Abstract
While significant advances have been made toward revealing the molecular mechanisms that influence breast cancer progression, much less is known about the associated cellular mechanical properties. To this end, we use particle-tracking microrheology to investigate the interplay among intracellular mechanics, three-dimensional matrix stiffness, and transforming potential in a mammary epithelial cell (MEC) cancer progression series. We use a well-characterized model system where human-derived MCF10A MECs overexpress either ErbB2, 14-3-3ζ, or both ErbB2 and 14-3-3ζ, with empty vector as a control. Our results show that MECs possessing ErbB2 transforming potential stiffen in response to elevated matrix stiffness, whereas non-transformed MECs or those overexpressing only 14-3-3ζ do no exhibit this response. We further observe that overexpression of ErbB2 alone is associated with the highest degree of intracellular sensitivity to matrix stiffness, and that the effect of transforming potential on intracellular stiffness is matrix-stiffness-dependent. Moreover, our intracellular stiffness measurements parallel cell migration behavior that has been previously reported for these MEC sublines. Given the current knowledge base of breast cancer mechanobiology, these findings suggest that there may be a positive relationship among intracellular stiffness sensitivity, cell motility, and perturbed mechanotransduction in breast cancer.
Introduction
Given that the vast majority of breast cancer-related deaths result from metastasis, understanding the interplay between the cellular microenvironment and breast cancer metastatic potential is critically important to the development of effective treatments and therapies for this disease. While significant progress has been achieved toward elucidating the molecular mechanisms that underlie breast cancer progression (1,2), quantitative characterization of the associated cellular mechanical properties remains largely incomplete. Breast tumors are more rigid (stiffer) than normal breast tissue (3), and cancer cell motility, a key aspect of metastasis, is in part governed by the stiffness of the extracellular matrix (ECM) (4). However, the system-wide relationships among ECM mechanical properties, transforming potential, and the intracellular mechanical state of breast cancer cells is even less well understood, particularly within the context of physiologically relevant, three-dimensional matrix environments.
A number of breast cancer biomarkers have been identified and linked to specific stages of the disease, notably including the ErbB2 (HER2/neu) and 14-3-3ζ proteins, both of whose overexpression has been correlated with poor prognoses (5,6). ErbB2 is a transmembrane receptor tyrosine kinase of the epidermal growth factor receptor family of proteins; it is involved in several signaling pathways that modulate cell growth, differentiation, and apoptosis, among other critical processes (7). Cells cultures that overexpress ErbB2 have been shown to exhibit hyperplasia (8), and they have also shown a capacity to overcome apoptotic signals (9). Strikingly, the ErbB2 protein is overexpressed in ∼25% of all invasive and metastatic breast cancers (6); its role in promoting metastasis of breast and other forms of cancer has rendered ErbB2 one of the most studied molecules in the field of cancer and a critical target for drug development (10). The 14-3-3ζ protein belongs to the larger family of seven 14-3-3 regulatory proteins that are widely expressed and involved in a variety of cellular homeostatic processes, including a general cell survival/anti-apoptotic mechanism (11). Recent mammary epithelial cell (MEC) in vitro studies have shown that 14-3-3ζ confers significant resistance to anoikis (12), a specific type of apoptosis that occurs when epithelial cells detach from extracellular ligands, thus promoting luminal filling and driving MECs toward transformation (13). Overexpression of 14-3-3ζ has additionally been shown to induce notable morphological irregularities that are characteristic of progression toward an invasive phenotype (12,14). Furthermore, analyses of patient biopsies indicate that >40% of advanced metastatic breast cancers overexpress the 14-3-3ζ protein (5).
Despite their capacities to confer oncogenic properties to non-transformed cells, overexpression of either ErbB2 or 14-3-3ζ alone is not sufficient to drive complete transformation in vitro (8,12). However, recent investigations have shown that their cooperative effect does promote progression from non-invasive carcinoma to invasive and metastatic breast cancer (14). Given the identified correlations between breast cancer biomarkers and metastatic progression and our current knowledge of cell-matrix interactions with respect to the cellular mechanical environment, the following fundamental questions remain unanswered for individual MECs in three-dimensional environments:
-
1.
Does the intracellular mechanical state adapt to matrix stiffness?
-
2.
Is this adaptability related to transforming potential?
And:
-
3.
Is there a relationship between intracellular stiffness and transforming potential given a determined matrix environment?
In this study, we quantitatively investigate these questions by employing particle-tracking microrheology (PTMR) to examine the effect of matrix stiffness on the intracellular stiffness of individual MECs that are embedded within three-dimensional matrices of varied elastic moduli. PTMR is a passive technique that avoids sample size and accessibility limitations that are presented by alternative rheological techniques (15), and it has been utilized extensively to probe the mechanical behavior of numerous biological organisms and materials (16,17). PTMR yields the time-dependent viscoelastic behavior of a material, as extracted from the thermally driven motions of small probes (tracer beads) embedded within the material (15,18). PTMR protocols have been established (19,20) and indeed have been used to link the cytoplasmic mechanical environment of living cells to the surrounding matrix mechanics (21). Here we use PTMR to examine human-derived MECs of varied transforming potential with respect to matrices formulated from Type I collagen, which is the primary structural component of the mammary stroma. Additionally, we examine the morphological differences exhibited among these MECs within three-dimensional matrices. In total, our studies provide novel insights into breast cancer mechanobiology by demonstrating that transforming potential couples with matrix stiffness to govern the intracellular mechanical state of MECs.
Materials and Methods
Cell culture
PTMR experiments were performed on stable sublines that were established as described previously (14) from the non-cancerous, human-derived MCF10A MEC line (provided by Dr. Robert Pauley of the Karmonos Cancer Institute, Detroit, MI). Cell lines were maintained in two-dimensional monolayer culture in DMEM/F12 growth media (22) within a humidified incubator at 37°C, 5% CO2 until the time of experimentation.
