Abstract
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disorder. The study of diverse mouse models of lupus has provided clues to the etiology of SLE. Spontaneous mouse models of lupus have led to identification of numerous susceptibility loci from which several candidate genes have emerged. Meanwhile, induced models of lupus have provided insight into the role of environmental factors in lupus pathogenesis as well as provided a better understanding of cellular mechanisms involved in the onset and progression of disease. The SLE-like phenotypes present in these models have also served to screen numerous potential SLE therapies. Due to the complex nature of SLE, it is necessary to understand the effect specific targeted therapies have on immune homeostasis. Furthermore, knowledge gained from mouse models will provide novel therapy targets for the treatment of SLE.
1. Introduction
The etiology of the autoimmune disease systemic lupus erythematosus (SLE) is considered to be a combination of multiple genetic and environmental factors whose amalgamation breaches the threshold of immune tolerance. SLE is, therefore, a complex multigenic disorder. The causative genetic factors of such disorders are believed to be allelic variants with minor functional contributions to the disease state and, individually, are not sufficient to cause overt disease. However, the combination of several of these susceptibility loci can lead to epistatic interactions that greatly enhance the overall contribution by each locus, whereby protective mechanisms are overcome and pathogenesis ensues. Hence, the capacity to tailor SLE treatments could be greatly enhanced upon the identification of the genetic variants capable of inducing autoimmunity and how their involvement can magnify SLE. Indeed, concerted efforts in human studies and mouse models have been made in that direction. The recent annotation of millions of single nucleotide polymorphisms (SNPs) in the human genome and the inception of high-throughput genotyping technologies has enabled the use of genome-wide association studies (GWAS) for this purpose. To date, SLE susceptibility has been associated to dozens of genes by GWAS [1]. While these associations offer important new insights into SLE etiology, there are logistical limitations to GWAS. Extremely large sample sets are required to achieve significant associations and extrinsic factors such as ethnicity, clinical history, and lifestyle must be considered in choosing these sets. Further, GWAS are capable of identifying genomic associations but do not characterize the function that an allelic variation confers. For this, other methods are required.
There are numerous murine models that have long been employed in an effort to understand the cellular and genetic requirements for SLE induction. The classic models of spontaneous lupus include the F1 hybrid between the New Zealand Black (NZB) and New Zealand White (NZW) strains (NZB/W F1) and its derivatives, the MRL/lpr, and BXSB/Yaa strains whereas induced models include the pristane-induced model and the chronic graft-versus-host-disease models (cGVHD). All of these models portray their own iterations of lupus-like diseases with a subset of symptoms akin to those observed in human SLE, namely, autoantibody production, lymphoid activation and hyperplasia, and lupus nephritis. In addition to spontaneous and induced lupus models, there is a plethora of genetically modified mouse models that goes beyond the scope of this paper (reviewed in [2]). Undoubtedly, genetically modified models will be used in the characterization of genes identified by GWAS, and in fact, many of these models already exist [3]. Here, we focus on what has been learned from spontaneous and induced mouse models of SLE and how they can be used to complement the recent advances in human studies. We also highlight how murine models have been used as tools to test therapies.
2. Classic Mouse Models of Spontaneous Lupus
2.1. NZB/W F1
The NZB/W F1 is the oldest classical model of lupus generated by the F1 hybrid between the NZB and NZW strains. Both NZB and NZW display limited autoimmunity, while NZB/W F1 hybrids develop severe lupus-like phenotypes comparable to that of lupus patients [4]. These lupus-like phenotypes include lymphadenopathy, splenomegaly, elevated serum antinuclear autoantibodies (ANA) including anti-dsDNA IgG, a majority of which are IgG2a and IgG3, and immune complex-mediated glomerulonephritis (GN) that becomes apparent at 5-6 months of age, leading to kidney failure and death at 10–12 months of age [4]. Unlike SLE patients and the MRL/lpr and BXSB/Yaa mouse models, NZB/W F1 mice lack autoantibodies against RNA-containing complexes. As for SLE patients, disease in the NZB/W F1 strain is strongly biased in favor of females, and this is at least in part due to estrogen levels. Indeed ovariectomy of NZB/W F1 mice not only delayed disease onset but also decreased autoantibody titer. Meanwhile, restoration of estradiol in ovariectomized NZB/W F1 mice reestablished high numbers of DNA-specific B cells, and thereby suggests a pathogenic role of estrogen in lupus [5]. The many contributions of the NZB/W F1 model to our understanding of lupus pathogenesis have been discussed in excellent reviews [2, 4] to which the reader is referred to.
2.1.1. NZM
An accidental backcross between NZB/W F1 and NZW followed by brother-sister matings generated 27 different recombinant inbred strains of New Zealand Mixed (NZM) mice among which NZM2328 and NZM2410 are now used as lupus models [6–8]. While both NZM2410 and NZM2328 strains exhibit a highly penetrant version of the NZB/W F1 disease with an earlier onset, NZM2410 differs from NZM2328 with a weaker gender bias [7]. The NZM2328 and 2410 strains share the same autoantibody specificities as NZB/W F1 mice. The homozygosity of the NZM strains and their potential enrichment for lupus susceptibility loci has facilitated genetic analyses, and arguably their greatest contribution has been to the understanding of the genetic basis of this disease.
2.1.2. Identification and Classification of Susceptibility Loci
In 1994, Morel et al. reported the first lupus linkage analysis which identified three NZM2410 susceptibility loci, Sle1-3, as being significantly correlated with GN development [7]. The analysis of these loci showed that disease was inherited as a threshold liability, that is, the probability of presenting disease by an individual increases with the number of susceptibility alleles it carries, a concept that has been confirmed in genetic analyses of the other lupus-prone strains. The NZM2410 model was also used to show the existence of NZW-derived suppressive loci, the strongest one, Sles1, being linked to the major histocompatibility complex (MHC) class II locus H-2z [9]. These results showed that the disease outcome for a given genome represents the integration of susceptibility and resistance loci and that lupus susceptible strains carry genomes in which the susceptibility loci outweigh the resistance loci. Resistance (or transgressive) loci have subsequently been identified in other models, including lupus models (e.g., see below Lmb3 in the MRL/lpr strain).
The phenotypes contributed by each NZM2410 susceptibility locus have been determined via detailed analysis of the immunological properties of congenic strains carrying the individual loci on a C57BL/6 (B6) genetic background in comparison to B6 mice [10–16]. Briefly, Sle1 mediates the loss of tolerance to nuclear antigens leading to the production of autoreactive B and T cells culminating in the secretion of antinuclear autoantibodies. Sle2 leads to B cell hyperactivity, elevated B-1 cell levels, and polyreactive IgM antibodies. Sle3 leads to decreased levels of activation-induced cell death in CD4+ T cells, with evidence that a non-T cell compartment is involved in the expression of this phenotype [15]. Interestingly, Sle1 largely overlaps with NZM2328 Cgnz1 and Agnz1 on chromosome 1 and Sle2 with NZM2328 Adaz1 on chromosome 4 [17]. The NZM2328 loci were, however, evaluated in congenic strains in which the susceptibility intervals were replaced by nonautoimmune genomic segments, which makes impossible to directly compare the phenotypes of the respective susceptibility loci between the two strains.
As predicted by the threshold liability findings from the linkage analysis, none of the NZM2410-derived Sle loci lead to full disease presentation. Instead, the stepwise combination of each congenic interval showed that the coexpression of these three major loci is necessary and sufficient for the development of fully penetrant disease [18]. The NZM2410 strain is to date the only autoimmune model in which the subset of loci that are necessary and sufficient for disease presentation has been identified. This achievement infused added significance to the identification of the genes corresponding to those three loci. A major obstacle towards the identification of these susceptibility genes has been revealed by the studies of the Sle congenic strains, which revealed that the original quantitative trait loci (QTLs) correspond to a cluster of susceptibility loci. This finding has been best demonstrated for Sle1, which is reviewed in detail in [3], corresponds to at least seven independent loci.
2.1.3. Identification of NZM2410 Susceptibility Genes
Progress towards the identification of lupus-susceptibility genes is ongoing in several models (see below), but it is by far the most advanced in NZM2410 as reviewed in [3]. Most of the genetic variants identified in these susceptibility genes are still in the process of formal validation, and the process by which they contribute to lupus pathogenesis is still largely unknown. However, variations in many of these genes has been directly associated with human lupus, which is by itself a strong validation of the mouse findings. The NZM2140 allele of Fcgr2b, which encodes for a negative regulator of B-cell signaling, contributes to an autoimmune phenotype [19] and a large number of studies have established an association between FCGR2B variants and human SLE [20]. Consequently, the involvement of Fcgr2b in lupus pathogenesis has been validated in both mice and humans at the genetic and functional levels. Similarly, polymorphisms in Cr2, which encodes the complement receptor type 2 that acts as a B-cell coreceptor, contribute to the Sle1c1 phenotypes [21, 22]. A common CR2 haplotype is more frequent in SLE patients than in healthy controls, and novel CR2 splice variants are expressed on the follicular dendritic cells of SLE patients [23, 24]. Sle1b corresponds to polymorphisms in four signaling lymphocytic activation molecule (SLAM) family member genes [25], including Ly108, which was directly implicated in the regulation of B-cell tolerance [26]. Variants of SLAMF3 (LY9) and SLAMF4 (CD244) have been associated with human SLE [27, 28]. Finally for the Sle1 subloci, Sle1a1 corresponds to the expression of a novel splice isoform of the Pbx1 gene that is associated with increased CD4+ T cell activation in both mice and humans (Cuda et al., in revision). Pbx1 function in T cells is unknown, and studies are ongoing to uncover the mechanisms linking Pbx1 expression and T cell phenotypes. The Sle3 locus induces a complex phenotype that includes myeloid cell-induced CD4+ T-cell activation [29] and mild GN [12]. Polymorphisms in kallikrein (Klk) genes, which are located in the Sle3 interval, have been associated with increased susceptibility to nephritis in the mouse and in SLE patients [30].
2.2. MRL/lpr
2.2.1. Derivation and Phenotype of the MRL/lpr Strain
The MRL strain was developed from several crosses of inbred strains. Specifically, most of the MRL genome was derived from the LG/J strain with minor contributions from C3H/Di, C57BL/6, and AKR/J [4]. Initial characterization of MRL substrains revealed one strain, termed MRL/lpr, which developed an SLE-like phenotype characterized by lymphadenopathy due to an accumulation of double negative (CD4− CD8−) and B220+ T-cells. These mice displayed an accelerated mortality rate, and unlike the NZB/W F1 mice, both males and females were significantly affected. In addition, the MRL/lpr substrain had high concentrations of circulating immunoglobulins, which included elevated levels of autoantibodies such as ANA, anti-ssDNA, anti-dsDNA, anti-Sm, and rheumatoid factors, resulting in large amounts of immune complexes [31]. This accelerated phenotype was attributed to a recessive autosomal mutation termed lymphoproliferation (lpr). The lpr mutation, located on chromosome 19, alters transcription of the Fas receptor [32].
