Abstract
Evidence suggests that individuals who are more obese may be more responsive to stress. Stress activates the sympathetic nervous system (SNS) and the adipose-tissue cytokine leptin stimulates SNS activity in animals. We examined the relationship between adiposity, leptin and physiological responses to acute laboratory stress in 67 women. We predicted that individuals with greater adiposity and/or higher plasma leptin would be more stress-responsive. Adiposity was unrelated to cardiovascular or neuroendocrine stress reactivity. However, women with larger waists had greater stress-induced increases in plasma leptin and interleukin-1 receptor antagonist (IL-1Ra). Similarly, women with higher basal leptin displayed greater stress-induced increases in heart rate and plasma interleukin-6, and larger decreases in heart rate variability and cardiac pre-ejection period. Heightened cardiovascular and inflammatory stress responses are predictive of future cardiovascular risk. Our findings suggest that the cytokines leptin and IL-1Ra may play a role in the association between obesity, stress and cardiovascular health.
Keywords: Psychological stress, Adiposity, Leptin, Cytokines, Cardiovascular reactivity
1. Introduction
The prevalence of obesity has risen sharply in recent years, reaching epidemic proportions world-wide. According to latest figures from the World Health Organisation approximately 1.6 billion adults are currently overweight (body mass index, BMI ≥25 kg m−2), and at least 400 million are clinically obese (BMI ≥30 kg m−2). These figures are estimated to reach an alarming 2.3 billion and >700 million by 2015 (WHO Global InfoBase, 2006). Obesity is a major risk factor for several chronic conditions including hypertension, cardiovascular disease (CVD), type 2 diabetes, and certain types of cancer and as such poses a major challenge to public health care (Lavie et al., 2009). Understanding the biological mechanisms linking obesity and health is therefore of fundamental importance.
Psychological stress is associated with an increased risk of hypertension and CVD and evidence emerging in the past decade suggests that individuals who are more obese may be more responsive to stress. Two of the principle pathways activated by stress are the hypothalamic–pituitary–adrenal axis, leading to elevations in circulating glucocorticoids and the sympathetic nervous system (SNS), resulting in increases in blood pressure, heart rate and circulating catecholamines (Black, 2006). Studies have shown that women with a larger waist circumference or waist–hip ratio have heightened cortisol responses to acute laboratory stress, as well as impaired dexamethasone suppression of cortisol (Epel et al., 2000; Pasquali et al., 2002). Similarly, central obesity has been associated with elevated or prolonged cardiovascular responses to acute stress in some but not all studies (Steptoe and Wardle, 2005; Goldbacher et al., 2005; Carroll et al., 2008; Davis et al., 1999; Waldstein et al., 1999; Barnes et al., 1998), and a recent report found larger cortisol and cardiovascular responses to public speaking stress in obese versus non-obese women (Benson et al., 2009).
The biological mechanisms linking obesity and stress reactivity are poorly understood. Adipose tissue is now recognised as a major endocrine organ that secretes signalling molecules playing a central role in inflammation, weight regulation and metabolic function including cytokines (Trayhurn, 2005). Circulating levels of the hormone-like cytokine leptin are markedly elevated in obese humans and animals and correlate with adiposity measures in non-obese healthy individuals (Considine et al., 1996; Trayhurn and Bing, 2006). Leptin is secreted into the blood stream in proportion to adipose tissue mass, and binds to receptors on specific hypothalamic nuclei to regulate energy balance by reducing appetite and stimulating SNS activity (Trayhurn and Bing, 2006). In rodents, acute systemic or central leptin infusion increases sympathetic nerve activity (SNA) to thermogenic tissues such as brown adipose tissue (BAT) as well as non-thermogenic organs including kidneys and adrenal glands (Ren, 2004). Similarly, chronic leptin infusion increases heart rate, arterial blood pressure and circulating catecholamines, and these effects are inhibited by α1 and β1/β2 adrenergic antagonists (Ren, 2004; da Silva et al., 2006). The relationship between leptin and SNS activity in humans is less clear. Elevated plasma leptin levels have been reported in patients with hypertension, compared to normotensive individuals (Thomopoulos et al., 2009). Similarly, cross-sectional studies have found positive associations between circulating leptin and sympathetic activity indexed by blood pressure, heart rate and heart rate variability in obese and lean normotensive humans (Bravo et al., 2006; Flanagan et al., 2007; Ma et al., 2009), and a recent prospective analyses of 489 normotensive men showed that individuals with high serum leptin levels had a 33% increased risk of developing hypertension over 8 years, independent of BMI (Galletti et al., 2008). Nevertheless, most intervention studies have found no effect of either acute or chronic leptin infusion on SNS activity in humans (Hukshorn et al., 2003; Brook et al., 2007; Mackintosh and Hirsch, 2001; Chan et al., 2007). It is conceivable that the sympatho-activating effects of leptin in humans may be more apparent under conditions of heightened SNS activation induced by factors such as psychological stress.
We set out to investigate the relationship between adiposity, leptin and physiological responses to acute psychological stress in a sample of healthy young women. We predicted that women with greater adiposity would have heightened or prolonged cardiovascular, neuroendocrine and inflammatory responses to stress. We also predicted that leptin would potentiate sympathetic cardiovascular reactivity to stress, so that women with higher basal plasma leptin levels would have larger cardiovascular stress responses.
