Abstract
We have investigated the effect of cholesterol and two abundant phytosterols (sitosterol and stigmasterol) on the voltage-dependent anion-selective channel (VDAC) purified from mitochondria of bean seeds (Phaseolus coccineus). These sterols differ by the degree of freedom of their lateral chain. We show that VDAC displays sensitivity to the lipid-sterol ratio and to the type of sterol found in the membrane. The main findings of this study are: 1), cholesterol and phytosterols modulate the selectivity but only stigmasterol alters the voltage-dependence of the plant VDAC in the range of sterol fraction found in the plant mitochondrial membrane; 2), VDAC unitary conductance is not affected by the addition of sterols; 3), the effect of sterols on the VDAC is reversible upon sterol depletion with 10 μM methyl-β-cyclodextrins; and 4), phytosterols are essential for the channel gating at salt concentration prevailing in vivo. A quantitative analysis of the voltage-dependence indicates that stigmasterol inhibits the transition of the VDAC in the lowest subconductance states.
Introduction
The voltage-dependent anion-selective channel (VDAC) was discovered ∼30 years ago (1) and is the major diffusion pathway for solutes through the outer mitochondrial membrane (2,3). With respect to physiology, it has been suggested that VDACs may play an important role in the regulation of mitochondrial metabolism by controlling the flux of small metabolites (e.g., ATP, ADP, malate) across the mitochondrial outer membrane (4) and that this channel participates to the early steps of programmed cell death (5).
Plant VDACs form a small multigene family, e.g., there are five genes in Arabidopsis thaliana (6–9). Some of these genes are highly and constitutively expressed in all plant tissues (6,10). The three-dimensional structure of recombinant proteins recently achieved by NMR spectroscopy and x-ray crystallography shows that the mouse and human VDAC1 consists of 19 transmembrane β-strands folded in a β-barrel (11–13). Spectroscopic investigations indicate that VDAC from plants and fungi share the same secondary structure (14,15), suggesting that this model might provide a general framework for eukaryotic VDAC of the various lineages.
The lipid composition of mitochondrial membranes changes in response to both internal and external cues and influence the mitochondrial functions (16). Information about the effect of membrane lipids on the VDAC structure and function is scarce. Both dioleoylphosphatidylethanolamine and cardiolipin modify the voltage-dependence of Neurospora crassa VDAC (17), indicating that phospholipids might regulate the function of the VDAC. Sterols are essential structural components of biological membranes. There is evidence that cholesterol is bound to mammalian mitochondrial VDAC (12,18,19) and sterols seem to be important for the folding of VDAC (12,20,21). Contrary to what is found in other organisms, plant membranes contain several sterols such as sitosterol, stigmasterol, campesterol, and cholesterol (but in very low amount) (22,23). Sitosterol and stigmasterol are the most abundant in plant membranes. A previous study on plant VDACs indicates that phytosterols are required to refold denaturated VDACs purified in organic solvents, and that the recovery of the channel activity varies with the type of sterol (24).
It has been suggested that a plant VDAC might also be targeted to the plasma membrane (25) but further experiments are required to confirm this localization (26). The lipid composition of the plasma membrane differs from that of the mitochondria, notably with respect to its sterol content. Moreover, in plants, the membrane sterol composition can also vary according to the ecophysiological conditions that prevail during growth and development (27). Thus, in plants, the sterol environment of membrane proteins can be quite variable and this raises the question of the effect of sterols on the function of membrane proteins. Here, we show that VDAC properties can be modulated by the type of sterol and its abundance in the membrane. We compare the effect of stigmasterol, sitosterol, and cholesterol. These sterols have an identical steroid ring but they differ by the structure of their side chain. As compared to cholesterol, both β-sitosterol and stigmasterol have an ethyl group branched in position 24. In addition, stigmasterol has a double-bond between carbons 22 and 23 (Fig. 1). We found that the selectivity and the voltage-dependence (but not single channel conductance) are differentially altered by phytosterols. We demonstrate that these effects are reversible upon removal of sterols with methyl-β-cyclodextrin.
Figure 1.
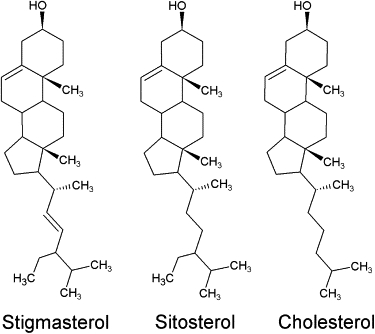
Chemical structure of the cholesterol and two phytosterols. Compared to the cholesterol structure, the sitosterol has an additional ethyl group and the stigmasterol has the same additional ethyl group and a double bond.
Materials and Methods
Materials
Seeds from Phaseolus coccineus var. “Streamline” were purchased from a local store. Percoll, chromatofocusing media (cat. Nos. PBE 94 and PB 96), were from Amersham Pharmacia Biotech (Piscataway, NJ). The soybean phospholipid extract was purchased from Avanti Polar Lipids (Alabaster, AL) and was chosen because it has a phospholipid composition close to that found in plant membranes which are characterized by a higher content in polyunsaturated acyl chains than those from animal or fungal membranes (16,28,29). Sterols and methyl-β-cyclodextrin were obtained from Sigma (St. Louis, MO). Octyl-POE was purchased from Bachem (Bubendorf, Switzerland).
VDAC purification
Membrane proteins were extracted as described previously (30). Seeds from P. coccineus were soaked in tap water for 18 h and mitochondrial membranes were isolated from the cotyledons by differential centrifugation steps and further purified on a 28% Percoll gradient. Two VDAC isoforms (VDAC32 and VDAC31) exist in mitochondria of bean seeds. Purification of the most abundant 32 kDa isoform (VDAC32) was achieved using the chromatofocusing technique (15).
Electrophysiology
We have reconstituted purified VDAC32 in planar lipid bilayers doped with various proportions of sterol similar to that found in mitochondria and plasma membranes. For stigmasterol, the fraction is given as a mass ratio of stigmasterol/phospholipids. For sitosterol and cholesterol, we used in each fraction the same number of moles as for stigmasterol. This permits us to compare the effect of equimolar quantities of sterols, but for cholesterol it introduces an error of ∼5% for the mass ratio written in the results. Lipids were dissolved in hexane to a final concentration of 2% (w/v). Planar lipid bilayers were formed by folding two lipid monolayers over a hole (110–150 μm in diameter) made in a 25-μm-thick Teflon partition that separated two Teflon experimental chambers. Before each experiment, the partition was treated with a solution of hexane/hexadecane (2.5%, v/v) to increase its oleophylicity and a new partition was used for each lipid composition. Ag/AgCl electrodes connected in series with a salt bridge (1 M KCl in 1% agar) were used to connect the experimental chambers to the electronic equipment. The trans compartment is defined as the one connected to the ground and the voltage was applied to the cis compartment. For channel reconstitution into a planar lipid bilayer, proteins were added to the cis compartment.
