using the ensemble, averages and standard deviations for the fitting parameters were calculated, with the standard deviation used to represent the error for the respective parameter.
Results
Preparation and characterization of diastereopure R5a-labeled DNA
In the study reported here, R5a was attached to pure Rp and Sp diastereomers at five CS sites (Fig. 1 B). Fig. 2 A shows two representative HPLC profiles that demonstrate diastereomer separation of either an unlabeled (e.g., CS2) or an R5a-labeled (CS7_R5a) DNA strand. All diastereomers used in this study were >98% pure as determined by AE HPLC runs after completion of purification. We note that the diastereomer separation protocol was laborious, generally requiring 2–3 weeks of work for each sample (see Methods). In addition, the degree of chromatographic resolution of Rp and Sp peaks varies significantly depending on the position of PS modification and the oligonucleotide sequence, rendering diastereomer separation at certain CS sites unattainable (e.g., CS5). This is consistent with the complexity of PS diastereomer separation reported in the literature (15–17).
Figure 2.
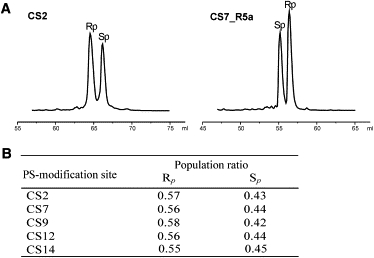
(A) Separations of Rp and Sp diastereomers by AE HPLC. The normalized traces shown correspond to initial sample loading of 5–6 nmol. (B) Rp versus Sp ratios determined by AE HPLC for the crude oligonucleotide mixtures supplied by the vendor.
Stereochemical configurations were assigned to chromatographic fractions based on a previous study in which a stereospecific nuclease digestion assay was used to determine diastereomer identity (11). As reported in that study, without R5a attached (e.g., CS2 (Fig. 2 A)), the Rp diastereomer elutes first, followed by the Sp, whereas after R5a coupling (e.g., CS7_R5a (Fig. 2 A)), the elution order is reversed, with Sp-R5a eluting first and Rp-R5a eluting later. Furthermore, for all oligonucleotides studied, the Rp diastereomer was present in slight excess over Sp in the mixtures supplied by the vendor, with the Rp/Sp ratio fluctuating between 60/40 and 50/50 (Fig. 2 B). This is consistent with other reports (18,19), and serves as an independent control of PS configuration assignment.
DNA structural perturbation due to Rp_R5a and Sp_R5a was assessed by thermal denaturation measurements. Differences in the standard-state free energy of duplex formation between R5a-labeled and wild-type DNA duplexes (ΔΔG°37°C) were determined to be 0.2–0.7 kcal/mol (Table S1 in the Supporting Material). At every site, differences between Rp- and Sp-R5a ΔΔG°37°C values fall in the 0–0.2 kcal/mol range, which is not significant compared to the error of ΔΔG°37°C (±0.2 kcal/mol). These results are consistent with previous studies of R5a attached to mixed diastereomers (7,9), and they confirm that both Rp- and Sp-R5a can be used to probe local environment in a DNA duplex without significantly affecting its native B-form conformation.
R5a reports different Rp and Sp spectra at a given DNA site
Fig. 3 shows X-band EPR spectra of Rp- and Sp-R5a at five sites of the CS duplex obtained in the presence of 34% (w/w) sucrose at 5°C (see Methods). At each site, the Rp-R5a spectrum is distinct from that of the Sp-R5a spectrum. The largest spectral differences between Rp- and Sp-R5a are found at CS2 and CS14 (Fig. 3). Unlike CS2_Sp_R5a, CS2_Rp_R5a gives a multicomponent spectrum, with a clearly visible bump at the low-field region (red arrow). This extra component indicates the presence of low-mobility nitroxide subpopulation(s), which may originate from site-specific interaction(s) between R5a and DNA (see Discussion). Affected by the immobilized subpopulation(s), the CS2_Rp_R5a lineshape shows amplitude reduction and linewidth increase in comparison with that of CS2_Sp_R5a, suggesting overall lower nitroxide mobility at the CS2_Rp diastereomer. Similar multiple-component features can be observed in CS14_Rp_R5a, although the spectrum has a more dominant high-mobility nitroxide population as compared to CS2_Rp_R5a (Fig. 3).
Figure 3.