Collagen matrix preparation and characterization
Matrices were formulated from high concentration Type I collagen (BD Biosciences, San Jose, CA), which was diluted to three concentrations of 2, 3, and 4 mg/mL. Equal parts collagen and neutralizing solution (100 mM HEPES in 2× PBS at pH 7.3) were mixed with a balance of 2 × 105 cells suspended in growth media to achieve the final concentration (23). Each matrix solution (1 mL) was then deposited across the surface of a 35-mm glass-bottom dish (MatTek, Ashland, MA). Matrix solutions were allowed to gel for 2 h at 37°C, 5% CO2, upon which 2 mL of growth media was deposited atop the three-dimensional matrices to provide cells with adequate nutrients during a subsequent 12 h incubation.
The matrix stiffness was quantified in terms of the bulk elastic modulus of the collagen gel (G′c). Measurements of G′c were performed on acellular collagen matrices after they were permitted to gel for 2 h at 37°C, 5% CO2. Four 1-mL samples of each gel were measured with a Physica MCR 300 rheometer (Anton Paar, Ashland, VA) operating in cone-and-plate mode and stressed at 0.1 μN·m oscillatory torque over a frequency (ω) range of 0.1–10 Hz. Measurements of elastic modulus for each gel concentration were averaged and then fit to a power-law equation of the form G′c = αωβ + δ. At ω = 10 Hz, we report G′c as 104, 271, and 391 Pa for collagen gels of concentration 2, 3, and 4 mg/mL, respectively. Thus, we modulate matrix stiffness by varying collagen concentration, which additionally has a negative correlation with matrix pore size and a positive correlation with ligand density (21). Numerous studies have shown that modulating concentration has a much greater affect on matrix mechanics-mediated cell responses than it does on ligand-mediated cell-matrix interactions (4,21,24,25). Thus, we examine our results with respect to changes in matrix stiffness (26).
Particle delivery and particle-tracking
Cell lines were cultured to confluency in a 10-cm dish and then embedded with 1.0-μm carboxylated, fluorescent polystyrene tracer beads (Molecular Probes, Eugene, OR) using a ballistic particle delivery system (BioRad Laboratories, Hercules, CA) (27). Next, cells were detached and resuspended at a concentration of 2 × 105 cells/mL in growth media. Suspended cells were mixed with Type I collagen (see above) and incubated for 12 h (post gelation) at 37°C, 5% CO2.
After incubation, xy-plane Brownian motions of individual tracer beads were imaged within cells in each three-dimensional matrix at 63× magnification for a period of 10 s at a frame rate of 10 Hz using an SP2 AOBS confocal microscope (Leica Microsystems, Bannockburn, IL). A total of 8–20 tracer beads were imaged in each culture. Only cells that were non-dividing and wholly suspended within the three-dimensional matrices (not attached to other cells or the glass-bottom surface) were selected for imaging. The microscope objective was maintained at 37°C with an objective heater (Bioptechs, Butler, PA). Imaris image analysis software (Bitplane, St. Paul, MN) was then utilized to create particle trajectories in the x,y plane for each tracer bead. For each cell line, the experiment was performed at least three times, yielding an average particle count of N = 40 and an average cell count of M = 29 per collagen matrix. Tracer beads used in the analyses were located ≥5 μm away from the nearest adjacent bead to avoid effects due to interparticle forces.
Intracellular rheology
Upon tracking the Brownian motions of individual tracer beads, we computed their ensemble-averaged, time-dependent mean-squared displacement (MSD),
| (1) |
where is the two-dimensional MSD, t is the elapsed time, and τ is the time lag. Because the ensemble-averaged one-dimensional MSD was found to be approximately equal to (data not shown), the total three-dimensional MSD was assumed to be isotropic and
The total ensemble-averaged MSD was then calculated over N and fit to a three-term power law of the form (average R2 of 0.97) using a built-in MATLAB (The MathWorks, Natick, MA) least-squares algorithm to smooth out trajectory noise.
For cells and other complex materials that exhibit both elastic and viscous behavior, the MSD at any time locally follows a power-law relationship, , where α is the diffusive exponent (18). For a passive material, the diffusive exponent can range anywhere from α = 0 for a purely elastic solid to α = 1 (simple diffusion) for a purely viscous liquid. Between these two viscoelastic extremes, embedded particle motion is described as subdiffusive (0 < α < 1) and reflects the relative contribution of the elastic and viscous components to the mechanical state of a material. However, because living cells are active materials, α reflects not only thermal energy (kBT), but also potential modes of active transport, which cannot be decoupled from thermal energy by particle-tracking measurements (28,29). For such cases, particles may exhibit superdiffusive motion (α > 1), and furthermore, an α < 1 is not a direct, absolute reflection of intracellular viscoelasticity. It has been shown previously that the time-dependent creep compliance of a passive material can be extracted directly from the MSD (30). For the case of living, active cells, the intracellular mechanical state has been described in terms of an effective creep compliance (31),
| (2) |
where a is the bead radius. Using Je, we describe the intracellular mechanical state in terms of an apparent elastic modulus (G′p) with the relationship (21)
| (3) |
Because G′p is defined at τ = 1 s and not computed from a plateau compliance, we utilize it to describe relative intracellular stiffness as opposed to an absolute measure of intracellular elasticity.
