Abstract
Adipose tissue metabolism is closely linked to insulin resistance, and differential fat distributions are associated with disorders like hypertension, diabetes, and cardiovascular disease. Adipose tissues vary in their impact on metabolic risk due to diverse gene expression profiles, leading to differences in lipolysis and in the production and release of adipokines and cytokines, thereby affecting the function of other tissues. In this paper, the roles of the various adipose tissues in obesity are summarized, with particular focus on mitochondrial function. In addition, we discuss how a functionally mitochondrial-targeted compound, the modified fatty acid tetradecylthioacetic acid (TTA), can influence mitochondrial function and decrease the size of specific fat depots.
1. Introduction
In a modern lifestyle people are chronically exposed to elevated amounts of lipids and nutrients, potentially leading to tissue dysfunction and disease [1]. The adipose tissue is highly involved in the development of metabolic disorders such as cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM). It is the body's largest storage site for triglycerides and plays an important role as an endocrine organ in energy homeostasis [2]. The adipose tissue is divided into specific regional depots with differences in structural organization, cellular size, and biological function [3]. The distribution of fat between these depots seems to be more important than the total adipose tissue mass for the risk of developing obesity-associated diseases (Figure 1).
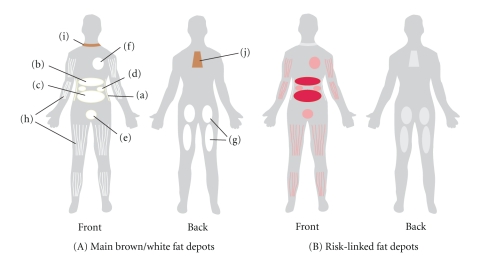
Figure 1.
(A) The main white adipose tissues (WATs) are abdominal subcutaneous adipose tissue (SAT, (a)), and visceral adipose tissue (VAT). VAT surrounds the inner organs and can be divided in omental (b), mesenteric (c), retroperitoneal ((d): surrounding the kidney), gonadal ((e): attached to the uterus and ovaries in females and epididymis and testis in men), and pericardial (f). The omental depot stars near the stomach and spleen and can expand into the ventral abdomen, while the deeper mesenteric depot is attached in a web-form to the intestine. The gluteofemoral adipose tissue (g) is the SAT located to the lower-body parts and is measured by hip, thigh, and leg circumference. WAT can also be found intramuscularly (h). Brown adipose tissue is found above the clavicle ((i): supraclavicular) and in the subscapular region (j). Although the mentioned subcutaneous and visceral adipose tissues are found in humans, depots (d) and (e) are mostly studied in rodents. (B) The adipose tissue depots that have been linked to risk of developing obesity-related diseases are indicated in red. The best-documented link to risk is found for the omental and mesenteric VAT.
Normally, fat storage in the form of neutral triglycerides takes place during food intake to be replaced by fat mobilization processes during fasting or situations with elevated energy demand (Figure 2(A)). Adipocytes produce a number of endocrine hormones that contribute to the regulation of this mechanism, such as adiponectin and leptin. As adipocytes play a key metabolic role as a source of fuel, they are required to respond acutely to changes in the nutritional levels. As a result they are tightly regulated by both hormonal (e.g., insulin) and sympathetic (e.g., adrenergic) stimulation [2].
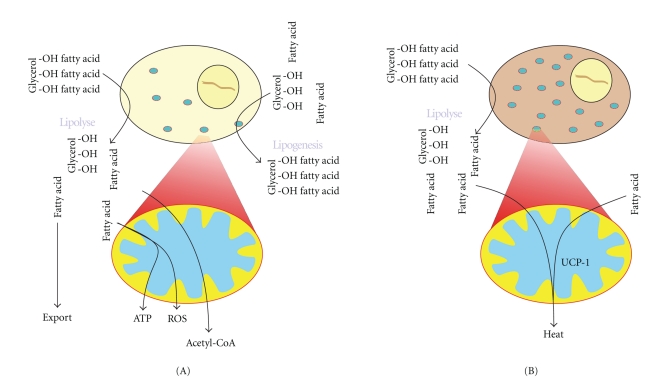
Figure 2.
(A) The role of white adipose tissue (WAT) is to store excess dietary fat in the form of triglycerides (TGs) and to release free fatty acids (FFAs) in times of starvation or energy demand. In addition WAT releases several important factors that regulate energy homeostasis. Lipolysis takes place in the cytosol and is also dependent on processes in the mitochondria. The resulting FFA can be released to the blood or directly used as an energy source by β-oxidation in the mitochondria. In lipogenesis, glycerol and acyl-CoA produced in the mitochondria are used to generate TG for storage in the adipocytes. (B) In brown adipose tissue (BAT) the FFAs are β-oxidized, and the resulting acetyl-CoA enters the Krebs cycle. The reduction potential is used to form a mitochondrial proton gradient that instead of producing ATP is released by uncoupling protein 1 (UCP1), a process that generates heat.
Metabolic syndrome is a group definition on several diseases connected to obesity. Subjects will display elevated levels of glucose and nonesterified fatty acids, also called free fatty acids (FFAs), and show symptoms of one or more afflictions amongst which hypertension, hypertriglyceridemia, diabetes, and obesity are the most usual. Lipotoxitcy and the accumulation of saturated fat in peripheral tissues are an important step in the development of metabolic syndrome. Fatty liver (liver steatosis) is found in a large proportion of individuals with body mass index (BMI) above 30. Liver steatosis contributes to insulin resistance and may lead to the development of nonalcoholic fatty liver diseases (NAFLDs), including nonalcoholic steatohepatitis (NASH) and cirrhosis [4].
T2DM is usually linked to obesity and lipid redistribution to nonadipose organs and tissues and is caused by a reduced sensitivity to insulin in muscle, liver, and adipose tissue [1]. During insulin resistance, an increase in insulin does not lead to a corresponding increased uptake of glucose in muscle and liver. As a compensatory mechanism insulin production is increased in pancreatic beta cells. T2DM occurs when the increased insulin production is no longer sufficient, or when lipid-induced beta cell malfunction takes place, particularly in genetically predisposed individuals. It is believed that high levels of circulating FFA and lipotoxic mechanisms can cause damage on β-cell and nonislet tissues.
CVD includes hypertension, atherosclerosis, heart disease, vascular disease, and stroke. Several of the high-risk factors for CVD result directly or indirectly from obesity and a malfunctioning adipose tissue: dyslipidemia, chronic inflammation, diabetes, and hypertension. Mitochondrial dysfunction could also contribute to CVD due to their role as targets and sources of reactive oxygen species (ROS), both in the formation of vascular lesions and in their involvement in the development of insulin resistance and T2DM [5].
A well-functioning adipose tissue with capacity to neutralize and store nutritional overload is therefore necessary to protect the body from peripheral insulin resistance. Two closely linked processes in adipose tissue are important for the development of metabolic disease: adipose tissue inflammation and adipose tissue hypertrophy. Adipose tissue inflammation due to the recruitment of T-cells and macrophages has been shown to contribute to insulin resistance in obese individuals. This inflammation leads to a disturbed adipokine-balance and an uncontrolled release of free fatty acids and inflammatory cytokines [6]. As a result, lipoprotein metabolism and insulin sensitivity in other organs will be affected. During obesity, the adipocyte size increases (hypertrophy), and they are eventually unable to store excess lipids even with enhanced adipocyte proliferation (hyperplasia). This redirects fatty acids to the liver promoting dyslipidemia, characterized by elevated plasma FFA, triglycerides (TGs), and small dense low-density lipoprotein (LDL), and the reduction of high-density lipoproteins (HDLi). In addition large adipocytes are characterized by an increased lipolysis, the breakdown of stored TG by lipases. This results in FFA and glycerol, which further increases plasma FFA-levels and upregulates TG synthesis in the liver [7]. A recent publication demonstrated that enlarged adipocytes in obese men had a reduced rate of FFA-delivery to the blood stream, resulting in normal systemic FFA concentrations. However, after meals the adipose tissue storage capacity was severely impaired, thus potentially causing ectopic lipid deposition [8].
How well the adipose tissue functions as a storage organ for excess energy intake and the distribution of fat and mitochondria between the different adipose depots may determine the large differences in health observed in obese individuals. Here, we will first focus on the importance of mitochondrial function in adipose tissue and then describe the different adipose tissue depots, in rodent animal models as well as humans (Figure 1), and their impact on the development of metabolic diseases. Pharmacological treatments that specifically target mitochondria have great potential in the treatment of obesity-related disorders, and their differential effect on adipose depots will be discussed.
