Abstract
It is well accepted that cotransporters facilitate water movement by two independent mechanisms: osmotic flow through a water channel in the protein and flow driven by ion/substrate cotransport. However, the molecular mechanism of transport-linked water flow is controversial. Some researchers believe that it occurs via cotransport, in which water is pumped along with the transported cargo, while others believe that flow is osmotic in response to an increase in intracellular osmolarity. In this letter, we report the results of a 200-ns molecular dynamics simulation of the sodium-dependent galactose cotransporter vSGLT. Our simulation shows that a significant number of water molecules cross the protein through the sugar-binding site in the presence as well as the absence of galactose, and 70–80 water molecules accompany galactose as it moves from the binding site into the intracellular space. During this event, the majority of water molecules in the pathway are unable to diffuse around the galactose, resulting in water in the inner half of the transporter being pushed into the intracellular space and replaced by extracellular water. Thus, our simulation supports the notion that cotransporters act as both passive water channels and active water pumps with the transported substrate acting as a piston to rectify the motion of water.
Main Text
The Na+-glucose cotransporter (SGLT1) and other cotransporters behave as channels for water and small hydrophilic solutes when expressed in oocytes (1). Passive water flow occurs in response to osmotic gradients in the presence or absence of Na+ or glucose (2), but water flow can occur against an osmotic gradient when SGLT is energized by a sodium or glucose gradient (3–5). The stoichiometry of water to Na+ and glucose transport for human SGLT1 (hSGLT1) is 260 water molecules for each glucose and 2 Na+ moved. The coupling ratio varies from 50 to 500 waters per cycle for different cotransporters (2,5). The interpretation of these observations is controversial with one group proposing water pumping (4,5) and another proposing an indirect osmotic mechanism, where an increase in the intracellular solute concentrations pull water into the cell across the transporter (6,7). Irrespective of the molecular mechanism, hSGLT1 plays a significant role in the transport of 6–8 liters of water a day across the intestine; and therapeutically, water transport associated with hSGLT1 activity is responsible for mitigating the adverse effects of acute diarrhea through the use of oral rehydration therapy.
Unfortunately, the structure of hSGLT1 is not known; however, the structure of the closely related transporter vSGLT was recently solved (8), allowing us to address issues of water transport for the first time. vSGLT is a sodium sugar transporter in Vibro parahaemolyticus, a marine bacterium. The Na+ electrochemical gradient drives the uptake of galactose (9), just as it does for human SGLT members. vSGLT has 14 transmembrane (TM) segments, and TM segments 1–10 form the core transport domain, which is common to a diverse family of ion coupled transporters (10). The vSGLT structure is an inward-occluded state with galactose bound in the center halfway across the membrane (Fig. 1). An inner hydrophilic cavity penetrates to just below the galactose and putative Na+ binding sites, and there is no clear pathway to the extracellular side. The structure is termed “occluded” because the galactose is hemmed in by two prominent hydrophobic gates: one to the extracellular space (M73, Y87, F424) and one to the intracellular hydrophilic cavity (Y263) (yellow residues in Fig. 1). In contrast to hSGLT1, the water transport properties of vSGLT are not well understood due to poor expression in systems used to examine water transport, i.e., Xenopus oocytes. However, vSGLT shares 32% sequence identity with hSGLT1, and we believe that analysis of vSGLT will shed light on the transport properties of all SGLT members.
Figure 1.
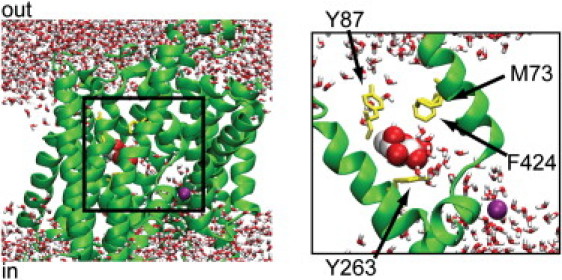
Architecture and hydration of the galactose binding site after equilibration. vSGLT (green) embedded in the membrane (not shown) with galactose (red and white) and Na+ (purple) in their respective binding sites. (Right panel) Magnification of the boxed region. A handful of water molecules (red and white) interact with galactose in its binding site as it is coordinated by TM1 (right) and TM6 (left).
We carried out a long unbiased molecular dynamics simulation of the vSGLT monomer embedded in a lipid bilayer with galactose and Na+ bound in their respective sites (see the Supporting Material for details). Similar to Li and Tajkhorshid (11), we observe that the Na+ dissociates and enters the intracellular space after 9 ns. After Na+ exit, galactose moves past the inner hydrophobic gate (Y263), enters the hydrophilic cavity, and ultimately enters the intracellular space at 110 ns. Once galactose exits the site, Y263 closes behind it and prevents reentry. Water plays a prominent role in this process. Even during the final 10 ns of equilibration, Fig. 1 shows that a few water molecules entered the binding site from the inside as observed by Zomot and Bahar (12). More water enters after Na+ release, and it disrupts galactose-protein interactions by competing for hydrogen bonds.