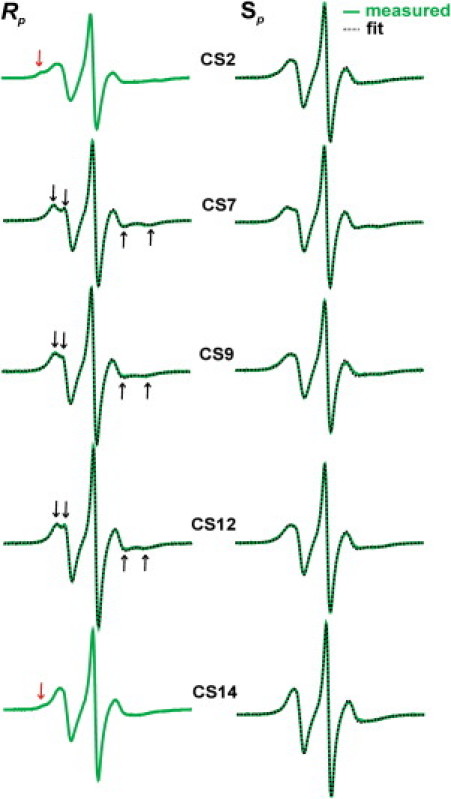
Rp- and Sp-R5a spectra measured at 5°C. Experimental spectra are shown as green solid lines, and the best-fit spectra obtained from simulations are shown as black dotted lines. For the Rp spectra, black arrows show the resolved hyperfine extrema, and red arrows indicate the low-mobility spectral components. Experimental spectra were corrected for residual amounts of free nitroxides (<3%, see Fig. S1).
In a similar way, the other three sites (CS7, CS9, and CS12) reveal clear differences between Rp and Sp spectra, though none of them show multiple spectral components. For example, the Rp spectra have splittings in low- and high-field peaks (Fig. 3, black arrows), which is a characteristic feature arising from incomplete averaging of the nitroxide hyperfine magnetic tensor. This is a signature of a nitroxide undergoing a restricted mode of motion. In the Sp spectra, splittings are absent at CS9 and CS12, and are barely discernable at CS7 (Fig. 3). This indicates reduced ordering of the nitroxide motions in these sites compared to the respective Rp sites. In addition, the amplitude of EPR lines is noticeably smaller in the Sp spectra, particularly at CS9 and CS12, which might suggest a lower rate for Sp-R5a rotational diffusion than for that of Rp-R5a.
To provide a quantitative description of Rp- and Sp-R5a dynamics, the observed spectra were simulated using the microscopic-order-macroscopic-disorder model, which describes the nitroxide motion within the macromolecular environment as an anisotropic rotational diffusion constricted by an ordering potential (14,20). Of the ten R5a spectra, eight were successfully simulated using a single-population approximation (see Methods), and the resulting best-fit spectra match well to the corresponding measured spectra (Fig. 3). A full list of fitting parameters is reported in Table S2. The multiple-component nature of CS2_Rp_R5a and CS14_Rp_R5a spectra renders it much more difficult to uniquely resolve their fitting parameters, and adequate fits have not been obtained.
Simulations yield two key parameters in describing R5a motions: 1), , which describes the rate of nitroxide rotational motions; and 2), S20, which provides a measure of local ordering. Fig. 4 A shows a comparison of and S20 between Rp-R5a and Sp-R5a at CS7, CS9, and CS12, where all diastereomer spectra have been simulated. The plots show that Rp-R5a has a higher degree of ordering (larger S20) and an increased diffusion rate compared to the corresponding parameters for Sp-R5a. We note that simulations treat nitroxide dynamics as a restricted Brownian rotational diffusion, and higher motional ordering does lead to an increase in diffusion rate due to reduction in the available diffusion space (21). Therefore, consistent with qualitative lineshape comparisons (i.e., linewidth, amplitude, and splitting), both S20 and indicate that at each site, Rp-R5a undergoes more restrictive motions than Sp-R5a. This likely originates from spatial constraints posted by the DNA (see Discussion).
Figure 4.
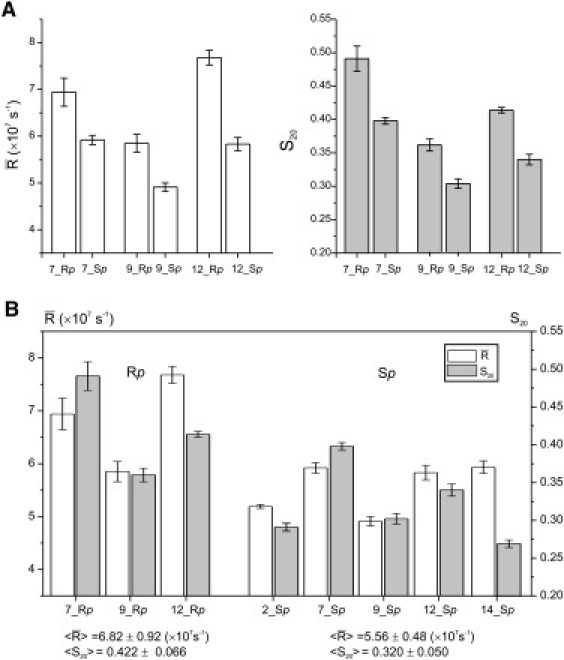
Motional parameters obtained from fitting 5°C spectra. (A) Pairwise comparisons between Rp- and Sp-R5a at the three sites lacking multiple components. (B) Comparisons between different sites for Rp- (right) and Sp-R5a (left), with average and standard deviation values listed below the respective series.