2. Reactive Oxygen Species during Obesity
An elevated metabolism during obesity results in increased production of reactive oxygen species (ROS). ROS damage is most likely involved in all features of metabolic syndrome. Increased ROS production is an early event in glucose intolerance, contributing to pancreatic β-cell dysfunction as well as liver steatosis. β-cells may be specially susceptible to ROS-damage due to their relatively low expression of free-radical-detoxifying enzymes compared to most other cell types [9].
There are indications that mitochondrial content and function in adipose tissue might be disrupted in metabolic disorders. It has been suggested that insulin resistance is linked to mitochondrial dysfunction [10, 11]. Furthermore, it is increasingly evident that oxidative capacity and thus mitochondria are fundamental to avoid the development of toxicity during excess energy intake. For instance, increase in mitochondrial ROS is observed in adipose tissue during obesity [12–14], and in skeletal muscle, the mitochondrial H2O2 emission is higher using fatty acids as substrates versus carbohydrate-based substrates [15, 16]. Two models of insulin resistance, induced by either tumor necrosis factor α (TNFα) or glucocorticoid treatment, were both shown to involve increased ROS levels [17]. Also, antioxidants attenuating mitochondrial H2O2 emission completely restored insulin sensitivity in skeletal muscle [16]. These findings show that mitochondria play a central role in the physiology leading to insulin resistance.
In addition, the NOX family of NADPH oxidases, located to the plasma membrane, cytosol and cytosolic membranes, is a major source of ROS generation. NOX2 is an energy transporter that catalyses the reduction of oxygen to O2− in an NADPH-dependent reaction. The involvement of NOX enzymes in the development of metabolic syndrome has been reviewed by Krause and coauthors [18, 19]. In low concentrations, ROS participates in the cell signaling as secondary messengers [20]. In response to insulin, NOX will release ROS that activates the distal insulin-signaling cascade, mobilizing glucose transporters to the surface of adipocytes. However in higher amounts it will have serious harmful effects on the cells, including decreased glucose uptake in adipocytes [21]. Several mouse obesity models have found an increase in oxidative stress in adipose tissue associated with overexpression of NADPH oxidase and repression of antioxidative enzymes such as catalase [13]. Also, mitochondrial proliferation is inhibited by increased ROS production [22, 23], indicating that ROS can modulate adipocyte generation.
3. Mitochondria in Adipose Tissue
Well-functioning mitochondria are essential in adipose tissue. Mature adipocytes require large amounts of ATP to maintain their activities such as lipolysis, fatty acid β-oxidation, and fatty acid synthesis. In the organism, mitochondria produce energy and are central in the fatty acid metabolism since more than 98% of fatty acids are oxidized in mitochondria [24]. Recent evidence indicates that a reduced mitochondrial function and altered biogenesis play important roles in the etiology of obesity, insulin resistance, and T2DM [25]. An impaired metabolic flexibility is the inability of an organism to adapt fuel oxidation to fuel availability. The ability to switch from fat to carbohydrate oxidation is usually impaired in subjects prone to obesity and in subjects with a family history of diabetes [26–29]. Indeed, there is strong evidence that defects in substrate switching cluster together with disturbances in mitochondrial content and/or function and might be a manifestation of an underlying mitochondrial disorder [30]. In this respect, it has been demonstrated that insulin-resistant offspring of patients with T2DM have reduced mitochondrial function, reduced ATP synthesis and accumulation of fat in skeletal muscle, the liver, and other cells [31].
3.1. Role of White Fat Mitochondria
The major adipose depots in the body consist of white adipose tissue (WAT). Mature white adipocytes require large amounts of ATP to maintain their diverse functions, and during adipogenesis mitochondrial biogenesis is an important process [20]. One of the main tasks of adipose tissue is to generate glycerol 3-phosphate and acetyl-CoA for esterification into TGs (lipogenesis), processes localized to the mitochondrial matrix as well as the cytosol, that require an abundant mitochondrial population [32, 33]. Mitochondria in WAT are also believed to be important for the regulation of lipolysis, the process of degrading TG into FFA and glycerol (Figure 2(A)). When inhibiting the mitochondrial respiratory chain or decreasing intracellular ATP by the use of mitochondrial uncouplers, lipolysis is inhibited [34]. Inhibition of the AMP-activated protein kinase (AMPK), which is an essential molecular sensor regulated by the ATP/AMP level in the cells, will decrease lipolysis stimulated by agonists of β-adrenoreceptors [35]. The FFAs produced by lipolysis can subsequently be utilized in the adipocytes as a source of energy, through β-oxidation in the mitochondria. Thus, mitochondria in white fat cells are involved in both de novo lipogenesis and lipolysis [36].
Depending on the anatomical position in the body, adipose tissue shows differences in metabolic activity due to different mitochondrial density. For example, rat epididymal (VAT) adipocytes have more mitochondria than inguinal (SAT) adipocytes [37]. The higher number of mitochondria per mg tissue in VAT than SAT is also observed in obese human individuals. Although mitochondrial respiratory flux per cell and per mitochondrial content was lower in VAT compared to SAT due to smaller cells, the visceral fat was bioenergetically more active and responsive to substrates of the electron transport chain [38]. In general, some studies show that obesity and T2DM in humans reduce mitochondrial number in white adipocytes [39], while others show a connection between mitochondrial number and lipogenesis, but not body mass index (BMI) or overall insulin sensitivity [40]. The link between mitochondrial dysfunction and T2DM is indicated by the reduced oxidative phosphorylation (OXPHOS) capacity in elderly subjects [41], and in obese subjects, where a reduced FA β-oxidation in several tissues including adipocytes will increase blood FFA levels and alter glucose uptake [10, 11]. Nonalcoholic steatohepatitis, a disease caused by lipid accumulation in the liver, is associated with mitochondrial dysfunction due to increased lipid peroxidation, alterations in mitochondrial ultrastructure, depletion in mtDNA, and low OXPHOS activity. These patients also commonly display abdominal obesity, diabetes, and hypertriglyceridemia with insulin resistance [42]. In rodent genetic or high-fat fed obesity models, the expression of genes involved in mitochondrial ATP production, energy uncoupling and other genes important for mitochondrial function was downregulated compared to lean control animals [43]. This indicates a strong impairment of mitochondrial biogenesis during obesity.
3.2. Role of Brown Fat Mitochondria
Although brown adipose tissue (BAT) is mainly found in newborns, recent studies have demonstrated its presence in adults [44, 45]. BAT originates from a different cellular lineage than WAT [46], but they share many features. However, mature BAT contains a higher number of mitochondria and lower numbers of lipid droplets. During cold temperatures and activation of the sympathetic nervous system [47], brown adipocyte lipolysis occurs (Figure 2(B)). Instead of being released for subsequent oxidation in organs such as liver and skeletal muscle, the resulting FFAs are oxidized in the BAT mitochondria and used to generate heat (see review in [48]). This process involves transport of FFA complexed to fatty acid binding proteins (FABPs) into the mitochondria by the carnitine shuttle system, where they are oxidized and used to generate heat by uncoupling protein 1 (UCP1), a process called nonshivering thermogenesis. This process is important in newborn humans and in rodents, but appears to be almost lost in adult humans. Although UCP1 is the hallmark of BAT, increased UCP1 expression and energy expenditure can also be seen in WAT under some conditions [49] and may be a desirable effect during the treatment of obesity. The increased expression of the Ucp1 gene in both BAT and WAT by a fat-specific aP2 promoter generated mice partially resistant to age-related obesity, both genetically and high-fat diet induced [50, 51]. The effect of BAT on obesity is further discussed below.
4. Transcriptional Regulation of Lipolysis and Lipogenesis
Peroxisome proliferator-activated receptors (PPARs) are important regulators of fatty acid metabolism. They serve as lipid sensors since they are activated by metabolic derivates of fatty acids in the body. There are three PPAR superfamily members PPARα, PPARγ, and PPARδ (also called PPARβ) that act upon ligand activation by controlling networks of target genes. It exceeds the scope of this paper to describe their function in detail, but the relevant PPARs will be mentioned briefly here. For a comprehensive review on the role of PPARs in metabolic syndrome, see Guri et al. [52].
