Abstract
Fibroblast growth factors (FGFs) that signal through FGF receptors (FGFRs) regulate a broad spectrum of biological functions, including cellular proliferation, survival, migration, and differentiation. The FGF signal pathways are the RAS/MAP kinase pathway, PI3 kinase/AKT pathway, and PLCγ pathway, among which the RAS/MAP kinase pathway is known to be predominant. Several studies have recently implicated the in vitro biological functions of FGFs for tissue regeneration. However, to obtain optimal outcomes in vivo, it is important to enhance the half-life of FGFs and their biological stability. Future applications of FGFs are expected when the biological functions of FGFs are potentiated through the appropriate use of delivery systems and scaffolds. This review will introduce the biology and cellular functions of FGFs and deal with the biomaterials based delivery systems and their current applications for the regeneration of tissues, including skin, blood vessel, muscle, adipose, tendon/ligament, cartilage, bone, tooth, and nerve tissues.
1. Introduction
Tissue engineering, with significant research inputs over the last decades, has emerged as a potential tool to regenerate damaged and diseased tissues [1]. As one of the key components in tissue engineering approach, growth factors provide chemical cues to stem cells, regulating their biological responses and tissue differentiation. While the basic biological functions of growth factors and their endogenic roles in tissue development and repair process have relatively been well studied, the use of growth factors in tissue engineering regime has recently gained great interest. Growth factors are a potential agent to target specific tissue reactions because of their regulatory roles in cellular functions, including adhesion, proliferation, migration, and differentiation in the epithelium, bone, and soft connective tissues and nerves.
Fibroblast growth factor (FGF) is a representative growth factor which has shown the potential effects on the repair and regeneration of tissues [2–6]. It was originally identified as a protein capable of promoting fibroblast proliferation and is now known to comprise 22 members. FGFs exert multiple functions through the binding into and activation of fibroblast growth factor receptors (FGFRs), and the main signaling through the stimulation of FGFRs is the RAS/MAP kinase pathway. With their potential biological functions, FGFs have been utilized for the regeneration of damaged tissues, including skin, blood vessel, muscle, adipose, tendon/ligament, cartilage, bone, tooth, and nerve. Then, the prospective source of FGF for the tissue regeneration is used with recombinant human FGF family. In fact, many previous studies administered the FGFs directly to the wound sites, like other growth factors. However, free-FGFs are readily degradable in vivo, leading to loss of biological activity and functions [7–9]. To gain satisfactory performance, FGFs are adsorbed onto or encapsulated within materials to secure biological activity in a sustained and controllable manner. Although many types of materials have been developed to carry FGFs and elicit their therapeutic efficacy in vitro and in vivo, more sustained, controlled, and targeted delivering system still remain a challenge.
Here, we review the cellular biology of FGFs and their functions in cell proliferation, migration, differentiation, and angiogenesis and address the current development of biomaterials-based delivery systems of FGFs and their applications for tissue regeneration, including skin, blood vessel, muscle, adipose, tendon/ligament, cartilage, bone, tooth, and nerve.
2. Biology of FGF
FGF, which was first discovered in pituitary extracts in 1973, is widely expressed in cells and tissues. Acidic FGF (FGF1) and basic FGF (FGF2) were originally isolated from the brain and pituitary gland as growth factors for fibroblasts. Since then, at least 22 distinct FGFs have been identified or isolated.
FGFs have been found in both vertebrates and invertebrates. Many FGF genes have been identified in vertebrates, including ten FGFs in zebrafish (FGF2–4, 6, 8, 10, 17a, 17b, 18, 24), six in Xenopus (FGF2–4, 8–10), 13 in chickens (FGF1–4, 8–10, 12, 13, 16, 18–20), 22 in mice (FGF1–18, 20–23) and humans (FGF1–14, 16–23), whereas only three Drosophila FGF genes and two Caenorhabditis elegans FGF genes have been observed in invertebrates [10]. Human FGFs contain 22 members: FGF1, FGF2, FGF3 (INT2), FGF4, FGF5, FGF6, FGF7 (KGF), FGF8 (AIGF), FGF9, FGF10, FGF11, FGF12, FGF13, FGF14, FGF16, FGF17, FGF18, FGF19, FGF20, FGF21, FGF22, and FGF23 [11].
The FGF family comprises 23 members, although there are only 18 FGFR ligands. Four family members do not bind with FGFR as FGF homologous factors (FGF11, FGF12, FGF13, and FGF14) and are more correctly referred to as FGF homologous factors. In addition, there is no human FGF15 gene; the gene orthologous to mouse FGF15 is FGF19 [12].