Live cell morphology characterization
Adherent cells in two-dimensional monolayer culture were stained with Cell Tracker Green CMFDA (Molecular Probes, Eugene, OR) and resuspended at a concentration of 5 × 105 cells/mL within three-dimensional collagen matrices. After gelation and addition of media as described above, matrices were further incubated for 12 h at 37°C, 5% CO2 before imaging. Only individual cells that were wholly suspended within the three-dimensional matrices were selected for imaging. Three samples of each collagen gel were imaged, with 15 cells imaged at random per condition of cell line and matrix stiffness. Confocal images of the cells were obtained using the LSM 5 Live (Carl Zeiss, Thornwood, NY). We quantify the morphology of the cells in terms of the sphericity (Ψ), which describes the shape of a three-dimensional object relative to that of a sphere. Sphericity is defined as the ratio of the surface area of a sphere (with the same volume as the given object) to the surface area of the given object; sphericity reaches a maximum value of 1 for a perfect sphere. Sphericity was computed using Imaris (Bitplane) iso-surface renderings of the reconstructed confocal images.
Results
ErbB2 transforming potential promotes intracellular stiffening in response to increased matrix stiffness
To explore the relationship between breast cancer transforming potential and the intracellular mechanical environment, we examined a well-characterized cancer progression series established from the non-transformed, human-derived MCF10A cell line. We analyzed four sublines, whose extent of transforming potential is determined by their growth traits and morphological features when permitted to form acinar structures in three-dimensional culture (14). The sublines consisted of
-
1.
10A.vec—a non-transformed control cell line.
-
2.
10A.ErbB2—a hyperplastic, partially transformed cell line that overexpressed ErbB2.
-
3.
10A.14-3-3ζ—a depolarized and morphologically abnormal, partially transformed cell line that overexpressed 14-3-3ζ.
And:
-
4.
10A.ErbB2.ζ—an invasive, fully transformed cell line that overexpressed both ErbB2 and 14-3-3ζ.
PTMR analyses showed that adjusting the matrix stiffness G′c had a notable effect on the Brownian dynamics of tracer beads embedded within MECs that exhibited the ErbB2 transformation profile (10A.ErbB2 and 10A.ErbB2.ζ), relative to control cells (10A.vec) and 10A.14-3-3ζ cells. This is illustrated for the 10A.ErbB2.ζ cell line (Fig. 1 A), in which the overall MSD magnitudes decrease as matrix stiffness increases; compare to the MSD magnitudes for the 10A.vec line (Fig. 1 B), which reveal no clear association with matrix stiffness. By extension, increasing the matrix stiffness accordingly yields an increase in intracellular apparent stiffness G′p for MECs that possess the ErbB2 transformation profile (Fig. 2, B and D), yet increasing the matrix stiffness does not invoke a significant intracellular stiffening response in non-transformed MECs or those exhibiting the 14-3-3ζ transformation profile alone (Fig. 2, A and C). Therefore, our results suggest that overexpression of ErbB2, either alone or in cooperation with 14-3-3ζ, confers individual MECs with the capacity to adapt their intracellular mechanical state to ECM mechanical cues.
Figure 1.

Matrix stiffness influences Brownian dynamics of tracer beads embedded within MECs that possess ErbB2 transforming potential. MSD of 1 μm tracer beads embedded within 10A.ErbB2.ζ (A) and 10A.vec (B) cells that reside within three-dimensional Type I collagen matrices of stiffness 104 (○), 271 (---), and 391 (—) Pa. Error bars omitted for figure clarity.
Figure 2.
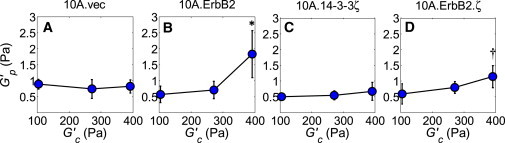
MECs that possess ErbB2 transforming potential stiffen in response to elevated matrix stiffness. Intracellular apparent stiffness G′p increases as matrix stiffness G′c increases for cell lines that overexpress ErbB2 (B and D) but does not significantly increase for 10A.vec (A) or 10A.14-3-3ζ cells (C). Error bars represent mean ± SE. P-values (∗, p ≤ 0.05; †, p ≤ 0.10) determined from t-tests for unpaired samples; p-values are with respect to the lowest intracellular stiffness of the given cell line.
ErbB2 is associated with increased intracellular mechanical sensitivity
Further comparative examination of our PTMR data shows that overexpression of ErbB2 alone is associated with the highest degree of intracellular mechanical sensitivity to matrix stiffness. This sensitivity is mitigated for cells that do not overexpress ErbB2 (10A.14-3-3ζ), but partially restored for cells that co-overexpress both ErbB2 and 14-3-3ζ. This is illustrated in Fig. 3 (wide bars), which shows the percent increase in intracellular apparent stiffness as the matrix stiffness is increased from 104 to 391 Pa for the sublines that exhibit transforming potential. In fact, previous analyses of these sublines showed a strikingly similar relationship between ErbB2 status and cell motility (quantified by trans-well migration): 10A.ErbB2 cells exhibited the highest degree of cell motility, followed by 10A.ErbB2.ζ cells and then by 10A.14-3-3ζ cells (Fig. 3, narrow bars) (14). Although the experimental system employed previously to examine motility was different than that utilized to investigate intracellular stiffness for this study, the two results suggest that there may be a link between motility and the intracellular mechanical state of individual MECs that possess transforming potential.
Figure 3.

ErbB2 sensitizes the intracellular mechanical state to matrix stiffness. Wide bars (red) represent the % increase in intracellular apparent stiffness G′p as matrix stiffness G′c is increased from 104 to 391 Pa. P-values (∗, p ≤ 0.05; †, p ≤ 0.10) determined from t-tests for unpaired samples; p-values reflect the significance of the highest intracellular stiffness relative to the lowest intracellular stiffness of the given cell line as reported in Fig. 2. Thin bars (14) represent the number of migrated cells counted per field of view, after a 6 h trans-well cell migration assay. Initial cell seeding count was maintained at 1 × 105 cells for all cell lines (migration data retrieved from (14)). Error bars represent mean ± SE.