PPARα is the most important PPAR in the liver and has many important target genes involved in fatty acid β-oxidation. During fasting, lipolysis is stimulated while lipogenesis is decreased. The increase in lipolysis provides FFAs that are used as energy source in other tissues [53]. The subsequent PPARα-regulated metabolism of fatty acids mainly takes place in the liver, through mitochondrial and peroxisomal β-oxidation, but the heart, adipose tissue, and skeletal muscle can also utilize FFA for energy.
The decrease in lipogenesis is on the other hand due to downregulation of the two main lipogenic transcription factors, PPARγ and steroyl regulatory element binding protein 1c (SREBP1c) [54, 55]. Two isoforms of PPARγ exist, of which PPARγ1 is expressed in a wide variety of tissues, while PPARγ2 is mainly expressed in adipose tissue [56]. PPARγ activation stimulates preadipocyte differentiation, promotes the storage of fatty acids in mature adipocytes [56], and can activate GLUT4, facilitating increased fatty acid synthesis from glucose [57]. SREBP1c is necessary for the insulin-stimulated increase in fatty acid synthesis [58]. Its activation has been shown to create ligands for PPARγ [59], as well as regulate PPARγ expression in cultured adipocytes [60]. Lipogenesis mainly takes place in the cytosol, but the activation of PPARγ using rosiglitazone has been shown to alter both mitochondrial density and morphology in adipocytes suggesting that PPARγ also controls mitochondrial functions [36]. In obese db/db mice, the adiponectin expression and mitochondrial content of white adipocytes are reduced, and these effects are reverted by the activation of PPARγ by rosiglitazone [61]. It is interesting to note that rosiglitazone can increase expression of mitochondrial genes/mitochondrial biogenesis in rodent obesity models [43].
5. Differential Role of Fat Depots
Although the total adipose tissue is important for the development of insulin resistance, it is believed that some fat depots are more linked to risk factors for disease than others. The main adipose depots of interest are found in the abdomen and can be divided into the SAT and VAT, and the visceral tissue can again be divided into omental and mesenteric, the latter being the more deeply buried depot surrounding the intestine (Figure 1). Some studies have also included a deep subcutaneous layer (dSAT). The distribution of SAT and VAT shows person-to-person variations and is dependent on several factors such as age, nutrition, sex, and the energy homeostasis of the individual adipose tissues [62]. Both human studies and rodent obesity models will be discussed below. Although several similarities exist, the differences in rodent and human adipose tissues entail caution when choosing which depots to study and when extrapolating information between species [63].
5.1. Visceral Fat
Particularly, visceral fat depots, including omental and mesenteric adipose tissue, represent a risk factor for the development of CVD and T2DM. Visceral adipose tissue mass correlates with development of insulin resistance, while total or subcutaneous tissue mass does not [62, 64, 65]. It has been thoroughly confirmed that the adipocytes of visceral fat tissue are more lipolytically active than subcutaneous adipocytes and thus contribute more to the plasma free fatty acid levels [62, 66]. This was found in particular in diabetic obese individuals, where it was linked to a significant upregulation of leptin and downregulation of adiponectin gene expression in mesenteric VAT compared to SAT and omental VAT [67].
The metabolic activity of a cell is dependent on its mitochondrial content, and it has been shown that, in rats, epididymal (VAT) adipocytes have more mitochondria than inguinal (SAT) adipocytes [37]. In adipose tissue from obese individuals undergoing bariatric surgery the relative OXPHOS activity was found to be higher in omental VAT than SAT [38]. Individuals with a polymorphism in the UCP1 promotor reducing UCP1 gene expression are prone to have a high BMI, in particular due to abdominal obesity [68]. Thus, the level of mitochondrial uncoupling and energy efficiency may have an effect on obesity in WAT as well as BAT. The higher expression level of beta-adrenergic receptors in VAT could contribute to the higher lipolytic activity [69]. In addition, insulin-stimulated glucose uptake was found to be higher in VAT compared to SAT [70]. As a result, excess visceral fat will enhance the level of free fatty acid delivered to the liver, thus increasing hepatic glucose and very low-density lipoprotein particles (VLDLs) output, and impair the hepatic insulin response.
The expression of PPARγ mRNA is increased in the adipose tissue of obese subjects [71], and it has been shown that while there was no difference in omental VAT and SAT, PPARγ was significantly higher expressed in mesenteric VAT and remarkably so in obese diabetic subjects [67]. This supports the involvement of PPARγ in mesenteric adipose tissue lipolysis. In rats, lipid synthesis is shown to be higher in internal adipose tissues compared to SAT [72]. A study comparing the gene expression pattern in rat retroperitoneal (rVAT), mesenteric VAT (mVAT), and inguinal SAT (iSAT) showed that the larger cells in rVAT expressed high amounts of the lipogenic transcription factors Pparγ and Srebp1c compared to mVAT and iSAT that showed a high expression of lipogenesis-related genes and a low expression of fatty acid oxidation-related genes [73]. They also showed that the genes involved in lipid metabolism changed more rapidly as a result of fasting in the internal rVAT depot compared to SAT [74].
5.2. Subcutaneous Fat
As SAT is less metabolically active than VAT, it may have better short-term and long-term storage capacity. Thus, this depot is important to accumulate TG in periods of excess energy intake and supply the organism with FFAs in periods of fasting, starvation, or exercise. Another suggested role of SAT is to be a buffer during intake of dietary lipids, thus protecting other tissues from lipotoxic effects [75].
In humans, SAT is anatomically divided by a stromal fascia (fascia superficialis) into superficial (sSAT) and deep subcutaneous adipose tissue (dSAT), with distinct histological features. While sSAT has not been linked to risk for T2DM, the size of dSAT depots is significantly linked to the fasting insulin level and insulin-stimulated glucose utilization, as is total fat and VAT [76, 77]. The association between dSAT and insulin resistance is particularly seen in male obese patients [77, 78]. When studying the expression and secretion of hormones and cytokines in lean subjects, dSAT was found to be more similar to VAT than sSAT [79]. Interestingly, SAT seem to have an exclusive role in leptin secretion, since it correlates with plasma leptin levels (in contrast to plasma insulin levels which correlate with inter abdominal fat [80]). Rat studies have shown that Pparγ2 expression is higher in male than in female SAT [81], indicating a gender-linked variation in this adipose depot.
5.3. Gluteofemoral Fat
The subcutaneous gluteofemoral fat tissue is measured by hip or thigh circumference, or leg adipose tissue mass. Accumulation of fat in this depot is believed to have a protective role against diabetes and cardiovascular disease [82]. Indeed, low amount of this tissue has been associated with an unfavorable lipid and glucose profile [83]. Likewise, an increase in gluteofemoral tissue size has been connected to increase in HDL-cholesterol and decrease in total- and LDL-cholesterol levels in several studies [83–85]. Gluteofemoral fat is also positively associated with adiponectin and leptin serum levels [86]. Tracing of specific lipid fraction in blood samples from veins draining femoral or abdominal fat showed that there was a lower metabolic flux from femoral fat [87] and that femoral fat had a preference for uptake of FFA and VLDL-TG compared to chylomicron-TG, thus accumulating recycled fat rather than dietary fat.
The gluteofemoral fat tissue may thus provide protection from ectopic deposition of excess fat. While the abdominal subcutaneous adipose tissue has a role as buffer during daily fatty acid intake [75], the gluteofemoral fat tissue may have an important role in TG storage. Since it shows less metabolic activity and is more lipolytically inert than upper-body adipose depots, it seems to be involved in the long-term sequestering of fatty acids.
5.4. Intermuscular Fat
Few studies have focused on intermuscular adipose tissue (IMAT), and little is known about its specific metabolic activities. However, due to its increased level in T2DM patients, IMAT has been suggested be a risk factor along with VAT for the development of obesity-related diseases [88]. The amount of IMAT seems to be associated with age and lack of activity [89], and increases the likelihood of metabolic abnormalities in elderly subjects with normal body weight. In one study, only one third of men and less than half of women with T2DM were obese, but in the normal weight subjects, high amounts of IMAT was associated with higher fasting insulin levels [90]. Also, IMAT correlates negatively with glucose infusion rate [91], and fasting glucose and total cholesterol level in Caucasians [92], indicating its involvement in the development of insulin resistance. The amount of IMAT seem to be hereditary since African-Americans, who are also prone to develop T2DM, have more of this fat type [93].