Voltage-dependence
The voltage-dependence of the VDAC32 was assessed from multichannel experiments using Colombini's (1) protocol. This consisted of a periodic symmetrical triangular voltage, 10 mHz in frequency and 70 mV in amplitude, from a model No. 39 waveform generator (Wavetek, San Diego, CA), that was applied across the membrane, and the current flowing through the membrane was amplified by mean of a BLM 120 amplifier (BioLogic, Claix, France). Data were filtered at 300 Hz (five-poles linearized Tchebichev filter), digitized at 44.4 kHz with a DRA 200 interface (BioLogic), and stored on CD for further processing using a homemade program written in the MATLAB environment (The MathWorks, Natick, MA). The probability of finding the VDAC in its fully open state was calculated following the standard procedure (1,31) using part of the wave corresponding to the channel reopening (17). The voltage-dependence of the probability of finding the VDAC in the fully open state can be described by a Boltzmann distribution as (1,17,31)
| (1) |
where Po(V) is the probability of occurrence of the fully open state, Vh is the voltage at which half of the channels are in their fully open states, n is a measure of the steepness of the voltage-dependence, V is the voltage applied across the membrane, e is the elementary electronic charge, k is the Boltzmann constant, and T is the absolute temperature. In the exponential, the plus-sign is used for the negative voltages and the minus-sign for the positive voltages. Po(V) was calculated from the relative change in membrane conductance,
| (2) |
where Gmax and Gmin are the maximal and the minimal conductance. Gmax is obtained at low applied voltages (|V| < 20 mV, where |V| is the modulus of V) when channels are in the fully open states and Gmin is calculated at high applied voltages (|V| > 50 mV) when channels have switched to a subconductance state. Combination of Eqs. 1 and 2 permits the direct calculation of n and Vh from the plot of the logarithmic version of Eq. 1 (32).
Ion selectivity
Salt solutions were prepared in molal concentrations corresponding to the desired activity. Molal activity was preferred over molar concentration because it is the relevant thermodynamic parameter. For KCl, 0.5, 0.1 and 0.05 molal activity is equal to a concentration of 0.81, 0.133, and 0.062 molal, respectively. Single channel experiments were used for ion selectivity experiments. The reversal potential (zero-current potential) was set to zero in presence of identical salt activity (0.5) on both sides of the membrane. Then, the cis compartment was perfused three times its volume with a solution of different salt activity and the change of reversal potential (Erev) was recorded. The ion selectivity was calculated using the Goldman-Hodgkin-Katz equation,
| (3) |
where R is the gas constant, T the absolute temperature, F the Faraday constant, α is the permeability ratio (PCl/PK), and and are the activity of ion species j in the trans and cis compartments, respectively.
Statistics
Data are shown as the mean ± standard error of the mean (N = number of experiments). The statistical significance between different means was estimated using either a t-test or the analysis of variance (ANOVA) at a level of p < 0.05.
Results
There are ∼10 ± 5% of phytosterols in plant mitochondrial membranes (16) whereas the plasma membrane may contain up to 50–60% phytosterols (33,34). It has been shown that VDAC can be found in the plasma membrane proteome of Arabidopsis and is targeted to the plasma membrane (25,26). Though these results require a confirmation, they suggest that VDAC might exist in membranes composed of various sterol fractions. This prompted us to study the effect of various composition of sterol.
Phytosterols regulate the channel selectivity
A single channel was reconstituted into a planar lipid bilayer in symmetrical 0.81 molal KCl and a 10 mV pulse voltage (2 s in length) was applied to the membrane. The single channel conductance was 3.42 ± 0.15 nS (N = 15). In the presence of a 10-fold KCl gradient the conductance drops by ∼35% to 2.23 ± 0.11 nS (N = 15). This conductance state measured in the presence of a KCl gradient is usually referred to the “open state” of the channel. In agreement with previous results (e.g., (30,35)), the conductance state that occurs most frequently in the presence of a KCl gradient is anion-selective. In the absence of sterol the VDAC selectivity ratio (α = PCl/PK) was low (α = 1.4). In the presence of stigmasterol or sitosterol the reversal potential reaches a maximum at ∼10% sterol, and then decreases when the sterol fraction is increased >30% (Fig. 2). This sterol-dependence is less effective in the presence of cholesterol which displays a maximum at a lipid fraction of 20%. In the range of sterol fractions found in plant mitochondria (∼10 ± 5%) (16), the channel selectivity increases according to the sterol sequence:
Figure 2.
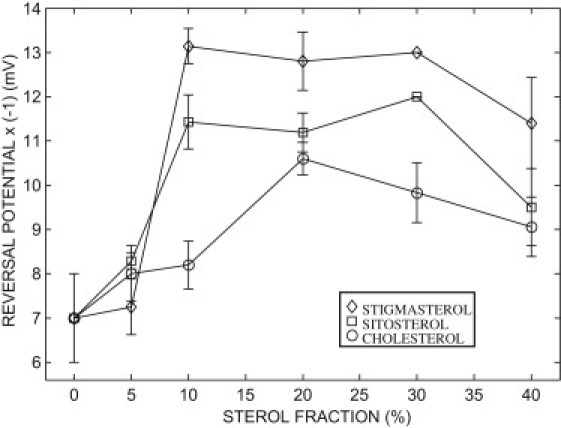
Effect of sterols on the VDAC selectivity. The anionic selectivity of the channel increases with the magnitude of the ordinate (reversal potential is multiplied by −1). The reversal potential was measured in the presence of a 10-fold KCl activity gradient (0.5:0.05 (0.81:0.062 molal); trans/cis). Data points are means of 6–11 independent experiments mean ± SE.