2. Methods
2.1. Sample
Sixty-nine female student volunteers were recruited from University College London. Participants were aged between 18 and 25 years, and were screened by structured interview to ensure that they were healthy, not taking any medication and had no previous history of any relevant physical or mental illness. They were instructed not to take antibiotics, ibuprofen or aspirin for 10 days prior to the study and to avoid caffeine, alcohol and excessive exercise during the 12 h prior to testing. On the day of the study, participants were advised to eat a low fat breakfast and were provided with a standardised low fat lunch consisting of vegetable couscous, fruit salad and fresh orange juice. Two participants were excluded from the final analyses since one was extremely obese (BMI 43.3) and another was greatly underweight (BMI 15.6). The study was approved by the joint University College London/University College London Hospital Committee on the Ethics of Human Research and all participants gave their informed consent. We chose to study a healthy population with a wide range of adiposity levels rather than specifically comparing obese versus non-obese individuals, since obese individuals are more likely to have co-morbid conditions such as diabetes or hypertension that may have influenced our measures.
2.2. Anthropometric measures
Anthropometric measures were taken by a research nurse at the beginning of the session. Height was measured to the nearest 0.1 cm using the Frankfort plane to standardise the measurement. Body weight was measured to the nearest 0.1 kg. Body mass index (BMI) was calculated as body weight in kilograms divided by height in metres squared. Waist circumference was measured midway between the lowest rib and ileac crest. Body fat mass was estimated using a Bodystat 1500 bioelectrical impedance body composition analyses device (Bodystat, Douglas, Isle of Man). Fat percentage was calculated as fat weight divided by total (fat + lean) body weight.
2.3. Autonomic and neuroendocrine measures
Cardiovascular data for the study were collected continuously and then averaged over specified 5 min trials. Blood pressure was monitored from the finger using a Portapres 2, a portable version of the Finpres device (TNO-TPD Biomedical Instrumentation, Amsterdam, The Netherlands). Heart rate and heart rate variability (HRV) were assessed by impedance cardiography (ICG; VU-AMS, Amsterdam, The Netherlands) as described previously (Willemsen et al., 1996). HRV was calculated as the root mean square of successive R-R interval differences (RMSSD). A reduction in RMSSD indicates a shift in cardiac sympathovagal balance towards sympathetic control over the rhythm of the heart (Malik, 1996). Cardiac pre-ejection period (PEP) was measured as an index of cardiac sympathetic drive (Sherwood et al., 1990). One minute ensemble averages were derived for the ICG for each minute of tasks and averaged. PEP was defined as the interval between R-wave and B-point plus a fixed Q–R interval of 48 ms. Cortisol was measured in saliva samples obtained throughout the session. Saliva was collected using Salivettes (Sarstedt, Inc. Leicester, UK) and stored at −80 °C prior to analyses. Salivary cortisol was analyzed using a commercially available time-resolved immunoassay with chemiluminescence detection (CLIA; IBL-Hamburg, Hamburg, Germany), at the Technical University of Dresden, Germany. This assay had a detection limit of 0.16 ng/ml and intra-and inter-assay coefficients of variation of <10 and <12% respectively.
2.4. Immune measures
For assessment of circulating cytokines, blood samples (10 ml) were drawn using a 21-gauge butterfly needle into Vacutainer tubes containing EDTA as an anti-coagulant, then centrifuged immediately at 1250 × g for 10 min at room temperature. The plasma layer was removed, aliquoted and stored at −80 °C until analyses. Plasma IL-6 levels were measured using a high-sensitivity two-site enzyme-linked immunosorbent assay (ELISA) from R & D Systems (Oxford, UK). This assay had a detection limit of 0.09 pg/ml with intra- and inter-assay coefficients of variation (CVs) of 5.3 and 9.2% respectively. Plasma IL-1Ra concentrations were measured using a commercial ELISA from R & D Systems, with a detection limit of 15 pg/ml and intra-and inter-assay CVs of <10%. Plasma leptin levels were assessed using a commercial ELISA from Ray Biotech at Insight Biotechnology Ltd. (Middlesex, UK). This assay had a detection limit of 6 pg/ml and intra-and interassay CVs of <10% and <12% respectively. Plasma samples were diluted 1:200 prior to leptin analyses.
2.5. Behavioural tasks
Mental stress was induced by two 5-min behavioural tasks, administered under time pressure. The first was a computerized Stroop task, involving the successive presentation of target color words (e.g. red) printed in a different color (e.g. green). The task was to press a computer key that corresponded to the position at the bottom of the screen of the name of the color in which the target word was printed. The second task was presented after an interval of 5 min and involved simulated public speaking. Participants were presented with a hypothetical scenario in which they had been wrongly accused of shoplifting, and were instructed to give a speech in their defense by addressing the camera directly in front of them. They were told that their speech would be video recorded and later judged for efficacy and fluency. The experimenter remained in the room with the participant during each of the tasks, and instructed them when to start and stop.