5.5. Epicardial Fat
Epicardial adipose tissue (EAT) is the visceral fat layer located around the heart and is believed to be important for the buffering of the coronary arteries, and in providing fatty acids as a source of energy for the cardiac muscle. The release of adiponectin and adrenomedullin could have a protective effect on the heart during metabolic or mechanical insults [94]. On the other hand, it has recently been shown that EAT will locally influence heart and vasculature through the secretion of pro-inflammatory cytokines and will contribute to coronary atherosclerosis [95–97]. Studies indicate that the amount of EAT can be related to carotid artery stiffness in obese patients with hypertension, while waist circumference shows no statistically significant link [98]. Age seem to determine the risk associated with epicardial fat, since there is no relationship between EAT thickness and insulin resistance and metabolic syndrome in obese children [99].
A study of patients undergoing coronary artery bypass surgery showed that the fat layer closest to the heart expressed 5-fold higher UCP1 mRNA than the more distal substernal fat, while UCP1 expression was undetectable in subcutaneous thoracic fat [100]. The UCP1 expression was influenced by age and body mass index, but showed no relationship to epicardial fat volume, waist circumference, metabolic syndrome or T2DM. This, along with the increased expression of brown adipocyte differentiation transcription factors PRDM16 and PGC-1α, indicate that epicardial fat may have a function similar to brown fat. This could possibly give an additional protection of the myocardium and coronary vessels from hypothermia, but its effect on obesity-linked disease is unknown.
5.6. Gonadal Fat
Gonadal VAT is one of the largest adipose depots in rodents and is found around the testis of males (epididymal) and around the ovaries of females (periovarian). This depot is specifically increased in rodents fed a cafeteria diet. Gonadal VAT has been shown to express more of Pparγ and Srebp1c as well as another key adipogenic transcription factor, CCAAT enhancer-binding protein alpha (C/EBP-alpha) compared to SAT [101]. Interestingly, PPARγ2 protein was found at a significantly higher level in female than in male rat perigonadal adipose tissue [81], indicating that sex hormones can affect the regulation of PPARγ2 in WAT. It is possible that this could contribute to the gender differences observed with different PPAR agonists.
5.7. BAT
The largest region of brown adipose tissue is found in the upper back region of rodents (interscapular BAT). In humans, small areas are found in the thorax region (supraclavicular), and in the chest and abdomen [44]. There are indications that the nonshivering heat generation is important for the development of obesity [48]. Mice lacking UCP1 demonstrates increased obesity with age when fed high-fat diets [102], and selective destruction of BAT leads to obesity and reduced energy expenditure and insulin resistance [103]. Interestingly, overnurished rat pups displayed excess weight gain, reduced thermogenic capacity, and lower levels of UCP1 as adults [104]. It seems that reduced amount and thermogenic capacity of BAT may contribute to a life-long predisposition for obesity. Although adult humans possess only a small percentage of BAT compared to WAT, the possibility to pharmacologically increase BAT amount or activity would be a potential treatment for obesity. Recent studies have demonstrated that the forced expression of transcription factors PRDM16 and C/EBP-beta is sufficient to generate brown fat cells from myoblastic precursors [105]. The activation of PPARγ by different agonists has been shown to induce the expression of brown adipose genes in white adipocytes [106], the differentiation of brown adipose progenitor cells [107], and the proliferation of BAT in rats and monkeys [108]. However, long-term activation of the sympathetic nervous system by drugs such as ephedrine and BAT proliferation with the PPARγ agonist the thiazolidinedione darglitazone have been linked to numerous side effects [108, 109].
6. Targeting Mitochondrial Function by Bioactive Components
6.1. The Effect of Dietary Supplementation with Bioactive Lipids
Adipose tissue plays a major role in the inflammation, insulin resistance, and dyslipidemia associated with obesity. It is therefore beneficial to use compounds that therapeutically target adipose tissue to avoid high-risk complications of obesity such as T2DM and CVD. Dietary lipids may influence the energy balance and are shown to have a differential effect on different fat depots. Studies in rats have shown that fish oil or n-3 polyunsaturated fatty acids (n-3 PUFA) intervention reduces epididymal and perirenal adipose tissues although the body weight stays constant [110, 111]. It is believed that fish oil can affect adipose tissue by the activation of PPARα and γ, leading to decreased lipolysis, improved lipid storage capacity in subcutaneous adipose tissue, as well as anti-inflammatory effects possibly due to inhibitory action on NF-κB [112]. It has been shown that fish oil at 15% (w/w) level of substitution in a 35% high-fat diet prevented fat accumulation in C57BL/6 mice, preferentially in abdominal fat depots [113–115]. This dose of fish oil also induced mitochondrial biogenesis in white fat, with a stronger effect in epididymal than in subcutaneous adipose tissue [114]. AMPK could be involved in this metabolic switch that increases adipocyte fatty acid metabolism, decreases lipolysis, and upregulates mitochondrial biogenesis [116]. The anti-inflammatory effect of n-3 PUFA in mice has been associated with increased adiponectin secretion [116, 117], possibly resulting from AMPK activation. This induction of adiponectin is probably mediated by PPARγ and is higher in epididymal fat than in SAT [118].
The artificially made 3-thia sulphur fatty acid tetradecylthioacetic acid (TTA) has a targeted effect on mitochondria and exhibit high potency compared to fish oil, both with regard to increased fatty acid β-oxidation and anti-inflammatory effects [119]. TTA thus shows great promise in the treatment of metabolic syndrome (Figure 3). It is a 16-carbon saturated fatty acid where a sulfur atom is inserted between the second and the third carbon in the β-position, thus making it unavailable for fatty acid β-oxidation. The mechanism of action of TTA is based mainly on the activation of PPARs, which induce mitochondrial biogenesis and enhance fatty acid oxidation in the liver [119–121]. This has been suggested to remodel WAT tissues due to drainage of fatty acids towards the liver. TTA was shown to specifically reduce epididymal fat in young obese Zucker (fa-fa) rats and epididymal and retroperitoneal fat in male Wistar rats fed a high-fat diet for 3 weeks [122]. To further study the effect of TTA-induced adipose tissue remodelling, Wistar rats were fed a high-fat lard diet (40% energy from fat) for 7 weeks, and the distribution of fat was studied by magnetic resonance imaging (MRI). The adipose tissue mass was significantly reduced in the TTA-fed rats, particularly in the perirenal and epididymal depots [123]. Interestingly, while most of the genes involved in lipolysis, fatty acid β-oxidation, mitochondrial biosynthesis, and immune response were unchanged by TTA in all studied adipose tissues, Ucp1 was highly increased at the mRNA level in epididymal and mesenteric depots [123]. Also, a de novo production of Ucp3 in the liver was detected both at the mRNA and protein level along with high induction of genes involved in β-oxidation [123]. The possible increased energy expenditure due to uncoupling of mitochondria combined with the enhanced hepatic fatty acid β-oxidation supports the hypothesis of hepatic lipid drainage. As a result, TTA treatment will channel TGs to the liver for β-oxidation and facilitate adipose tissue lipolysis. The higher metabolic activity of the VAT tissues could explain the specific reduction of these risk-linked depots, perhaps also influenced by their increase in BAT-like features (Figure 2). Although the effect of TTA is mostly due to activation of hepatic mitochondrial genes, activation of some adipose genes involved in β-oxidation was observed. The lipogenic genes acetyl-CoA carboxylase alpha (Acaca) and fatty acid synthase (Fasn) were induced by TTA in perirenal and epididymal VAT [123]. In a directly comparable long-term rat study, the 3-n PUFA eicosapentaenoic acid (EPA) was found to increase Acaca and Fasn in VAT while Cpt2 mRNA-level was increased in mesenteric VAT, indicating an increase in genes involved in fatty acid degradation also with marine oils [124]. Ucp1 was on the other hand increased only by TTA. Similar results have been found in human adipocytes (not published results). TTA and EPA were able to significantly increase the gene expression of AMPK, CPT2, UCP1, and acyl-coA oxidase 1 (ACOX1), but TTA induced a higher level of AMPK and UCP1. Importantly, AMPK is believed to function as a metabolic switch resulting in upregulation of FA oxidation in parallel with reduced maloyl-CoA level and downregulated lipogenesis and TG biosynthesis.