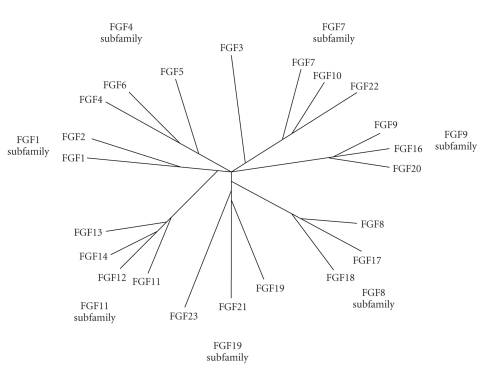
By phylogenetic analysis, the human FGF gene family can be divided into seven subfamilies: FGF1, FGF4, FGF7, FGF8, FGF9, FGF11, and FGF19 (Figure 1). The FGF1, FGF4, FGF7, FGF8, FGF9, FGF11, and FGF19 subfamilies comprise FGF1 and 2, FGF4, 5, and 6, FGF3, 7, 10, and 22, FGF8, 17, and 18, FGF9, 16, and 20, FGF11, 12, 13, and 14, and FGF19, 21, and 23, respectively. In contrast to phylogenetic analysis, gene location analysis indicates that the human FGF gene family can be divided into six subfamilies: FGF1/2/5, FGF3/4/6/19/21/23, FGF7/10/22, FGF8/17/18, FGF9/16/20, and FGF11/12/13/14. Members of the FGF8, FGF9, and FGF11 subfamilies are similar to those of the FGF7/10/22, FGF8/17/18, FGF9/16/20, and FGF11/12/13/14 subfamilies in the gene location analysis [13].
Figure 1.
Phylogenetic tree of human FGF family [13]. Human FGF gene family can be divided into seven subfamilies containing two to four members each. Branch lengths are proportional to the evolutionary distance between each gene.
The molecular weight of FGFs in vertebrates varies from 17 to 34 kDa, while the Drosophila FGF is 84 kDa. All members of the family share a conserved sequence of 120 amino acids that show 16%–65% sequence identity [14].
FGFs have various biological functions both in vivo and in vitro, including roles in mitogenesis, cellular migration, differentiation, angiogenesis, and wound healing.
FGFs exert their physiological roles through binding FGFR and regulate developmental pathways, controlling events such as mesoderm patterning in the early embryo through development of multiple organ systems. The mammalian FGF family is composed of 18 ligands that elicit their actions through four highly conserved transmembrane tyrosine kinase receptors (FGFR1, FGFR2, FGFR3, and FGFR4) [12]. Four FGFRs have been identified in humans and mice and encode receptor tyrosine kinases (ca. 800 amino acids) that contain an extracellular ligand-binding domain with three immunoglobulin domains (I, II, and III), a transmembrane domain, and a split intracellular tyrosine kinase domain [15]. FGFRs are expressed on many different cell types and regulate key cell behaviors, such as proliferation, differentiation, and survival, which make FGF signaling susceptible to subversion by cancer cells. Unlike other growth factors, FGFs act in concert with heparin or heparan sulfate proteoglycan (HSPG) to activate FGFRs and induce the pleiotropic responses that lead to a variety of cellular responses induced by this large family of growth factors [14].
Recent studies of FGF or FGFR have focused on mutations related to disease. Several germline FGF mutations have been identified in human disease, including loss-of-function mutations, and gain-of-function mutations. For instance, loss-of-function in FGF3 is involved with hereditary deafness, leading to total inner ear agenesis in humans [16]. Degradation of FGF8 by loss-of-function leads to kallmann's syndrome (KAL1), a developmental disorder characterized by anosmia and hypogonadism [17]. FGF10 loss-of-function causes lacrimo-auditory-dento-digital (LADD) syndrome, which is characterized by hearing loss, dental anomalies, and lacrimal and salivary gland hypoplasia [18]. Gain-of-function mutations in FGF23 have been identified in hypophosphataemic rickets [19].
Table 1 summarizes the location, receptor, and therapeutic application of the FGF family.
Table 1.