Cyclodextrins are a family of cyclic oligomer of glucose. The β-cyclodextrin subfamily consists of seven glucose units. Their hydrophobic core can bind hydrophobic molecules whereas their hydrophilic outer surface made them soluble in aqueous solution. These compounds have been widely used on animal cells and liposomes to deplete membranes with cholesterol (13). At low concentration (<10 mM), the methyl-β-CD has negligible binding to other lipids (36,37). To check the reversibility of the effect of sterols on the channel selectivity, a single VDAC was reconstituted into a planar lipid bilayer containing 15% of stigmasterol in a symmetric 0.81 molal KCl. Then, the cis compartment was perfused with a solution of 0.062 molal KCl. Several aliquots of methyl-β-CD were successively added in the cis compartment to increase its concentration up to 10 μM. After stirring, the reversal potential was recorded at the stationary state (usually reached within 10 min) for each methyl-β-CD concentration (Fig. 3 A and Fig. S1 in the Supporting Material). In agreement with the results of Fig. 2 in the presence of 15% stigmasterol, the reversal potential was −12.33 ± 0.67 mV (N = 9). Starting from 5 μM methyl-β-CD, the reversal potential becomes less negative and reaches a steady value of −7.0 ± 0 mV (N = 9) at 9 μM, which corresponds to the value expected in the absence of stigmasterol (Fig. 2). Similar results were obtained with sitosterol and cholesterol (Fig. S1). Voltage step of 10 mV were used to measure the single channel conductance in the absence and in the presence of 10 μM methyl-β-CD. These data were compared to the single channel conductance measured in the absence of sterol (Fig. 3 B). Our results indicate that the conductance of the most probable open state recorded in asymmetric KCl solution is not affected by stigmasterol.
Figure 3.
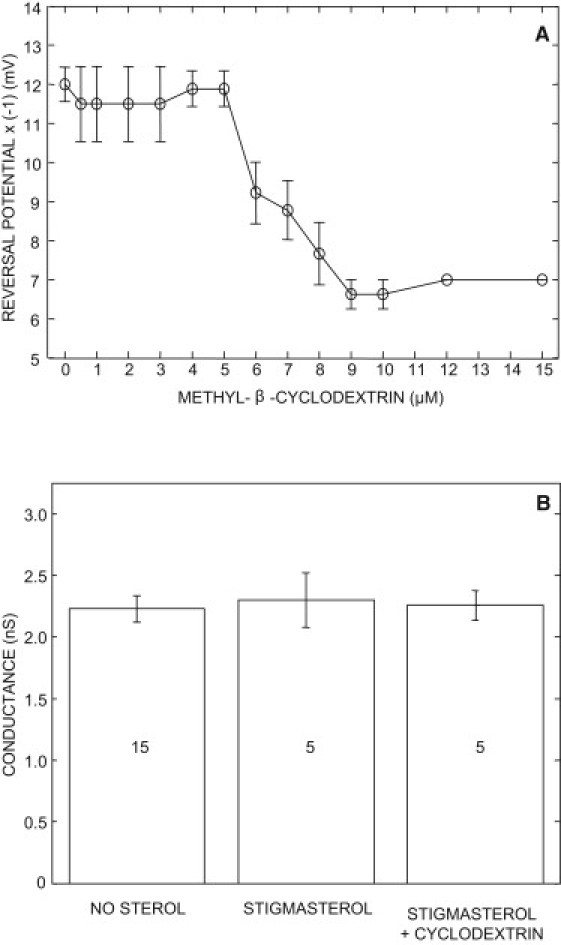
Effect of methyl-β-CD (A) on the reversal potential (four replicates) and (B) on the single channel open conductance. The lipid bilayer contained 15% stigmasterol. Numbers in the bar graph indicate the sample size. The data were collected in the presence of a 10-fold KCl activity gradient (0.5:0.05 (0.81:0.062 molal); trans/cis). The conductance values are not significantly different (t-test, p < 0.05).
These results indicate that phytosterols can modulate the selectivity of the VDACs in the range of the sterol fraction found in mitochondria. It is worth noting that cholesterol, which is a minor sterol in plants, has a relatively weak effect on the plant VDAC selectivity. At high sterol fraction (>30%), sterols condense, which leads to the formation of sterol-rich and sterol-depleted membrane domains (38,39). This might explain the decrease of selectivity observed at high sterol fraction.
Voltage-dependence
The voltage-dependence of the VDAC was investigated on multichannel experiments. Between 10 and 100 VDAC32 were reconstituted into the planar lipid bilayer. Fig. 4 A shows a typical example of the current flowing through the membrane during a triangular voltage cycle. Usually, 10–20 successive voltage waves can be applied to the membrane without alteration of the channel properties. Averaging these data provides a good estimate of the I/V curve of the VDAC in one experiment, avoiding the current fluctuations inherent to the gating activity in membrane containing few channels. At each voltage, the conductance is given by the ratio of the current and the voltage. The membrane conductance displayed a typical bell-shaped voltage-dependence (Fig. 4 B). The value of the parameters characterizing the voltage-dependence (n and Vh) was obtained from the plot of the logarithmic version of Eq. 1 for positive and negative voltages (Fig. 4, C and D).
Figure 4.
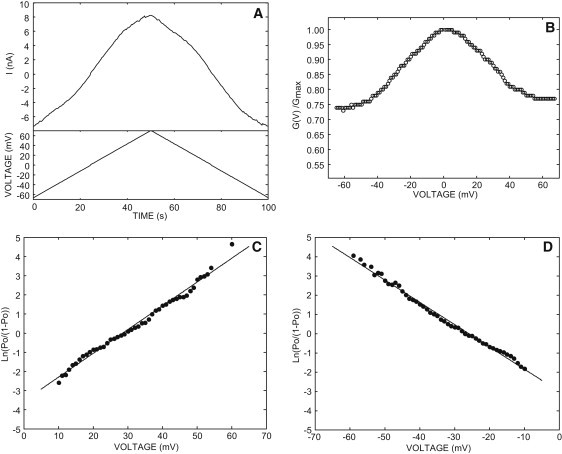
Typical example of the VDAC voltage-dependence. (A) Current flowing through 50 channels during a voltage cycle. (B) The symmetric bell-shaped voltage-dependence of the VDAC corresponding to the reopening phase of the current cycle measured between [−70, 0] and [+70, 0] mV. Linearization of the Boltzmann function (Eq. 1) for V > 0 (C) or V < 0 (D). A best fit was drawn through the linear portion of each curve using a linear regression analysis. The slope of the line yields the value n and the intercept with the abscissa gives Vh (see Table 1). The VDAC was reconstituted in a lipid bilayer containing 20% stigmasterol in the presence of 0.81 molal KCl on both sides of the membrane.
The voltage-dependence was measured in symmetric 0.81 molal KCl, in the absence and in the presence of 20% sterols. In the presence of stigmasterol, the minimal conductance state is significantly higher (P < 0.05) than the control curve (no sterol) whereas sitosterol and cholesterol have no effect (Fig. 5 A). This effect is reversible. When stigmasterol was removed from the lipid bilayer by the addition of 10 μM methyl-β-CD then the open probability decreased (Fig. 5 B). In agreement with the results of Fig. 5 A, the methyl-β-CD has no effect on the VDAC minimal conductance state in the presence of sitosterol (Fig. 5 C) or cholesterol (Fig. 5 D). This indicates that the methyl-β-CD has no effect by itself on the VDAC voltage-dependence. Similar results were obtained in the presence of 5% sterols (stigmasterol, sitosterol, and cholesterol) indicating that sterols can restore the voltage-dependence in the range of the sterol fraction found in plant mitochondria (Fig. S2).