2.2.2. Effect of Fas Signaling Deficiency in Disease Development
Fas is a surface-bound receptor belonging to the tumor necrosis factor receptor (TNF-R) family that induces apoptosis upon interacting with its ligand, FasL [33]. The defects in Fas transcription are caused by an insertion of the retrotransposon ETn which leads to aberrant, nonfunctional transcripts due to alternative splicing of the Fas gene [34]. Studies on B-cells and T-cells from MRL/lpr mice, which both express Fas, confirmed a defect in apoptosis due to the lack of functional Fas receptor [35]. Furthermore, a mutation on the FasL gene, termed generalized lymphoproliferative disease (gld), was shown to cause an autoimmune disorder similar to the lpr mutation [36, 37]. This recessive mutation, located on chromosome 1, caused a single amino acid change (Leu > Phe) which prevented FasL from binding to its receptor. Therefore, a deficiency in Fas signaling results in an SLE-like phenotype due to defective Fas-mediated apoptosis. In humans, defective Fas signaling can lead to the development of autoimmune lymphoproliferative syndrome (ALPS) which shares many symptoms with SLE although GN is uncommon in ALPS patients [38].
The SLE symptoms attributed to Fas deficiency in MRL/lpr mice are dependent on both CD4+ T-cells and B-cells as their absence decreased autoimmunity [39, 40]. Further studies on Fas-deficient T-cells using the (C57BL/6 × MRL)F1 autoimmune strain resulted in lymphopenia as activated T-cells upregulated FasL, promoting the apoptosis of peripheral lymphocytes [41]. Meanwhile, in the presence of the lpr mutation, the nonautoimmune C3H/HeJ and C57BL/6J strains developed lymphoproliferation and had a reduced lifespan [42]. In addition, high levels of IgM and IgG antibodies, in particular autoantibodies against DNA and IgG, were detected in these strains. Conditional deletion of Fas in T-cells, B-cells, or both T-cells and B-cells in nonautoimmune C57BL/6 mice resulted in higher levels of autoantibodies but lymphoproliferation was not observed. To reconstitute the phenotype seen in lpr mice, Fas deletion had to be present in both lymphoid and nonlymphoid cells [41]. In particular, Fas deficiency in dendritic cells (DCs) led to systemic autoimmunity as it induced elevated ANA, splenomegaly, and accumulation of mature DCs [43].
2.2.3. Effect of the MRL Genetic Background in Disease Development
Expression of the lpr mutation in either the C3H/HeJ and C57BL/6J strains did not result in nephritis [44]. In contrast, Fas-sufficient, MRL+/+ mice displayed the pathological effects of nephritis though onset of symptoms was significantly delayed when compared to MRL/lpr mice. Therefore, the MRL background genes play an important role in the development of disease. Characterization of (MRL-Faslpr × C57BL/6-Faslpr)F2 mice revealed four susceptibility loci, Lmb1–Lmb4, that were linked to anti-dsDNA autoantibodies and glomerulonephritis [45]. In particular, studies on congenic mice showed that Lmb3, located on the telomeric region of chromosome 7, had a significant effect on lymphoproliferation and enhanced T-cell proliferation while T-cell apoptosis was reduced [46]. Recently, a single nonsense mutation (C > T) in the Coronin-1A gene, located within the Lmb3 susceptibility locus, was identified in the Scripp substrain of C57BL/6 and it resulted in suppression of disease when crossed to MRL/lpr [47]. Coronin-1A binds F-actin and prevents its assembly and depolymerization. Nonfunctional Coronin-1A, from the mutant Lmb3 locus, was associated with increased T-cell apoptosis and reduced T-cell activation and proliferation. Further characterization of the MRL background should reveal additional candidate genes that promote autoimmunity in MRL/lpr mice.
2.3. BXSB/Yaa
2.3.1. Derivation and Phenotype of the BXSB/Yaa Strain
Lymphoproliferation was observed in males of an (C57BL6/J × SB/Le)F1 intercross and its backcross to SB/Le. A recombinant inbred strain, BXSB/Mp, was derived from this backcross, and nearly 100% of BXSB mice develop a lupus-like disease that was much more severe with earlier onset in males [48, 49]. The disease phenotypes include secondary lymphoid tissue hyperplasia, immune complex-mediated GN, monocytosis, hypergammaglobulinemia, antinuclear and antierythrocytic autoantibodies, and high-serum retroviral glycoprotein gp70 titers [48, 50]. Mean survival is roughly 5 months in males and 14 months in females, and the major cause of death is proliferative GN [48, 50].
2.3.2. Effect of the Y Chromosome in Disease Development
The fact that disease is greatly accelerated in BXSB males suggested the presence of a susceptibility element on the Y chromosome or that sex hormones play a role in this model. Orchiectomy did not delay onset or severity in BXSB males, while ovariectomy did not exacerbate disease in females, ruling out a hormonal effect [51]. Moreover, consomic studies showed that the BXSB-derived Y chromosome exacerbated disease when combined with either NZW or MRL genetic backgrounds [52, 53]. Thus, an element, termed Y-linked autoimmune accelerator (Yaa), was deemed to be responsible for the acute disease in BXSB males.
It is now known that Yaa is due to a translocation of the telomeric end of the X chromosome to the Y chromosome, resulting in the duplication of at least 16 genes and a ~2-fold expression in many of these genes [54, 55]. Among these is Toll-like Receptor 7 (Tlr7), which encodes an innate pattern recognition receptor found in B cells and antigen presenting cells (APCs). TLR7 is activated by viral ssRNA and signals through the myeloid differentiation primary response gene 88 (MyD88) adaptor resulting in nuclear factor NF-κβ activation and expression of type I IFN (IFN I) and other proinflammatory cytokines [56]. Importantly, it has been shown that TLR signaling can act as signal 2 in B cell activation and that those interactions with endogenous RNA ligands are sufficient for autoreactive B cell activation [57, 58]. Additionally, disease activity is extremely sensitive to TLR7 expression. Where a 2-fold increase by the Yaa translocation only results in overt disease in the presences of other susceptibility alleles, greater increases in expression result in a Tlr7 dose-responsive disease that does not require other lupus susceptibility loci [59]. Moreover, deletion of the endogenous copy of Tlr7 from the X chromosome abrogates Yaa-induced monocytosis, lymphoid activation, splenomegaly, GN, and mortality [59–61]. Thus, Tlr7 gene duplication has been deemed to be the major functional contributor to the Yaa phenotype. Shen et al. have recently reported a human allele of the TLR7 gene with associated risk for SLE development in males [62]. This human susceptibility allele increased the expression of TLR7 and of IFN I-regulated genes in blood monocytes. In this way, TLR7 dose increases lupus susceptibility in both mice and humans.
It is also likely that there is a minor contribution from other genes in the translocated Yaa interval, since endogenous Tlr7 deletion does not eliminate all Yaa-induced phenotypes. Lupus prone Yaa+·Tlr7− mice still display some humoral abnormalities, increased autoantibody production, and a decreased marginal zone (MZ) B cell compartment as compared to lupus prone Yaa−·Tlr7+ mice [60, 61].
2.3.3. Effect of the BSXB Genetic Background in Disease Development
While the Yaa allele is certainly the most distinguishable feature of the BSXB model, it is not the whole story. In fact, the Yaa mutation alone is not sufficient to cause disease. This is clearly illustrated by the fact that nonlupus prone strains, such as B6 and CBA, that are consomic for the BSXB Y chromosome do not develop disease [52, 63]. The BXSB MHC (H-2b/b) locus plays a dominant role in disease activity since congenic BSXB.H-2d/d mice have a less severe syndrome, while BSXB.H-2b/d and BSXB.H-2b/b mice have the full disease [64]. However, as stated above, B6·Yaa (H-2b/b) mice have no malady. Therefore, there are non-MHC-linked autosomal elements in the BSXB genome that contribute to disease development.
Quantitative trait linkage analyses have identified BXSB susceptibility loci on chromosomes 1, 3, and 13 (Bxs1-6) [65, 66]. These loci are all derived from the parental SB/Le strain except for Bxs5 on chromosome 3, which is a transgressive allele derived from B10 [49]. Bxs1-4 are all located on chromosome 1 and studies using congenic mice confirmed that these loci confer lupus phenotypes [67]. Interestingly, Bsx3 overlaps with Sle1 and seems to have the most robust effect. This suggests that like Sle1, Bsx3 may itself contain multiple susceptibility loci, some of which might be allelic. Of the four loci located on chromosome 1, only Bsx2 has a proposed candidate gene. RNA and protein levels of the innate scavenger receptor Marco (macrophage receptor with collagenous structure) are reduced in the macrophages of Bxs2-containing congenic mice. This leads to a defect in clearance of apoptotic material, which is generally associated with the development of systemic autoimmunity [68].
Bxs6 on chromosome 13 is linked to increased serum gp70 and anti-gp70 autoantibody. The presence of Yaa greatly enhances Bxs6 resulting in nephritis due to deposition of the associated gp70 immune complexes (gp70IC) in glomeruli. Gp70 is a glycoprotein subunit encoded by the viral envelope env gene of endogenous retroviruses that is produced by hepatocytes as an acute phase protein [69]. Though not proven conclusively, there is a good deal of evidence for a role of endogenous retroviruses in lupus pathogenesis (reviewed in [70]). Bsx6 overlaps with two other loci, NZW-derived Sgp3 [71] and 129-derived Gv1 [72], which are also involved in the regulation of serum gp70 levels. This positional and functional relationship between these three loci suggests that they may be caused by the same gene(s). Further, TLR7 activation causes Sgp3-mediated upregulation of serum gp70, in much the same way that Yaa enhances of Bxs6-mediated upregulation of serum gp70 [73]. Sgp3 has been the most extensively analyzed and has been recently narrowed down to a 5.42 Mb region in which 21 of the 30 genes encode Krüppel-associated box zinc-finger proteins (KRAB-ZFP) that are capable of suppressing proviral transcription through the heterochromatization viral integration sites [70]. KRAB-ZFPs are, therefore, the primary candidate genes for Sgp3 and likely for Bxs6.
3. Induced Models of Lupus
3.1. The Pristane-Induced Lupus Model
Unlike the mouse models mentioned above, in which genetic factors play the major role, induced mouse models develop lupus after exposure to certain environmental triggers. Intraperitoneal injections of pristane (2, 6, 10, and 14 tetramethylpentadecane, TMPD), an isoprenoid alkane found at high concentration in mineral oil, is a standard method to obtain ascitic fluid enriched in monoclonal antibodies. However, pristane administration in BALB/c mice induces autoantibodies characteristic of lupus, such as antiribonucleoprotein (RNP) antibodies (anti-Su, anti-Sm, and anti-U1RNP), anti-DNA, and anti-histone, to a level comparable to that found in MRL/lpr mice [74, 75]. Pristane-treated mice also have immune-complex deposition in the kidney leading to severe proteinuria and nephritis [75]. Besides the BALB/c strain, almost all mouse strains are susceptible, to a variable extent, to the pristane-induced production of antibody and lupus manifestations [76], confirming that environment has a considerable importance in lupus. Like human SLE patients and NZB/W F1 model, pristine-induced lupus is more severe in females than in males, at least in SJL/J strain [77].