Matrix stiffness couples with transforming potential to determine intracellular stiffness
Next, we compare the intracellular mechanical state of MECs with respect to transforming potential for a given matrix environment. PTMR results indicate that within the softest matrix (Fig. 4 A), intracellular apparent stiffness is reduced for partially transformed 10A.14-3-3ζ cells and fully transformed 10A.ErbB2.ζ cells (relative to 10A.vec cells), while in a matrix of moderate stiffness (Fig. 4 B), none of the cell sublines show a significant difference in intracellular apparent stiffness. In the stiffest matrix (Fig. 4 C), however, ErbB2 status is the dominant factor that influences G′p. In this matrix environment, cells overexpressing ErbB2 alone exhibit the highest intracellular stiffness, followed by cells co-overexpressing both ErbB2 and 14-3-3ζ. A closer analysis of this data reveals another important observation: increasing the matrix stiffness progressively shifts the difference in intracellular apparent stiffness between non-transformed cells (10A.vec) and fully invasive cells (10A.ErbB2.ζ) (Fig. 4 D). In the softest matrix (104 Pa), non-transformed MECs are notably stiffer than fully invasive ones. However, as the matrix stiffness increases to 271 Pa, this differential reverses, becomes only slight in magnitude, and becomes statistically insignificant, while in the stiffest matrix (391 Pa), the reversed differential becomes more pronounced. To summarize, Table 1 provides a compilation of the relevant parameters associated with the sublines examined here. Overall, these results show that the interplay between transforming potential and the intracellular mechanical state of MECs is not a simple, singular association. Rather, it is a matrix stiffness-dependent phenomenon that may have an important bearing on how we characterize the mechanical state of healthy and cancerous breast tissues.
Figure 4.

Matrix stiffness affects the relationship between transforming potential and intracellular stiffness. Intracellular apparent stiffness G′p of MCF10A sublines for matrices of stiffness 104 (A), 271 (B), and 391 (C) Pa. Error bars represent mean ± SE. (D) Difference between G′p of 10A.vec cells and G′p of 10A.ErbB2.ζ cells as a function of matrix stiffness G′c.. P-values (∗, p ≤ 0.05; †, p ≤ 0.10) determined from t-tests for unpaired samples; p-values are with respect to intracellular stiffness of 10A.vec cells.
Table 1.
Summary of relevant parameters associated with MCF10A cancer progression series
| Subline | Cancer progression | Sphericity | Motility (%) (14) | Stiffness sensitivity (% increase) | Stiffness differential (+/−) |
|---|---|---|---|---|---|
| 10A.vec | Non-transformed | 0.98 | 0.26 | — | — |
| 10A.ErbB2 | Partially transformed | 0.97∗∗ | 1.00 | 221∗ | — |
| 10A.14-3-3ζ | Partially transformed | 0.90∗∗∗ | 0.44 | 33 | — |
| 10A.ErbB2.ζ | Fully transformed | 0.79∗∗∗ | 0.82 | 93† | + For high G′c− For low G′c |
Sphericity is that of single cells embedded wholly within 3D Type I collagen matrices of stiffness 391 Pa. P-values (∗∗∗, p ≤ 0.001; ∗∗, p ≤ 0.01) determined from t-tests for unpaired samples; p-values are with respect to the sphericity of 10A.vec cells. Motility is normalized by the number of migrated 10A.ErbB2 cells counted per field of view, following a 6 h trans well cell migration assay (data retrieved (14)). Stiffness sensitivity is quantified as % increase in intracellular apparent stiffness as matrix stiffness is increased from 104 to 391 Pa. P-values (∗, p ≤ 0.05; †, p ≤ 0.10) determined from t-tests for unpaired samples; p-values compare the intracellular stiffness of cells within a matrix of stiffness 391 Pa to cells within a matrix of stiffness 104 Pa. Stiffness differential, notated as positive (+) or negative (−), is the difference in intracellular apparent stiffness between 10A.vec cells and that of 10A.ErbB2.z cells.
Single MEC morphology in three-dimensional matrices is dictated by protein expression
Previous observation of monolayer cultures have shown that the two-dimensional morphology of the present MCF10A sublines is correlated with expression of biomarkers: non-transformed (10A.vec) and hyperproliferative (10A.ErbB2) sublines exhibit a cobblestone-like morphology, whereas the depolarized and fully invasive cell lines (those overexpressing 14-3-3ζ) exhibit an elongated, spindle-like morphology on flat substrates (14). Similarly, three-dimensional acinar structures for 10A.vec and 10A.ErbB2 cells exhibit a defined boundary (9,14), whereas those of 10A.14-3-3ζ and 10A.ErbB2.ζ are characterized by an abnormal morphology with a less-defined boundary (12,14). Our observations of individual MCF10A cell morphology in three-dimensional matrices is consistent with prior findings for two-dimensional cultures and three-dimensional aggregate structures; individual 10A.vec and 10A.ErbB2 cells exhibit a round, spherical morphology in three-dimensional collagen matrices, whereas individual 10A.14-3-3ζ and 10A.ErbB2.ζ cells exhibit an elongated, irregular shape (Fig. 5, A–D).
Figure 5.
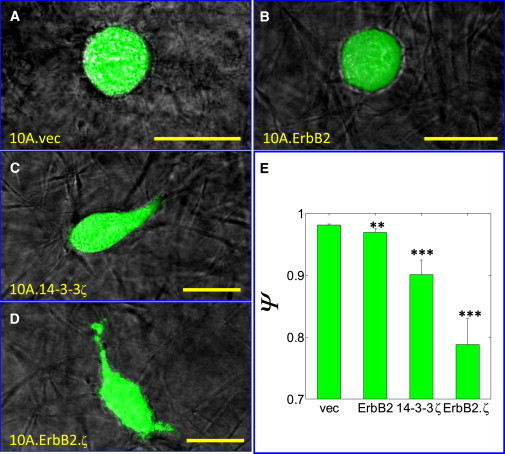
14-3-3ζ alters MCF10A single-cell morphology in three-dimensional matrices. Images of live, individual 10A.vec (A), 10A.ErbB2 (B), 10A.14-3-3ζ (C), and 10A.ErbB2.ζ (D) cells embedded wholly within three-dimensional Type I collagen matrices of stiffness 391 Pa. Scale bar = 20 μm. (E) Sphericity Ψ of MCF10A sublines embedded wholly within three-dimensional Type I collagen matrices of stiffness 391 Pa. Error bars represent mean ± SE. P-values (∗∗∗, p ≤ 0.001; ∗∗, p ≤ 0.01) determined from t-tests for unpaired samples; p-values are with respect to the sphericity of 10A.vec cells.