A known issue in EPR simulation at a single frequency is degeneracy, in which different parameter sets may yield simulated spectra that fit comparably to a particular experimental spectrum (22). In studies reported here, correlation coefficients between variable fitting parameters were controlled to be <0.9, which is deemed acceptable (23). Parameter uncertainties were assessed by the respective standard deviation values (see Methods, Table S2, and Table S3) and were found comparable to those reported in the literature (22). These controls support the use of and S20 to reveal relative differences in R5a dynamics between DNA sites. However, absolute values of and S20 are likely systematically biased, as the rigid-limit g and A tensors were not accurately determined using a single X-band frequency, and details in the motional model (e.g., axially symmetric rotational diffusion and values of diffusion tilt angles) cannot be independently validated. Analyses based on the absolute and S20 values require further work, such as studies employing multiple EPR frequencies (24).
In summary, differences observed between diastereomer spectra at each of the five CS sites indicate that rotational dynamics of the R5a probe are differentially affected by Rp and Sp local DNA environment.
Site-specific variations in Rp-R5a and Sp-R5a spectra
Variations between different DNA sites can be clearly observed within the respective series of Rp- or Sp-R5a spectra (Fig. 3). Rp-R5a spectra can be separated into two groups, with group 1 including CS7, CS9, and CS12 and group 2 including CS2 and CS14. Spectra in group 1 show well-resolved splittings of the low- and high-field peaks (Fig. 3, black arrows), suggesting a common mode of motion (i.e., a homogeneous nitroxide population undergoing restricted anisotropic diffusion). However, R5a mobility (i.e., rate and ordering) varies from site to site, as evident qualitatively from lineshape comparisons (Fig. 3) and quantitatively from simulations (Fig. 4 B). Specifically, CS7_Rp_R5a shows best-resolved splittings at low- and high-field regions and the largest S20, both reporting the highest degree of nitroxide ordering. CS12_Rp_R5a has the largest line amplitudes and the narrowest center line, and spectral simulations yield the biggest value, suggesting fast rotational dynamics of the probe. In group 2 (CS2 and CS14), spectra show no low- and high-field splitting, but clearly reveal shoulders in the low-field region (Fig. 3, red arrows), indicating the presence of heterogeneous nitroxide populations. In these spectra, their center- and low-field lines are unevenly broadened and diminished, rendering it difficult to directly compare Rp-R5a mobility at CS2 and CS14 with the other Rp sites.
Unlike Rp-R5a, all five Sp-R5a spectra feature a single nitroxide population. Lineshape comparisons (Fig. 3) and simulations (Fig. 4 B) report site-dependent variations in Sp-R5a motions. For example, CS7_Sp_R5a is the only Sp spectrum that shows discernable splittings, and it has the largest S20 value, both reporting a high degree of nitroxide ordering. CS14_Sp_R5a has noticeably smaller width and larger amplitude of spectral lines, and simulations yield a combination of low ordering (small S20) and fast rate (large ), indicating the highest overall nitroxide mobility within the Sp series.
A global comparison of all measured spectra also reveals that Rp-R5a exhibits a larger degree of spectral variations between the DNA sites. Distributions of and S20 values are broader for the Rp series as compared to the Sp series(Fig. 4 B). In addition, some Rp spectra show multiple-component features, whereas all Sp spectra are single-component (Fig. 3). Together, the data suggest that Rp-R5a mobility, as compared with that of Sp-R5a, varies more from one DNA site to the other.
Temperature dependence of Rp-R5a and Sp-R5a spectra
R5a spectra at sites CS2, CS7, and CS9 were obtained at other temperatures (−5°C, 15°C, and 25°C) (Fig. 5) and found to vary in both a stereo- and site-specific manner. The observed spectral variations generally agree with those obtained at 5°C. At all temperatures, a shoulder at the low-field peak appears in the CS2_Rp spectrum (Fig. 5 A, arrows), indicating the presence of multiple nitroxide population(s). This multiple-component feature is absent in spectra of CS2_Sp, as well as those of CS7 and CS9. Due to the low-mobility nitroxide population(s), CS2_Rp spectra have broader center lines than the corresponding CS2_Sp spectra, thus appearing to report an overall lower R5a mobility. Furthermore, for CS7 and CS9, two common features are revealed by lineshape comparisons (Fig. 5 A) and spectral fittings (Fig. 5 B, Table S3, and Fig. S2). First, as measured by S20, at a given DNA site, Rp-R5a undergoes more restricted motions compared to Sp-R5a. Second, both Rp- and Sp-spectra suggest that site CS7 is characterized by more confined (larger S20) and faster (higher ) nitroxide rotational diffusion than site CS9.
Figure 5.