Figure 5.
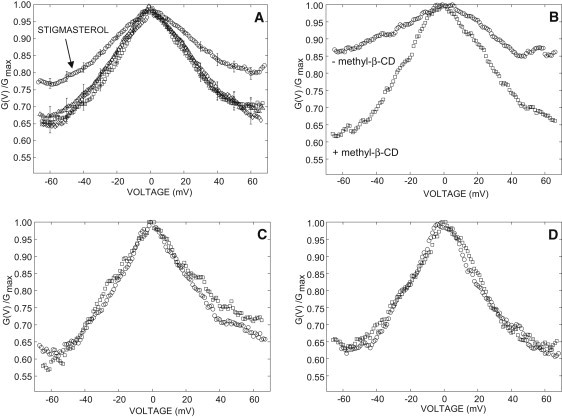
Effect of sterols on the VDAC voltage-dependence. (A) Planar lipid bilayer contained no sterol (diamond) or 20% sitosterol (triangle), cholesterol (square), or stigmasterol (circle). Data points are means of 11–13 independent experiments mean ± SE. For the sake of clarity, the standard error is shown every 10 mV. (B) Voltage-dependence measured before (circle) and after (square) addition of 10 μM methyl-β-CD to a planar lipid bilayer containing 15% stigmasterol. (C) Voltage-dependence measured before (circle) and after (square) addition of 10 μM methyl-β-CD to a planar lipid bilayer containing 15% sitosterol. (D) Voltage-dependence measured before (circle) and after (square) addition of 10 μM methyl-β-CD to a planar lipid bilayer containing 20% cholesterol. Experiments were performed in the presence of 0.81 molal KCl on both side of the membrane.
As shown in Table 1, parameters n and Vh that characterize, respectively, the steepness and the broadness of the voltage-dependence, are not significantly affected by sterols. In a previous work on denaturated VDAC (with organic solvents), it was shown that the reconstitution of plant VDACs into a planar lipid bilayer can be achieved, as long as the protein is incubated beforehand with phytosterols (24). Contrary to the results obtained with these denaturated VDACs, our results indicate that the conductance of the fully open state is unaffected (P < 0.05) by the sterols. The fully open state conductance measured in symmetrical 0.81 molal KCl was
in the absence and in the presence of 20% stigmasterol, sitosterol, and cholesterol, respectively. This discrepancy might arise from the imperfect folding of the VDAC in the work of Carbonara et al. (24), due to the relative inefficiency of phytosterols to compensate for the denaturating effect of organic solvents used in their work. Thus, our results indicate that the effect of stigmasterol on the voltage-dependence does not arise from a change either in the gating or in the maximal open conductance of each channel. At voltages larger than |V| = 50 mV, the minimum relative conductance (G(V)/Gmax) is constant and is significantly increased in the presence of stigmasterol. At this voltage, each VDAC32 can close to different subconducting states (30). Thus, this change in voltage-dependence indicates that the lowest conductance levels achieved in the absence of sterols (or in the presence of either cholesterol or sitosterol) are not accessible in the presence of stigmasterol.
Table 1.
Effect of 20% (w/w) sterols on the steepness parameter (n) of the voltage-dependence and on the voltage at which half of the channels are in the fully open state (Vh)
| n (V > 0) | n (V < 0) | Vh (V > 0) | Vh (V < 0) | |
|---|---|---|---|---|
| No sterol | 2.59 ± 0.14 (N = 14) | 2.32 ± 0.10 (N = 14) | 29.26 ± 1.63 (N = 14) | −28.28 ± 1.19 (N = 14) |
| Stigmasterol | 2.54 ± 0.12 (N = 13) | 2.36 ± 0.15 (N = 13) | 28.33 ± 1.28 (N = 13) | −26.62 ± 1.34 (N = 13) |
| Sitosterol | 2.53 ± 0.12 (N = 11) | 2.49 ± 0.15 (N = 11) | 27.68 ± 1.59 (N = 11) | −26.00 ± 1.14 (N = 11) |
| Cholesterol | 2.53 ± 0.12 (N = 12) | 2.79 ± 0. 17 (N = 12) | 24.63 ± 1.28 (N = 12) | −23.76 ± 1.09 (N = 12) |
Experiments were performed in 0.81 molal KCl. No statistical difference (ANOVA) exists between either n values or Vh values (p < 0.05). N = number of experiments.
All the functional studies on plant VDAC channels (and almost all studies on VDAC from other organisms) were performed in high (1 M) KCl concentration solutions and it is generally implicitly assumed that the functional properties of the VDAC recorded in 1 M KCl can be extrapolated to conditions found in vivo. To check this hypothesis, we performed an experiment in 0.133 molal KCl. Contrary to what we found in 0.81 molal KCl, in the absence of sterol the VDAC voltage-dependence is strongly inhibited (Fig. 6 A). The voltage-dependence increases linearly with the ionic concentration (Fig. 6 B). However, in the presence of both 0.133 molal KCl and 15% sterols (Fig. 7), the voltage-dependence was recovered. This effect is reversible: the voltage-dependence is lost upon removal of the membrane sterols by addition of 10 μM methyl-β-CD on the cis side (Fig. 7). Similar results were obtained in the presence of 5% sterols (Fig. S2). In agreement with the result of Fig. 5 (0.81 molal KCl) at voltages larger than |V| = 50 mV, the minimum relative conductance (G(V)/Gmax) is constant, and it is significantly higher in the presence of stigmasterol than in the presence of sitosterol or cholesterol—therefore indicating that the differential effect of phytosterols on the voltage-dependence is not altered by the ionic strength of the solution. These results further support our conclusion, according to which sterols are required for the proper function of the VDAC and that phytosterols have a differential effect on the voltage-dependence.
Figure 6.