3.1.1. Cytokines in Pristane-Induced Lupus
Inflammatory cytokines have critical effects in pristane-induced lupus. By using BALB/c IL-6−/− mice, it was found that anti-ssDNA, dsDNA, and chromatin antibodies are highly IL-6-dependent whereas anti-RNP/Sm and anti-Su antibodies have no obvious correlation with IL-6 levels [78]. Interestingly, anti-dsDNA antibodies generally develop after renal disease, which indicates that these autoantibodies do not participate in the development of nephritis in this model. When pristane was administered to lupus-prone NZB/W F1 mice, it greatly accelerated the disease and lead to large amounts of IL-12, anti-Sm, anti-Su, and anti-RNP autoantibody production, implying a link between IL-12 and these anti-RNP autoantibodies [79]. This was confirmed by the fact that IL-12 deficiency prevented pristane-treated mice from anti-RNP/Su IgG production and nephritis [80]. These studies revealed that the production of different autoantibodies may be induced by different cytokine pathways and contribute differently to end-organ disease. In another study, IFN-γ deficiency significantly protected pristane-treated BALB/c mice from autoantibody production and renal disease whereas IL-4 deficiency has no effect [81]. Thus, pristane-induced lupus may be attributable to a shift of the T-helper cell subsets, Th1-Th2, balance.
Overexpression of IFN-I and interferon-stimulated genes (ISGs, also called “interferon signature”) has been closely associated with ANA production and disease severity in SLE patients [82]. Pristane-induced lupus is the only animal model that shows an interferon signature. Indeed, studies in pristane-induced lupus have revealed that IFN-I receptor deficiency (IFNAR−/− or IFNAR2−/−) completely erased the interferon signature, eliminated anti-RNP, anti-Sm, and anti-dsDNA autoantibodies, reduced anti-ssDNA and anti-histone IgG, and significantly improved GN [83, 84]. IFN-I may contribute to autoantibody production by regulating TLR7/9 expression and activation in B cells [84]. IFN-I also seems to regulate another autoantibody mediator, IL-12, as IFNAR−/− mice fail to elevate IL-12 level following pristane treatment [83].
Instead of plasmacytoid dendritic cells (pDCs) which have been identified as the major producers of IFN-I in human lupus [85], an accumulation of peritoneal immature Ly6Chigh monocytes is the major source of IFN-I in pristane-treated mice. Depletion of this cell population rapidly abolished the interferon signature and autoantibody production in pristane-treated mice [86]. IFN-I in turn mediates the recruitment of Ly6Chigh immature monocytes to the peritoneum through the upregulation of chemokines such as CCL2, CCL7, and CCL12. IFN-1 also prevents the maturation of monocytes, with a twofold effect: firstly, immature monocytes are a major source of IFN-I. Therefore, blocking of monocyte maturation results in a vicious feedback loop of IFN-1 production, monocyte recruitment, and block in an immature state. Secondly, failure of monocyte maturation into phagocytic Ly6Clow monocytes/macrophages leads to an impaired clearance of cell debris, which is an important stimulus of inflammation and autoantibody production [87]. Thus, IFN-I production by Ly6Chigh monocytes may be a major cause of chronic inflammation and SLE.
Pristane induces IFN-I production exclusively through the TLR7/MyD88 pathway [88]. TLR7 deficiency not only abolishes pristane-induced IFN-I production but also eliminates autoantibody production and renal disease, indicating a key role of TLR7/MyD88/IFN-I pathway in this model [88, 89]. FcγR recognizes the Fc portion of extracellular IgG and is proposed to mediate the intake of immune complex that subsequently activates endosomal TRLs, such as TLR7 [90]. However, IFN-I and autoantibody production are FcγR-independent in pristane-induced lupus [88]. Therefore, the modulation of TLR7 by pristane does not rely on immune complexes. The exact mechanism of pristane-mediated TLR7 activation remains to be identified.
3.1.2. Other Manifestations of Pristane-Induced Lupus
In addition to nephritis, hemorrhagic pulmonary capillaritis and arthritis have also been identified in pristane-treated mice [91]. 14 days after a single dose of pristane injection, bronchoalveolar lavage (BAL) fluid becomes hemorrhagic with elevated neutrophil counts and high levels of IL-6 and IL-10. Perivascular infiltrates of immune cells and endothelial injury are also found in the lung of pristine-treated mice [91]. Some SLE patients also develop pulmonary capillaritis [92], and whether pristane-induced hemorrhagic pulmonary capillaritis can serve as a valid model for human lupus lung disease remains to be verified. The arthritis symptoms in pristane-induced lupus include synovial hyperplasia, periostitis, and progressive marginal erosions [93, 94]. Pristane-induced arthritis is tumor necrosis factor (TNF) α mediated, as treatment with neutralizing anti-TNF-α antibody ameliorates the arthritis symptoms [95]. The broader extension of end-organ targets as well as the preeminent role plays by IFN I make pristane-induced lupus arguably a model of choice for mechanistic studies of human lupus.
3.2. Induced Chronic Graft-versus-Host Disease
3.2.1. Highlights and Advantages of the Parent into F1 Method
Various models of induced graft-versus-host disease (GVHD) have been used as models of lupus. As with the pristane-induced model, these models require only a single injection of donor cells to induce a lupus-like syndrome. The disease process is reproducible, with severity correlating to the number of allografted cells. Because this method induces a rapid disease onset from a known starting point, the study of lupus induction is greatly facilitated as compared to the spontaneous models of lupus. As an example, autoantibodies are detectable as early as 10–14 days after induction in these models whereas it can be several weeks to months after birth for the same phenotype to develop in spontaneous lupus models. Further, since the donor T cells undergo activation and expansion, they are relatively easy to observe by flow cytometry, allowing the study of the effect of various modifications on the donor as well as the host cells.
In the parent into F1 (P → F1) model, lymphocytes are transferred into a semiallogenic recipient such that the donor cells react to the host, and the host is tolerant to the donor allograft. (reviewed in [96]). Depending on the model used, an acute or a chronic GVHD (aGVHD or cGVHD) ensues. In most models of P → F1, such as B6 into (B6 × DBA)F1, aGVHD is induced by donor CD8+ T cells, which manifests as severe lymphopenia of host cells as well as strain-specific connective tissue diseases. Host B cells are most sensitive to this CTL killing and may be almost completely ablated after 2 weeks, followed by host APCs, then CD4+ T cells, and finally host CD8+ T cells. Conversely, DBA into (B6 × DBA)F1 (DBA → BDF1) results in cGVHD, a donor CD4+-mediated response resulting in massive host polyclonal B cell activation, expansion, and immunoglobulin production. This chronic condition exhibits several similarities to SLE, including ANA, lupus-specific autoantibodies, and immune complex-mediated GN. Another feature of DBA → BDF1 is that the lupus pathogenesis in this model is more severe in females than males allowing the study of gender-biased lupus etiology [97, 98]. Additionally, DBA → BDF1 allows for identification of CTL-promoting treatments in vivo, such as with agonist anti-CD40 antibody, IL12, and antagonist anti-CD80 antibody [99–101].
The induction of a cGVHD instead of aGVDH response is caused by a diminished capacity for DBA CD8+ T cells to produce a CTL response to the F1 host. Correspondingly, B6 into (B6 × DBA)F1 will result in cGVHD if the donor cells are depleted of CD8+ T cells. The ensuing lupus phenotypes include ANA, anti-dsDNA and anti-RBC autoantibodies, and GN. The major advantage of B6(-CD8) → F1 over the DBA → BDF1 model is that genetic modification of the donor cells can be examined due to the plethora of knockout and transgenic B6 mice. To this extent, this model has demonstrated that partial CTL deficiency due to the lack of perforin (B6·Pfp−/− → F1) or Fas/FasL (B6·gld → F1) was sufficient to induce a CD4+-mediated cGVHD phenotype.
B6(-CD8) → F1 was also used as a complement of B6 → F1 (not depleted of CD8+ T cells) to study in vivo mechanisms that mediate the overall donor CD8+ T cell versus CD4+ T cell response, and thus result in aGVHD versus cGVHD. One such study demonstrated that TNFα blockade inhibited the early induction of CTLs and skewed T cell cytokine profile to antibody promoting IL-4, IL-6, and IL-10, resulting in an antibody mediated cGVHD response [102]. This novel finding revealed the potential of TNFα-blocking treatments to instigate humoral autoimmunity. Another study described a positive role in B6(-CD8) → F1 cGVHD and a negative role in B6 → F1 aGVHD for the TNF family member, TRAIL, highlighting its role in the promotion of lupus [103].
3.2.2. B6·H-2bm12← → B6 cGVHD
When B6·H-2bm12 lymphocytes, whose MHC II locus confers a 3 amino acid substitution in H-2b, are transferred into B6 hosts with the wild-type H-2b locus, cGVHD develops. The lupus-like phenotypes in this model include ANA, anti-dsDNA, antichromatin, and anti-Sm autoantibodies, and GN [104]. Since the MHC disparity between donor and host is limited to class II in this model, CD8+ T cell depletion of donor lymphocytes is not required. Also, since this model works equally well with B6·H-2bm12 → B6 and B6 → B6·H-2bm12, donor and host gene modifications can be examined by the use of knockout and transgenic mice.
Although cGVHD has been shown to be mediated through donor CD4+ T cells and host B cells, an interesting facet of cGVHD was revealed using this model. The observation that a response did not occur in CD4−/− hosts indicated a role for endogenous CD4+ T cells [189]. Further, B cells raised in such an environment had intrinsic defects in their ability to produce an alloresponse [190]. It has been recently reported that B cells require “nurturing” during early ontogeny in the form of IL-4 [191]. In lieu of endogenous CD4+ T cell nurturing, large doses of rIL-4 or agonist anti-CD40 antibody in CD4−/− hosts during this critical developmental period were also sufficient for B cells to develop with alloreactive potential.
4. Therapeutic Studies for SLE on Mouse Models
Mouse models of SLE have been used not only to dissect disease mechanisms but also to test potential therapies. In addition to validating targets, testing drugs on mouse models of SLE has proved indispensable before they go to clinical trials. We have summarized the major therapeutic studies that have been conducted on mice models of SLE in Table 1. Several components of the immune system, such as cytokines, B-cells, T-cells, and hormones have been identified as potential targets for therapy, which have been tested in mouse models of lupus, most often in a preventive rather than therapeutic mode. Some of the most successful therapeutic approaches have lead to clinical trials, several of which are currently in progress. Among them, Belimumab, a humanized monoclonal antibody directed against B-cell activating factor (BAFF), is the first biologic treatment that has met clinical trial end points and has been recommended for SLE treatment. BAFF regulates the survival of B-cells. It was determined that lupus-prone mice and SLE patients have elevated levels of BAFF. Additionally, nonautoimmune transgenic mice overexpressing BAFF showed lupus-like symptoms [130]. Targeting BAFF receptors (BAFF-R, TACI, or BCMA) in the NZM2410 and MRL/lpr lupus mouse models was effective in reducing disease symptoms [125, 132]. Murine studies targeting the BAFF pathway were followed by clinical trials targeting BAFF in SLE patients. The success of Belimumab in clinical trials is considered a breakthrough in the search for new therapies for SLE [126, 127]. This example serves to illustrate the important role mouse models play in the development of effective treatments for this complex human disease.
Table 1.