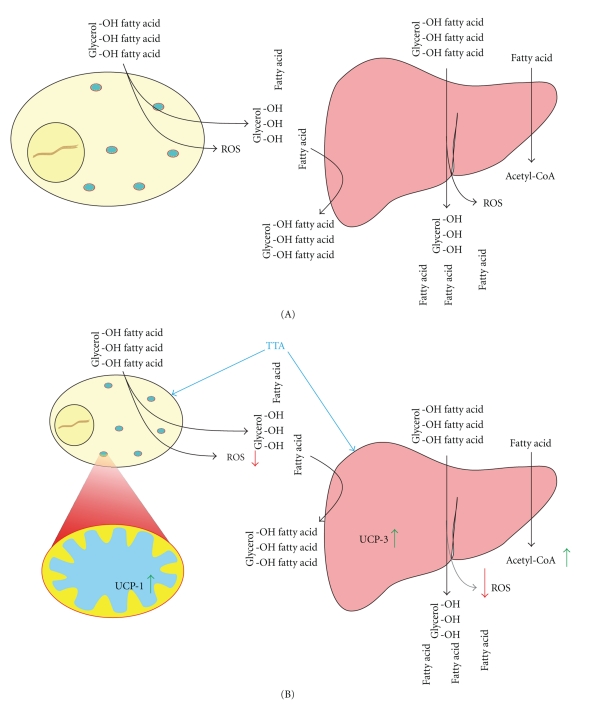
Figure 3.
(A) During obesity, the adipocytes will have reduced lipid storage capacity, leading to increased lipolysis and release of free fatty acids (FFAs), inflammatory agents, and disturbed adipokine release. The FFAs will be repackaged to triglycerides (TGs) in the liver where they are released as very low-density lipoprotein particles (VLDL). Together this has secondary effects on organs such as skeletal muscle and pancreas, as well as liver, and can result in hyperlipidemia, tissue lipid deposition, mitochondrial malfunction, insulin resistance, increased insulin production, and pancreatic β-cell disruption. (B) The effect of tetradecylthioacetic acid-(TTA-) treatment on metabolic syndrome. In the liver, TTA treatment will increase the degradation of FFA, by the induction of both mitochondrial and peroxisomal genes involved in β-oxidation. The excess FFA released from adipose tissue during metabolic syndrome (hyperlipidemia) is drained from the blood, thereby reducing TG, cholesterol, and FFA levels. In addition the TTA-induced increase in uncoupling protein 3 (UCP3) could increase energy expenditure, as well as function as a protection from the excess ROS production observed with obesity and high levels of FA degradation. The effect of TTA on liver is probably due to PPARα-mediated mechanisms, while the effect on adipose tissue may arrive from PPARγ as well as PPARα activation. In adipose tissue, the main effect of TTA is an increase in the brown adipose tissue marker Ucp1 in visceral adipose tissue (VAT) indicating higher energy expenditure and heat production. The higher metabolic activity of VAT compared to SAT will cause it to be the major source of FFA during increased hepatic β-oxidation. Together, this may explain the specific reduction of these risk-linked adipose depots with TTA-treatment.
The mitochondrial gene pyruvate kinase (Pklr) was upregulated by TTA specifically in perirenal VAT [123], indicating mitochondrial biogenesis in this fat depot. This is important as the generation of new mitochondria is severely compromised in several obesity-models [36, 43]. In accordance, a coactivator central for mitochondrial biogenesis, PPAR coactivator 1α (Pgc-1α) was reduced in a rodent obesity model [125] and in morbidly obese humans [126]. Although TTA reduces dyslipidemia in diabetic patients [121], a direct effect on glucose metabolism and insulin sensitivity is more disputed [121, 123, 127]. A reduced amount of risk-related adipose tissue in obese individuals would however be important to reduce the likelihood of developing disease. The efficient reduction of plasma FFA and TG by TTA, seen in both human and animal studies [128], may also prevent ectopic lipid deposition and lipotoxic effects.
TTA induces mitochondrial biogenesis in liver, but the effect on mitochondrial respiration has not been fully elucidated. Although the number of mitochondria increases in the liver during TTA-treatment, the hepatocytes are enlarged, and the respiration per mg tissue stays constant (not published results). Little work has been done to measure oxygen consumption/OXPHOS in adipose tissue, probably due to the difficulties caused by a low mitochondrial density in adipocytes compared to skeletal muscle [129]. Some studies have successfully determined mitochondrial OXPHOS in cells or organelles isolated from both brown and white adipose tissues [130], and VAT and SAT have been compared using similar techniques [38]. Information on mitochondrial OXPHOS activity will be of importance when testing for improved adipose tissue function during treatment with mitochondrial-targeted compounds.
6.2. Regulation of Gene Expression in the Treatment of Obesity-Related Diseases
PPAR is the target of many agents that restore insulin sensitivity. However, they do not necessarily decrease adipose tissue size. Thiazolidinediones (TZDs), such as pioglitazone and rosiglitazone, activate PPARγ and are associated with increased body weight due to the ability to stimulate preadipocyte differentiation [131, 132]. In particular the retroperitoneal VAT [133, 134] and intermuscular fat infiltration was increased by TZDs [134]. Studies show that the activation of PPARα by fenofibrates or oleoyletanolamide reduces adiposity and increases lipolysis [132], while a pan-PPAR ligand, LM 4156, had no effect on adipose tissue size [134]. Another study in accordance with this showed that a PPARα agonist reduced feed intake and body weight gain but had less effect on glucose intolerance (GI), a PPARγ agonist improved GI and adiponectin release but enhanced feed intake and body weight gain, while an agent with dual PPAR-activation demonstrated a combined, more beneficial effect [135]. TTA activates both PPARα and γ, the former with higher efficiency [136–138], and this may partly explain the effect on lipid degradation in the liver, as well as the reduced weight gain and hepatic induction of UCP3 primarily associated with PPARα activation [132, 139]. However, we have shown the existence of PPAR dependent and independent induction of UCP2 by TTA [140]. Therefore, mechanisms not involving PPAR-activation may also be important for the effects seen with TTA.
6.3. ROS Generation and TTA as an Antioxidant
There is mounting evidence that the increased release of H2O2, mainly originating from mitochondria, may be an important step in the development of insulin resistance in different tissues during high-fat intake [15, 16]. Mitochondrial damage is linked to insulin resistance and could be a secondary effect of the increase in ROS [141]. A number of studies have shown that treatment with antioxidants will both improve oxidative stress and restore insulin sensitivity [141–143]. While antioxidants studied so far have had little effect on human atherogenesis, it could be worthwhile to investigate mitochondrial-targeted antioxidants in the treatment of CVD [5].
TTA mainly affects mitochondrial functions and is a very potent antioxidant [144, 145] and anti-inflammatory agent [120, 146], both of which may be important for its reduction of dyslipedemia in diabetic patients [121]. TTA inhibits the lipoprotein oxidation in rats, indicating that its effects as an antioxidant may influence the development of atherosclerosis [145]. It also reduces the stenosis development after balloon angioplasty injury of rabbit iliac arteries [147]. Recently we have shown downregulated expression of PPAR target genes and reduced mitochondrial fatty acid oxidation in the liver of mice transgenic for hTNFα [148]. TTA enhanced the hepatic fatty acid oxidation in these animals (in preparation). Moreover, TTA reduced the hepatic gene expression of TNFα and visfatin [123].
7. Conclusions
The different adipose depots have specific roles based on their level of lipolysis and rate of TG storage. It is becoming clear that while abdominal visceral adipose tissue increases the risk of obesity-related disease, subcutaneous adipose tissue, especially located to the lower-body parts, protects from lipotoxic effects through short-term and long-term storage of TGs. In rodent studies, intake of bioactive lipids gives specific reduction of risk-related adipose tissues, and this targeted effect may be due to the higher metabolic activity of these depots. Treatments that increase energy expenditure through “mild uncoupling” of mitochondria and fatty acid β-oxidation show great promise in the treatment of obesity. The artificial fatty acid TTA is an inducer of these processes. Enhanced expression of uncoupling proteins together with an induction of hepatic β-oxidation suggests that TTA may increase energy consumption via increased uncoupling in liver and/or WAT. In addition its activity as an antioxidant and anti-inflammatory agent will have a direct positive effect on the diseases linked to obesity, such as T2DM and CVD.
Abbreviations
- AMPK:
Adenosine monophosphate-activated protein kinase
- BAT:
Brown adipose tissue
- CPT:
Carnitine palmitoyltransferase
- CVD:
Cardiovascular disease
- DMT2:
Diabetes mellitus type 2
- dSAT:
Deep subcutaneous adipose tissue
- EAT:
Epicardial adipose tissue
- FFA:
Free fatty acid
- IMAT:
Intermuscular adipose tissue
- ME:
Mesenteric adipose tissue
- MRI:
Magnetic resonance imaging
- NOX:
NADPH oxidase
- OXPHOS:
Oxidative phosphorylation
- PPAR:
Peroxisome proliferator-activated receptor
- PUFA:
Polyunsaturated fatty acid
- ROS:
Reactive oxygen species
- SAT:
Subcutaneous adipose tissue
- sSAT:
Superficial subcutaneous adipose tissue
- TG:
Triglyceride
- TTA:
Tetradecylthioacetic acid
- UCP:
Uncoupling protein
- VAT:
Visceral adipose tissue
- WAT:
White adipose tissue.