This morphology is quantified in terms of sphericity (Ψ), which we find highest for 10A.vec cells (nearly perfectly spherical) and lowest for 10A.ErbB2.ζ cells (Fig. 5 E). Imaging did not show a consistent sphericity dependence on matrix stiffness, which may seem contrary to previously published morphological studies of MECs within three-dimensional matrices (3,32,33); however, these prior studies examined morphology of cell aggregates, and cells were observed over much longer periods of exposure to chronically elevated matrix stiffness. The lack of sphericity dependence on matrix stiffness may in part reflect cellular matrix metalloproteinase (MMP) secretion, which is similar among the four sublines examined here (14). MMP secretion may counter the effect of ECM structural barriers that would otherwise be imposed on the cell bodies by increased collagen content, thereby allowing the cells to roughly maintain their shape in the presence of density-induced matrix stiffness. Given that the cells are permitted to migrate freely through the collagen matrix in this experimental design, MMP secretion coupled with the amoeboid mode of motility may further enable the cells to maintain their sphericity, even as matrix pore size decreases (34,35).
Discussion
The rheological behavior of a single cell is governed by the constituency of its cytoplasm (36), the forces exerted on it by the surrounding ECM (37), and the integrins that connect its cytoskeleton to the ECM (38). Increasingly, cancer cells are becoming the focus of studies that explore the effect of the extracellular environment on cellular homeostasis (3,39) and cellular viscoelasticity (21,40). While significant progress has been achieved in discovering some of the molecular mechanisms and signaling pathways that underlie breast and other cancers (7,11), much less is known about the associated cellular mechanical properties. It is has long been established that cancerous breast tissue exhibits elevated stiffness compared to healthy mammary tissues (3); however, the relationship between the external cellular mechanical environment and the intracellular mechanical state of breast cancer is not understood. The mechanical interplay between external and internal cellular compartments is further confounded by the stage of breast cancer progression and may also have a significant bearing on breast cancer cell migration. In order to investigate the interplay among three-dimensional matrix mechanics, the internal cellular mechanical state, and transforming potential in breast cancer, we have utilized PTMR to gauge the relative intracellular stiffness of MECs within three-dimensional Type I collagen matrices. By examining a breast cancer progression series of sublines that derive from a single MEC parent line, we are able to offer direct comparisons among cells of varying transforming potential.
Clarifying breast cancer progression at a fundamental level (the interaction of a cell with its surrounding microenvironment) will improve the basis for understanding and addressing cancer at the systems level, as evidenced by the recent, growing body of single cancer cell investigations (3,21,39,41). The in vivo extracellular microenvironment consists of several components, including but not limited to cells, ECM proteins, vessels, etc., all of which possess distinct mechanical properties and individual roles in tissue homeostasis; the relative balance and significance of these components depend upon the extent of cancer progression. In this study, we have focused on probing the intracellular mechanical state of MECs that have the capacity to freely navigate the ECM, which is physiologically relevant to individual MECs that have invaded their local ductal basement membrane and may exhibit motility within the ECM. We examined single cells that are wholly engaged with the matrix (but unattached to other cells) in order to experimentally control the degree and type of cell surface attachment. Accordingly, the MECs examined here form cell-matrix attachments via β1 integrins.
Our results suggest that overexpression of the metastatic biomarker ErbB2 (either alone or in cooperation with 14-3-3ζ) grants MECs a mechanical adaptability that is not displayed by their non-cancerous counterparts (10A.vec) or those overexpressing only 14-3-3ζ. MECs possessing the ErbB2 transformation profile stiffen in response to increased three-dimensional matrix stiffness (Fig. 2, B and D), whereas the non-transformed control cells and 10A.14-3-3ζ cells do not show a consistent or significant intracellular stiffening response to increased three-dimensional matrix stiffness (Fig. 2, A and C). In the mature, resting (non-pregnant and non-lactating) in vivo mammary gland, healthy luminal MECs are nonmotile and line the ductal basement membrane as a cell layer that is surrounded by an outer myoepithelial cell layer (42); however, MECs that overexpress ErbB2 exhibit adaptive characteristics that drive epithelial to mesenchymal transition (EMT) (43). Overexpression of ErbB2 has been associated with cellular depolarization, as well as hyperproliferation-induced luminal filling (8). A dense lumen inherently transmits a relatively high stress to individual MECs (37); nevertheless, MECs at this early stage of breast cancer progression are still capable of proliferating and may ultimately migrate through a dense lumen to invade the basement membrane. Given their ability to thrive and exert protrusive, migratory force in an environment of elevated mechanical stress, it follows that the associated sublines examined here (10A.ErbB2 and 10A.ErbB2.ζ) would be capable of wielding an internal mechanical state that allows them to resist an increase in ECM stress, a phenomenon that has been termed mechanoreciprocity (37).