Summary of treatments tested in mouse models of lupus, their targets, proposed mechanisms, outcomes in the mouse, and follow up in clinical trials.
| Targets | Treatment | Mouse model | Mechanisms | Main outcomes | Ref | Clinical trial |
|---|---|---|---|---|---|---|
| Targeting cytokines | ||||||
| IL-6 | IL-6R mAb | MRL/lpr | Nephritis↓, anti-dsDNA Ab↓ | [105] | Tocilizumab* | |
| IL-6R mAb | NZB/W F1 | Inhibits IgG class switch | Survival↑, IgG ANA↓, proteinuria↓ | [107] | A Phase I trial showed both decreased disease activity and side effects [106] | |
| IL-6 mAb | NZB/W F1 | Suppresses autoreactive B cell and T cell | Nephritis↓, anti-dsDNA Ab↓ | [108] | ||
| IL-10 | IL-10 inhibitor (AS101) | NZB/W F1 | Decreases IL-10, increases TNF-α | Delayed onset, anti-DNA Ab↓, proteinuria↓, glomerular deposition↓ | [109] | Antagonizing anti- IL-10 mAb A small-scale trial showed efficacy. [110] |
| IL-10 mAb | NZB/W F1 | Neutralizes IL-10, increases TNF-α | Delayed onset, survival↑, ANA↓ | [111] | ||
| rIL-10 | MRL/lpr | Suppresses Th1 response | IgG2a anti-dsDNA↓ | [112] | ||
| AAV-IL-10 (low exp.) | B6·Sle1·Sle2·S le3 |
Suppresses Th1 response | ANA↓, GN↓, T cell activation↓ | [113] | ||
| TNF-α | rTNF-α | NZB/W F1 | Decreases cellular response | Delayed onset, survival↑ | [114] |
Infliximab A small-scale trial showed both efficacy and side effects [115] Etanercept Phase II trial for discoid lupus in progress |
| IFN-α | IFN-α kinoid vaccination | NZB/W F1 | Induces anti IFN-α neutralizing Ab | Delayed onset, survival↑, proteinuria↓ | [116] |
MEDI-545 (sifalimumab) Phase I trial showed positive result [117] rhuMab IFN-α (Rontalizumab) Phase I trial showed positive result [118] |
| IFN-β | IFN-β | MRL/lpr | Reduces both cellular and humoral responses and cytokine production | Survival↑, proteinuria↓, skin lesions↓, ANA↓, splenomegaly↓ | [119] | |
| IFN-γ | IFN-γ cDNA vaccination | MRL/lpr | Induces anti IFN-γ neutralizing Ab | Survival↑, lymphoid hyperplasia↓, ANA↓, GN↓ | [120] |
AMG 811 Phase I trial in progress |
| IFN-γ mAb | NZB/W F1 | Survival↑, proteinuria↓, anti-DNA Ab↓ | [121] | |||
| IL-17 | Rock2 inhibitor (Fasudil) | MRL/lpr | Inhibits IRF4 phosphorylation and reduces IL-17 and IL-21 production | Anti-dsDNA Ab↓, glomerular deposition↓, proteinuria↓ | [122] | |
| IL-21 | IL-21R·Fc | MRL/lpr | Decreases T cell numbers and alters B cell functions | Total Ig ↓, anti-dsDNA Ab↓, proteinuria↓ skin lesions↓, lymphadenopathy↓ | [123] | |
| IL-18 | IL-18 cDNA vaccination | MRL/lpr | Induces anti-IL-18 neutralizing Ab | Survival↑, GN↓, IFN- γ↓ | [124] | |
| BAFF | Adenovirus-BAFF-R-Ig | NZM2410 | Depletes B cell, decreases T cell activation and myeloproliferation | Survival↑, proteinuria↓, splenomegaly↓, reverses disease | [125] | Belimumab |
| Anti-BAFF-Ig | B6·lyn−/− | Nephritis↓, | [129] | Phase III met primary complex end point. [126, 127]. | ||
| BAFF and April | Human TACI-Ig | NZB/WF1 | Blocks BAFF and April signaling | Survival↑, proteinuria↓, no significant change in anti-dsDNA Ab level | [130] |
Atacicept Phase II terminated [128] |
| Adenovirus-TACI-Ig (w/ or w/o CTLA4-Ig co-administration) | NZB/W F1 | Blocks BAFF and April signaling splenic B cell depletion | Survival↑, delayed onset,, ANA↓, proteinuria↓, nephritis↓, reverses disease only when CTLA4-Ig coadministered | [131] | ||
| Adenovirus-TACI-Fc | MRL/lpr, NZB/W F1, |
Blocks BAFF and April signaling | In MRL/lpr: survival↑, GN↓, proteinuria↓,In NZB/W F1: anti-TACI antibodies neutralized TACI-Fc | [132] | ||
| Adenovirus-TACI-Ig | NZM2410 | Blocks on BAFF and April signaling; | Survival↑, proteinuria↓, splenomegaly↓, reverses disease | [125] | ||
| B cell depletion | ||||||
| CD20 | Anti-CD20 mAb | MRL/lpr | ADCC, CDC | Serum Ab and ANA↓, nephritis↓, proteinuria↓, dermatitis↓ | [133] |
Rituximab Failed to reach primary end point [134, 135] |
| Anti-CD20 mAb | NZB/W F1 | ADCC, CDC | Treatment on 12–28-wk-old mice ameliorates disease, while treatment on 4-wk old mice hastens disease | [137] |
Ocrelizumab Phase III terminated [136] |
|
| CD22 | Anti-CD22 mAb | NZB/W F1 | ADCC, targets inhibitory receptor | Able to deplete several B cell subsets. No significant effect on improving survival | [137] |
Epratuzumab Phase IIb trial showed positive result [138, 139] |
| CD79 | Anti-CD79 α and Anti-CD79 β mAb | MRL/lpr | Survival↑, IgG antichromatin↓, skin lesions↓ Inflammatory infiltrates↓ | [140] | ||
| Proteasome | Proteasome inhibitor bortezomib | NZB/W F1 and MRL/lpr | Eliminates both short-lived and long-lived plasma cells | Survival↑, ANA↓, GN↓ | [141] | |
| Targeting T cell-APC interactions | ||||||
| CTLA4-Ig | NZB/W F1 | Inhibits B7-CD28 interaction | Survival↑, ANA↓ | [142, 143] |
Abatacept The ACCESS trial is in progress [144] |
|
| CD28-B7 | Adenovirus-CTLA4-Ig | NZB/W F1 | Inhibits B7-CD28 interaction | Survival↑, ANA↓, GN↓ | [145] | |
| CTLA4-Ig transgene | B6 lyn−/− | Inhibits B7-CD28 interaction | Myeloid hyperplasia↓, splenomegaly↓ IgG ANA↓, renal disease unaffected | [146] | ||
| CTLA4-Ig and anti-gp39 | NZB/W F1 | Simultaneously inhibits B7-CD28 and CD40/gp39 interaction | Survival↑, ANA↓, GN↓, effects are more significant than CTLA4-Ig alone | [143] | ||
| Targeting T follicular helper cells | ||||||
| CD3 | Nasal anti-CD3 Ab | NZB/W F1 | Downregulates the expression of IL17 and IL21 by Tfh cells | ANA↓, GN↓ | [147] | |
| ICOS-B7RP-1 | anti-B7RP-1 Ab | NZB/W F1 | Downregulates ICOS and reduces Tfh cell number | Survival↓, anti-dsDNA Ab↓, proteinuria↓ | [148] | |
| Targeting other receptors | ||||||
| FcgRIIB | FcgRIIB expressing retrovirus | NZM2410, BXSB, B6Fcgr2b−/− | Restores FcgRIIB level | ANA↓, immune complex deposition↓, proteinuria↓, GN↓, lung pathology↓ | [149] | |
| TLR7/TLR9 | Immunoregulatory sequence (IRS) 954 | NZB/W F1 | Reduces the production of IFN-α | Survival↑, ANA↓, proteinuria↓, GN↓, | [150] | |
| Programmed death-1 (PD-1) | Neutralizing PD-1Ab | NZB/W F1 | Promotes suppressive CD8+ T cells | Survival↑, delayed nephritis, IgG↓, anti-dsDNA IgG↓ | [151] | |
| Targeting cell signaling | ||||||
| Syk | Syk inhibitor R788 | NZB/W F1 | Blocks B cell receptor (BCR) and FcR signaling | Survival↑, proteinuria↓, renal disease↓ | [152] | |
| Syk inhibitor R788 | MRL/lpr or BAK/BAX−/− | Blocks BCR and FcR signaling | Skin lesions↓ lymphadenopathy and splenomegaly↓, renal disease↓ | [153] | ||
| PI3K γ | AS605240 compound | MRL/lpr | Reduces T and B cell activation | Survival↑, renal infiltrates↓, GN↓ | [154] | |
| mTOR | Rapamycin | NZB/W F1 | Immunosuppression | Survival↑, anti-dsDNA Ab↓, splenomegaly↓, albuminuria↓ | [155] |
Rapamycin A small scale trial showed efficacy [156] |
| Cellular therapies | ||||||
| Regulatory B cells (Breg) | Anti-CD40 injection | MRL/lpr | Agonistic anti-CD40 expands Breg | Survival↑, anti-dsDNA Ab↓, proteinuria↓, skin lesions↓ | [157] | |
| Regulatory T cells (Treg) | Adoptive transfer of ex vivo expanded Treg | NZB/W F1 | T cell suppression | Survival↑, renal IC deposition↓, proteinuria↓, GN↓ | [158, 159] | |
| Umbilical cord mesenchymal stem cells (MSC) |
MRL/lpr | immunosuppressive and anti-inflammatory properties | anti-dsDNA Ab↓, Proteinuria↓, renal disease↓ | [160] | A small-scale trial showed positive result [161] | |
| Umbilical cord mesenchymal stem cells (MSC) |
NZB/W F1 | immunosuppressive and anti-inflammatory properties | Survival↑, anti-dsDNA Ab↓, proteinuria↓, renal disease↓ | [162] | ||
| Bone marrow derived mese- nchymal stem cell (BM-MSC) |
NZB/W F1 | Inhibition of B cell proliferation and differentiation dependent on IFN-γ | Glomerular IC deposition↓, lymphocytic infiltration↓, glomerular proliferation↓, anti-dsDNA Ab and proteinuria unaffected | [163] | ||
| Sex hormone modulation | ||||||
| Estrogen | Nafoxidine | NZB/W F1 | Delayed onset, survival↑, ANA↓, proteinuria↓ | [164] |
Tamoxifen A small-scale trial showed no effect [165] |
|
| Estrogen | Tamoxifen | NZB/W F1 | Reduces IgG3 ANA production | Survival↑, IgG3 ANA↓, proteinuria↓, thrombocytopenia↓, glomerular deposits↓ | [166, 167] |
Fulvestrant (estrogen receptor downregulator) A small-scale trial showed some efficacy [168] |
| Estrogen | Raloxifene | NZB/W F1 | Inhibits B cell function | Anti-DNA Ab↓, kidney damage↓ | [169] | |
| Estrogen | Indole-3-carbinol | NZB/W F1 | Blocks B cell and T cell differentiation | Survival↑, ANA↓, nephritis↓ | [170] | |
| Androgen | Androgen treatment on castrated female | NZB/W F1 | Unspecified | Survival↑, IgG ANA↓ | [171] |
Dehydroepiandrosterone Little clinical effect [172] |
| Androgen | Androgen treatment | NZB/W F1, MRL/lpr | Unspecified | Survival↑, anti-DNA Ab↓, | [173] | |
| Peptide-based immunotherapy | ||||||
| Laminin | Laminin derived peptides | MRL/lpr | Laminin derived peptides competitively bind with ANA and prevent their deposition in the kidney | Survival↑, proteinuria↓, GN↓ | [174] | |
| HSC chaperone protein | U1-70K snRNP derived peptide administered in saline (P140) | MRL/lpr | Impairs autoimmune T cell response | Survival↑, proteinuria↓, anti-dsDNA Ab↓ | [175, 176] |
P140 A phase IIa study showed positive result [177] |
| Autoreactive B and T cells | Histone peptide H2B10-33, H416-39, H471-94 | (SWR/ NZB)F1 | Impairs autoimmune B and T cell | Survival↑, proteinuria↓, ANA↓ | [178] | |
| Suppressive CD8+ T cells | pCONS | NZB/W F1 | Promotes suppressive CD8+ T cells by regulating Foxp3 and PD-1 | Survival↑, nephritis↓, ANA↓ | [179–181] | |
| Unclear | CDR1 of human anti-DNA Ab (hCDR1) | NZB/W F1 | Modulation of cytokines, regulatory T cells and T cell apoptosis | ANA↓, nephritis↓, CNS symptoms↓ | [182] |
hCDR1 A small-scale trial showed positive result [182] |
| Other therapies | ||||||
| Histone deacetylase | Histone deacetylase inhibitors TSA and SAHA | MRL/lpr | Decreases transcription of key cytokines involved in SLE | Splenomegaly↓, proteinuria↓, renal disease↓ | [183] | |
| Topoisomerase I | Topoisomerase I Inhibitor irinotecan | NZB/W F1 | Prevents anti-dsDNA Ab from binding to dsDNA | Survival↑, proteinuria↓ subendothelial immune deposit↓ | [184] | |
| ACE | ACE inhibitor Captopril | NZB/W F1 and MRL/lpr | Reduces TGF- β, IL-4 and IL-10 production | Delayed onset, proteinuria↓, renal lesion↓ | [185] | |
| HMG-CoA reductase | HMG-CoA reductase inhibitor Statin | NZB/W F1 | Immunomodulatory effects on B and T cells and APCs | ANA↓, serum urea↓, proteinuria↓, nephritis↓ | [186] | |
| ACE and HMG-CoA reductase | Coadministration of Statin and ACE inhibitor Imidapril | MRL/lpr | Synergistic effect | Survival↑, ANA↓, proteinuria↓, glomerular deposits↓, renal monocyte attractant CCL2/MCP-1↓ | [187] | |
| CCL2/MCP-1 | Spiegelmer mNOX-E36 | MRL/lpr | mNOX-E36 blocks CCL2 without induction of IFN-α production | Survival↑, nephritis↓, skin lesion↓, peribronchial inflammation↓ | [188] | |
*Drug names are shown in italic font.