References
- 1.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annual Review of Biochemistry. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 2.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. Journal of Lipid Research. 2007;48(6):1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frühbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods in Molecular Biology. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Puddu P, Puddu GM, Cravero E, de Pascalis S, Muscari A. The emerging role of cardiovascular risk factor-induced mitochondrial dysfunction in atherogenesis. Journal of Biomedical Science. 2009;16:112–120. doi: 10.1186/1423-0127-16-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Current Diabetes Reports. 2009;9(1):26–32. doi: 10.1007/s11892-009-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Hormone and Metabolic Research. Supplement. 1988;19:26–29. [PubMed] [Google Scholar]
- 8.McQuaid SE, Hodson L, Neville MJ, et al. Down-regulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60(1):47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Biology and Medicine. 1996;20(3):463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 10.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 11.Maassen JA. Mitochondria, body fat and type 2 diabetes: what is the connection? Minerva Medica. 2008;99(3):241–251. [PubMed] [Google Scholar]
- 12.Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-δ activation in adipocytes of obese, insulin-resistant mice. American Journal of Physiology. 2003;285(2):E295–E302. doi: 10.1152/ajpendo.00044.2003. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis JM, Grimsrud PA, Wright WS, et al. Downregulation of adipose glutathione S-tansferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. The Journal of Biological Chemistry. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 16.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. The Journal of Clinical Investigation. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 18.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 19.Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochemical Society Transactions. 2008;36(5):920–929. doi: 10.1042/BST0360920. [DOI] [PubMed] [Google Scholar]
- 20.Wilson-Fritch L, Burkart A, Bell G, et al. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Molecular and Cellular Biology. 2003;23(3):1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein BJ, Kalyankar M, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54(2):311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrière A, Fernandez Y, Rigoulet M, Pénicaud L, Casteilla L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Letters. 2003;550(1-3):163–167. doi: 10.1016/s0014-5793(03)00862-7. [DOI] [PubMed] [Google Scholar]
- 23.Carrière A, Carmona MC, Fernandez Y, et al. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. The Journal of Biological Chemistry. 2004;279(39):40462–40469. doi: 10.1074/jbc.M407258200. [DOI] [PubMed] [Google Scholar]
- 24.Rogge MM. The role of impaired mitochondrial lipid oxidation in obesity. Biological Research for Nursing. 2009;10(4):356–373. doi: 10.1177/1099800408329408. [DOI] [PubMed] [Google Scholar]
- 25.Højlund K, Mogensen M, Sahlin K, Beck-Nielsen H. Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinology and Metabolism Clinics of North America. 2008;37(3):713–731. doi: 10.1016/j.ecl.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Gaster M, Rustan AC, Aas V, Beck-Nielsen H. Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes. 2004;53(3):542–548. doi: 10.2337/diabetes.53.3.542. [DOI] [PubMed] [Google Scholar]
- 27.Blaak EE. Metabolic fluxes in skeletal muscle in relation to obesity and insulin resistance. Best Practice and Research: Clinical Endocrinology and Metabolism. 2005;19(3):391–403. doi: 10.1016/j.beem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. The Journal of Clinical Investigation. 2005;115(7):1699–1702. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ukropcova B, McNeil M, Sereda O, et al. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. The Journal of Clinical Investigation. 2005;115(7):1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. The New England Journal of Medicine. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franckhauser S, Muñoz S, Pujol A, et al. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51(3):624–630. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- 33.Olswang Y, Cohen H, Papo O, et al. A mutation in the peroxisome proliferator-activated receptor γ-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(2):625–630. doi: 10.1073/pnas.022616299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fassina G, Dorigo P, Gaion RM. Equilibrium between metabolic pathways producing energy: a key factor in regulating lipolysis. Pharmacological Research Communications. 1974;6(1):1–21. doi: 10.1016/s0031-6989(74)80010-x. [DOI] [PubMed] [Google Scholar]
- 35.Daval M, Diot-Dupuy F, Bazin R, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. The Journal of Biological Chemistry. 2005;280(26):25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 36.Wilson-Fritch L, Nicoloro S, Chouinard M, et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. The Journal of Clinical Investigation. 2004;114(9):1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deveaud C, Beauvoit B, Salin B, Schaeffer J, Rigoulet M. Regional differences in oxidative capacity of rat white adipose tissue are linked to the mitochondrial content of mature adipocytes. Molecular and Cellular Biochemistry. 2004;267(1-2):157–166. doi: 10.1023/b:mcbi.0000049374.52989.9b. [DOI] [PubMed] [Google Scholar]
- 38.Kraunsoe R, Boushel R, Hansen CN, et al. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. Journal of Physiology. 2010;588(12):2023–2032. doi: 10.1113/jphysiol.2009.184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54(5):1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 40.Kaaman M, Sparks LM, van Harmelen V, et al. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007;50(12):2526–2533. doi: 10.1007/s00125-007-0818-6. [DOI] [PubMed] [Google Scholar]
- 41.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pessayre D, Fromenty B, Mansouri A. Mitochondrial injury in steatohepatitis. European Journal of Gastroenterology and Hepatology. 2004;16(11):1095–1105. doi: 10.1097/00042737-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Rong JX, Qiu Y, Hansen MK, et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56(7):1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 44.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 45.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. The New England Journal of Medicine. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 46.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. American Journal of Physiology. 1999;276(6):R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 48.Mattson MP. Perspective: does brown fat protect against diseases of aging? Ageing Research Reviews. 2010;9(1):69–76. doi: 10.1016/j.arr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochemical Journal. 2006;398(2):153–168. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopecky J, Clarke G, Enerbäck S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. The Journal of Clinical Investigation. 1995;96(6):2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopecky J, Hodny Z, Rossmeisl M, Syrovy I, Kozak LP. Reduction of dietary obesity in aP2-Ucp transgenic mice: physiology and adipose tissue distribution. American Journal of Physiology. 1996;270(5, part 1):E768–E775. doi: 10.1152/ajpendo.1996.270.5.E768. [DOI] [PubMed] [Google Scholar]
- 52.Guri AJ, Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptors: bridging metabolic syndrome with molecular nutrition. Clinical Nutrition. 2006;25(6):871–885. doi: 10.1016/j.clnu.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22(7-8):830–844. doi: 10.1016/j.nut.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. The Journal of Biological Chemistry. 2001;276(41):37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 55.Gosmain Y, Dif N, Berbe V, et al. Regulation of SREBP-1 expression and transcriptional action on HKII and FAS genes during fasting and refeeding in rat tissues. Journal of Lipid Research. 2005;46(4):697–705. doi: 10.1194/jlr.M400261-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Boon Yin K, Najimudin N, Muhammad TST. The PPARγ coding region and its role in visceral obesity. Biochemical and Biophysical Research Communications. 2008;371(2):177–179. doi: 10.1016/j.bbrc.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Wu Z, Xie Y, Morrison RF, Bucher NLR, Farmer SR. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. The Journal of Clinical Investigation. 1998;101(1):22–32. doi: 10.1172/JCI1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cagen LM, Deng X, Wilcox HG, Park EA, Raghow R, Elam MB. Insulin activates the rat sterol-regulatory-element-binding protein 1c (SREBP-1c) promoter through the combinatorial actions of SREBP, LXR, Sp-1 and NF-Y cis-acting elements. Biochemical Journal. 2005;385(1):207–216. doi: 10.1042/BJ20040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(8):4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fajas L, Schoonjans K, Gelman L, et al. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Molecular and Cellular Biology. 1999;19(8):5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koh EH, Park JY, Park HS, et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56(12):2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- 62.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocrine Reviews. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 63.Casteilla L, Pénicaud L, Cousin B, Calise D. Choosing an adipose tissue depot for sampling. Factors in selection and depot specificity. Methods in Molecular Biology. 2008;456:23–38. doi: 10.1007/978-1-59745-245-8_2. [DOI] [PubMed] [Google Scholar]