2.6. Laboratory procedure
All sessions were run in the afternoon in a light and temperature-controlled laboratory, and participants were tested individually. They were provided with water but were not allowed to eat during testing. Anthropometric measures were obtained and the impedance cardiogram was fitted for continuous assessment of heart rate, HRV and cardiac PEP. Participants were then seated comfortably and fitted with finger cuffs so that blood pressure (BP) could be monitored using the Portapres-2. A venous cannula was inserted into the lower arm for blood sampling, and they were left to rest for 30 min. Cardiovascular measures (BP, heart rate, HRV and cardiac PEP) were recorded for the last 5 min of the baseline rest period, then a saliva sample was obtained and a baseline blood sample was drawn. At this time, participants were asked to rate their subjective feelings of stress on a 7-point Likert scale ranging from 1 = low to 7 = high. Next, the two tasks were administered in a fixed order. Five-minute recordings of cardiovascular activity were made during each of the tasks and cortisol samples and subjective stress ratings were obtained after each task. Following the tasks, a second blood sample was taken and participants rested for a further 45 min. Additional recordings of cardiovascular activity were obtained at 10–15, 25–30 and 40–45 min post-tasks, and a third blood sample was drawn at 45 min. Further measures of subjective stress and cortisol were obtained at 15, 30 and 45 min post-task.
2.7. Statistical analyses
First, we tested whether the tasks used in our study were indeed stressful, by assessing participants’ subjective and physiological responses to these tasks using repeated measures analyses of variance (ANOVA). The repeated measures analyses of subjective stress and salivary cortisol involved six trials (baseline, post-Stroop, post-speech, 15, 30 and 45 min post-tasks). Similarly, analyses of heart rate, HRV, cardiac PEP and blood pressure involved six trials (baseline, Stroop, speech, 10–15, 25–30 and 40–45 min post-tasks), while the analyses of plasma cytokines involved three trials (baseline, immediately post-tasks and 45 min post-task). The Greenhouse-Geisser correction of degrees of freedom was applied when sphericity assumptions were violated. The distribution of plasma leptin was skewed so data were square root transformed before analyses. Heart rate variability (RMSSD) data were also skewed and were log transformed, however raw values are presented for comparability with other studies. Post hoc tests were conducted using Tukey's least significant difference (LSD) test.
We then investigated the association between adiposity measures and physiological stress responses using a multiple linear regression approach. Three aspects of physiological response were analyzed: baseline levels, stress reactivity (computed as the change in levels between baseline and stress) and stress recovery (computed as the change between baseline and 45 min post-task). Age, smoking status and ethnicity were included in all models as covariates. In regressions involving reactivity and recovery measures, baseline levels of the relevant dependent measure (HR, HRV, etc.) were included as additional covariates in the model. The same regression approach was used to assess the relationship between basal plasma leptin levels and physiological stress responses. This time adiposity measures (BMI, waist circumference and percentage body fat) were also included as covariates, since adiposity is a major determinant of leptin levels and is associated with stress reactivity. Results are presented as standardised regression coefficients (β) with standard errors (s.e.). Associations between adiposity (or leptin) measures and reactivity are illustrated by displaying the mean cardiovascular and inflammatory responses of individuals in the lower and higher tertiles of waist circumference or plasma leptin, adjusted for covariates.
3. Results
3.1. Participant characteristics
Sample characteristics at baseline are presented in Table 1. Participants were relatively young with a mean age of 21. The majority were White non-smokers. All were normotensive and had glycated haemoglobin (HbA1c) levels in the normal range. Although they were not overweight on average, there were large individual differences in adiposity measures; BMI ranged from 18.4 to 34.0 kg m−2, waist circumference ranged from 57.0 to 95.0 cm and percentage body fat ranged from 10.1 to 40.5%. Participants also varied widely in basal plasma leptin levels, ranging from 5.7 to 105.5 ng/ml, with a mean concentration of 35.7 ng/ml, s.d. 22.0. These levels are in the expected physiological range for non-fasting women (Rosenbaum et al., 1996).
Table 1.
Participant characteristics (n = 67).
| Mean | s.d. | Range | |
|---|---|---|---|
| Age | 21.3 | 2.1 | 18–25 |
| Smoker (%) | 17.9 | ||
| Ethnicity (% white) | 66.7 | ||
| Weight (kg) | 62.0 | 10.3 | 47.3–93.8 |
| Waist (cm) | 70.3 | 7.9 | 57.0–95.0 |
| BMI (kg/m2) | 23.2 | 3.1 | 18.4–34.0 |
| Body fat (%) | 25.7 | 5.4 | 10.1–40.5 |
| Subjective stress rating | 2.0 | 1.1 | 1.0–6.0 |
| Salivary cortisol (nmol/l) | 5.5 | 2.5 | 1.8–13.7 |
| Systolic BP (mmHg) | 111.5 | 10.1 | 90.0–132.0 |
| Diastolic BP (mmHg) | 65.0 | 8.8 | 41.3–88.0 |
| Heart rate (bpm) | 72.2 | 8.7 | 51.8–91.7 |
| HRV (ms) | 56.0 | 29.1 | 19.4–174.2 |
| Cardiac PEP (ms) | 123.2 | 8.8 | 106.8–148.0 |
| Plasma leptin (ng/ml) | 35.7 | 22.0 | 5.7–105.5 |
| Plasma IL-6 (pg/ml) | 0.71 | 0.46 | 0.27–1.72 |
| Plasma IL-1Ra (pg/ml) | 176.9 | 73.0 | 98.7–480.1 |
| HbA1c | 4.77 | 0.27 | 3.9–5.2 |
Abbreviations: BP, blood pressure; HbA1c, glycated haemoglobin; HRV, heart rate variability; PEP, pre-ejection period; IL-6, interleukin-6; IL-1Ra, interleukin-1 receptor antagonist.