Recent investigations have established ErbB2 overexpression as a significant effector of mechanotransduction when MECs are exposed to a stiffened extracellular collagen matrix. Mammary acini cultured in ribose-induced, cross-linked-stiffened three-dimensional matrices have been shown to exhibit stiffness-induced altered morphology; yet, these organoids only developed invasive protrusions into the stiffened matrices once their ErbB2 oncogenes were activated (44). Moreover, ErbB2 activation did not induce cellular invasiveness when the ECM stiffness was not elevated. The same study determined that inhibition of β1 integrin signaling prevented ErbB2-induced invasive behavior within stiff matrices, which strongly suggests that ErbB2 cooperates with integrin-sensed extracellular mechanical cues to influence cancer progression (44). This notion is consistent with the findings of this study, which show that ErbB2 overexpression is associated with increased intracellular stiffness sensitivity to collagen matrix stiffness, for which the β1 integrin is the primary mechanosensor. Additionally, studies have established that focal adhesion (FA) kinase (FAK), a key regulator of the FA integrin clustering site, is critical to ErbB2-associated oncogenic transformation (45,46) and that FAK promotes transformation and invasion in response to elevated collagen matrix stiffness (32). In conjunction with these prior studies, our results further implicate a strong connection between β1 integrins and ErbB2 overexpression for cells within mechanically perturbed extracellular environments.
The lack of intracellular mechanical adaptability observed for non-cancerous MECs may in part explain their susceptibility to a relatively high stress matrix environment. Several studies performed on non-transformed MECs show that prolonged exposure to elevated matrix stiffness induces abnormal morphology and acinar organization (3,32,33), hyperproliferation (8), and aberrant signaling and gene expression (32,46–48), all of which drive eventual malignant transformation. On the other hand, constitutively activated mechanotransduction that characterizes transformed MECs (37) is consistent with the intracellular stiffening that we find of the fully transformed, invasive cells in this study. While the 10A.14-3-3ζ cells did not exhibit an appreciable intracellular mechanical response to changes in matrix stiffness (Fig. 2 C), cells that overexpressed both 14-3-3ζ and ErbB2 (10A.ErbB2.ζ) did show a significant response (Fig. 2 D). Therefore, relevance of the 10A.14-3-3ζ cells is twofold. First, the mechanical non-responsiveness of 10A.14-3-3ζ cells serves as a negative control for the 10A.ErbB2.ζ response, which further implicates ErbB2 as a significant regulator of the cellular mechanical environment with regard to cancer progression. Second, the mechanical non-responsiveness of 10A.14-3-3ζ cells further demonstrates the distinct contributions of 14-3-3ζ and ErbB2 to cancer progression. Previous studies show that ErbB2 promotes hyperproliferation (8), while 14-3-3ζ promotes resistance to apoptosis and EMT via downregulation of p53 and E-cadherin (12,14). Additionally, this study now suggests that overexpression of ErbB2 confers intracellular mechanical sensitivity to matrix rigidity, while overexpression of 14-3-3ζ confers individual cells with morphological irregularity that is associated with invasive phenotypes in three-dimensional matrices (32,33).
Among the MECs that possess transforming potential, we find that overexpression of ErbB2 alone is associated with the greatest intracellular mechanical sensitivity to matrix stiffness. As the matrix stiffness increases from 104 to 391 Pa, the percent increase in intracellular stiffness is highest for 10A.ErbB2 cells, followed by 10A.ErbB2.ζ cells, and then by 10A.14-3-3ζ cells. Owing to prior studies that examined migratory propensity of these MCF10A sublines (14), our data suggests that transformation profile (overexpression of ErbB2 and/or 14-3-3ζ) may produce a similar effect on both cell motility and intracellular stiffness sensitivity (Fig. 3). Trans-well migration and two-dimensional wound healing assays showed that cell motility was found to exhibit a comparable relationship with ErbB2 status: motility was highest for cells overexpressing ErbB2 alone, lowest for cells overexpressing 14-3-3ζ but not ErbB2, and moderate for cells co-overexpressing both biomarkers (14). This parallel of motility and stiffness sensitivity is also consistent with recent investigations that reveal ErbB2 regulation of FA turnover via the Src-FAK signaling pathway (46). Given the connection among enhanced epidermal growth factor receptor signaling and upregulated β1 integrin activity (48), the role of β1 integrins in relaying force between ECM collagen fibers and the cytoskeleton (38), and the involvement of FAs in cell migration and cell contractility (49), ErbB2 influence on intracellular stiffness sensitivity suggests yet another link that relates cell stiffness, cell motility, and matrix elasticity to one another. Computational models predict that for a given cell type, cell migration speed will gradually increase as three-dimensional matrix stiffness increases and porosity decreases, until the matrix stiffness reaches a critical value at which migration speed decreases thereafter, due to relatively high cell adhesivity and steric resistance presented by matrix ligands (50,51). Future examination of this phenomenon for the cancer progression series examined in this study may provide significant insights into the connections among intracellular stiffness, matrix stiffness, and cell motility, and even more importantly, how these relationships are influenced by transforming potential with respect to a purely three-dimensional extracellular environment.
Examining single cells within three-dimensional matrices (integrin-mediated attachments) may provide insight into the state of cells attached to other cells (cadherin-mediated attachments) as well. Epithelial cells that form single cell layers along inner surfaces of hollow structures possess a free, unengaged surface; in contrast, cells that form attachments around their entire surface, whether mediated by integrins or cadherins, experience an inherently more constrained intracellular environment (21). However, given that both β1 integrins and cadherins transmit tension through the cytoskeletal network, we should expect to see similar trends in intracellular stiffness as a function of matrix stiffness when cells form cadherin-mediated cell-cell attachments as well. The degree of measured intracellular stiffness sensitivity expected for cells that form cell-cell attachments would likely depend, in part, on the extent to which the cells are attached to other cells versus ECM. For example, a cell that is attached mostly to other cells and only minimally to ECM would likely show a muted sensitivity to matrix stiffness because relatively little cell surface area is available for matrix engagement. Alternatively, a cell that is attached mostly to ECM and only minimally to other cells may exhibit a higher intracellular stiffness sensitivity to matrix stiffness, relative to a cell of the former case.