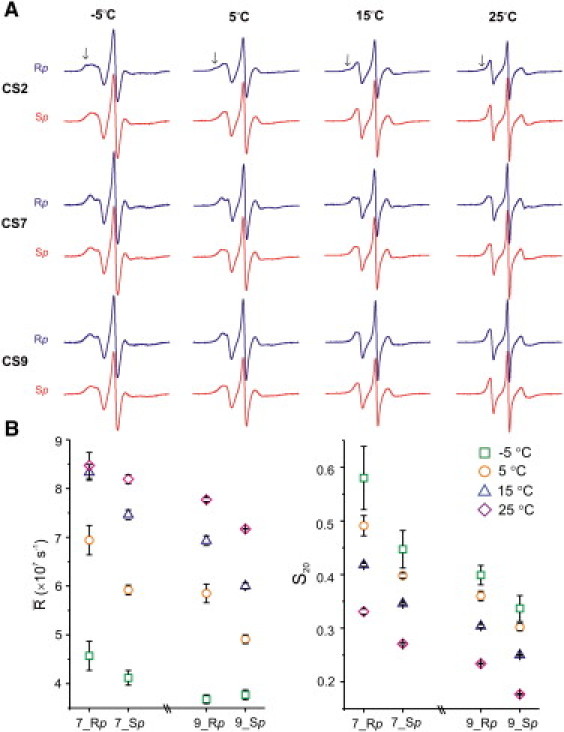
Temperature dependence of Rp- and Sp-R5a spectra. (A) Measured spectra with the Y-scale adjusted for each temperature set. Black arrows indicate the low-mobility spectral components. Spectra were corrected for residual amounts of free nitroxides (<3%). (B) Plots of motional parameters obtained from fitting CS7 and CS9 spectra at different temperatures.
Overall, the data indicate that the major trends observed using 5°C spectra are present at lower and higher temperatures. We note that temperature changes alter behaviors of R5a as well as that of the parent DNA molecule. Further analyses of temperature-dependent data sets (e.g., S20 and ) may provide better understanding of the complex dynamical modes contributing to overall nitroxide motions. This will be pursued in the future.
A linear combination of Rp-R5a and Sp-R5a spectra reproduces the mixed-diastereomer spectrum
In previous studies, it has been assumed that a spectrum of R5a attached to mixtures of PS diastereomers is an average of the individual Rp and Sp spectra (7). To test this hypothesis, a composite spectrum at a given site was computed by adding the 5°C Rp- and Sp-R5a spectra with coefficients corresponding to the ratio of Rp/Sp oligonucleotides observed in the mixtures (Fig. 2 B). At all five sites, the resulting composite spectra match well to the corresponding mixed-diastereomer spectra (Fig. 6 A), reproducing all prominent spectral features, such as peak splittings (e.g., CS7) and low-field bumps (e.g., CS2 and CS14). Given that the mixed-diastereomer spectra were obtained two to three years before those of the individual Rp- and Sp-R5a, the degree of agreement between the composite and measured spectra well exceeded our expectations, unambiguously demonstrating that the mixed-diastereomer spectrum is a linear combination of the individual Rp and Sp spectra.
Figure 6.
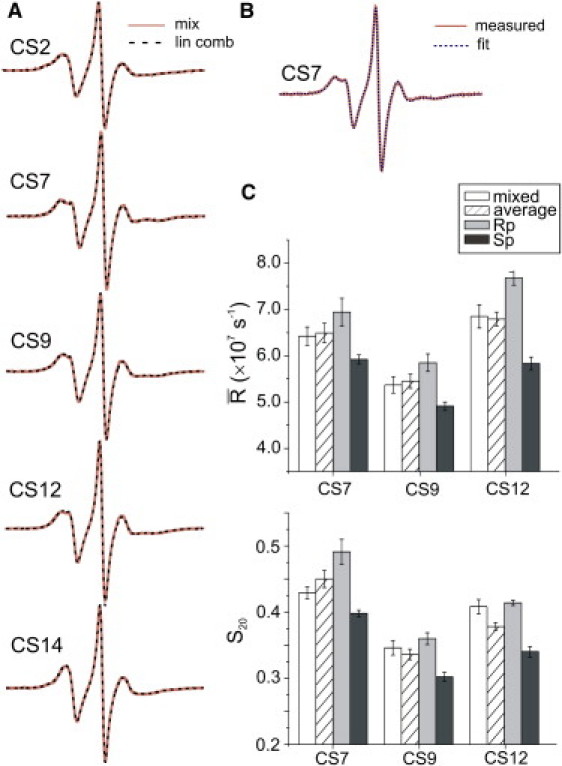
(A) Comparisons between measured spectra of R5a attached to mixed diastereomers (solid orange lines) and those computed by weighted average of the individual Rp- and Sp-R5a spectra (dashed black lines). All spectra were measured at 5°C. (B) An example of a mixed-diastereomer spectral simulation. (C) Comparisons of motional parameters obtained from fitting experimental spectra of mixed diastereomers (open bars), Rp (gray bars) and Sp (black bars). Weighted averages of Rp and Sp parameters (hatched bars) are also included.
Furthermore, the mixed-diastereomer spectra of CS7, CS9, and CS12 at 5°C were successfully simulated assuming that only one nitroxide population is present (Fig. 6 B, Fig. S3, and Table S2). The resulting fitting parameters fall between those obtained from the individual diastereomers, and they agree surprisingly well with the weighted averages of the corresponding Rp and Sp parameters (Fig. 6 C). We note that two diastereomers cannot interconvert without breaking and reforming a bridging P-O bond; therefore, fast exchange between the two species is unlikely at the EPR timescale. Simulation results indicate that mixed-diastereomer spectra at these sites can be adequately represented using a homogenous nitroxide population, whose dynamic behavior is characterized by the weighted average of Rp- and Sp-R5a.