Physiological effects of the human FGF gene.
| Gene | Location | Receptor | Therapeutic application | Ref. |
|---|---|---|---|---|
| FGF1 | 5q31.3 | FGFR 1b, 1c, 2b, 2c, 3b, 3c, 4 | Cardiovascular disease | [32] |
| FGF2 | 4q27 | FGFR 1b, 1c, 2c, 3c, 4 | Cardiovascular disease, cancer | [33–36] |
| FGF3 | 11q13.3 | FGFR 1b, 2b | Not established | |
| FGF4 | 11q13.3 | FGFR 1c, 2c, 3c, 4 | Stable angina | [37] |
| FGF5 | 4q21.21 | FGFR 1c | Hair growth | [38] |
| FGF6 | 12p13.32 | FGFR 1c, 2c, 4 | Not established | |
| FGF7 | 15q21.2 | FGFR 2b | Oral mucositis | [39] |
| FGF8 | 10q24.32 | FGFR 3c, 4 | Not established | |
| FGF9 | 13q12.11 | FGFR 2c, 3b, 3c, 4 | Not established | |
| FGF10 | 5p12 | FGFR 1b, 2b | Not established | |
| FGF11 | 17p13.1 | Intracytoplasmic | Not established | |
| FGF12 | 3q28 | Not identified | Not established | |
| FGF13 | Xq26.3 | Not identified | Not established | |
| FGF14 | 13q33.1 | Not identified | Not established | |
| FGF16 | Xq21.1 | FGFR 4 | Not established | |
| FGF17 | 8p21.3 | FGFR 2c, 3c, 4 | Not established | |
| FGF18 | 5q35.1 | FGFR 2c, 3c, 4 | Osteoarthritis, cartilage | [40] |
| FGF19 | 11q13.3 | FGFR 4 | Diabetes | [41] |
| FGF20 | 8p22 | Not identified | Parkinson's disease | [42] |
| FGF21 | 19q13.32 | Not identified | Diabetes | [43] |
| FGF22 | 19p13.3 | FGFR 2b | Not established | |
| FGF23 | 12p13.32 | FGFR 3c | Hypophosplataemia | [44] |
3. FGF Signaling
FGFs act as signal molecules that bind and activate FGFRs. Activated FGFRs mediate signaling by recruiting specific molecules that bind to phosphorylated tyrosine at the cytosolic part of the receptor, triggering a number of signaling pathways leading to specific cellular responses. These then serve as docking sites for the recruitment of SH2 (Src homology-2) or PTB (phosphotyrosine binding) domains of adaptors docking proteins or signaling enzymes. Signaling complexes are formed and recruited to the active receptors resulting in a cascade of phosphorylation events [20]. The best understood pathways are the RAS/MAP kinase pathway, PI3 kinase/AKT pathway, and PLCγ pathway. Figure 2 schematically describes the three pathways of the FGF signal, the RAS/MAP kinase pathway, PI3 kinase/AKT pathway, and PLCγ pathway.
Figure 2.
FGF signal pathway. FGFs stimulate tyrosine phosphorylation of the docking protein FRS, followed by forming the GRB2-SHP2-GAB-1 complex resulting in activation of RAS-MAP kinase pathway and PI3 kinase/AKT pathway. In PLCγ pathway, activated PLCγ hydrolyzes phosphatidylinositol, generating IP3 and DAG and results in the activation of PKC. FRS2: fibroblast growth factor receptor substrate 2, GRB: guanine nucleotide exchange factor, SOS: son of sevenless, RAS: monomeric G-protein, RAF: kinase, MEK: kinase, MKP1: MAP kinase phosphatase, PIP2: phosphatidylinositol (4,5)-bisphosphate, IP3: inositol triphosphate, DAG: diacylglycerol, PKC: protein kinase C.
3.1. RAS/MAP Kinase Pathway
Mitogen-activated protein (MAP) kinases are serine/threonine-specific protein kinases that respond to extracellular stimuli (mitogens) and regulate various cellular activities such as gene expression, mitosis, differentiation, and cell survival/apoptosis [21]. c-Jun N-terminal kinase (JNK), extracellular signal regulated kinase (ERK), and p38 mitogen-activated kinase are examples of effectors MAP kinase [22]. Interestingly, the activation of ERK 1/2 and p38 in response to FGF has been observed in all cell types, while the activities of other signal transduction pathways vary depending on the cell type.
To date, the main pathway of the FGF signal is the RAS/MAP kinase pathway, which contains many signaling proteins. A key event in the FGF signaling pathway is phosphorylation of the tyrosine residues of the docking protein, fibroblast growth factor receptor substrate 2α (FRS2α), which provides new binding sites for direct or indirect recruitment of proteins that are responsible for both activation and attenuation of signaling [23, 24]. FRS2α recruits a complex consisting of an adaptor protein, the guanine nucleotide exchange factor 2 (GRB2), the son of sevenless (SOS), the tyrosine phosphatase (SHP2), and the docking protein, GRB2-associated binding protein 1 (GAB1). Formation of the FRS2 signaling complex results in activation of RAS/MAP kinase [25] as well as PI3 kinase/AKT pathways [26]. The RAS-MAP kinase pathway has been implicated in cell growth and differentiation in many studies [11].