3.2. Subjective, cardiovascular and neuroendocrine responses to stress
Participants rated tasks as stressful with a mean score of 4.64 ± 1.1 and 4.42 ± 1.4 during the Stroop and speech task respectively. Subjective stress ratings returned to baseline levels during recovery, falling below baseline at 45 min post-task (F (3, 196) = 174.4, p < 0.001). Participants’ blood pressure and heart rate increased significantly during the tasks, with mean increases of 13.3 mmHg (F (3, 167) = 38.2, p < 0.001) and 10.3 mmHg (F (4, 221) = 44.5, p < 0.001) in systolic BP and diastolic BP respectively, and an average rise of 11.5 bpm in heart rate (F (2, 149) = 116.3, p < 0.001). At the same time, tasks induced a substantial decrease in participants’ HRV and cardiac PEP; HRV decreased by 18.6 ms on average during tasks (F (2, 149) = 42.4, p < 0.001), and cardiac PEP decreased by 6.0 ms on average (F (2, 131) = 44.7, p < 0.001). There were large individual differences in all cardiovascular responses. For example, changes in heart rate ranged from −1.8 bpm to +30.3 bpm, changes in HRV ranged from −99.4 ms to +4.8 ms, and changes in cardiac PEP ranged from −28.0 ms to +13.0 ms, during tasks. In addition, there was a small but significant (14.5%) rise in salivary cortisol in response to tasks, returning to baseline levels during the rest period (F (2, 122) = 9.96, p < 0.001).
3.3. Cytokine stress responses
Tasks induced a small but significant increase in participants’ plasma levels of IL-6 (F (1, 78) = 21.1, p < 0.001) and leptin (F (2, 105) = 26.3, p < 0.001), with maximum levels detected at 45 min post-tasks. Plasma levels of IL-6 increased by 37% on average at 45 min, with responses ranging from −0.45 pg/ml to +1.72 pg/ml. Similarly, plasma leptin levels increased by 14% on average at 45 min, with responses ranging from −6.7 ng/ml to +20.2 ng/ml. IL-1Ra levels were not altered by stress in the sample as a whole. However, there were large individual differences in this response, with changes in plasma IL-1Ra ranging from −148.1 pg/ml to +124.2 pg/ml at 45 min post-task. Plasma concentrations of IL-1Ra and leptin were positively correlated immediately post-stress (r = 0.30, p = 0.023) and at 45 min post-stress (r = 0.32, p = 0.020).
3.4. Adiposity and cardiovascular, neuroendocrine and immune measures
There was a significant relationship between adiposity and diastolic BP. Baseline diastolic BP was associated with BMI, waist circumference and percentage body fat (β = 0.29–0.37, s.e. = 0.13–0.14, all p < 0.05), independent of age, ethnicity and smoking status. Similarly, stress-induced increases in diastolic BP at 45 min were related to percentage body fat (β = 0.33, s.e. = 0.13, p = 0.014) and waist (β = 0.26, s.e. = 0.14, p = 0.059 ns trend), independent of age, ethnicity, smoking and baseline diastolic BP. There were no associations between adiposity and any other cardiovascular or neuroendocrine measures.
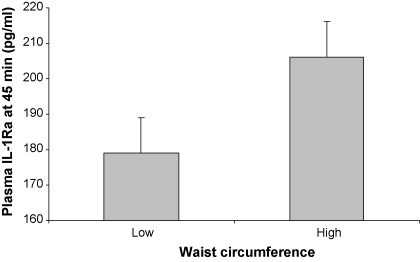
Basal plasma cytokine levels were related to adiposity, independent of age, ethnicity and smoking. Baseline leptin was associated with BMI, waist and percentage body fat (β = 0.49–0.62, s.e. = 0.11–0.13, all p < 0.001). Similarly, IL-6 levels were related to BMI and percentage body fat (β = 0.28–0.29, s.e. = 0.13–0.14, p < 0.05), and basal plasma IL-1Ra was associated with waist (β = 0.41, s.e. = 0.13, p = 0.003). There was also a significant relationship between central adiposity and cytokine stress responses. Waist circumference was associated with individual differences in stress-induced increases in plasma leptin immediately post-task (β = 0.34, s.e. = 0.15, p = 0.022) and stress-induced increases in plasma IL-1Ra at 45 min post-task (β = 0.32, s.e. = 0.15, p = 0.040), independent of age, ethnicity, smoking and baseline levels of the respective cytokine. The relationship between waist and IL-1Ra responses is illustrated in Fig. 1, showing mean plasma IL-1Ra concentrations in individuals in the lowest and highest tertiles of waist circumference. People with greater central adiposity had 15% higher IL-1Ra levels at 45 min post-task.
Fig. 1.
Mean plasma concentrations of interleukin-1 receptor antagonist (IL-1Ra) at 45 min post-tasks, in relation to waist circumference. Waist circumference was divided into tertiles and data are presented for individuals with low (<67 cm) and high (>70 cm) waist measures. Values are adjusted for age, ethnicity, smoking status and baseline plasma IL-1Ra. Error bars are SEM.