Discussion
Spectral variations reported here clearly demonstrate stereo- and site-dependent coupling between R5a rotational motions and local DNA environment. Neither Rp-R5a nor Sp-R5a severely perturbs the native conformation of the parent DNA molecule, so R5a can be used to probe DNA local structure and dynamics in a stereospecific fashion. In the following text, we first examine how DNA local structure may differentially affect Rp- and Sp-R5a, giving rise to observed spectral variations. We then analyze the sensitivity of Rp- and Sp-R5a to local DNA motions and, finally, discuss advantages and limitations of using mixed versus pure diastereomers in studying DNA.
Modulations of Rp-R5a and Sp-R5a by DNA local structure
In previous studies of R5a attached to mixed diastereomers, the observed site-specific spectral variations were analyzed in terms of two modes of coupling between DNA local environment and nitroxide dynamics (7). First, DNA three-dimensional structure may restrain, in a site-specific manner, internal motions of R5a, which are defined as torsional rotations about bonds connecting the pyrroline ring to the phosphate of the DNA backbone (Fig. 1 A). Second, site-specific dynamical modes of DNA can be physically transmitted to the nitroxide via the motions of the phosphorus atom. Analyses presented below suggest that constraints on R5a internal motions, which arise primarily from DNA-defined allowable rotamer space and site-specific DNA-nitroxide interactions, may account for spectral variations observed between Rp- and Sp-R5a at a given site.
A common feature present at all sites under the temperatures studied is the greater restriction of rotational diffusion for Rp-R5a compared to Sp-R5a (Figs. 3 and 4). Molecular modeling clearly shows that an Rp diastereomer directs the nitroxide pyrroline ring toward the major groove of the DNA helix, and an Sp diastereomer directs it away from the helix and into the solvent (Fig. 1 C). In a previous study (7), we used a modeling tool (NASNOX) to generate ensembles of sterically allowable Rp- and Sp-R5a rotamers at a given site of the CS duplex and estimated the allowable rotamer space with an S parameter, which was computed based on the angular distribution of the nitroxide N-O bond vectors (see Table 2 of our previous study (7)). It was reported that at every CS site studied, Rp-R5a has a larger S parameter than that of Sp-R5a (<S> being 0.538 ± 0.022 for Rp and 0.496 ± 0.001 for Sp), indicating smaller directional variations of the N-O vector and thus more restricted sterically allowable rotamer space for Rp-R5a. This is in agreement with the higher degree of motional ordering (larger S20) reported by Rp-R5a at each DNA site (Figs. 3 and 4). We also note that Rp sites show an apparently broader distribution of S values (7), indicating larger variability in the allowable rotamer space for Rp-R5a. This agrees with the broader distributions of S20 and in the Rp series (Fig. 4 B).
In addition to macromolecule-imposed structural constrains, nitroxide internal motions may be affected by site-specific DNA/nitroxide interactions. It has been speculated that Rp-R5a is more susceptible to these interactions, as it is located closer to the DNA (7,12). This is now strongly supported by the observation that immobilized nitroxide subpopulations, a signature of nitroxide-DNA interactions, are observed exclusively in CS2_Rp_R5a and CS14_Rp_R5a spectra but are absent in the corresponding Sp-R5a spectra (Fig. 3). Modeling studies suggest that at CS2, selected Rp-R5a rotamers may interact with the methyl group of the neighboring T2 (Fig. 7, A and B), and similar interactions are also feasible at CS14. These interactions, however, are not likely for the corresponding Sp-R5a, as the Sp diastereomer directs the nitroxide ring away from the DNA surface (Fig. 7, C and D). It is interesting that at CS9, which has a neighboring T9, an immobilized spectral component is not observed, indicating an absence of R5a/T-methyl interactions. Further studies are needed to confirm such R5a/T-methyl interactions and to explore their dependence on location (duplex interior versus terminus) and/or surrounding nucleotide sequence.
Figure 7.
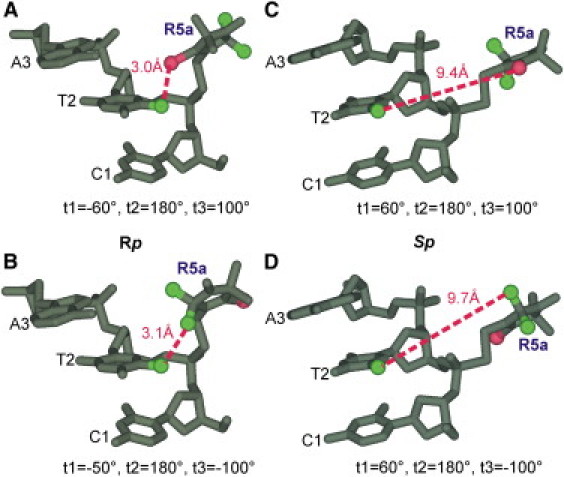
Spatial orientations of Rp and Sp nitroxides at CS2. Molecular models were generated based on the NMR structure using WebLab ViewerPro 3.7 (Molecular Simulations, Orange, CA). During modeling, torsion angles t2 and t3 were set to fixed values (t2 = 180° and t3 = +100° or −100°) based on MD simulations (data not shown), whereas t1 was varied to avoid any steric collisions between R5a and DNA. For Rp-R5a (A and B), the particular rotamers shown give the closest distance (dotted line) between C5-methyl of T2 and functional groups of the R5a pyrroline ring, C4-Br (A) or C2-methyls (B). For Sp-R5a (C and D), none of these pyrroline moieties can be positioned closer than 9 Å to the C5-methyl of T2, and the rotamers shown have t1 arbitrarily set to 60°.