A major limitation for drug trials in humans is that although physiological parameters are collected, it is difficult to determine changes in internal organs, such as the lymphoid organs and the kidneys. For example, the anti-CD20 antibody (Rituximab) is able to deplete B cells in humans through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) [192]. However, this data was mainly obtained from human peripheral blood B cells, which represents only 2% of the total B cells in the body [193]. Studies in mice have shown that unlike the blood, the spleen, bone marrow, and peritoneal cavity provide a reservoir for B cells in which they are more resistant to drug depletion [133, 194, 195]. Therefore, depletion efficacy seen in human blood does not necessarily reflect a global depletion. This may be one of the reasons why two randomized, double-blind, phase II/III trials failed to show an effect of Rituximab over placebo in SLE patients [134, 135]. Moreover, drug efficacy may be different between lupus-prone and lupus-resistant mice. Anti-CD20 depletion of B cells has been shown to be much more efficient in BALB/c than in MRL/lpr mice [133]. Therefore, it is crucial to test potential SLE therapies and drugs directly in lupus-prone mice.
There are obvious limitations in evaluating lupus therapies in mouse models. SLE targets the skin, joints, kidneys, and many other organs in humans whereas mouse models of SLE are characterized mainly by the development of nephritis. This is probably due to the fact that the genetic background is far more complicated in lupus patients than in mouse models. Some lupus mice may develop lupus-like symptoms other than renal disease, such as joint swelling and skin rash in a portion of MRL/lpr mice, and pulmonary symptoms in pristane-induced mice (see above). However, no mouse model can mimic the complexity of human lupus. Thus, drugs validated in mouse models may only be effective for a subset of symptoms or patients. To mimic the genetic and pathological heterogeneity in humans, it is crucial to use various mouse models in therapeutic studies. It has been shown that same treatment can have variable effects on different mouse models. For example, treatment with a soluble form of the BAFF receptor (BAFF-R-Ig) induces complete and enduring remission of established nephritis in NZM2410 mice [125], while it is unable to reverse nephritis in NZB/W F1 model unless a soluble form of cytotoxic T-lymphocyte antigen 4 (CTLA4-Ig) is coadministered to block T cell coactivation [131]. This difference is probably due to the fact that nephritic NZB/W F1 mice produce more inflammatory mediators in the kidney than do nephritic NZM2410 mice [125]. Thus, the genetic background should be taken into account when testing therapies on mouse models, and multiple models should be used to ensure the applicability of drugs on the more complicated human genetic background.
5. Summary
Murine models are useful tools for the study of the etiology of lupus. A multitude of models exist, each sharing a subset of attributes with SLE observed in humans. In addition, each model affords the study of different aspects of lupus pathogenesis. For example, the cGVHD and pristane-induced models allow for the study of the mechanisms involved at the onset of the disease, while the spontaneous models allow for probing of the genetic causes of disease. NZB/W F1 and DBA → DBF1 can be used to study phenotypes in a gender-biased context similar to that seen in humans. All models are valid candidates for the study of therapeutic interventions.
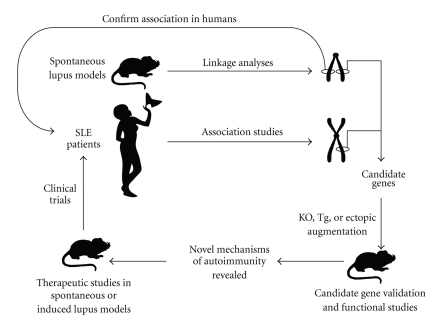
To date, the use of murine models has identified several novel candidate genes, and some of them have been associated to SLE in humans. Further value in these models is demonstrated by that fact that these genes were not initially identified in human SLE studies. On the other hand, recent genome-wide human studies have identified an array of genes, many of which have no currently described role in lupus. To this effect, murine models allow for forward genetics to identify novel genes that can then be probed using a candidate gene approach to discover their human susceptibility allele counterparts (Figure 1). Conversely, genes identified by human studies can be translated to genetically modified murine models where their roles in lupus pathogenesis can be followed in a forward genetics manner. Both methodologies have proven their usefulness and have the shared goal of identifying novel pathways and targets for disease intervention.
Figure 1.
Integration of human and murine studies for new SLE drug discovery. Study of human SLE patients and mouse models of lupus has led to the identification of potential therapeutic targets. In-depth studies in murine models are undertaken to validate the association of the potential targets with disease symptoms. The efficacy of targeted treatments are first tested on murine models of lupus prior to the initiation of human clinical trials.
Abbreviations
- ADCC:
Antibody dependent cell-mediated cytotoxicity
- aGVHD:
Acute graft-versus-host-disease
- ALPS:
Autoimmune lymphoproliferative syndrome
- ANA:
Antinuclear antibody
- APC:
Antigen presenting cells
- anti-RNP antibodies:
Anti-ribonucleoprotein antibodies: anti-Su, anti-Sm, and anti-U1RNP
- BAFF-R-Ig:
B-cell-activating factor receptor immunoglobulin
- CCL:
Chemokine C-C motif ligand
- CDC:
Complement-dependent cytotoxicity
- cGVHD:
Chronic graft-versus-host-disease
- Cr2:
Complement receptor type 2 CR2 (humans)
- CTLA-4-Ig:
Cytotoxic T-lymphocyte antigen 4 immunoglobulin
- CTL:
Cytotoxic T cell
- DC:
Dendritic cell
- Fcgr2b:
Fc receptor for IgG2b FCGR2B (humans)
- gld:
Generalized lymphoproliferative disease
- GN:
Glomerulonephritis
- gp70:
Glycoprotein 70
- GWAS:
Genome wide association studies
- H-2:
Murine MHC II
- IFNAR:
Interferon alpha receptor
- IFN I:
Type I interferon
- IL:
Interleukin
- ISG:
Interferon-stimulated gene
- Klk:
Kallikrein
- KRAB-ZFP:
Krüppel-associated box zinc-finger proteins
- Marco:
Macrophage receptor with collagenous structure
- MHC:
Major histocompatibility complex
- MyD88:
Myeloid differentiation primary response gene 88
- MZ:
Marginal zone
- NF-κβ:
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NZB/W F1:
First generation offspring from cross between NZB and NZW
- NZB:
New Zealand black
- NZM:
New Zealand mixed
- NZW:
New Zealand white
- Pbx1:
Pre-B-cell leukemia transcription factor 1
- pDCs:
Plasmacytoid dendritic cells
- QTL:
Quantitative trait loci
- SLAM:
Signaling lymphocytic activation molecule
- SLE:
Systemic lupus erythematosus
- SNP:
Single nucleotide polymorphism
- Th1:
T helper 1
- Th2:
T helper 2
- tlr7:
Toll-like receptor 7
- TMPD:
Pristane (2, 6, 10, 14 tetramethylpentadecane)
- TNF:
Tumor necrosis factor
- TNF-R:
Tumor necrosis factor receptor
- TRAIL:
Tumor necrosis factor-related apoptosis-inducing ligand
- RBC:
Red blood cell
- Yaa:
Y-linked autoimmune acceleration.