- 64.Chowdhury B, Sjostrom L, Alpsten M, Kostanty J, Kvist H, Lofgren R. A multicompartment body composition technique based on computerized tomography. International Journal of Obesity. 1994;18(4):219–234. [PubMed] [Google Scholar]
- 65.Hoffstedt J, Arner P, Hellers G, Lönnqvist F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. Journal of Lipid Research. 1997;38(4):795–804. [PubMed] [Google Scholar]
- 66.Hajer GR, van Haeften TW, Visseren FLJ. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European Heart Journal. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 67.Yang YK, Chen M, Clements RH, Abrams GA, Aprahamian CJ, Harmon CM. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cellular Physiology and Biochemistry. 2008;22(5-6):531–538. doi: 10.1159/000185527. [DOI] [PubMed] [Google Scholar]
- 68.Sramkova D, Krejbichova S, Vcelak J, et al. The UCP1 gene polymorphism A-3826G in relation to DM2 and body composition in Czech population. Experimental and Clinical Endocrinology and Diabetes. 2007;115(5):303–307. doi: 10.1055/s-2007-977732. [DOI] [PubMed] [Google Scholar]
- 69.Arner P, Hellstrom L, Wahrenberg H, Bronnegard M. Beta-adrenoceptor expression in human fat cells from different regions. The Journal of Clinical Investigation. 1990;86(5):1595–1600. doi: 10.1172/JCI114880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Virtanen KA, Lönnroth P, Parkkola R, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. Journal of Clinical Endocrinology and Metabolism. 2002;87(8):3902–3910. doi: 10.1210/jcem.87.8.8761. [DOI] [PubMed] [Google Scholar]
- 71.Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, et al. Peroxisome proliferator-activated receptor gene expression in human tissues: effects of obesity, weight loss, and regulation by insulin and glucocorticoids. The Journal of Clinical Investigation. 1997;99(10):2416–2422. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jamdar SC. Glycerolipid biosynthesis in rat adipose tissue. Influence of adipose-cell size and site of adipose tissue on triacylglycerol formation in lean and obese rats. Biochemical Journal. 1978;170(1):153–160. doi: 10.1042/bj1700153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palou M, Priego T, Sánchez J, Rodríguez AM, Palou A, Picó C. Gene expression patterns in visceral and subcutaneous adipose depots in rats are linked to their morphologic features. Cellular Physiology and Biochemistry. 2009;24(5-6):547–556. doi: 10.1159/000257511. [DOI] [PubMed] [Google Scholar]
- 74.Palou M, Sánchez J, Priego T, Rodríguez AM, Picó C, Palou A. Regional differences in the expression of genes involved in lipid metabolism in adipose tissue in response to short- and medium-term fasting and refeeding. Journal of Nutritional Biochemistry. 2010;21(1):23–33. doi: 10.1016/j.jnutbio.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Frayn K. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 76.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. American Journal of Physiology. 2000;278(5):E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 77.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 78.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. American Journal of Physiology. 2002;283(6):E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 79.Walker GE, Verti B, Marzullo P, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity. 2007;15(8):1933–1943. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 80.Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51(4):1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 81.Kadowaki K, Fukino K, Negishi E, Ueno K. Sex differences in PPARγ expressions in rat adipose tissues. Biological and Pharmaceutical Bulletin. 2007;30(4):818–820. doi: 10.1248/bpb.30.818. [DOI] [PubMed] [Google Scholar]
- 82.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. International Journal of Obesity. 2010;34(6):949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 83.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48(2):301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 84.Seidell JC, Pérusse L, Després JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. American Journal of Clinical Nutrition. 2001;74(3):315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 85.Terry RB, Stefanick ML, Haskell WL, Wood PD. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism. 1991;40(7):733–740. doi: 10.1016/0026-0495(91)90093-c. [DOI] [PubMed] [Google Scholar]
- 86.Buemann B, Astrup A, Pedersen O, et al. Possible role of adiponectin and insulin sensitivity in mediating the favorable effects of lower body fat mass on blood lipids. Journal of Clinical Endocrinology and Metabolism. 2006;91(5):1698–1704. doi: 10.1210/jc.2005-1062. [DOI] [PubMed] [Google Scholar]
- 87.McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes. 2010;59(10):2465–2473. doi: 10.2337/db10-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gallagher D, Kelley DE, Yim JE, et al. Adipose tissue distribution is different in type 2 diabetes. American Journal of Clinical Nutrition. 2009;89(3):807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. Journal of Nutrition, Health and Aging. 2010;14(5):362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 91.Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. Journal of Magnetic Resonance Imaging. 2009;29(6):1340–1345. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 92.Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. International Journal of Obesity. 2007;31(9):1400–1405. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. American Journal of Clinical Nutrition. 2005;82(6):1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Hormone and Metabolic Research. 2008;40(7):442–445. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 95.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obesity Reviews. 2007;8(3):253–261. doi: 10.1111/j.1467-789X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 96.Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Current Pharmaceutical Design. 2007;13(21):2180–2184. doi: 10.2174/138161207781039670. [DOI] [PubMed] [Google Scholar]
- 97.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. American Heart Journal. 2007;153(6):907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. European Journal of Echocardiography. 2009;10(4):549–555. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 99.Mazur A, Ostański M, Telega G, Malecka-Tendera E. Is epicardial fat tissue a marker of metabolic syndrome in obese children? Atherosclerosis. 2010;211(2):596–600. doi: 10.1016/j.atherosclerosis.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 100.Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. Journal of Clinical Endocrinology and Metabolism. 2009;94(9):3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 101.Rodríguez E, Ribot J, Rodríguez AM, Palou A. PPAR-γ2 expression in response to cafeteria diet: gender- and depot-specific effects. Obesity Research. 2004;12(9):1455–1463. doi: 10.1038/oby.2004.182. [DOI] [PubMed] [Google Scholar]
- 102.Kontani Y, Wang Y, Kimura K, et al. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4(3):147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 103.Hamann A, Flier JS, Lowell BB. Obesity after genetic ablation of brown adipose tissue. Zeitschrift für Ernahrungswissenschaft. 1998;37(supplement 1):1–7. [PubMed] [Google Scholar]
- 104.Xiao QX, Williams SM, Grayson BE, et al. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology. 2007;148(9):4150–4159. doi: 10.1210/en.2007-0373. [DOI] [PubMed] [Google Scholar]
- 105.Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-β transcriptional complex. Nature. 2009;460(7259):1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vernochet C, Peres SB, Davis KE, et al. C/EBPα and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor ³ agonists. Molecular and Cellular Biology. 2009;29(17):4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrovic N, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARγ agonist. American Journal of Physiology. 2008;295(2):E287–E296. doi: 10.1152/ajpendo.00035.2008. [DOI] [PubMed] [Google Scholar]
- 108.Aleo MD, Lundeen GR, Blackwell DK, et al. Mechanism and implications of brown adipose tissue proliferation in rats and monkeys treated with the thiazolidinedione darglitazone, a potent peroxisome proliferator-activated receptor-γ agonist. Journal of Pharmacology and Experimental Therapeutics. 2003;305(3):1173–1182. doi: 10.1124/jpet.102.042648. [DOI] [PubMed] [Google Scholar]
- 109.Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. The New England Journal of Medicine. 2000;343(25):1833–1838. doi: 10.1056/NEJM200012213432502. [DOI] [PubMed] [Google Scholar]