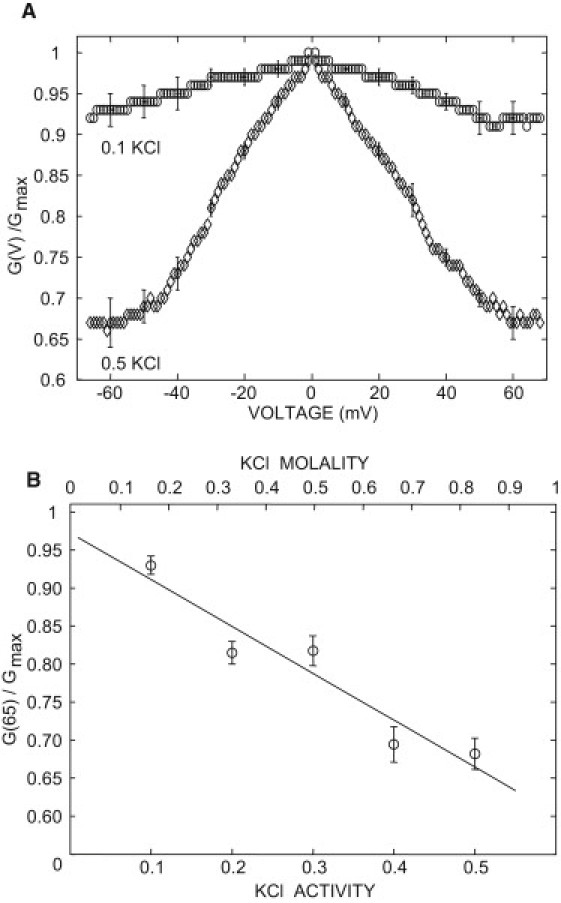
Effect of the ionic strength on the VDAC voltage-dependence in the absence of sterols. (A) In symmetrical 0.5 KCl activity (=0.81 molal) (diamond) or 0.1 KCl activity (=0.133 molal) (circle) . (B) Change in the relative conductance measured at 65 mV.
Figure 7.
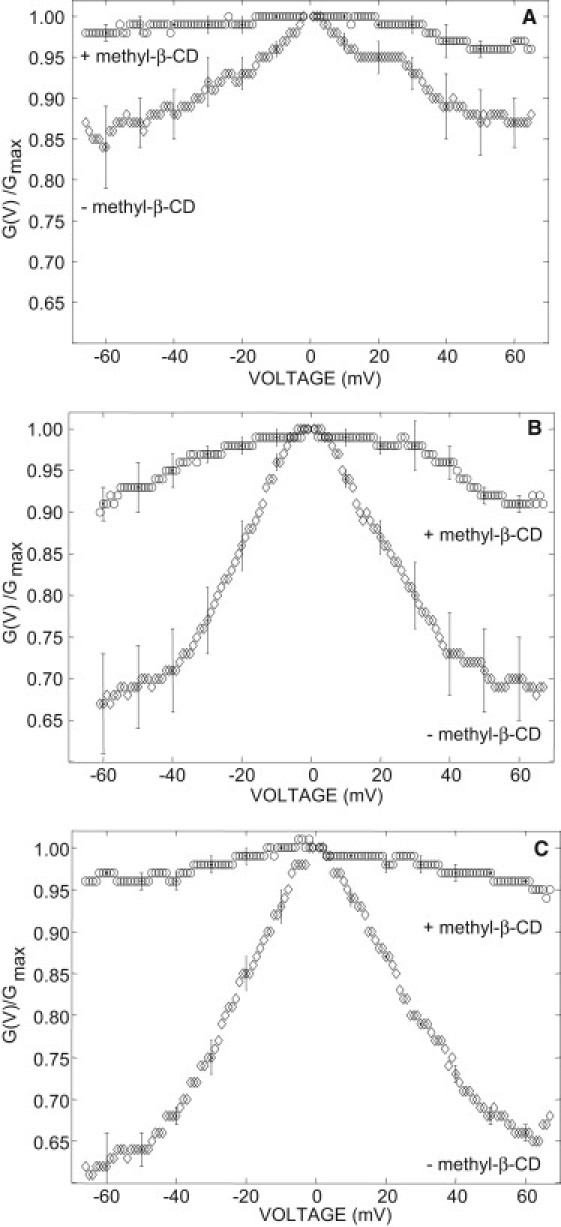
Effect of methyl-β-CD on the VDAC voltage-dependence measured in 0.133 molal KCl. (A) In the presence of 15% stigmasterol. (B) In the presence of 15% sitosterol. (C) In the presence of 15% cholesterol. (Circle) In the presence of methyl-β-CD. (Diamond) In the absence of methyl-β-CD.
Discussion
Although there is little data about the effect of lipids on VDAC, the effect of sterol has been better characterized in mammalian plasma membrane where cholesterol is known to alter membrane protein function (40–43). Biochemical and genetic experiments on sterol-deficient mutants in plants show the pleiotropic role for sterols in regulating plant growth and development as well as cellular signaling (44–49). It has previously been shown that plant sterols affect the water permeability of lipid bilayers (50) and the function of the plasma membrane H+-ATPase (51,52), and that cholesterol induces a reversible closure of a low conductance unselective vacuolar channel (53). However, the molecular mechanism underlying the effects of phytosterols on ion transport in plants is not elucidated.
Our results indicate a differential effect of phytosterols on the selectivity and voltage-dependence of the VDAC from plants. Stigmasterol, sitosterol, and cholesterol were used because they differ by only a slight modification in their lateral chain. Therefore, any difference in the effect of these sterols must originate in the hydrophobic core of the lipid bilayer. We have shown that 5% cholesterol has no effect on the VDAC selectivity. Thus, we can reasonably assume that, in vivo, it will have no significant regulatory effect on VDAC because of its very low abundance (<1%) in plant membranes (54).
The VDAC displays a bell-shaped voltage-dependence. Only stigmasterol affects the voltage-dependence of the VDAC in high KCl concentration. Parameters such as n and V0, that describe, respectively, the steepness and the broadness of the voltage-dependence, are not significantly affected by sterols. However, in the presence of stigmasterol, the constant tail value of G(V)/Gmax calculated at high voltages (|V| ≥ 50 mV) significantly increased. These results indicate that the lowest levels of conductance achieved in the absence of sterols (or in the presence of either cholesterol or sitosterol) are not accessible in the presence of stigmasterol.
Two general mechanisms have been proposed for the regulation of membrane protein function by sterols:
-
1.
A change in the physical properties of the membrane.
-
2.
A sterol-protein interaction.
Modifications of the physical properties of the lipid bilayer such as an increase in microviscosity and stiffness occur when the sterol fraction is increased. They mostly result from alterations of lipid packing. Moreover, sterol-rich lipid domains may exist due to the mutual solubility properties of lipids or as a consequence of specific lipid-protein interactions.