Lax et al. [24] showed that FGF signals induce a MAP kinase mediated negative feedback loop that causes threonine phosphorylation of FRS2a, leading to a reduction of its tyrosine phosphorylation and decreased recruitment of GRB2. Receptor tyrosine kinases also induce negative signals via activation of the sprouty proteins that inhibit the recruitment of GRB2-SOS complexes to FRS2 and the receptor and attenuate the RAS-MAP kinase pathway. Members of the Sef and MAP kinase phosphatase families are other negative modulators of FGF signaling, while XFLRT3, a member of a leucine-rich-repeat transmembrane protein family, is a novel positive modulator. Expression of XFLRT3 is induced by FGF and down-regulated after inhibition of FGF signaling [27]. Thus, FGF signaling is modulated by both positive and negative mechanisms, and subtle modulations in the signal are important determinants of the biological response during development.
3.2. PI3 Kinase/AKT Pathway
Similar to the RAS/MAP kinase pathway, the phosphoinositide 3 (PI3) kinase/AKT pathway is initiated by forming an FRS2 signaling complex. Next, GAB1 protein links activated FGF receptors with PI3 kinase. GAB1 consists of a pleckstrin homology domain, a proline-rich region, and multiple tyrosine phosphorylation sites that serve as binding sites for the SH2 domains. The p110 catalytic subunit of PI3 kinase is in a complex with an adaptor protein (p85) that has two SH2 domains; thus, p85 binds to phosphorylated tyrosine residues in GAB1 adaptor protein. Phosphoinositide-dependent kinase and the anti-apoptotic protein kinase AKT are activated downstream of the PI3 kinase [26].
The PI3 kinase/AKT pathway is implicated in cell survival and cell fate determination, as well as the PI3 kinase/aPKC signaling cascade in cell polarity control [11]. Böttcher et al. [27] showed that GAB1 is required for stimulation of the AKT pathway by FGF.
3.3. PLCγ Pathway
One of the target molecules for activated FGFR is phospholipase C gamma (PLCγ), which binds to the phosphorylated Tyr-766 of the receptor and then becomes tyrosine phosphorylation of PLCγ, resulting in PLCγ activation. Activated PLCγ hydrolyzes phosphatidylinositol, generating inositol triphosphate (IP3) and diacylglycerol (DAG) [28]. IP3 is a cellular second messenger that facilitates the release of calcium from the endoplasmic reticulum. Increased levels of calcium in the cytosol and DAG together activate protein kinase C (PKC). The physiological relevance of this pathway is not obvious since its disruption does not abolish either mitogenesis [29] or cell differentiation [30]. However, some data indicate that it may be necessary for adhesion, at least in some cell types [31].
4. Biological Functions of FGFs
As stated above, FGFs exert their physiological roles by binding to high affinity tyrosine kinase FGFRs on the surface of the target cell. Therefore, the function of FGFs depends on the FGF signal pathway between the FGF family and FGFRs. Many studies have reported that FGFs have functions such as cell proliferation, migration, differentiation, and angiogenesis in various cells and tissues. Table 2 summarizes the function of FGFs.
Table 2.
Functions of fibroblast growth factors.