While we did not observe a matrix-stiffness-dependent effect on single-cell morphology of the MCF10A sublines examined here, analyzing the relationship between transforming potential and intracellular stiffness of MECs in three-dimensional matrices did yield an important paradigm: this interplay is matrix-stiffness-dependent and cannot be relegated to a universal association (Table 1). Our PTMR data shows that within the softest matrix environment, intracellular stiffness of partially transformed 10A.14-3-3ζ cells is less than that of non-transformed cells, but that intracellular stiffness is recovered to some degree for fully invasive cells (Fig. 4 A). Conversely, ErbB2 expression is the dominating factor that affects intracellular stiffness within the stiffest of matrices (Fig. 4 C).
Matrix stiffness also affects the differential in intracellular stiffness between non-cancerous and fully invasive MECs, whereby non-cancerous cells are notably stiffer in softer matrices, while this differential reverses in more rigid matrices (Fig. 4 D). This result is interesting in light of the fact that normal, resting mammary tissue is relatively compliant, with a shear modulus that is ∼1 × 102 Pa (44). The stiffness of mammary ECM adjacent to tumors is elevated, with a modulus that is approximately twice (2 × 102 Pa) that of normal tissue, and the modulus of mammary tumor masses increases even further to ∼9 × 102 Pa (44). Our studies probe the intracellular mechanical response as the ECM progressively stiffens from a typical, resting mechanical state (from 104 to 391 Pa). Our results indicate that within a relatively normal, soft matrix environment, MECs that possess transforming potential may not be capable of fully manifesting the survival advantages (hyperproliferative and antiapoptotic mechanisms) that have been associated with perturbed matrix stiffness-induced mechanotransduction, as evidenced by their relatively low level of intracellular stiffness (Fig. 4 A). Yet, within relatively stiffer matrices (Fig. 4, B and C), the intracellular stiffness of these cells approaches and even exceeds that of non-transformed cells for the case of 10A.ErbB2 cells within the stiffest matrix. This again emphasizes the synergism among elevated matrix stiffness, FAs, integrins, and increased motility in driving breast cancer progression, as all of these elements affect the cytoskeletal organization that underlies intracellular stiffness (52).
In summary, our studies provide novel insights into the subject matter of breast cancer mechanobiology by demonstrating that transforming potential couples with matrix stiffness to dictate the intracellular mechanical state of MECs. The interactions between ErbB2 and 14-3-3ζ are complex, and further clarification of these connections may arise from additional future studies that examine their individual and cooperative roles in three-dimensional motility and in EMT-related signal transduction. Several significant earlier investigations have shaped the present understanding by examining MEC aggregate morphology within three-dimensional matrices (33), global mammary tissue stiffness (3), two-dimensional MEC traction forces (3), two-dimensional indentation analyses of MECs (53), MEC FA activity (46), and MEC β1 integrin signaling (54). Via PTMR, we have added to this knowledge base by directly probing the intracellular space of MECs embedded within three-dimensional matrices, which have been shown to recapitulate fundamental properties of MECs that do not manifest in two-dimensional cultures (23,48,55). An improved understanding of internal MEC rheology holds wide-reaching potential that may eventually influence the design of targeted drug transport as well as evolve the systems biology framework of cancer. With continued investigations that focus on simulating in vivo environments of MECs, the field of breast cancer biology will continue to advance and ultimately reduce the impact of breast cancer on human mortality.
Acknowledgments
This work was made possible by the UNCF/Merck Graduate Science Research Dissertation Fellowship (to E.L.B.), a UT Seed Grant from the Charles Tate Foundation (to M.H.Z. and D.Y.), and National Institutes of Health grant No. 1R01CA132633 (to M.H.Z.).
References
- 1.Hondermarck H., Vercoutter-Edouart A.S., Peyrat J.P. Proteomics of breast cancer for marker discovery and signal pathway profiling. Proteomics. 2001;1:1216–1232. doi: 10.1002/1615-9861(200110)1:10<1216::AID-PROT1216>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Ligresti G., Libra M., Stivala F. Breast cancer: molecular basis and therapeutic strategies [Review] Mol. Med. Rep. 2008;1:451–458. [PubMed] [Google Scholar]
- 3.Paszek M.J., Zahir N., Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Zaman M.H., Trapani L.M., Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neal C.L., Yao J., Yu D. 14-3-3ζ overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69:3425–3432. doi: 10.1158/0008-5472.CAN-08-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon D.J., Godolphin W., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 7.Yu D.H., Hung M.C. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115–6121. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 8.Muthuswamy S.K., Li D.M., Brugge J.S. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debnath J., Mills K.R., Brugge J.S. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 10.Ross J.S., Fletcher J.A., Bloom K.J. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8:307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 11.Porter G.W., Khuri F.R., Fu H. Dynamic 14-3-3/client protein interactions integrate survival and apoptotic pathways. Semin. Cancer Biol. 2006;16:193–202. doi: 10.1016/j.semcancer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Danes C.G., Wyszomierski S.L., Yu D. 14-3-3ζ down-regulates p53 in mammary epithelial cells and confers luminal filling. Cancer Res. 2008;68:1760–1767. doi: 10.1158/0008-5472.CAN-07-3177. [DOI] [PubMed] [Google Scholar]
- 13.Grossmann J. Molecular mechanisms of “detachment-induced apoptosis—Anoikis”. Apoptosis. 2002;7:247–260. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- 14.Lu J., Guo H., Yu D. 14-3-3ζ cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell. 2009;16:195–207. doi: 10.1016/j.ccr.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason T.G., Ganesan K., Kuo S.C. Particle tracking microrheology of complex fluids. Phys. Rev. Lett. 1997;79:3282–3285. [Google Scholar]