3.5. Basal leptin and cardiovascular, neuroendocrine and immune measures
Plasma levels of leptin at baseline were significantly related to participants’ cardiovascular and inflammatory stress responses, independent of age, smoking, adiposity and baseline measures of the respective dependent variable. Specifically, basal leptin was associated with stress-induced increases in heart rate (β = 0.53, s.e. = 0.18, p = 0.006) and with decreases in HRV (β = −0.44, s.e. = 0.18, p = 0.015) and cardiac PEP (β = −0.51, s.e. = 0.17, p = 0.004) during tasks, independent of covariates. There was also a positive association between basal leptin levels and stress-induced increases in IL-6 at 45 min (β = 0.35, s.e. = 0.17, p = 0.042). These effects are illustrated in Fig. 2 (A–D). Women in the highest versus lowest tertile of basal leptin had a 2.2-fold greater IL-6 response to stress at 45 min. Similarly, during tasks, women in the highest leptin tertile had 187% greater increases in heart rate, 86% greater reductions in HRV and >11-fold larger decreases in cardiac PEP. All associations remained significant when controlling for ethnicity (p < 0.05). There was no relationship between basal leptin and measures of diastolic or systolic BP, salivary cortisol or plasma IL-1Ra.
Fig. 2.
Basal circulating leptin and cardiovascular and inflammatory stress responses. Mean stress-induced changes in (A) plasma interleukin-6 (IL-6), (B) heart rate, (C) heart rate variability and (D) cardiac PEP in relation to baseline plasma leptin levels. Leptin levels were skewed and were square root transformed prior to analyses. Baseline plasma leptin was divided into tertiles and data are presented for individuals with low (<4.8 ng/ml) and high (>6.4 ng/ml) transformed leptin levels. Values are adjusted for age, smoking status, BMI, waist circumference, % body fat, and baseline levels of the appropriate dependent variable. Error bars are SEM. RMSSD, root mean square of successive R–R interval differences; PEP, pre-ejection period.
4. Discussion
This study examined the relationship between adiposity, circulating leptin and physiological responses to acute laboratory stress in women. Contrary to our predictions, we found no evidence of an association between adiposity measures and either cardiovascular or neuroendocrine reactivity to stress. However, women with greater total adiposity had impaired post-stress recovery in diastolic BP. In addition, there was a positive relationship between central adiposity and individual differences in cytokine stress responses, such that women with a larger waist circumference had greater stress-induced increases in plasma levels of IL-1Ra and leptin. In line with our hypotheses there was a significant association between participants’ basal plasma leptin levels and cardiovascular reactivity to stress, such that women with higher leptin levels displayed greater increases in heart rate and greater decreases in heart rate variability and cardiac PEP during tasks. Interestingly, women with elevated basal leptin also displayed larger stress-induced increases in the inflammatory cytokine IL-6.
The lack of association between adiposity and cardiovascular reactivity is somewhat surprising. However, not all previous studies have found a positive relationship between adiposity and cardiovascular stress reactivity, and the majority of those that did were either carried out in small samples of fewer than 25 participants or in samples that were not representative of the general population and did not adjust for confounding variables (Davis et al., 1999; Barnes et al., 1998; Waldstein et al., 1999; Goldbacher et al., 2005). Similar to our observations, a more recent analysis of 225 middle-aged British civil servants found no association between total or central adiposity and cardiovascular stress reactivity, but found a positive relationship between these measures and impaired post-stress recovery of diastolic blood pressure, independent of traditional risk factors (Steptoe and Wardle, 2005). Furthermore, the largest study to date carried out in 1647 adults from the West of Scotland Twenty-07 study, found a negative association between adiposity and cardiovascular reactivity such that those with greater BMI and waist–hip ratio as well as those categorised as obese displayed smaller heart rate reactions to stress (Carroll et al., 2008). The lack of association between adiposity and cortisol reactivity in our study is difficult to interpret, since the tasks used were only moderately stressful and cortisol responses were very small. More socially evaluative tasks eliciting more robust changes in cortisol may be required to adequately assess this relationship.
The positive association between central adiposity and cytokine stress responses is interesting and adds to the existing literature relating obesity and stress reactivity in humans. White adipose tissue (WAT), in particular visceral WAT, is a major source of leptin and IL-1Ra and the expression of these cytokines is markedly increased in WAT of obese humans and animals (Juge-Aubry et al., 2003; Considine et al., 1996). Accordingly, serum levels of leptin and IL-1Ra are elevated approximately 4-fold and 7-fold respectively in obese individuals, where they are positively correlated with BMI and % body fat (Considine et al., 1996; Meier et al., 2002). Unlike other adipose tissue depots, visceral WAT has a rich blood and nerve supply and a high abundance of glucocorticoid and beta-adrenergic receptors (Black, 2006). Glucocorticoids stimulate leptin gene expression in human and mouse adipocytes in vitro and increase circulating leptin levels in humans (Trayhurn and Bing, 2006; Masuzaki et al., 1997). Conversely β-adrenergic agonists inhibit leptin gene expression in adipocytes and lower circulating leptin levels in vivo (Trayhurn and Bing, 2006) whereas they increase plasma IL-1Ra levels in humans (Sondergaard et al., 2000). The higher expression of cytokines in visceral WAT coupled with a greater density of stress hormone receptors, may explain why we found relationships between cytokine responses and central but not total adiposity (Black, 2006). Together with the existing evidence, our results suggest that adipose tissue may be an important source of stress-responsive cytokines.