We observe hundreds of water molecules move through vSGLT via the galactose-binding site (see Fig. S1 in the Supporting Material). Fig. 2 shows the cumulative number of outward (yellow curve) and inward (black curve) permeation events, where a successful event is defined as a water molecule that starts in the upper or lower bulk solution and then enters the opposite bath by passing through vSGLT. The number of events per nanosecond increases from 0 to 3 (0–100 ns) to 3 to 10 (100–200 ns) after galactose exits (Fig. 2 A).
Figure 2.
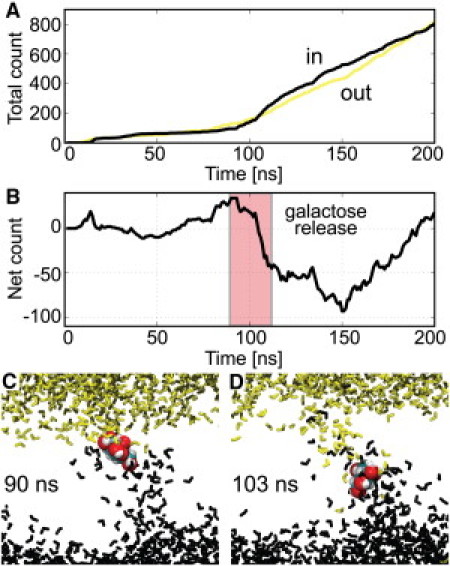
Water permeates vSGLT via the galactose binding site. (A) Cumulative outward (yellow) and inward (black) water movement through the transporter. (B) Net water movement through the transporter. Inward events pass from top to bottom and are negative. (C and D) Snapshots at 90 ns just before galactose unbinding (C) and at 103 ns just before exit from the hydrophilic cavity (D). Water starting above galactose is yellow and water below is black.
The net water movement fluctuates around zero with an amplitude of 10–20 molecules up until ∼100 ns (Fig. 2 B), which is what we expect due to the periodicity in the z direction and the lack of an osmotic gradient across the membrane. However, as galactose exits the binding site between 90 and 110 ns, there is a sharp, inward flow of 70–80 waters into the intracellular space.
Is this a natural fluctuation or a deterministic pumping event correlated with substrate unbinding? To answer this, we asked whether the motion of the water is correlated with the motion of galactose by color-coding all of the water molecules during galactose dissociation based on whether they started above (yellow) or below (black) the galactose. As can be seen from the 90-ns and 103-ns configurations (Fig. 2, C and D), the majority of water in the inner half of the transporter (black) moves out of the cavity ahead of the galactose and is replaced by waters from the extracellular space (yellow) which move in behind the galactose.
The physical dimension of galactose is comparable to the inner hydrophilic cavity, and we reasoned that as galactose exits it may act as a Brownian piston rectifying the motion of water. Galactose exits inwardly because it is blocked on the extracellular side by the outer gate, and then during the simulation it is rectified again by Y263, as described above. There is very little energy expended to move the water, as indicated by our analysis in the Supporting Material, and therefore, galactose acts as a slow rectifying wall.
Next, we calculated the osmotic permeability, pf, of vSGLT from our equilibrium simulation using the theory developed by Zhu et al. (13) (also see the Supporting Material). This constant relates the water flux through the transporter to the pressure or osmolite gradient across the membrane. Over the entire simulation, pf is 4.1 × 10−13 cm3/s. The experimental estimate of pf for rabbit SGLT1 is 4.5 × 10−16 cm3/s (14)–900 times lower than calculated here. However, our simulation is 200,000 times shorter than the estimated 40-ms turnover time for hSGLT1 (3), which implies that there are states along the transport cycle that are not being sampled. It is known that the water permeability is state-dependent (2,15); thus, the experimental value is an average over both low and high permeability states along the reaction cycle, and we believe that the unbinding step studied here may be a high water permeability state. Therefore, limited simulation of the entire cycle most likely accounts for the discrepancy between our calculations and experiment.
Finally, to gain further insight into the nature of transport we tracked tagged waters as they crossed vSGLT and calculated the corresponding water diffusion permeability, pd, and the ratio of pf/pd. A large ratio indicates that movement through the pore is highly cooperative while ratios near 1 are common for large pores (16). Our simulations give a pf/pd ratio of 3.4, which is in good agreement with experimental values for other transporters (17), and much less than experimental and computational values of ∼13 reported for aquaporins (16,18). Another difference compared to aquaporins is that water movement through vSGLT appears to be uncoordinated while the precise alignment of water molecules in aquaporins blocks proton passage (19); thus, protons may permeate the sugar site of vSGLT, which may partially explain why some family members can also operate on pH gradients (20).