Taken together, Rp-R5a is positioned toward the DNA major groove, its motions are more constrained and sensitive to site-dependent variations in the local structure. Consequently, Rp-R5a is more adapted to report site-specific structural features, and may be valuable in probing subtle DNA conformational changes, such as those induced by ligand-binding or changes of salt and solvent.
Modulation of Rp-R5a and Sp-R5a by DNA local motions
The analyses above suggest that Sp-R5a is not prone to site-specific DNA-nitroxide contacts and experiences mostly invariant rotamer space from one DNA site to another, variations in local DNA motions are therefore expected to be the main factor giving rise to Sp-R5a spectral differences. In a previous study, we suggested that R5a mobility reports DNA flexibility that may correlate with thermodynamic stability determined by location (duplex interior versus terminus) and flanking sequence of the labeling site (7). Within the Sp_R5a series (Figs. 3 and 4), nitroxide rotational diffusion is most restricted at CS7, consistent with the low DNA flexibility (i.e., local DNA motion) expected at a site located at the duplex center and flanked by a thermodynamically stable -GC- dinucleotide (25,26). Likewise, the highest Sp-R5a mobility observed at CS14 correlates with an increased DNA flexibility presumed at a terminal site flanked by a less stable -CT- dinucleotide.
Although the stability of the nearest neighbor is sufficient to account for Sp_R5a data at CS7 and CS14, sequence beyond the flanking dinucleotide may also affect DNA flexibility and R5a dynamics. The two 5′-terminal sites CS14 and CS2, which share the same trinucleotide neighboring sequence (5′-CTA-3′ (Fig. 1 B)), feature distinct Sp-R5a spectra (Fig. 3). Nitroxide mobility at CS14 is clearly higher than that at CS2 (Fig. 4 B), suggesting different DNA backbone flexibility at these two sites. CS2 and CS14 are also different in their Rp spectra (Fig. 3), indicating subtle differences in the DNA groove structure. This suggests that R5a probes can report variations of local DNA structure (Rp effect) and dynamics (Sp effect) induced by flanking sequence(s) beyond the nearest neighbor. Previous studies of mixed diastereomers did reveal different R5a spectra at CS14 and CS2, but a detailed analysis was hampered by the multiple-component features (7). Resolving the individual Rp and Sp spectra clearly yields more information on local DNA environment differences at CS2 and CS14.
We also note that the 3′-terminal site CS12 features higher S20 than the duplex interior site CS9 (Fig. 4), an indication of more restricted nitroxide motions at CS12. This is consistent with a previous observation that nitroxide rotational motions at the duplex termini may not always be less restricted than those in the duplex interior (7). CS12 and CS9 differ in both position and sequence (Fig. 1 B), which may collectively contribute to DNA thermodynamic stability in a complex fashion. Overall, although certain correlations between Sp-R5a motions and DNA thermodynamic stability can be discerned from current data, an expanded database is needed for a detailed understanding of the correlation between nitroxide mobility (e.g., S20 and ) and DNA motional behaviors.
As compared to Sp-R5a, Rp-R5a is expected to sense a similar mode(s) of DNA local motions, as both use three single bonds to connect the pyrroline ring to the DNA backbone. In our studies, Rp- and Sp-R5a show a consistent trend in nitroxide ordering (S20(CS7) > S20(CS12) > S20(CS9) (Fig. 4 B) but deviate in one of the two pairwise comparisons of diffusion rate (for Rp, (CS12) > (CS7) and for Sp, (CS12) = (CS7) (Fig. 4 B)). We believe this deviation arises from differences in nitroxide internal motions, which may vary from site to site for Rp-R5a but are presumably constant for the entire Sp series. Studies are underway to investigate contributions of nitroxide internal motions to Rp and Sp spectra.
Taken together, local DNA motions are likely the main factor contributing to variations in the Sp spectra, rendering Sp-R5a better suited for probing DNA local flexibility.