Notably, adipose tissue is heterogeneous and contains a stromal vascular cell population including monocytes, as well as adipocytes (Weisberg et al., 2003). Leptin receptors are expressed on monocytes, and leptin stimulates monocyte production of other cytokines including IL-1Ra and IL-6 by binding to these receptors (La Cava and Matarese, 2004; Gabay et al., 2001). It is thus conceivable that basal or stress-induced increases in leptin may drive IL-1Ra and IL-6 responses to stress. This may explain why we found an association between waist circumference and leptin immediately post-stress whereas waist was associated with IL-1Ra levels at 45 min. Supporting a relationship between these two cytokines, plasma concentrations of leptin and IL-1Ra were positively correlated both immediately post-stress and at 45 min. Although adiposity was not related to IL-6 stress responses, women with higher basal leptin levels displayed larger increases in IL-6 following tasks, indicating that elevated leptin levels may drive this inflammatory response.
The observed relationship between basal plasma leptin levels and cardiovascular reactivity to stress is consistent with previous reports in humans and animals supporting a sympatho-activating effect of leptin (Ren, 2004; da Silva et al., 2006; Thomopoulos et al., 2009; Bravo et al., 2006; Flanagan et al., 2007; Ma et al., 2009). Although human leptin infusion studies have generated inconsistent results, our findings suggest that leptin may potentiate SNS activity during stress. The effects of leptin on SNS activity are thought to be driven mainly by a central mechanism involving the long form of the leptin receptor (OB-Rb) and melanocortin-3/4 receptors (MC3/4 R) in the hypothalamic arcuate nucleus (ARC). In rodents, microinjection of leptin into the ARC increases arterial pressure and sympathetic outflow to BAT and kidneys, whereas lesions to the ARC blunt the thermogenic SNA response to leptin (Rahmouni and Morgan, 2007). Similarly, central pharmacological blockade or genetic ablation of MC3/4R in rodents abolishes the hypertensive effects of chronic leptin infusion (da Silva et al., 2009). In addition, leptin may promote sympathetic activity via direct, non-neural pathways. Leptin receptors have been detected in the heart and endothelium of blood vessels (Bjorbaek and Kahn, 2004), and a strong independent association between circulating leptin and heart rate was observed in heart transplant patients with sympathetic denervation, supporting a direct effect of leptin on heart rate conceivably through cardiac leptin receptors (Winnicki et al., 2001). The sympatho-activating effects of leptin may be more apparent in women. Women are generally more ‘adipose’ than men, with greater total body fat and significantly higher leptin levels both pre- and post-menopause (Rosenbaum et al., 1996). Furthermore, two recent studies found a significant correlation between circulating leptin levels, sympathetic autonomic activity and hypertension in women but not in men (Ma et al., 2009; Flanagan et al., 2007), and women display greater cardiovascular reactivity to certain types of acute laboratory stress (Schmaus et al., 2008).
Heightened or prolonged sympathetic reactivity to acute stress has been shown to predict future hypertension in initially normotensive samples as well as an increased risk of cardiac events in patients with documented cardiovascular disease (Treiber et al., 2003; Flaa et al., 2008). Although few studies have examined the prospective relationship between acute cytokine responses and cardiovascular risk, we previously showed that IL-6 responses to laboratory stress were predictive of 3-year elevations in BP in men and women from the Whitehall II cohort (Brydon and Steptoe, 2005). IL-6 and leptin have a number of pro-atherogenic effects including upregulation of endothelial cell adhesion molecules and promoting smooth muscle cell proliferation and platelet aggregation, and elevated circulating levels of these cytokines are associated with a heightened risk of CVD (Gualillo et al., 2007). Similarly IL-1Ra has a number of metabolic effects that promote obesity and insulin resistance (Meier et al., 2002; Juge-Aubry et al., 2003). Therefore acute cardiovascular and cytokine stress responses may become clinically relevant if repeated on a long-term basis.
This investigation was carried out in a sample of young, predominately white females and results may not generalise to other populations. Cytokines were measured in non-fasting plasma samples, and there is some evidence that ingestion of food with a high carbohydrate or fat content can alter circulating leptin levels in animals (Houseknecht and Spurlock, 2003). However, most human studies show only a long-term dietary effect of chronic overfeeding on leptin (Houseknecht and Spurlock, 2003). A significant proportion (17.9%) of our participants smoked and smoking is known to affect cardiovascular function and cytokine levels. However, results remained significant when controlling for this factor. Heart rate and HRV are regulated by both parasympathetic vagal withdrawal as well as increases in sympathetic nerve activity, and it would have been useful to include more direct measures of sympathetic activity such as catecholamines. Nevertheless, cardiac PEP is a strong indicator of sympathetic nerve activation (Sherwood et al., 1990). We also did not control for menstrual cycle. However differences in physiological stress reactivity across the menstrual cycle have been inconsistent (Weidner and Helmig, 1990). The multiple statistical analyses performed increases the likelihood of type-1 errors in our results. Lastly, the cross-sectional nature of the study prevents conclusions about the causal direction of associations between adiposity, leptin and stress-reactivity. Since leptin and IL-1Ra play an important role in weight regulation and adipose tissue physiology, stress-induced increases in these cytokines may also impact obesity levels (Trayhurn and Bing, 2006; Meier et al., 2002).
Nevertheless, our findings throw some light on the potential mechanisms linking obesity, stress and cardiovascular risk in women. Since visceral WAT is a major source of cytokines, central adiposity may increase cardiovascular risk by facilitating a pro-atherogenic response to stress. Similarly, by driving sympathetic and inflammatory stress responses, leptin may promote the development of future cardiovascular disease. Further experiments are required to confirm our observations in a larger sample including equal numbers of both genders and to test whether there is a prospective association between adiposity or leptin, stress reactivity and cardiovascular risk in humans.