Sterols are known to decrease molecular motion and to increase lipid packing, and therefore, to increase the effective membrane viscosity. The water permeability of a lipid bilayer is correlated to the lipid ordering (effective membrane viscosity). The water permeability decreases as the order parameter of the acyl chain increases. Sterols increase the ordering of saturated phospholipid bilayers prepared in the liquid-crystalline state and decrease their water permeability. The ordering effect of sterols arises notably from an increase of attractive van der Waals interactions between lipids molecules. The effect of phytosterols on saturated phospholipids indicates that they are less efficient than cholesterol for reordering the lipids. The efficiency sequence is cholesterol > sitosterol ≥ stigmasterol (55–57). Phospholipids of plant origin are characterized by a high content in polyunsaturated fatty acyl chains. Sitosterol is the most efficient phytosterol to increase the ordering of soybean phospholipid and to reduce its water permeability, whereas stigmasterol at a lipid ratio of 15% has no effect (50,58). The differential effect of phytosterols on lipid bilayer is not consistent with that reported on the VDAC. Notably, because 15% stigmasterol has no physicochemical effect on bilayer formed from phospholipids of plant origin (rich in polyunsaturated acyl chains), we can reasonably conclude that its effect on the VDAC properties arises from a lipid-protein interaction. Stigmasterol has a relatively high rigidity due to the methyl group and is double-bound in its lateral chain. Upon interaction with the VDAC, this might impede large displacement (reorganization) of β-strands required to reach the lowest conductance states at high voltages.
The differential effect itself, of the various sterols tested, is different when analyzing the probability of opening or the selectivity. However, in both cases, stigmasterol has the strongest effect—suggesting that this phytosterol might have the highest affinity for the VDAC. This hypothesis assumes that the different phytosterols will compete for the same site. There is evidence that several cholesterol molecules can bind to mammalian VDAC (18), and the high-resolution structure obtained by NMR indicates several region for cholesterol binding to the exterior of VDAC. Thus, we cannot exclude that plant VDACs might have different binding sites for different phytosterols. The sterols used in this work differ mainly in their lateral carbon chain, indicating that the differential effect observed must involve a region of the VDAC32 structure located in the core of the membrane.
Despite the fact that VDAC selectivity is governed by the electrostatic profile in the channel diffusion pore (59–61), we show that sterols modulate the selectivity. Sterols are known to change the thickness of the lipid bilayer, which results from a minimization of mechanical and electrostatic constrain in the lipid bilayer (62). It thereby creates a mismatch between the hydrophobic thickness of the VDAC and the thickness of the hydrophobic core of the lipid bilayer at the vicinity of the VDAC. Thus, a change of the VDAC topology might arise from minimization of the hydrophobic mismatch between the VDAC and the lipid bilayer due to the binding of sterol to the VDAC. We can then hypothesize that the binding of sterol to the barrel might change the topology (strands tilt, strand twist) of at least some strands and therefore alters the orientation of charged residues inside the diffusion pore, which will change the charge density inside the channel and thus the selectivity of the channel. In support of this hypothesis, it has been shown that cholesterol can bind to the outer side of the mammalian VDAC barrel, notably with the Cα-carbon of charged residues pointing toward the diffusion pore (12,18,19) and that bacterial porins (also folded in a β-barrel) change their orientational ordering of the β-barrels and their assembly in the membrane as a function of the membrane thickness (63). Due to the different degree of freedom of the lateral chain of sterols studied here, we can reasonably assume that each sterol will have a different effect on the hydrophobic mismatch upon binding to VDAC.
The effect of the ionic strength on the voltage-dependence shown in Fig. 6 cannot be due to sterols because there is no sterol in the lipid bilayer in these experiments. Moreover, in the presence of sterols, the voltage-dependence is the same at 0.8 and 0.133 molal KCl (see Fig. S2 or compare Fig. 5, B–D, recorded at 0.8 molal with Fig. 7, A–C, recorded at 0.133 molal, in the absence of methyl-β-cyclodextrin). This effect likely results from a change in the phospholipid-protein interaction: the phospholipid headgroups and their neighboring water molecules may form electrostatic interactions with the VDAC. The change of ionic strength of the solution alters the screening of phospholipid and protein charges and thus modifies the lipid-protein interactions, which might alter the voltage-dependence. Our results show that sterols restore the voltage-dependence at low ionic strength, suggesting that, upon binding to the barrel, sterols might displace phospholipids in the first shell surrounding the VDAC, and thus disrupt the phospholipids-VDAC interactions—giving rise to an effect similar to the electrostatic screening.
The found decrease of voltage gating with KCl concentration was not observed for VDAC from other sources (31,64,65). Although we cannot rule out that this is a difference between VDAC from plants and other sources, it most probably reflects a difference between isoforms. The number of isoforms differs between organisms: one in Neurospora, two in yeast, three in animals, and between five (Arabidopsis) and nine (Populus) in fully sequenced plant genomes. It would be unexpected that all these isoforms have identical function and regulation. Differences in the function and regulation among isoforms of plants, yeast, and human have already been reported (66–69) and thus, the effect of KCl concentration reported here might be an isoform specificity. In symmetrical 0.133 molal KCl, the gating parameters n = 2.42 ± 0.39 (N = 13) and Vh = 30.94 ± 3.40 mV (N = 13) are not significantly different from the values recorded in 0.81 molal KCl (Table 1). The value of the fully open state conductance measured on single channel experiments was 0.59 ± 0.01 nS (N = 36) in 0.133 molal KCl. This decrease of conductance is correlated to the decrease of concentration: in both 0.133 and 0.81 molal KCl, the conductance per unit concentration is ∼4 nS/molal. This indicates that the ionic strength has no effect on the gating and on the maximal open conductance of each channel, but does affect their minimal conducting state.
In summary, plant VDAC undergoes a reversible regulation of selectivity and voltage-dependence at sterol fraction similar to that found in mitochondria. Our results indicate that phytosterols are essential for the proper function of plant VDACs at KCl concentrations that prevails in vivo, and they suggest that phytosterols might have direct interaction with the VDAC.
Acknowledgments
We thank Dr. M. Prevost for fruitful discussions.
F.H. is a Research Director from the Fund for Scientific Research (FRS-FNRS). S.C. thanks the FRS-FNRS for its financial support during her stay at the laboratory of Structure et Fonction des Membranes Biologiques de l'Université Libre de Bruxelles. This work was supported by the joint research program Tournesol of the Communauté Française de Belgique and the Ministère Français des Affaires Étrangères.