- 110.Belzung F, Raclot T, Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. American Journal of Physiology. 1993;264(6):R1111–R1118. doi: 10.1152/ajpregu.1993.264.6.R1111. [DOI] [PubMed] [Google Scholar]
- 111.Rustan AC, Hustvedt BE, Drevon CA. Dietary supplementation of very long-chain n-3 fatty acids decreases whole body lipid utilization in the rat. Journal of Lipid Research. 1993;34(8):1299–1309. [PubMed] [Google Scholar]
- 112.Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Medicine. 2008;34(9):1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 113.Ruzickova J, Rossmeisl M, Prazak T, et al. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;39(12):1177–1185. doi: 10.1007/s11745-004-1345-9. [DOI] [PubMed] [Google Scholar]
- 114.Flachs P, Horakova O, Brauner P, et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce β-oxidation in white fat. Diabetologia. 2005;48(11):2365–2375. doi: 10.1007/s00125-005-1944-7. [DOI] [PubMed] [Google Scholar]
- 115.Kuda O, Jelenik T, Jilkova Z, et al. N-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia. 2009;52(5):941–951. doi: 10.1007/s00125-009-1305-z. [DOI] [PubMed] [Google Scholar]
- 116.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clinical Science. 2009;116(1):1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 117.Rossi AS, Lombardo YB, Lacorte JM, et al. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. American Journal of Physiology. 2005;289(2):R486–R494. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]
- 118.Neschen S, Morino K, Rossbacher JC, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-γ-dependent mechanism in mice. Diabetes. 2006;55(4):924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 119.Berge RK, Tronstad KJ, Berge K, et al. The metabolic syndrome and the hepatic fatty acid drainage hypothesis. Biochimie. 2005;87(1):15–20. doi: 10.1016/j.biochi.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Dyroy E, Yndestad A, Ueland T, et al. Antiinflammatory effects of tetradecylthioacetic acid involve both peroxisome proliferator-activated receptor α-dependent and -independent pathways. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(7):1364–1369. doi: 10.1161/01.ATV.0000171982.57713.96. [DOI] [PubMed] [Google Scholar]
- 121.Løvås K, Røst TH, Skorve J, et al. Tetradecylthioacetic acid attenuates dyslipidaemia in male patients with type 2 diabetes mellitus, possibly by dual PPAR-α/δ activation and increased mitochondrial fatty acid oxidation. Diabetes, Obesity and Metabolism. 2009;11(4):304–314. doi: 10.1111/j.1463-1326.2008.00958.x. [DOI] [PubMed] [Google Scholar]
- 122.Madsen L, Guerre-Millo M, Flindt EN, et al. Tetradecylthioacetic acid prevents high fat diet induced adiposity and insulin resistance. Journal of Lipid Research. 2002;43(5):742–750. [PubMed] [Google Scholar]
- 123.Wensaas AJ, Rustan AC, Rokling-Andersen MH, et al. Dietary supplementation of tetradecylthioacetic acid increases feed intake but reduces body weight gain and adipose depot sizes in rats fed on high-fat diets. Diabetes, Obesity and Metabolism. 2009;11(11):1034–1049. doi: 10.1111/j.1463-1326.2009.01092.x. [DOI] [PubMed] [Google Scholar]
- 124.Rokling-Andersen MH, Rustan AC, Wensaas AJ, et al. Marine n-3 fatty acids promote size reduction of visceral adipose depots, without altering body weight and composition, in male Wistar rats fed a high-fat diet. British Journal of Nutrition. 2009;102(7):995–1006. doi: 10.1017/S0007114509353210. [DOI] [PubMed] [Google Scholar]
- 125.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Foundation Symposium. 2007;287:60–63. [PubMed] [Google Scholar]
- 126.Semple RK, Crowley VC, Sewter CP, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1α is reduced in the adipose tissue of morbidly obese subjects. International Journal of Obesity. 2004;28(1):176–179. doi: 10.1038/sj.ijo.0802482. [DOI] [PubMed] [Google Scholar]
- 127.Madsen L, Frøyland L, Grav HJ, Berge RK. Up-regulated Δ9-desaturase gene expression by hypolipidemic peroxisome-proliferating fatty acids results in increased oleic acid content in liver and VLDL: accumulation of a Δ9-desaturated metabolite of tetradecylthioacetic acid. Journal of Lipid Research. 1997;38(3):554–563. [PubMed] [Google Scholar]
- 128.Fredriksen J, Ueland T, Dyrøy E, et al. Lipid-lowering and anti-inflammatory effects of tetradecylthioacetic acid in HIV-infected patients on highly active antiretroviral therapy. European Journal of Clinical Investigation. 2004;34(10):709–715. doi: 10.1111/j.1365-2362.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 129.Hallgren P, Korsback S, Sjostrom L. Measurements of adipose tissue respiration in a closed chamber using an oxygen sensor: methodological considerations. Journal of Lipid Research. 1986;27(9):996–1005. [PubMed] [Google Scholar]
- 130.Cannon B, Nedergaard J. Studies of thermogenesis and mitochondrial function in adipose tissues. Methods in Molecular Biology. 2008;456:109–121. doi: 10.1007/978-1-59745-245-8_8. [DOI] [PubMed] [Google Scholar]
- 131.Mori Y, Murakawa Y, Okada K, et al. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care. 1999;22(6):908–912. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]
- 132.Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochemical and Biophysical Research Communications. 2000;271(2):445–450. doi: 10.1006/bbrc.2000.2647. [DOI] [PubMed] [Google Scholar]
- 133.de Souza CJ, Eckhardt M, Gagen K, et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50(8):1863–1871. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- 134.Fissoune R, Pellet N, Chaabane L, Contard F, Guerrier D, Briguet A. Evaluation of adipose tissue distribution in obese fa/fa Zucker rats by in vivo MR imaging: effects of peroxisome proliferator-activated receptor agonists. MAGMA. 2004;17(3–6):229–235. doi: 10.1007/s10334-004-0088-y. [DOI] [PubMed] [Google Scholar]
- 135.Fernandes-Santos C, Carneiro RE, de Souza Mendonca L, Aguila MB, Mandarim-de-Lacerda CA. Pan-PPAR agonist beneficial effects in overweight mice fed a high-fat high-sucrose diet. Nutrition. 2009;25(7-8):818–827. doi: 10.1016/j.nut.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 136.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raspé E, Madsen L, Lefebvre AM, et al. Modulation of rat liver apolipoprotein gene expression and serum lipid levels by tetradecylthioacetic acid (TTA) via PPARα activation. Journal of Lipid Research. 1999;40(11):2099–2110. [PubMed] [Google Scholar]
- 138.Westergaard M, Henningsen J, Svendsen ML, et al. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. Journal of Investigative Dermatology. 2001;116(5):702–712. doi: 10.1046/j.1523-1747.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 139.Lanni A, Mancini FP, Sabatino L, et al. De novo expression of uncoupling protein 3 is associated to enhanced mitochondrial thioesterase-1 expression and fatty acid metabolism in liver of fenofibrate-treated rats. FEBS Letters. 2002;525(1–3):7–12. doi: 10.1016/s0014-5793(02)02828-4. [DOI] [PubMed] [Google Scholar]
- 140.Grav HJ, Tronstad KJ, Gudbrandsen OA, et al. Changed energy state and increased mitochondrial β-oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 (UCP-2) expression. Evidence for peroxisome proliferator-activated receptor-α independent induction of UCP-2 expression. The Journal of Biological Chemistry. 2003;278(33):30525–30533. doi: 10.1074/jbc.M303382200. [DOI] [PubMed] [Google Scholar]
- 141.Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. The Journal of Clinical Investigation. 2008;118(2):789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maddux BA, See W, Lawrence Jr. JC, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat l6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes. 2001;50(2):404–410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 143.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxidants and Redox Signaling. 2005;7(7-8):1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 144.Muna ZA, Bolann BJ, Chen X, Songstad J, Berge RK. Tetradecylthioacetic acid and tetradecylselenoacetic acid inhibit lipid peroxidation and interact with superoxide radical. Free Radical Biology and Medicine. 2000;28(7):1068–1078. doi: 10.1016/s0891-5849(00)00196-9. [DOI] [PubMed] [Google Scholar]
- 145.Muna ZA, Gudbrandsen OA, Wergedahl H, Bohov P, Skorve J, Berge RK. Inhibition of rat lipoprotein oxidation after tetradecylthioacetic acid feeding. Biochemical Pharmacology. 2002;63(6):1127–1135. doi: 10.1016/s0006-2952(01)00934-0. [DOI] [PubMed] [Google Scholar]
- 146.Berge RK, Skorve J, Tronstad KJ, Berge K, Gudbrandsen OA, Grav H. Metabolic effects of thia fatty acids. Current Opinion in Lipidology. 2002;13(3):295–304. doi: 10.1097/00041433-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 147.Kuiper KKJ, Muna ZA, Erga KS, et al. Tetradecylthioacetic acid reduces stenosis development after balloon angioplasty injury of rabbit iliac arteries. Atherosclerosis. 2001;158(2):269–275. doi: 10.1016/s0021-9150(01)00417-8. [DOI] [PubMed] [Google Scholar]
- 148.Glosli H, Gudbrandsen OA, Mullen AJ, et al. Down-regulated expression of PPARα target genes, reduced fatty acid oxidation and altered fatty acid composition in the liver of mice transgenic for hTNFα. Biochimica et Biophysica Acta. 2005;1734(3):235–246. doi: 10.1016/j.bbalip.2005.02.011. [DOI] [PubMed] [Google Scholar]