Acknowledgments
This research was funded by the British Heart Foundation, Cancer Research UK and the Medical Research Council, England, UK. I am grateful to Professors Andrew Steptoe and Jane Wardle for their contributions to the study design, and Dr. Katie 0’Donnell, Dr. Caroline E. Wright, Dr. Andrew Wawrzyniak and Bev Murray for their assistance with data collection.
References
- Barnes V.A., Treiber F.A., Davis H., Kelley T.R., Strong W.B. Central adiposity and hemodynamic functioning at rest and during stress in adolescents. International Journal of Obesity. 1998;22:1079–1083. doi: 10.1038/sj.ijo.0800730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Arck P.C., Tan S., Mann K., Hahn S., Janssen O.E., Schedlowski M., Elsenbruch S. Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 2009;34:181–189. doi: 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C., Kahn B.B. Leptin signaling in the central nervous system and the periphery. Recent Progress in Hormone Research. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- Black P.H. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Medical Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bravo P.E., Morse S., Borne D.M., Aguilar E.A., Reisin E. Leptin and hypertension in obesity. Vascular Health Risk Management. 2006;2:163–169. doi: 10.2147/vhrm.2006.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R.D., Bard R.L., Bodary P.F., Eitzman D.T., Rajagopalan S., Sun Y., Depaoli A.M. Blood pressure and vascular effects of leptin in humans. Metabolic Syndrome and Related Disorders. 2007;5:270–274. doi: 10.1089/met.2006.0023. [DOI] [PubMed] [Google Scholar]
- Brydon L., Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. Journal of Hypertension. 2005;23:1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- Carroll D., Phillips A.C., Der G. Body mass index, abdominal adiposity, obesity, and cardiovascular reactions to psychological stress in a large community sample. Psychosomatic Medicine. 2008;70:653–660. doi: 10.1097/PSY.0b013e31817b9382. [DOI] [PubMed] [Google Scholar]
- Chan J.L., Mietus J.E., Raciti P.M., Goldberger A.L., Mantzoros C.S. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clinical Endocrinology. 2007;66:49–57. doi: 10.1111/j.1365-2265.2006.02684.x. [DOI] [PubMed] [Google Scholar]
- Considine R.V., Sinha M.K., Heiman M.L., Kriauciunas A., Stephens T.W., Nyce M.R., Ohannesian J.P., Marco C.C., McKee L.J., Bauer T.L., Caro J.F. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- da Silva A.A., do Carmo J.M., Freeman J.N., Tallam L.S., Hall J.E. A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes. 2009;58:1749–1756. doi: 10.2337/db08-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A.A., Tallam L.S., Liu J., Hall J.E. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2006;291:R1275–R1282. doi: 10.1152/ajpregu.00187.2006. [DOI] [PubMed] [Google Scholar]
- Davis M.C., Twamley E.W., Hamilton N.A., Swan P.D. Body fat distribution and hemodynamic stress responses in premenopausal obese women: a preliminary study. Health Psychology. 1999;18:625–633. doi: 10.1037//0278-6133.18.6.625. [DOI] [PubMed] [Google Scholar]
- Epel E.S., McEwen B., Seeman T., Matthews K., Castellazzo G., Brownell K.D., Bell J., Ickovics J.R. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Flaa A., Eide I.K., Kjeldsen S.E., Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18-year follow-up study. Hypertension. 2008;52:336–341. doi: 10.1161/HYPERTENSIONAHA.108.111625. [DOI] [PubMed] [Google Scholar]
- Flanagan D.E., Vaile J.C., Petley G.W., Phillips D.I., Godsland I.F., Owens P., Moore V.M., Cockington R.A., Robinson J.S. Gender differences in the relationship between leptin, insulin resistance and the autonomic nervous system. Regulatory Peptides. 2007;140:37–42. doi: 10.1016/j.regpep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Gabay C., Dreyer M., Pellegrinelli N., Chicheportiche R., Meier C.A. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. Journal of Clinical Endocrinology and Metabolsim. 2001;86:783–791. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- Galletti F., D’Elia L., Barba G., Siani A., Cappuccio F.P., Farinaro E., Iacone R., Russo O., De Palma D., Ippolito R., Strazullo P. High-circulating leptin levels are associated with greater risk of hypertension in men independently of body mass and insulin resistance: results of an eight-year follow-up study. Journal of Clinical Endocrinology and Metabolism. 2008;93:3922–3926. doi: 10.1210/jc.2008-1280. [DOI] [PubMed] [Google Scholar]
- Goldbacher E.M., Matthews K.A., Salomon K. Central adiposity is associated with cardiovascular reactivity to stress in adolescents. Health Psychology. 2005;24:375–384. doi: 10.1037/0278-6133.24.4.375. [DOI] [PubMed] [Google Scholar]
- Gualillo O., Gonzalez-Juanatey J.R., Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends in Cardiovascular Medicine. 2007;17:275–283. doi: 10.1016/j.tcm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Houseknecht K.L., Spurlock M.E. Leptin regulation of lipid homeostasis: dietary and metabolic implications. Nutrition Research Reviews. 2003;16:83–96. doi: 10.1079/NRR200256. [DOI] [PubMed] [Google Scholar]
- Hukshorn C.J., Menheere P.P., Westerterp-Plantenga M.S., Saris W.H. The effect of pegylated human recombinant leptin (PEG-OB) on neuroendocrine adaptations to semi-starvation in overweight men. European Journal of Endocrinology. 2003;148:649–655. doi: 10.1530/eje.0.1480649. [DOI] [PubMed] [Google Scholar]