Supporting Material
References
- 1.Schein S.J., Colombini M., Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J. Membr. Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- 2.Lemasters J.J., Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. Biochim. Biophys. Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Rostovtseva T.K., Tan W.Z., Colombini M. On the role of VDAC in apoptosis: fact and fiction. J. Bioenerg. Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- 4.Liu M.Y., Colombini M. Regulation of mitochondrial respiration by controlling the permeability of the outer-membrane through the mitochondrial channel, VDAC. Biochim. Biophys. Acta. 1992;1098:255–260. doi: 10.1016/s0005-2728(05)80344-5. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heiden M.G., Li X.X., Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 6.Al Bitar F., Roosens N., Homble F. Sequence analysis, transcriptional and posttranscriptional regulation of the rice VDAC family. Biochim. Biophys. Acta. 2003;1625:43–51. doi: 10.1016/s0167-4781(02)00590-0. [DOI] [PubMed] [Google Scholar]
- 7.Wandrey M., Trevaskis B., Udvardi M.K. Molecular and cell biology of a family of voltage-dependent anion channel porins in Lotus japonicus. Plant Physiol. 2004;134:182–193. doi: 10.1104/pp.103.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Pinto V., Messina A. Gene family expression and multitopological localization of eukaryotic porin/voltage dependent anion-selective channel (VDAC): intracellular trafficking and alternative splicing. In: Benz R., editor. Bacterial and Eukaryotic Porins. Wiley-VCH; Weinheim, Germany: 2005. pp. 309–337. [Google Scholar]
- 9.Elkeles A., Devos K.M., Breiman A. Multiple cDNAs of wheat voltage-dependent anion channels (VDAC): isolation, differential expression, mapping and evolution. Plant Mol. Biol. 1995;29:109–124. doi: 10.1007/BF00019123. [DOI] [PubMed] [Google Scholar]
- 10.Al Bitar F., Roosens N., Homble F. Expression of the rice VDAC isoform2: histochemical localization and expression level. Biochim. Biophys. Acta. 2002;1579:133–141. doi: 10.1016/s0167-4781(02)00532-8. [DOI] [PubMed] [Google Scholar]
- 11.Ujwal R., Cascio D., Abramson J. The crystal structure of mouse VDAC1 at 2.3 Å resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiller S., Garces R.G., Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayrhuber M., Meins T., Zeth K. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannella C.A. Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications. J. Struct. Biol. 1998;121:207–218. doi: 10.1006/jsbi.1997.3954. [DOI] [PubMed] [Google Scholar]
- 15.Abrecht H., Goormaghtigh E., Homble F. Structure and orientation of two voltage-dependent anion-selective channel isoforms—an attenuated total reflection Fourier-transform infrared spectroscopy study. J. Biol. Chem. 2000;275:40992–40999. doi: 10.1074/jbc.M006437200. [DOI] [PubMed] [Google Scholar]
- 16.Daum G. Lipids of mitochondria. Biochim. Biophys. Acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- 17.Rostovtseva T.K., Kazemi N., Bezrukov S.M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 2006;281:37496–37506. doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- 18.De Pinto V., Benz R., Palmieri F. Interaction of non-classical detergents with the mitochondrial porin. Eur. J. Biochem. 1989;183:179–187. doi: 10.1111/j.1432-1033.1989.tb14911.x. [DOI] [PubMed] [Google Scholar]
- 19.Bay D.C., O'Neil J.D., Court D.A. The influence of sterols on the conformation of recombinant mitochondrial porin in detergent. Biochem. Cell Biol. 2008;86:539–545. doi: 10.1139/O08-132. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller R., Freitag H., Neupert W. A water-soluble form of porin from the mitochondrial outer membrane of Neurospora crassa. Properties and relationship to the biosynthetic precursor form. J. Biol. Chem. 1985;260:8188–8193. [PubMed] [Google Scholar]
- 21.Popp B., Schmid A., Benz R. Role of sterols in the functional reconstitution of water-soluble mitochondrial porins from different organisms. Biochemistry. 1995;34:3352–3361. doi: 10.1021/bi00010a026. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann M.A., Benveniste P. Plant membrane sterols—isolation, identification, and biosynthesis. Methods Enzymol. 1987;148:632–650. [Google Scholar]
- 23.Benveniste P. Biosynthesis and accumulation of sterols. Annu. Rev. Plant Biol. 2004;55:429–457. doi: 10.1146/annurev.arplant.55.031903.141616. [DOI] [PubMed] [Google Scholar]
- 24.Carbonara F., Popp B., Benz R. The role of sterols in the functional reconstitution of water-soluble mitochondrial porins from plants. J. Bioenerg. Biomembr. 1996;28:181–189. doi: 10.1007/BF02110649. [DOI] [PubMed] [Google Scholar]
- 25.Marmagne A., Rouet M.A., Ephritikhine G. Identification of new intrinsic proteins in Arabidopsis plasma membrane proteome. Mol. Cell. Proteomics. 2004;3:675–691. doi: 10.1074/mcp.M400001-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Ephritikhine G., Ferro M., Rolland N. Plant membrane proteomics. Plant Physiol. Biochem. 2004;42:943–962. doi: 10.1016/j.plaphy.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Veron B., Billard C., Hartmann M.A. Sterol composition of Phaeodactylum tricornutum as influenced by growth temperature and light spectral quality. Lipids. 1996;31:989–994. doi: 10.1007/BF02522694. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida S., Uemura M. Lipid composition of plasma membranes and tonoplasts isolated from etiolated seedlings of mung bean (Vigna radiata L.) Plant Physiol. 1986;82:807–812. doi: 10.1104/pp.82.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohn M., Heinz E., Lnthje S. Lipid composition and fluidity of plasma membranes isolated from corn (Zea mays L.) roots. Arch. Biochem. Biophys. 2001;387:35–40. doi: 10.1006/abbi.2000.2224. [DOI] [PubMed] [Google Scholar]
- 30.Abrecht H., Wattiez R., Homble F. Purification and characterization of two voltage-dependent anion channel isoforms from plant seeds. Plant Physiol. 2000;124:1181–1190. doi: 10.1104/pp.124.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colombini M. Voltage gating in the mitochondrial channel, VDAC. J. Membr. Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- 32.Wunder U.R., Colombini M. Patch-clamping VDAC in liposomes containing whole mitochondrial membranes. J. Membr. Biol. 1991;123:83–91. doi: 10.1007/BF01993966. [DOI] [PubMed] [Google Scholar]
- 33.Wu J., Seliskar D.M., Gallagher J.L. The response of plasma membrane lipid composition in callus of the halophyte Spartina patens (Poaceae) to salinity stress. Am. J. Bot. 2005;92:852–858. doi: 10.3732/ajb.92.5.852. [DOI] [PubMed] [Google Scholar]
- 34.Cowan D.S.C., Cooke D.T., Hall J.L. Lipid and sterol composition of plasma membranes isolated from stele and cortex of maize roots. J. Exp. Bot. 1993;44:991–994. [Google Scholar]