We have shown that water diffuses across vSGLT using the sugar pathway. The computed pf is high compared to that for hSGLT1, but this probably reflects that we are simulating only a portion of the transport cycle. However, the pf/pd ratio of 3.4 suggests a narrow functional water pore through the transporter. We observe that 70–80 water molecules are pushed in the direction of galactose movement during substrate exit, which is consistent with the hypothesis that cotransporters behave as water pumps.
Acknowledgments
We thank Joshua Adelman, Om Choudhary, and Keith Callenberg for valuable discussions.
Simulations were performed through grant No. MCB-080011 to M.G. and S.C. (from TeraGrid). E.M.W. was supported by grant No. DK19567 and J.A. by grant No. GM078844, both grants from the National Institutes of Health, Bethesda, MD. M.G. is an Alfred P. Sloan Research Fellow.
Contributor Information
Ernest M. Wright, Email: ewright@mednet.ucla.edu.
Michael Grabe, Email: mdgrabe@pitt.edu.
Supporting Material
References and Footnotes
- 1.Wright E.M., Loo D.D., Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology (Bethesda) 2004;19:370–376. doi: 10.1152/physiol.00026.2004. [DOI] [PubMed] [Google Scholar]
- 2.Loo D.D., Hirayama B.A., Wright E.M. Passive water and ion transport by cotransporters. J. Physiol. 1999;518:195–202. doi: 10.1111/j.1469-7793.1999.0195r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loo D.D., Zeuthen T., Wright E.M. Cotransport of water by the Na+/glucose cotransporter. Proc. Natl. Acad. Sci. USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loo D.D.F., Wright E.M., Zeuthen T. Water pumps. J. Physiol. 2002;542:53–60. doi: 10.1113/jphysiol.2002.018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeuthen T. Water-transporting proteins. J. Membr. Biol. 2010;234:57–73. doi: 10.1007/s00232-009-9216-y. [DOI] [PubMed] [Google Scholar]
- 6.Duquette P.P., Bissonnette P., Lapointe J.Y. Local osmotic gradients drive the water flux associated with Na+/glucose cotransport. Proc. Natl. Acad. Sci. USA. 2001;98:3796–3801. doi: 10.1073/pnas.071245198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagnon M.P., Bissonnette P., Lapointe J.Y. Glucose accumulation can account for the initial water flux triggered by Na+/glucose cotransport. Biophys. J. 2004;86:125–133. doi: 10.1016/S0006-3495(04)74090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faham S., Watanabe A., Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turk E., Kim O., Wright E.M. Molecular characterization of Vibrio parahaemolyticus vSGLT: a model for sodium-coupled sugar cotransporters. J. Biol. Chem. 2000;275:25711–25716. doi: 10.1074/jbc.M003127200. [DOI] [PubMed] [Google Scholar]
- 10.Abramson J., Wright E.M. Structure and function of Na+-symporters with inverted repeats. Curr. Opin. Struct. Biol. 2009;19:425–432. doi: 10.1016/j.sbi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Tajkhorshid E. Ion-releasing state of a secondary membrane transporter. Biophys. J. 2009;97:L29–L31. doi: 10.1016/j.bpj.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zomot E., Bahar I. The sodium/galactose symporter crystal structure is a dynamic, not so occluded state. Mol. Biosyst. 2010;6:1040–1046. doi: 10.1039/b927492h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu F., Tajkhorshid E., Schulten K. Collective diffusion model for water permeation through microscopic channels. Phys. Rev. Lett. 2004;93:224501. doi: 10.1103/PhysRevLett.93.224501. [DOI] [PubMed] [Google Scholar]
- 14.Zampighi G.A., Kreman M., Wright E.M. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J. Membr. Biol. 1995;148:65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- 15.MacAulay N., Gether U., Zeuthen T. Passive water and urea permeability of a human Na+-glutamate cotransporter expressed in Xenopus oocytes. J. Physiol. 2002;542:817–828. doi: 10.1113/jphysiol.2002.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu F., Tajkhorshid E., Schulten K. Theory and simulation of water permeation in aquaporin-1. Biophys. J. 2004;86:50–57. doi: 10.1016/S0006-3495(04)74082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelstein A. John Wiley & Sons; New York: 1987. Water Movement through Lipid Bilayers, Pores, and Plasma Membranes. [Google Scholar]
- 18.Mathai J.C., Mori S., Agre P. Functional analysis of aquaporin-1 deficient red cells. The Colton-null phenotype. J. Biol. Chem. 1996;271:1309–1313. doi: 10.1074/jbc.271.3.1309. [DOI] [PubMed] [Google Scholar]
- 19.Tajkhorshid E., Nollert P., Schulten K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–530. doi: 10.1126/science.1067778. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama B.A., Loo D.D., Wright E.M. Protons drive sugar transport through the Na+/glucose cotransporter (SGLT1) J. Biol. Chem. 1994;269:21407–21410. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