References
- 1.Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes and Immunity. 2009;10(5):373–379. doi: 10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kono DH, Theofilopoulos AN. Genetics of SLE in mice. Springer Seminars in Immunopathology. 2006;28(2):83–96. doi: 10.1007/s00281-006-0030-7. [DOI] [PubMed] [Google Scholar]
- 3.Morel L. Genetics of SLE: evidence from mouse models. Nature Reviews Rheumatology. 2010;6(6):348–357. doi: 10.1038/nrrheum.2010.63. [DOI] [PubMed] [Google Scholar]
- 4.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Advances in Immunology. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 5.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F mice. Journal of Experimental Medicine. 1978;147(6):1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE. Differences in expression of lupus nephritis in New Zealand Mixed H-2(z) homozygous inbred strains of mice derived from New Zealand Black and New Zealand White mice: origins and initial characterization. Laboratory Investigation. 1993;68(4):419–426. [PubMed] [Google Scholar]
- 7.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1(3):219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 8.Waters ST, Fu SM, Gaskin F, et al. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clinical Immunology. 2001;100(3):372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 9.Morel L, Tian XH, Croker BP, Wakeland EK. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 1999;11(2):131–139. doi: 10.1016/s1074-7613(00)80088-6. [DOI] [PubMed] [Google Scholar]
- 10.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosu pathogenesis: Sle2 on murine chromosome 4 leads to B cell hyperactivity. Journal of Immunology. 1997;159(1):454–465. [PubMed] [Google Scholar]
- 11.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnuclesomes. Journal of Clinical Investigation. 1998;101(6):1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of Sle pathogenesis: Sle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. Journal of Immunology. 1999;162(11):6492–6502. [PubMed] [Google Scholar]
- 13.Morel L, Mohan C, Yu Y, et al. Functional dissection of systemic lupus erythematosus using congenic mouse strains. Journal of Immunology. 1997;158(12):6019–6028. [PubMed] [Google Scholar]
- 14.Sobel ES, Mohan C, Morel L, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. Journal of Immunology. 1999;162(4):2415–2421. [PubMed] [Google Scholar]
- 15.Sobel ES, Morel L, Baert R, Mohan C, Schiffenbauer J, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis: evidence for functional expression of Sle3/5 by non-T cells. Journal of Immunology. 2002;169(7):4025–4032. doi: 10.4049/jimmunol.169.7.4025. [DOI] [PubMed] [Google Scholar]
- 16.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. Journal of Immunology. 2002;169(5):2694–2700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 17.Waters ST, McDuffie M, Bagavant H, et al. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. Journal of Experimental Medicine. 2004;199(2):255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morel L, Croke BP, Blenman KR, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman ZSM, Niu H, Perry D, Wakeland E, Manser T, Morel L. Expression of the autoimmune Fcgr2b NZW allele fails to be upregulated in germinal center B cells and is associated with increased IgG production. Genes and Immunity. 2007;8(7):604–612. doi: 10.1038/sj.gene.6364423. [DOI] [PubMed] [Google Scholar]
- 20.Lee YH, Ji JD, Song GG. Fcγ receptor IIB and IIIB polymorphisms and susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysis. Lupus. 2009;18(8):727–734. doi: 10.1177/0961203309104020. [DOI] [PubMed] [Google Scholar]
- 21.Boackle SA, Holers VM, Chen X, et al. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2001;15(5):775–785. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Perry D, Boackle SA, et al. Several genes contribute to the production of autoreactive B and T cells in the murine lupus susceptibility locus Sle1c. Journal of Immunology. 2005;175(2):1080–1089. doi: 10.4049/jimmunol.175.2.1080. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Boackle SA, Hanvivadhanakul P, et al. Association of a common complement receptor 2 haplotype with increased risk of systemic lupus erythematosus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(10):3961–3966. doi: 10.1073/pnas.0609101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas KB, Windels DC, Zhao J, et al. Complement receptor 2 polymorphisms associated with systemic lupus erythematosus modulate alternative splicing. Genes and Immunity. 2009;10(5):457–469. doi: 10.1038/gene.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wandstrat AE, Nguyen C, Limaye N, et al. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21(6):769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Kumar KR, Li L, Yan M, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312(5780):1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 27.Graham DSC, Vyse TJ, Fortin PR, et al. Association of LY9 in UK and Canadian SLE families. Genes and Immunity. 2008;9(2):93–102. doi: 10.1038/sj.gene.6364453. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki A, Yamada R, Kochi Y, et al. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nature Genetics. 2008;40(10):1224–1229. doi: 10.1038/ng.205. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Liu X, Xie C, et al. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. Journal of Clinical Investigation. 2005;115(7):1869–1878. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K, Li QZ, Delgado-Vega AM, et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. Journal of Clinical Investigation. 2009;119(4):911–923. doi: 10.1172/JCI36728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews B, Eisenberg RA, Theofilopoulos AN. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. Journal of Experimental Medicine. 1978;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson ML, Rao JK, Gilkeson GS, et al. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. Journal of Experimental Medicine. 1992;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30(2):180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu JL, Drappa J, Parnassa A, Elkon KB. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. Journal of Experimental Medicine. 1993;178(2):723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reap EA, Leslie D, Abrahams M, Eisenberg RA, Cohen PL. Apoptosis abnormalities of splenic lymphocytes in autoimmune lpr and gld mice. Journal of Immunology. 1995;154(2):936–943. [PubMed] [Google Scholar]
- 36.Takahashi T, Tanaka M, Brannan CI, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 37.Lynch DH, Watson ML, Alderson MR, et al. The mouse fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994;1(2):131–136. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 38.Teachey DT, Seif AE, Grupp SA. Advances in the management and understanding of autoimmune lymphoproliferative syndrome (ALPS) British Journal of Haematology. 2010;148(2):205–216. doi: 10.1111/j.1365-2141.2009.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabs DA, Kuppers RC, Saboori AM, et al. Effects of early and late treatment with anti-CD4 monoclonal antibody on autoimmune disease in MRL/MP-lpr/lpr mice. Cellular Immunology. 1994;154(1):66–76. doi: 10.1006/cimm.1994.1057. [DOI] [PubMed] [Google Scholar]
- 40.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. Journal of Experimental Medicine. 1994;180(4):1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. Journal of Experimental Medicine. 2004;199(10):1355–1365. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izui S, Kelley VE, Masuda K. Induction of various autoantibodies by mutant gene lpr in several strains of mice. Journal of Immunology. 1984;133(1):227–233. [PubMed] [Google Scholar]
- 43.Stranges PB, Watson J, Cooper CJ, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26(5):629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley VE, Roths JB. Interaction of mutant lpr gene with background strain influences renal disease. Clinical Immunology and Immunopathology. 1985;37(2):220–229. doi: 10.1016/0090-1229(85)90153-9. [DOI] [PubMed] [Google Scholar]
- 45.Vidal S, Kono DH, Theofilopoulos AN. Loci predisposing to autoimmunity in MRL-Fas(lpr) and C57BL/6-Fas(lpr) mice. Journal of Clinical Investigation. 1998;101(3):696–702. doi: 10.1172/JCI1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santiago-Raber ML, Haraldsson MK, Theofilopoulos AN, Kono DH. Characterization of reciprocal Lmb1-4 interval MRL-Fas and C57BL/6-Fas congenic mice reveals significant effects from Lmb3. Journal of Immunology. 2007;178(12):8195–8202. doi: 10.4049/jimmunol.178.12.8195. [DOI] [PubMed] [Google Scholar]
- 47.Haraldsson MK, Louis-Dit-Sully CA, Lawson BR, et al. The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity. 2008;28(1):40–51. doi: 10.1016/j.immuni.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews B, Eisenberg RA, Theofilopoulos AN. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. Journal of Experimental Medicine. 1978;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maibaum MA, Haywood MEK, Walport MJ, Morley BJ. Lupus susceptibility loci map within regions of BXSB derived from the SB/Le parental strain. Immunogenetics. 2000;51(4-5):370–372. doi: 10.1007/s002510050632. [DOI] [PubMed] [Google Scholar]
- 50.Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis and Rheumatism. 1979;22(11):1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- 51.Eisenberg RA, Dixon FJ. Effect of castration on male-determined acceleration of autoimmune disease in BXSB mice. Journal of Immunology. 1980;125(5):1959–1961. [PubMed] [Google Scholar]
- 52.Hudgins CC, Steinberg RT, Klinman DM. Studies of consomic mice bearing the Y chromosome of the BXSB mouse. Journal of Immunology. 1985;134(6):3849–3854. [PubMed] [Google Scholar]
- 53.Merino R, Shibata T, De Kossodo S, Izui S. Differential effect of the autoimmune Yaa and Ipr genes on the acceleration of lupus-like syndrome in MRL/MpJ mice. European Journal of Immunology. 1989;19(11):2131–2137. doi: 10.1002/eji.1830191124. [DOI] [PubMed] [Google Scholar]
- 54.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312(5780):1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 55.Subramanian S, Tus K, Li QZ, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Landenberg P, Bauer S. Nucleic acid recognizing Toll-like receptors and autoimmunity. Current Opinion in Immunology. 2007;19(6):606–610. doi: 10.1016/j.coi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 58.Lau CM, Broughton C, Tabor AS, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. Journal of Experimental Medicine. 2005;202(9):1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deane JA, Pisitkun P, Barrett RS, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27(5):801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fairhurst AM, Hwang SH, Wang A, et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. European Journal of Immunology. 2008;38(7):1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santiago-Raber ML, Kikuchi S, Borel P, et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. Journal of Immunology. 2008;181(2):1556–1562. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- 62.Shen N, Fu Q, Deng Y, et al. Sex-specific association of X-linked toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15838–15843. doi: 10.1073/pnas.1001337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izui S, Higaki M, Morrow D, Merino R. The Y chromosome from autoimmune BXSB/MpJ mice induces a lupus-like syndrome in (NZW x C57BL/6)F male mice, but not in C57BL/6 male mice. European Journal of Immunology. 1988;18(6):911–915. doi: 10.1002/eji.1830180612. [DOI] [PubMed] [Google Scholar]
- 64.Merino R, Fossati L, Lacour M, Lemoine R, Higaki M, Izui S. H-2-1inked control of the Yaa gene-induced acceleration of lupus-like autoimmune disease in BXSB mice. European Journal of Immunology. 1992;22(2):295–299. doi: 10.1002/eji.1830220202. [DOI] [PubMed] [Google Scholar]
- 65.Hogarth MB, Slingsby JH, Allen PJ, et al. Multiple lupus susceptibility loci map to chromosome 1 in BXSB mice. Journal of Immunology. 1998;161(6):2753–2761. [PubMed] [Google Scholar]
- 66.Haywood MEK, Hogarth MB, Slingsby JH, et al. Identification of intervals on chromosomes 1, 3, and 13 linked to the development of lupus in BXSB mice. Arthritis and Rheumatism. 2000;43(2):349–355. doi: 10.1002/1529-0131(200002)43:2<349::AID-ANR14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 67.Haywood MEK, Rogers NJ, Rose SJ, et al. Dissection of BXSB lupus phenotype using mice congenic for chromosome 1 demonstrates that separate intervals direct different aspects of disease. Journal of Immunology. 2004;173(7):4277–4285. doi: 10.4049/jimmunol.173.7.4277. [DOI] [PubMed] [Google Scholar]