- Juge-Aubry C.E., Somm E., Giusti V., Pernin A., Chicheportiche R., Verdumo C., Rohner-Jeanrenaud F., Burger D., Dayer J.-M., Meier C.A. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes. 2003;52:1104–1110. doi: 10.2337/diabetes.52.5.1104. [DOI] [PubMed] [Google Scholar]
- La Cava A., Matarese G. The weight of leptin in immunity. Nature Reviews Immunology. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Lavie C.J., Milani R.V., Ventura H.O. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. Journal of the American College of Cardiology. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Ma D, Feitosa M.F., Wilk J.B., Laramie J.M., Yu K., Leiendecker-Foster C., Myers R.H., Province M.A., Borecki I.B. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. 2009;53:473–479. doi: 10.1161/HYPERTENSIONAHA.108.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh R.M., Hirsch J. The effects of leptin administration in non-obese human subjects. Obesity. 2001;9:462–469. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- Malik M. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Masuzaki H., Ogawa Y., Hosoda K., Miyawaki T., Hanaoka I., Hiraoka J., Yasuno A., Nishimura H., Yoshimasa Y., Nishi S., Nakao K. Glucocorticoid regulation of leptin synthesis and secretion in humans: elevated plasma leptin levels in Cushing's syndrome. Journal of Clinical Endocrinology and Metabolism. 1997;82:2542–2547. doi: 10.1210/jcem.82.8.4128. [DOI] [PubMed] [Google Scholar]
- Meier C.A., Bobbioni E., Gabay C., Assimacopoulos-Jeannet F., Golay A., Dayer J.M. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? Journal of Clinical Endocrinology and Metabolism. 2002;87:1184–1188. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- Pasquali R., Ambrosi B., Armanini D., Cavagnini F., Uberti E.D., Del Rio G., De Pergola G., Maccario M., Mantero F., Marugo M., Rotella C.M., Vettor R. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose–response study. Journal of Clinical Endocrinology and Metabolism. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan D.A. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- Ren J. Leptin and hyperleptinemia—from friend to foe for cardiovascular function. Journal of Endocrinology. 2004;181:1–10. doi: 10.1677/joe.0.1810001. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Nicolson M., Hirsch J., Heymsfield S.B., Gallagher D., Chu F., Leibel R.L. Effects of gender, body composition, and menopause on plasma concentrations of leptin. Journal of Clinical Endocrinology and Metabolism. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- Schmaus B.J, Laubmeier K.K., Boquiren V.M., Herzer M., Zakowski S.G. Gender and stress: differential psychophysiological reactivity to stress reexposure in the laboratory. International Journal of Psychophysiology. 2008;69:101–106. doi: 10.1016/j.ijpsycho.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Sherwood A., Allen M.T., Fahrenberg J., Kelsey R.M., Lovallo W.R., Van Doornen L.J. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sondergaard S.R., Ostrowski K., Ullum H., Pedersen B.K. Changes in plasma concentrations of interleukin-6 and interleukin-1 receptor antagonists in response to adrenaline infusion in humans. European Journal of Applied Physiology. 2000;83:95–98. doi: 10.1007/s004210000257. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Wardle J. Cardiovascular stress responsivity, body mass and abdominal adiposity. International Journal of Obesity. 2005;29:1329–1337. doi: 10.1038/sj.ijo.0803011. [DOI] [PubMed] [Google Scholar]
- Thomopoulos C., Papadopoulos D.P., Papazachou O., Bratsas A., Massias S., Anastasiadis G., Perrea D., Makris T. Free leptin is associated with masked hypertension in nonobese subjects: a cross-sectional study. Hypertension. 2009;53:965–972. doi: 10.1161/HYPERTENSIONAHA.108.128041. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiologica. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Bing C. Appetite and energy balance signals from adipocytes. Philosophical Transactions of the Royal Society Biological Sciences. 2006;361:1237–1249. doi: 10.1098/rstb.2006.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber F.A., Kamarck T., Schneiderman N., Sheffield D., Kapuku G., Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Waldstein S.R., Burns H.O., Toth M.J., Poehlman E.T. Cardiovascular reactivity and central adiposity in older African Americans. Health Psychology. 1999;18:221–228. doi: 10.1037//0278-6133.18.3.221. [DOI] [PubMed] [Google Scholar]
- Weidner G., Helmig L. Cardiovascular stress reactivity and mood during the menstrual cycle. Women Health. 1990;16:5–21. doi: 10.1300/J013v16n03_02. [DOI] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen G.H., De Geus E.J., Klaver C.H., Van Doornen L.J., Carroll D. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 1996;33:184–193. doi: 10.1111/j.1469-8986.1996.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Winnicki M., Phillips B.G., Accurso V., van De B.P., Shamsuzzaman A., Patil K., Narkiewicz K., Somers V.K. Independent association between plasma leptin levels and heart rate in heart transplant recipients. Circulation. 2001;104:384–386. doi: 10.1161/hc2901.094150. [DOI] [PubMed] [Google Scholar]
- World Health Organization Global InfoBase Online, September 2006. Fact sheet No. 311. http://www.who.int/mediacentre/factsheets/fs311/en.