Mixed versus pure diastereomers: advantages and limitations in probing the DNA environment
The results presented here clearly demonstrate that at each site, a mixed-diastereomer spectrum is a weighted sum of individual Rp and Sp spectra (Fig. 6) and therefore reports collective DNA features present at both diastereomers. At CS2 and CS14, the mixed-diastereomer spectra are able to report multiple nitroxide populations. At CS7, CS9, and CS12, simulations of mixed-diastereomer spectra using a single R5a population revealed nitroxide motional parameters falling between those of Rp- and Sp-R5a (Fig. 6 C). As a consequence, one recovers a trend of relative nitroxide mobility (in the case of , CS12 ≥ CS7 > CS9, and for S20, CS7 > CS12 > CS9) that follows those of the individual Rp- and Sp-R5a. Overall, a mixed-diastereomer spectrum is a composite that provides information on the gross features of the DNA local environment: it reports the average amplitude and rate of R5a motions at the labeling site and reveals potential site-specific DNA-nitroxide interactions. It is clear that SDSL studies should start with the mixed diastereomers. This will allow a fast and convenient scanning of a given oligonucleotide sequence and reveal collective features of DNA local environment at specific sites. It will also identify sites, such as those showing multiple spectral components, where subsequent diastereopure analyses might be particularly informative.
Although interpreting a mixed-diastereomer spectrum may be more complicated, mixtures of Rp and Sp oligonucleotides are commercially available, and R5a can be easily placed at any nucleotide position within a target DNA and RNA molecule, including those of high molecular weight (9). On the other hand, preparation of diastereopure PS oligonucleotides remains very challenging, and may not be feasible for large nucleic acid molecules (>50 nucleotides). Here, diastereomer separation was achieved at five sites of the CS DNA, but was not successful at a number of other positions. This highlights a long-standing practical issue in obtaining diastereopure PS oligonucleotides. Although there have been a number of reports since the early 1990s on preparation of diastereopure PS oligonucleotides (27–29), those studies were limited to small-scale works and often required a combination of diastereomer separation and/or multistep reactions. In addition, the success of diastereomer separation is rather unpredictable (16,17), as it is strongly influenced by many factors, including oligonucleotide size, nucleotide sequence, and location of modification. As we have demonstrated here the feasibility of probing DNA in a stereospecific manner, further developments are needed to provide an efficient and facile method of diastereomer separation for a broad range of oligonucleotide systems.
Conclusions
Data reported here establish that Rp- and Sp-R5a, when separated, provide site- and stereospecific information at the single-nucleotide level, thus serving as unique spectroscopic probes for investigating the stereomeric local environment in DNA. Furthermore, a mixed-diastereomer spectrum is shown to be a weighted average of the individual Rp- and Sp-R5a spectra, and it provides information on the gross features of the DNA local environment. Although the work reported here focused on lineshape analyses of Rp-R5a and Sp-R5a attached to a B-form DNA, future explorations of other nucleic acid structures (e.g., A-form duplexes) and EPR measurements (e.g., relaxation and internitroxide distance) should be fruitful. A major remaining obstacle is the difficulty of preparing PS-modified oligonucleotides that are diastereopure independent of their length, sequence, and position of modification. Further development of methods that overcome this hurdle would greatly benefit nucleic acid studies using R5a as well as other spectroscopic probes.
Acknowledgments
Research supported by the National Institutes of Health (GM069557) and the National Science Foundation (MCB 054652).
Supporting Material
References
- 1.Altenbach C., Flitsch S.L., Hubbell W.L. Structural studies on transmembrane proteins. 2. Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry. 1989;28:7806–7812. doi: 10.1021/bi00445a042. [DOI] [PubMed] [Google Scholar]
- 2.Fanucci G.E., Cafiso D.S. Recent advances and applications of site-directed spin labeling. Curr. Opin. Struct. Biol. 2006;16:644–653. doi: 10.1016/j.sbi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Sowa G.Z., Qin P.Z. Site-directed spin labeling studies on nucleic acid structure and dynamics. Prog. Nucleic Acids Res. Mol. Biol. 2008;82:147–197. doi: 10.1016/S0079-6603(08)00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Cekan P., Qin P.Z. Studying RNA using site-directed spin-labeling and continuous-wave electron paramagnetic resonance spectroscopy. Methods Enzymol. 2009;469:303–328. doi: 10.1016/S0076-6879(09)69015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin P.Z., Butcher S.E., Hubbell W.L. Quantitative analysis of the isolated GAAA tetraloop/receptor interaction in solution: a site-directed spin labeling study. Biochemistry. 2001;40:6929–6936. doi: 10.1021/bi010294g. [DOI] [PubMed] [Google Scholar]