- 68.Mũoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nature Reviews Rheumatology. 2010;6(5):280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 69.Hara I, Izui S, Dixon FJ. Murine serum glycoprotein gp70 behaves as an acute phase reactant. Journal of Experimental Medicine. 1982;155(2):345–357. doi: 10.1084/jem.155.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baudino L, Yoshinobu K, Morito N, Santiago-Raber M-L, Izui S. Role of endogenous retroviruses in murine SLE. Autoimmunity Reviews. 2010;10(1):27–34. doi: 10.1016/j.autrev.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 71.Santiago ML, Mary C, Parzy D, et al. Linkage of a major quantitative trait locus to Yaa gene-induced lupus-like nephritis in (NZW × C57BL/6)F1 mice. European Journal of Immunology. 1998;28(12):4257–4267. doi: 10.1002/(SICI)1521-4141(199812)28:12<4257::AID-IMMU4257>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 72.Oliver PL, Stoye JP. Genetic analysis of Gv1, a gene controlling transcription of endogenous murine polytropic proviruses. Journal of Virology. 1999;73(10):8227–8234. doi: 10.1128/jvi.73.10.8227-8234.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baudino L, Yoshinobu K, Dunand-Sauthier I, Evans LH, Izui S. TLR-mediated up-regulation of serum retroviral gp70 is controlled by the Sgp loci of lupus-prone mice. Journal of Autoimmunity. 2010;35(2):153–159. doi: 10.1016/j.jaut.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. Journal of Experimental Medicine. 1994;180(6):2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Satoh M, Kumar A, Kanwar YS, Reeves WH. Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(24):10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satoh M, Richards HB, Shaheen VM, et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clinical and Experimental Immunology. 2000;121(2):399–405. doi: 10.1046/j.1365-2249.2000.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith DL, Dong X, Du S, Oh M, Singh RR, Voskuhl RR. A female preponderance for chemically induced lupus in SJL/J mice. Clinical Immunology. 2007;122(1):101–107. doi: 10.1016/j.clim.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. Journal of Experimental Medicine. 1998;188(5):985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshida H, Satoh M, Behney KM, et al. Effect of an exogenous trigger on the pathogenesis of lupus in (NZB × NZW)F1 mice. Arthritis and Rheumatism. 2002;46(8):2235–2244. doi: 10.1002/art.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calvani N, Satoh M, Croker BP, Reeves WH, Richards HB. Nephritogenic autoantibodies but absence of nephritis in Il-12p35-deficient mice with pristane-induced lupus. Kidney International. 2003;64(3):897–905. doi: 10.1046/j.1523-1755.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 81.Richards HB, Satoh M, Jennette JC, Croker BP, Yoshida H, Reeves WH. Interferon-γ is required for lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney International. 2001;60(6):2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 82.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Current Opinion in Immunology. 2006;18(6):676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Nacionales DC, Kelly-Scumpia KM, Lee PY, et al. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis and Rheumatism. 2007;56(11):3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thibault DL, Graham KL, Lee LY, Balboni I, Hertzog PJ, Utz PJ. Type I interferon receptor controls B-cell expression of nucleic acid-sensing Toll-like receptors and autoantibody production in a murine model of lupus. Arthritis Research and Therapy. 2009;11(4, article no. R112) doi: 10.1186/ar2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rönnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17(5):394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee PY, Weinstein JS, Nacionales DC, et al. A novel type i IFN-producing cell subset in murine lupus. Journal of Immunology. 2008;180(7):5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee PY, Li YI, Kumagai Y, et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. American Journal of Pathology. 2009;175(5):2023–2033. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee PY, Kumagai Y, Li YI, et al. TLR7-dependent and FcγR-independent production of type I interferon in experimental mouse lupus. Journal of Experimental Medicine. 2008;205(13):2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Savarese E, Steinberg C, Pawar RD, et al. Requirement of toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis and Rheumatism. 2008;58(4):1107–1115. doi: 10.1002/art.23407. [DOI] [PubMed] [Google Scholar]
- 90.Barrat FJ, Meeker T, Gregorio J, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. Journal of Experimental Medicine. 2005;202(8):1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chowdhary VR, Grande JP, Luthra HS, David CS. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology. 2007;46(9):1405–1410. doi: 10.1093/rheumatology/kem117. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen VA, Gotwald T, Prior C, Obermoser G, Sepp N. Acute pulmonary edema, capillaritis and alveolar hemorrhage: pulmonary manifestations coexistent in antiphospholipid syndrome and systemic lupus erythematosus? Lupus. 2005;14(7):557–560. doi: 10.1191/0961203305lu2107cr. [DOI] [PubMed] [Google Scholar]
- 93.Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Pristane-induced arthritis. The immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis and Rheumatism. 1989;32(8):1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- 94.Potter M, Wax JS. Genetics of susceptibility to pristane-induced plasmacytomas in BALB/cAn: reduced susceptibility in BALB/cJ with a brief description of pristane-induced arthritis. Journal of Immunology. 1981;127(4):1591–1595. [PubMed] [Google Scholar]
- 95.Beech JT, Thompson SJ. Anti-tumour necrosis factor therapy ameliorates joint disease in a chronic model of inflammatory arthritis. British Journal of Rheumatology. 1997;36(10):p. 1129. doi: 10.1093/rheumatology/36.10.1129. [DOI] [PubMed] [Google Scholar]
- 96.Via CS. Advances in lupus stemming from the parent-into-F1 model. Trends in Immunology. 2010;31(6):236–245. doi: 10.1016/j.it.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grader-Beck T, Casciola-Rosen L, Lang TJ, Puliaev R, Rosen A, Via CS. Apoptotic splenocytes drive the autoimmune response to poly(ADP-ribose) polymerase 1 in a murine model of lupus. Journal of Immunology. 2007;178(1):95–102. doi: 10.4049/jimmunol.178.1.95. [DOI] [PubMed] [Google Scholar]
- 98.Lang TJ, Nguyen P, Papadimitriou JC, Via CS. Increased severity of murine lupus in female mice is due to enhanced expansion of pathogenic T cells. Journal of Immunology. 2003;171(11):5795–5801. doi: 10.4049/jimmunol.171.11.5795. [DOI] [PubMed] [Google Scholar]
- 99.Puliaev R, Puliaeva I, Welniak LA, et al. CTL-promoting effects of CD40 stimulation outweigh B cell-stimulatory effects resulting in B cell elimination and disease improvement in a murine model of lupus. Journal of Immunology. 2008;181(1):47–61. doi: 10.4049/jimmunol.181.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Via CS, Rus V, Cately MK, Finkelman FD. IL-12 stimulates the development of acute graft-versus-host disease in mice that normally would develop chronic, autoimmune graft-versus-host disease. Journal of Immunology. 1994;153(9):4040–4047. [PubMed] [Google Scholar]
- 101.Lang TJ, Nguyen P, Peach R, Gause WC, Via CS. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. Journal of Immunology. 2002;168(8):3786–3792. doi: 10.4049/jimmunol.168.8.3786. [DOI] [PubMed] [Google Scholar]
- 102.Via CS, Shustov A, Rus V, Lang T, Nguyen P, Finkelman FD. In vivo neutralization of TNF-α promotes humoral autoimmunity by preventing the induction of CTL. Journal of Immunology. 2001;167(12):6821–6826. doi: 10.4049/jimmunol.167.12.6821. [DOI] [PubMed] [Google Scholar]
- 103.Rus V, Nguyen V, Puliaev R, et al. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. Journal of Immunology. 2007;178(6):3962–3972. doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 104.Morris SC, Cohen PL, Eisenberg RA. Experimental induction of systemic lupus erythematosus by recognition of foreign Ia. Clinical Immunology and Immunopathology. 1990;57(2):263–273. doi: 10.1016/0090-1229(90)90040-w. [DOI] [PubMed] [Google Scholar]
- 105.Kiberd BA. Interleukin-6 receptor blockage ameliorates murine lupus nephritis. Journal of the American Society of Nephrology. 1993;4(1):58–61. doi: 10.1681/ASN.V4158. [DOI] [PubMed] [Google Scholar]
- 106.Illei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis and Rheumatism. 2010;62(2):542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/WF mice. Clinical and Experimental Immunology. 1998;112(3):397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang B, Gardner DB, Griswold DE, Bugelski PJ, Song XYR. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119(3):296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalechman Y, Gafter U, Da JP, Albeck M, Alarcon-Segovia D, Sredni B. Delay in the onset of systemic lupus erythematosus following treatment with the immunomodulator AS101: association with IL-10 inhibition and increase in TNF-α levels. Journal of Immunology. 1997;159(6):2658–2667. [PubMed] [Google Scholar]
- 110.Proudman SM, Conaghan PG, Richardson C, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic Lupus erythematosus. Arthritis and Rheumatism. 2000;43(8):1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 111.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. Journal of Experimental Medicine. 1994;179(1):305–310. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yin Z, Bahtiyar G, Zhang NA, et al. IL-10 regulates murine lupus. Journal of Immunology. 2002;169(4):2148–2155. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- 113.Blenman KRM, Duan B, Xu Z, et al. IL-10 regulation of lupus in the NZM2410 murine model. Laboratory Investigation. 2006;86(11):1136–1148. doi: 10.1038/labinvest.3700468. [DOI] [PubMed] [Google Scholar]
- 114.Gordon C, Ranges GE, Greenspan JS, Wofsy D. Chronic therapy with recombinant tumor necrosis factor-α in autoimmune NZB/NZW F mice. Clinical Immunology and Immunopathology. 1989;52(3):421–434. doi: 10.1016/0090-1229(89)90157-8. [DOI] [PubMed] [Google Scholar]
- 115.Aringer M, Houssiau F, Gordon C, et al. Adverse events and efficacy of TNF-alpha blockade with infliximab in patients with systemic lupus erythematosus: long-term follow-up of 13 patients. Rheumatology (Oxford) 2009;48(11):1451–1454. doi: 10.1093/rheumatology/kep270. [DOI] [PubMed] [Google Scholar]
- 116.Zagury D, Buanec HL, Mathian A, et al. IFNα kinoid vaccine-induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(13):5294–5299. doi: 10.1073/pnas.0900615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wallace DJ, Petri M, Olsen N, et al. MEDI-545, an anti-interferon alpha monoclonal antibody, shows evidence of clinical activity in systemic lupus erythematous. Arthritis & Rheumatism. 2007;56(supplement 9):S526–S527. [Google Scholar]
- 118.McBride JM, Wallace DJ, Yao Z, et al. Dose-dependent modulation of interferon regulated genes with administration of single and repeat doses of Rontalizumab in a phase I, placebo controlled, double blind, dose escalation study in SLE. Arthritis & Rheumatism. 2009;60:S775–S776. [Google Scholar]
- 119.Schwarting A, Paul K, Tschirner S, et al. Interferon-β: a therapeutic for autoimmune lupus in MRL-Fas mice. Journal of the American Society of Nephrology. 2005;16(11):3264–3272. doi: 10.1681/ASN.2004111014. [DOI] [PubMed] [Google Scholar]
- 120.Lawson BR, Prud’homme GJ, Chang Y, et al. Treatment of murine lupus with cDNA encoding IFN-γR/Fc. Journal of Clinical Investigation. 2000;106(2):207–215. doi: 10.1172/JCI10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jacob CO, Van Der Meide PH, McDevitt HO. In vivo treatment of (NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to γ interferon. Journal of Experimental Medicine. 1987;166(3):798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Biswas PS, Gupta S, Chang E, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. Journal of Clinical Investigation. 2010;120(9):3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. Journal of Immunology. 2007;178(6):3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 124.Bossù P, Neumann D, Del Giudice E, et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(2):14181–14186. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis and Rheumatism. 2010;62(5):1457–1468. doi: 10.1002/art.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petri MF, Merrill J, Wallace DJ, et al. Four-year experience of belimumab, a BlyS-specific inhibitor, in systemic lupus erythematosus (SLE) patients. Arthritis & Rheumatism. 2009;60 [Google Scholar]
- 127.GlaxoSmithKline press release. GlaxoSmithKline and Human Genome Sciences announce positive results in second of two phase 3 trials of benlysta in systemic lupus erythematosus. 2009, http://us.gsk.com/html/media-news/pressreleases/2009/2009_pressrelease_10121.htm.
- 128.Smith RM, Clatworthy MR, Jayne DRW. Biological therapy for lupus nephritis-tribulations and trials. Nature Reviews Rheumatology. 2010;6(9):547–552. doi: 10.1038/nrrheum.2010.117. [DOI] [PubMed] [Google Scholar]
- 129.Scapini P, Hu Y, Chu C-L, et al. Myeloid cells, BAFF, and IFN-γ establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. Journal of Experimental Medicine. 2010;207(8):1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 131.Ramanujam M, Wang X, Huang W, et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. Journal of Immunology. 2004;173(5):3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 132.Liu W, Szalai A, Zhao L, et al. Control of spontaneous B lymphocyte autoimmunity with adenovirus-encoded soluble TACI. Arthritis and Rheumatism. 2004;50(6):1884–1896. doi: 10.1002/art.20290. [DOI] [PubMed] [Google Scholar]
- 133.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. Journal of Immunology. 2007;179(5):3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 134.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis and Rheumatism. 2010;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sanz I, Lee FEH. B cells as therapeutic targets in SLE. Nature Reviews Rheumatology. 2010;6(6):326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.US National Library of Medicine. A study to evaluate ocrelizumab in patients with nephritis due to systemic lupus erythematosus (BeLONG) 2010, http://clinicaltrials.gov/ct2/show/NCT00626197.