- 16.Daniels B.R., Masi B.C., Wirtz D. Probing single-cell micromechanics in vivo: the microrheology of C. elegans developing embryos. Biophys. J. 2006;90:4712–4719. doi: 10.1529/biophysj.105.080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason T.G., Gisler T., Weitz D.A. Rheology of F-actin solutions determined from thermally driven tracer motion. J. Rheol. 2000;44:917–928. [Google Scholar]
- 18.Wirtz D. Particle-tracking microrheology of living cells: principles and applications. Annu. Rev. Biophys. 2009;38:301–326. doi: 10.1146/annurev.biophys.050708.133724. [DOI] [PubMed] [Google Scholar]
- 19.Panorchan P., Lee J.S.H., Wirtz D. Probing cellular mechanical responses to stimuli using ballistic intracellular nanorheology. Methods Cell Biol. 2007;83:115–140. doi: 10.1016/S0091-679X(07)83006-8. [DOI] [PubMed] [Google Scholar]
- 20.Tseng Y., Kole T.P., Wirtz D. Micromechanical mapping of live cells by multiple-particle-tracking microrheology. Biophys. J. 2002;83:3162–3176. doi: 10.1016/S0006-3495(02)75319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker E.L., Bonnecaze R.T., Zaman M.H. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys. J. 2009;97:1013–1021. doi: 10.1016/j.bpj.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debnath J., Muthuswamy S.K., Brugge J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 23.Wozniak M.A., Keely P.J. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol. Proced. Online. 2005;7:144–161. doi: 10.1251/bpo112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engler A., Bacakova L., Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung T., Georges P.C., Janmey P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 26.Lutolf M.P., Hubbell J.A. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.S.H., Panorchan P., Wirtz D. Ballistic intracellular nanorheology reveals ROCK-hard cytoplasmic stiffening response to fluid flow. J. Cell Sci. 2006;119:1760–1768. doi: 10.1242/jcs.02899. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman B.D., Massiera G., Crocker J.C. The consensus mechanics of cultured mammalian cells. Proc. Natl. Acad. Sci. USA. 2006;103:10259–10264. doi: 10.1073/pnas.0510348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weihs D., Mason T.G., Teitell M.A. Bio-microrheology: a frontier in microrheology. Biophys. J. 2006;91:4296–4305. doi: 10.1529/biophysj.106.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J.Y., Viasnoff V., Wirtz D. Compliance of actin filament networks measured by particle-tracking microrheology and diffusing wave spectroscopy. Rheol. Acta. 1998;37:387–398. [Google Scholar]
- 31.Weihs D., Mason T.G., Teitell M.A. Effects of cytoskeletal disruption on transport, structure, and rheology within mammalian cells. Phys. Fluids. 2007;19:103102. doi: 10.1063/1.2795130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provenzano P.P., Inman D.R., Keely P.J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provenzano P.P., Inman D.R., Keely P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008 doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedl P., Wolf K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulrich T.A., Jain A., Kumar S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials. 2010;31:1875–1884. doi: 10.1016/j.biomaterials.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 36.Heidemann S.R., Wirtz D. Towards a regional approach to cell mechanics. Trends Cell Biol. 2004;14:160–166. doi: 10.1016/j.tcb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Paszek M.J., Weaver V.M. The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 38.Baker E.L., Zaman M.H. The biomechanical integrin. J. Biomech. 2010;43:38–44. doi: 10.1016/j.jbiomech.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulrich T.A., de Juan Pardo E.M., Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou H.W., Li Q.S., Lim C.T. Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices. 2009;11:557–564. doi: 10.1007/s10544-008-9262-8. [DOI] [PubMed] [Google Scholar]
- 41.Bloom R.J., George J.P., Wirtz D. Mapping local matrix remodeling induced by a migrating tumor cell using three-dimensional multiple-particle tracking. Biophys. J. 2008;95:4077–4088. doi: 10.1529/biophysj.108.132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howard B.A., Gusterson B.A. Human breast development. J. Mammary Gland Biol. Neoplasia. 2000;5:119–137. doi: 10.1023/a:1026487120779. [DOI] [PubMed] [Google Scholar]
- 43.Hebner C., Weaver V.M., Debnath J. Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu. Rev. Pathol. Mech. Dis. 2008;3:313–339. doi: 10.1146/annurev.pathmechdis.3.121806.151526. [DOI] [PubMed] [Google Scholar]
- 44.Levental K.R., Yu H.M., Weaver V.M. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benlimame N., He Q., Alaoui-Jamali M.A. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J. Cell Biol. 2005;171:505–516. doi: 10.1083/jcb.200504124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y., Benlimame N., Alaoui-Jamali M.A. Regulation of focal adhesion turnover by ErbB signaling in invasive breast cancer cells. Br. J. Cancer. 2009;100:633–643. doi: 10.1038/sj.bjc.6604901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim I.Y., Yong H.Y., Moon A. Overexpression of ErbB2 induces invasion of MCF10A human breast epithelial cells via MMP-9. Cancer Lett. 2009;275:227–233. doi: 10.1016/j.canlet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Wang F., Weaver V.M., Bissell M.J. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelham R.J., Jr., Wang Y.L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harley B.A.C., Kim H.D., Gibson L.J. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys. J. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaman M.H., Kamm R.D., Lauffenburger D.A. Computational model for cell migration in three-dimensional matrices. Biophys. J. 2005;89:1389–1397. doi: 10.1529/biophysj.105.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mofrad M.R.K. Rheology of the cytoskeleton. Annu. Rev. Fluid Mech. 2009;41:433–453. [Google Scholar]
- 53.Li Q.S., Lee G.Y.H., Lim C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008;374:609–613. doi: 10.1016/j.bbrc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 54.Weaver V.M., Petersen O.W., Bissell M.J. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barcellos-Hoff M.H., Aggeler J., Bissell M.J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