- 35.Pavlov E., Grigoriev S.M., Kinnally K.W. The mitochondrial channel VDAC has a cation-selective open state. Biochim. Biophys. Acta. 2005;1710:96–102. doi: 10.1016/j.bbabio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Leventis R., Silvius J.R. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys. J. 2001;81:2257–2267. doi: 10.1016/S0006-3495(01)75873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsamaloukas A., Szadkowska H., Heerklotz H. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys. J. 2005;89:1109–1119. doi: 10.1529/biophysj.105.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKersie B.D., Thompson J.E. Influence of plant sterols on the phase properties of phospholipid bilayers. Plant Physiol. 1979;63:802–805. doi: 10.1104/pp.63.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali M.R., Cheng K.H., Huang J. Assess the nature of cholesterol-lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 2007;104:5372–5377. doi: 10.1073/pnas.0611450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romanenko V.G., Rothblat G.H., Levitan I. Sensitivity of volume-regulated anion current to cholesterol structural analogues. J. Physiol. 2004;123:77–87. doi: 10.1085/jgp.200308882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pucadyil T.J., Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog. Lipid Res. 2006;45:295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Epand R.M. Proteins and cholesterol-rich domains. Biochim. Biophys. Acta. 2007;1778:1576–1582. doi: 10.1016/j.bbamem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Abi-Char J., Maguy A., Hatem S.N. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J. Physiol. 2007;582:1205–1217. doi: 10.1113/jphysiol.2007.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Men S.Z., Boutte Y., Grebe M. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- 45.Clouse S.D. Arabidopsis mutants reveal multiple roles for sterols in plant development. Plant Cell. 2002;14:1995–2000. doi: 10.1105/tpc.140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clouse S.D. Plant development: a role for sterols in embryogenesis. Curr. Biol. 2000;10:R601–R604. doi: 10.1016/s0960-9822(00)00639-4. [DOI] [PubMed] [Google Scholar]
- 47.Lindsey K., Pullen M.L., Topping J.F. Importance of plant sterols in pattern formation and hormone signaling. Trends Plant Sci. 2003;8:521–525. doi: 10.1016/j.tplants.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Zabrouskov V., Knowles N. Changes in lipid molecular species and sterols of microsomal membranes during aging of potato (Solanum tuberosum L.) seed-tubers. Lipids. 2002;37:309–315. doi: 10.1007/s11745-002-0896-0. [DOI] [PubMed] [Google Scholar]
- 49.Mikami K., Murata N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid Res. 2003;42:527–543. doi: 10.1016/s0163-7827(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 50.Schuler I., Milon A., Hartmann M.A. Differential-effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 1991;88:6926–6930. doi: 10.1073/pnas.88.16.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grandmougin A., Hartmann M.A., Benveniste P. Plasma-membrane ATPase from maize roots—effect of sterol composition. Plant Physiol. Biochem. 1988;26:217. [Google Scholar]
- 52.Grandmougin-Ferjani A., Schuler Muller I., Hartmann M.A. Sterol modulation of the plasma membrane H+-ATPase activity from corn roots reconstituted into soybean lipids. Plant Physiol. 1997;113:163–174. doi: 10.1104/pp.113.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaile A. Cholesterol-modulated ion channel activity in tonoplast vesicles: a planar lipid bilayer study. J. Exp. Bot. 1997;48:979–983. [Google Scholar]
- 54.Nomura T., Kitasaka Y., Yokota T. Brassinosteroid/sterol synthesis and plant growth as affected by Lka and Lkb mutations of pea. Plant Physiol. 1999;119:1517–1526. doi: 10.1104/pp.119.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halling K.K., Slotte J.P. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim. Biophys. Acta. 2004;1664:161–171. doi: 10.1016/j.bbamem.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Bernsdorff C., Winter R. Differential properties of the sterols cholesterol, ergosterol, sitosterol, trans-7-dehydrocholesterol, stigmasterol and lanosterol on DPPC bilayer order. J. Chem. Phys. B. 2003;107:10658–10664. [Google Scholar]
- 57.Hodzic A., Rappolt M., Pabst G. Differential modulation of membrane structure and fluctuations by plant sterols and cholesterol. Biophys. J. 2008;94:3935–3944. doi: 10.1529/biophysj.107.123224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krajewski-Bertrand M.A., Milon A., Hartmann M.A. Deuterium-NMR investigation of plant sterol effects on soybean phosphatidylcholine acyl chain ordering. Chem. Phys. Lipids. 1992;63:235–241. [Google Scholar]
- 59.Blachly-Dyson E., Peng S.Z., Forte M. Probing the structure of the mitochondrial channel, VDAC, by site-directed mutagenesis: a progress report. J. Bioenerg. Biomembr. 1989;21:471–483. doi: 10.1007/BF00762519. [DOI] [PubMed] [Google Scholar]
- 60.Blachly-Dyson E., Peng S., Forte M. Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science. 1990;247:1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]
- 61.Peng S., Blachly-Dyson E., Colombini M. Large scale rearrangement of protein domains is associated with voltage gating of the VDAC channel. Biophys. J. 1992;62:123–135. doi: 10.1016/S0006-3495(92)81799-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Killian J.A. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta. 1998;1376:401–415. doi: 10.1016/s0304-4157(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 63.Ramakrishnan M., Qu J., Marsh D. Orientation of β-barrel proteins OmpA and FhuA in lipid membranes. Chain length dependence from infrared dichroism. Biochemistry. 2005;44:3515–3523. doi: 10.1021/bi047603y. [DOI] [PubMed] [Google Scholar]
- 64.Gincel D., Silberberg S.D., Shoshan-Barmatz V. Modulation of the voltage-dependent anion channel (VDAC) by glutamate. J. Bioenerg. Biomembr. 2000;32:571–583. doi: 10.1023/a:1005670527340. [DOI] [PubMed] [Google Scholar]
- 65.Levadny V., Colombini M., Aguilella V.M. Electrostatics explains the shift in VDAC gating with salt activity gradient. Biophys. J. 2002;82:1773–1783. doi: 10.1016/S0006-3495(02)75528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blachly-Dyson E., Forte M. VDAC channels. IUMBM Life. 2001;52:113–118. doi: 10.1080/15216540152845902. [DOI] [PubMed] [Google Scholar]
- 67.Blachly-Dyson E., Song J.M., Forte M. Multicopy suppressors of phenotypes resulting from the absence of yeast VDAC encode a VDAC-like protein. Mol. Cell. Biol. 1997;17:5727–5738. doi: 10.1128/mcb.17.10.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 2004;256:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 69.Elkeles A., Breiman A., Zizi M. Functional differences among wheat voltage-dependent anion channel (VDAC) isoforms expressed in yeast. Indication for the presence of a novel VDAC-modulating protein? J. Biol. Chem. 1997;272:6252–6260. doi: 10.1074/jbc.272.10.6252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