- 137.Haas KM, Watanabe R, Matsushita T, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. Journal of Immunology. 2010;184(9):4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dörner T, Kaufmann J, Wegener WA, Teoh N, Goldenberg DM, Burmester GR. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Research and Therapy. 2006;8(3, article no. R74) doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.UCB press release. UCB and Immunomedics announce positive results for epratuzumab phase IIb study in systemic lupus erythematosus (SLe) 2009, http://www.ucb.com/media-room/newsdetail/?det=1337304.
- 140.Li Y, Chen F, Putt M, et al. B cell depletion with anti-CD79 mAbs ameliorates autoimmune disease in MRL/lpr mice. Journal of Immunology. 2008;181(5):2961–2972. doi: 10.4049/jimmunol.181.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nature Medicine. 2008;14(7):748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 142.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265(5176):1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 143.Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. Journal of Immunology. 1997;159(7):3104–3108. [PubMed] [Google Scholar]
- 144.US National Library of Medicine. Abatacept and cyclophosphamide combination therapy for lupus nephritis (ACCeSS) 2010, http://clinicaltrials.gov/ct2/show/NCT00774852.
- 145.Mihara M, Tan I, Chuzhin Y, et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. Journal of Clinical Investigation. 2000;106(1):91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oracki SA, Tsantikos E, Quilici C, et al. CTLA4Ig alters the course of autoimmune disease development in Lyn−/− mice. Journal of Immunology. 2010;184(2):757–763. doi: 10.4049/jimmunol.0804349. [DOI] [PubMed] [Google Scholar]
- 147.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+CD25+LAP+ regulatory T Cell and is associated with down-regulation of IL-17+CD4+ICOS+CXCR5+ follicular helper T cells. Journal of Immunology. 2008;181(9):6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu YIL, Metz DP, Chung J, Siu G, Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. Journal of Immunology. 2009;182(3):1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 149.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307(5709):590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 150.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffmann RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. European Journal of Immunology. 2007;37(12):3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 151.Wong M, La Cava A, Singh RP, Hahn BH. Blockade of programmed death-1 in young (New Zealand Black × New Zealand White)F1 mice promotes the activity of suppressive CD8+ T cells that protect from lupus-like disease. The Journal of Immunology. 2010;185(11):6563–6571. doi: 10.4049/jimmunol.0903401. [DOI] [PubMed] [Google Scholar]
- 152.Bahjat FR, Pine PR, Reitsma A, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis and Rheumatism. 2008;58(5):1433–1444. doi: 10.1002/art.23428. [DOI] [PubMed] [Google Scholar]
- 153.Deng GM, Liu L, Bahjat FR, Pine PR, Tsokos GC. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis and Rheumatism. 2010;62(7):2086–2092. doi: 10.1002/art.27452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barber DF, Bartolomé A, Hernandez C, et al. PI3Kγ inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nature Medicine. 2005;11(9):933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 155.Lui SL, Tsang R, Chan KW, et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrology Dialysis Transplantation. 2008;23(9):2768–2776. doi: 10.1093/ndt/gfn216. [DOI] [PubMed] [Google Scholar]
- 156.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 2006;54(9):2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. Journal of Immunology. 2009;182(6):3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. Journal of Immunology. 2006;177(3):1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 159.Scalapino KJ, Daikh DI. Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of Ex vivo expanded regulatory T cells. PLoS One. 2009;4(6) doi: 10.1371/journal.pone.0006031. Article ID e6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gu Z, Akiyama K, Ma X, et al. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010;19(13):1502–1514. doi: 10.1177/0961203310373782. [DOI] [PubMed] [Google Scholar]
- 161.Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Annals of the Rheumatic Diseases. 2010;69(8):1423–1429. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 162.Chang J, Hung S, Wu H, et al. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. doi: 10.3727/096368910X520056. Cell Transplantation. In press. [DOI] [PubMed] [Google Scholar]
- 163.Schena F, Gambini C, Gregorio A, et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis and Rheumatism. 2010;62(9):2776–2786. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 164.Duvic M, Steinberg AD, Klassen LW. Effect of the anti-estrogen, nafoxidine, on NZB/W autoimmune disease. Arthritis and Rheumatism. 1978;21(4):414–417. doi: 10.1002/art.1780210403. [DOI] [PubMed] [Google Scholar]
- 165.Sturgess AD, Evans DTP, Mackay IR, Riglar A. Effects of the oestrogen antagonist tamoxifen on disease indices in systemic lupus erythematosus. Journal of Clinical and Laboratory Immunology. 1984;13(1):11–14. [PubMed] [Google Scholar]
- 166.Sthoeger ZM, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZB×NZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Annals of the Rheumatic Diseases. 2003;62(4):341–346. doi: 10.1136/ard.62.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu WM, Lin BF, Su YC, Suen JL, Chiang BL. Tamoxifen decreases renal inflammation and alleviates disease severity autoimmune NZB/W F1 mice. Scandinavian Journal of Immunology. 2000;52(4):393–400. doi: 10.1046/j.1365-3083.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 168.Abdou NI, Rider V, Greenwell C, Li X, Kimler BF. Fulvestrant (Faslodex), an estrogen selective receptor downregulator, in therapy of women with systemic lupus erythematosus. Clinical, serologic, bone density, and T cell activation marker studies: a double-blind placebo-controlled trial. Journal of Rheumatology. 2008;35(5):797–803. [PubMed] [Google Scholar]
- 169.Zhang Y, Saha S, Rosenfeld G, Gonzalez J, Pepeljugoski KP, Peeva E. Raloxifene modulates estrogen-mediated B cell autoreactivity in NZB/W F1 mice. Journal of Rheumatology. 2010;37(8):1646–1657. doi: 10.3899/jrheum.090911. [DOI] [PubMed] [Google Scholar]
- 170.Yan XJ, Qi M, Telusma G, et al. Indole-3-carbinol improves survival in lupus-prone mice by inducing tandem B- and T-cell differentiation blockades. Clinical Immunology. 2009;131(3):481–494. doi: 10.1016/j.clim.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 171.Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. Journal of Clinical Investigation. 1977;59(6):1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moszkorzová L, Lacinová Z, Marek J, Musilová L, Dohnalová A, Dostál C. Hyperprolactinaemia in patients with systemic lupus erythematous. Clinical and Experimental Rheumatology. 2002;20(6):807–812. [PubMed] [Google Scholar]
- 173.Steinberg AD, Roths JB, Murphy ED. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr mice. Journal of Immunology. 1980;125(2):871–873. [PubMed] [Google Scholar]
- 174.Amital H, Heilweil M, Ulmansky R, et al. Treatment with a laminin-derived peptide suppresses lupus nephritis. Journal of Immunology. 2005;175(8):5516–5523. doi: 10.4049/jimmunol.175.8.5516. [DOI] [PubMed] [Google Scholar]
- 175.Monneaux F, Lozano JM, Patarroyo ME, Briand JP, Muller S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MRL/lpr mice. European Journal of Immunology. 2003;33(2):287–296. doi: 10.1002/immu.200310002. [DOI] [PubMed] [Google Scholar]
- 176.Page N, Schall N, Strub J-M, et al. The spliceosomal phosphopeptide P140 controls the lupus disease by interacting with the HSC70 protein and via a mechanism mediated by γδ T cells. PLoS One. 2009;4(4, article e5273) doi: 10.1371/journal.pone.0005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muller S, Monneaux F, Schal N, et al. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: results of an early phase II clinical trial. Arthritis and Rheumatism. 2008;58(12):3873–3883. doi: 10.1002/art.24027. [DOI] [PubMed] [Google Scholar]
- 178.Kaliyaperumal A, Michaels MA, Datta SK. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides: tolerance spreading impairs pathogenic function of autoimmune T and B cells. Journal of Immunology. 1999;162(10):5775–5783. [PubMed] [Google Scholar]
- 179.Ozaki Y, Amakawa R, Ito T, et al. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis and Rheumatism. 2001;44(2):432–441. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 180.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3 -expressing, apoptosis-resistant, TGFβ-secreting CD8+ T cell suppressors. Journal of Immunology. 2005;175(11):7728–7737. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 181.Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB × NZW)F mice by differentially regulating Foxp3 and PD1 molecules. Journal of Immunology. 2008;180(4):2069–2080. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- 182.Mozes E, Sharabi A. A novel tolerogenic peptide, hCDR1, for the specific treatment of systemic lupus erythematosus. Autoimmunity Reviews. 2010;10(1):22–26. doi: 10.1016/j.autrev.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 183.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. Journal of Clinical Investigation. 2003;111(4):539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Frese-Schaper M, Zbaeren J, Gugger M, Monestier M, Frese S. Reversal of established lupus nephritis and prolonged survival of New Zealand Black x New Zealand White mice treated with the topoisomerase I inhibitor irinotecan. Journal of Immunology. 2010;184(4):2175–2182. doi: 10.4049/jimmunol.0903153. [DOI] [PubMed] [Google Scholar]
- 185.De Albuquerque DA, Saxena V, Adams DE, et al. An ACE inhibitor reduces Th2 cytokines and TGF-β1 and TGF-β2 isoforms in murine lupus nephritis. Kidney International. 2004;65(3):846–859. doi: 10.1111/j.1523-1755.2004.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lawman S, Mauri C, Jury EC, Cook HT, Ehrenstein ME. Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand black/white F1 mice. Journal of Immunology. 2004;173(12):7641–7646. doi: 10.4049/jimmunol.173.12.7641. [DOI] [PubMed] [Google Scholar]
- 187.Shimazu H, Kinoshita K, Hino S, et al. Effect of combining ACE inhibitor and statin in lupus-prone mice. Clinical Immunology. 2010;136(2):188–196. doi: 10.1016/j.clim.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 188.Kulkarni O, Pawar RD, Purschke W, et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. Journal of the American Society of Nephrology. 2007;18(8):2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- 189.Chen F, Maldonado MA, Madaio M, Eisenberg RA. The role of host (endogenous) T cells in chronic graft-versus-host autoimmune disease. Journal of Immunology. 1998;161(11):5880–5885. [PubMed] [Google Scholar]
- 190.Choudhury A, Maldonado MA, Cohen PL, Eisenberg RA. The role of host CD4 T cells in the pathogenesis of the chronic graft-versus-host model of systemic lupus erythematosus. Journal of Immunology. 2005;174(12):7600–7609. doi: 10.4049/jimmunol.174.12.7600. [DOI] [PubMed] [Google Scholar]
- 191.Choudhury A, Cohen PL, Eisenberg RA. B cells require “nurturing” by CD4 T cells during development in order to respond in chronic graft-versus-host model of systemic lupus erythematosus. Clinical Immunology. 2010;136(1):105–115. doi: 10.1016/j.clim.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nature Reviews Immunology. 2010;10(5):301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 193.Yazawa N, Hamaguchi Y, Poe JC, Tedder TF. Immunotherapy using unconjugated CD19 monoclonal antibodies in animal models for B lymphocyte malignancies and autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(42):15178–15183. doi: 10.1073/pnas.0505539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hamaguchi Y, Uchida J, Cain DW, et al. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. Journal of Immunology. 2005;174(7):4389–4399. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 195.DiLillo DJ, Hamaguchi Y, Ueda Y, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. Journal of Immunology. 2008;180(1):361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]