- 6.Qin P.Z., Haworth I.S., He H. Measuring nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nat. Protoc. 2007;2:2354–2365. doi: 10.1038/nprot.2007.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popova A.M., Kálai T., Qin P.Z. Site-specific DNA structural and dynamic features revealed by nucleotide-independent nitroxide probes. Biochemistry. 2009;48:8540–8550. doi: 10.1021/bi900860w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant G.P.G., Boyd N., Qin P.Z. Motions of the substrate recognition duplex in a group I intron assessed by site-directed spin labeling. J. Am. Chem. Soc. 2009;131:3136–3137. doi: 10.1021/ja808217s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Q., Kusnetzow A.K., Qin P.Z. Site-directed spin labeling measurements of nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nucleic Acids Res. 2006;34:4722–4730. doi: 10.1093/nar/gkl546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q., Kusnetzow A.K., Qin P.Z. Nanometer distance measurements in RNA using site-directed spin labeling. Biophys. J. 2007;93:2110–2117. doi: 10.1529/biophysj.107.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant G.P.G., Popova A., Qin P.Z. Diastereomer characterizations of nitroxide-labeled nucleic acids. Biochem. Biophys. Res. Commun. 2008;371:451–455. doi: 10.1016/j.bbrc.2008.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price E.A., Sutch B.T., Haworth I.S. Computation of nitroxide-nitroxide distances in spin-labeled DNA duplexes. Biopolymers. 2007;87:40–50. doi: 10.1002/bip.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leporc S., Mauffret O., Fermandjian S. An NMR and molecular modelling analysis of d(CTACTGCTTTAG). d(CTAAAGCAGTAG) reveals that the particular behaviour of TpA steps is related to edge-to-edge contacts of their base-pairs in the major groove. Nucleic Acids Res. 1999;27:4759–4767. doi: 10.1093/nar/27.24.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earle K.A., Budil D.E. Calculating slow-motion ESR spectra of spin-labeled polymers. In: Schlick S., editor. Advanced ESR Methods in Polymer Reaserch. John Wiley and Sons; New York: 2006. pp. 53–83. [Google Scholar]
- 15.Kanehara H., Mizuguchi M., Makino K. Isolation of oligodeoxynucleoside phosphorothioate diastereomers by the combination of DEAE ion-exchange and reversed-phase chromatography. Nucleosides Nucleotides. 1996;15:399–406. [Google Scholar]
- 16.Thorogood H., Grasby J.A., Connolly B.A. Influence of the phosphate backbone on the recognition and hydrolysis of DNA by the EcoRV restriction endonuclease. A study using oligodeoxynucleotide phosphorothioates. J. Biol. Chem. 1996;271:8855–8862. doi: 10.1074/jbc.271.15.8855. [DOI] [PubMed] [Google Scholar]
- 17.Subach F.B., Miuller S., Gromova E.S. Preparation of DNA-duplexes, containing internucleotide thiophosphate groups in various positions of the recognition site for the EcoRII restriction endonuclease. Bioorg. Khim. 2003;29:623–631. doi: 10.1023/b:rubi.0000008898.42854.7c. [DOI] [PubMed] [Google Scholar]
- 18.Wilk A., Stec W.J. Analysis of oligo(deoxynucleoside phosphorothioate)s and their diastereomeric composition. Nucleic Acids Res. 1995;23:530–534. doi: 10.1093/nar/23.3.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheruvallath Z.S., Sasmor H., Ravikumar V.T. Influence of diastereomeric ratios of deoxyribonucleoside phosphoramidites on the synthesis of phosphorothioate oligonucleotides. Nucleosides Nucleotides Nucleic Acids. 2000;19:533–543. doi: 10.1080/15257770008035005. [DOI] [PubMed] [Google Scholar]
- 20.Meirovitch E., Nayeem A., Freed J.H. Analysis of protein-lipid interactions based on model simulations of electron spin resonance spectra. J. Phys. Chem. B. 1984;88:3454–3465. [Google Scholar]
- 21.Martínez M.C., García de la Torre J. Brownian dynamics simulation of restricted rotational diffusion. Biophys. J. 1987;52:303–310. doi: 10.1016/S0006-3495(87)83217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Columbus L., Kálai T., Hubbell W.L. Molecular motion of spin labeled side chains in α-helices: analysis by variation of side chain structure. Biochemistry. 2001;40:3828–3846. doi: 10.1021/bi002645h. [DOI] [PubMed] [Google Scholar]
- 23.Budil D.E., Lee S., Freed J.H. Nonlinear-least-squares analysis of slow-motion EPR spectra in one and two dimensions using a modified Levenberg-Marquardt algorithm. J. Magn. Reson. A. 1996;120:155–189. [Google Scholar]
- 24.Zhang Z., Fleissner M.R., Freed J.H. Multifrequency electron spin resonance study of the dynamics of spin labeled T4 lysozyme. J. Phys. Chem. B. 2010;114:5503–5521. doi: 10.1021/jp910606h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SantaLucia J., Jr. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc. Natl. Acad. Sci. USA. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florian J., Sponer J., Warshel A. Thermodynamic parameters for stacking and hydrogen bonding of nucleic acid bases in aqueous solution: ab initio/Langevin dipoles study. J. Phys. Chem. B. 1999;103:884–892. [Google Scholar]
- 27.Fidanza J.A., Ozaki H., McLaughlin L.W. Site-specific labeling of DNA sequences containing phosphorothioate diesters. J. Am. Chem. Soc. 1992;114:5509–5517. [Google Scholar]
- 28.Nawrot B., Rebowska B., Stec W.J. New approach to the synthesis of oligodeoxyribonucleotides modified with phosphorothioates of predetermined sense of P-chirality. Tetrahedron Lett. 2005;46:6641–6644. [Google Scholar]
- 29.Oka N., Yamamoto M., Wada T. Solid-phase synthesis of stereoregular oligodeoxyribonucleoside phosphorothioates using bicyclic oxazaphospholidine derivatives as monomer units. J. Am. Chem. Soc. 2008;130:16031–16037. doi: 10.1021/ja805780u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