| Function | Subfamily related to the function | Target cell | Ref. |
|---|---|---|---|
| Cell proliferation | FGF1, FGF2 | Preadipocyte | [45–48] |
| Endothelial cell, epithelial cell, | |||
| fibroblast cell, neural stem cell | |||
| FGF4 | Trophoblast stem cell | [49] | |
| FGF7, FGF10 | Epithelial cell | [50, 51] | |
| FGF18 | Osteoblast, chondrocytes, osteoclast | [52] | |
| Cell migration | FGF2 | Astrocyte, myogenic cell | [47, 54] |
| FGF4 | Myogenic cell | [54] | |
| FGF7 | Epithelial cell, keratinocyte | [55] | |
| FGF8 | Neural crest cell | [56] | |
| Cell differentiation | FGF1, FGF2 | Neuroepithelial | [48, 52] |
| FGF7 | Keratinocyte | [57] | |
| FGF20 | Monkey stem cell | [42] | |
| Angiogenesis | FGF1, FGF2 | Endothelial cell | [61] |
4.1. Cell Proliferation
Cell proliferation by FGFs has been reported in many cell types, including endothelial cells, stem cells, and epithelial cells. FGF1 is a proliferative factor for human preadipocytes that may be important to the overall regulation of human adipogenesis [45]. In addition, FGF1 leads to an increase in the proliferation of IEC-6, Caco-2, and HT-29 cell lines with FGF2 and FGF7 [46]. FGF2 induces cell proliferation after flia-specific gene transfer in mice [47] and stimulates the proliferation and survival of neuroepithelial cells isolated from the telencephalon and mesencephelon of E10 mice [48]. FGF4 knockout mouse embryos experience postimplantation lethality owing to the necessity of FGF4 for trophoblast proliferation [49]. FGF7 (called human KGF) is related to the epithelial cell growth [50]. FGF10 play a role in the pathogenesis of prostate cancer via facilitation of epithelial cell proliferation [51]. FGF18 has also been shown to stimulate the proliferation of cultured mouse primary osteoblasts, osteoblastic MC3T3-E1 cells, primary chondrocytes, and prechondrocytic ATDC5 cells, although it inhibited the differentiation and matrix synthesis of these cells [52]. Interestingly, some FGFs stimulate proliferation of cancer cells as well as normal cells.
4.2. Cell Migration
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing, and immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to and toward specific external signals in a process known as chemotaxis. Cell migration of FGFs varies with subfamilies. Apparently, both FGF1 and FGF2 play important roles in the migration of cochlear ganglion neurons in mice [53]. FGF2 induces cell migration after flia-specific gene transfer in mice [47] and stimulates cell migration of mouse embryonic limb myogenic cells such as FGF4 [54]. FGF7 is known to stimulate migration and plasminogen activity (PA) of normal human keratinocytes [55]. Similar to FGF2, FGF8 is a potent chemoattractant in the migration of mesencephalic neural crest cells [56].
4.3. Cell Differentiation
In developmental biology, cellular differentiation is the process by which a less specialized cell becomes a more specialized cell type. Differentiation occurs numerous times during the development of multicellular organisms as they change from a single zygote to a complex system of tissues and cell types. Differentiation is common in adults as well. Specifically, adult stem cells divide and create fully-differentiated daughter cells during tissue repair and normal cell turnover. Differentiation dramatically changes the size, shape, membrane potential, metabolic activity, and responsiveness of a cell to signals.
Cell differentiation of FGFs also varies with subfamilies. FGF1 and FGF2 play important roles in the initial differentiation of cochlear ganglion neurons in mice [29]. Moreover, FGF2 stimulates the differentiation of neuroepithelial cells into mature neurons and glia [48]. FGF7 is essential for the morphogenesis of suprabasal keratinocytes and establishment of the normal program of keratinocyte differentiation [57]. Exogenous FGF20 stimulates the differentiation of monkey stem cells into dopaminergic neurons after treatment in vitro [42].
4.4. Angiogenesis
Angiogenesis is the process of the formation of new blood vessels from pre-existing vessels. This process plays a key role in various physiological and pathological conditions such as embryonic development, wound repair, inflammation, and tumor growth [58]. Angiogenesis is a multistep process that begins with the degradation of the basement membrane by activated endothelial cells that migrate and proliferate, leading to the formation of solid endothelial cell sprouts into the stromal space. Next, vascular loops are formed and capillary tubes develop with the formation of tight junctions and deposition of new basement membrane [59].
Numerous inducers of angiogenesis have been identified, including members of the vascular endothelial growth factor (VEGF) family, angiopoietins, transforming growth factor-alpha and -beta (TGF-alpha and beta), platelet-derived growth factor (PDGF), tumor necrosis factor-alpha (TNF-alpha), interleukins, chemokines, and members of the fibroblast growth factor (FGF) family [60]. However, only a limited number of the 22 members of the FGF family have been investigated for their angiogenic potential in vitro and in vivo [61]. The angiogenic properties of FGF1 and FGF2 are well known. Specifically, FGF1 and FGF2 induce the promotion of endothelial cell proliferation and the physical organization of endothelial cells into tube-like structures. Thus, they promote angiogenesis. FGF1 and FGF2 are more potent angiogenic factors than vascular endothelial growth factor (VEGF) or platelet-derived growth factor (PDGF). FGF4 also has angiogenic properties.
5. Delivery Systems for FGFs
Due to their specific biological functions and roles, FGFs have the potential for application to induce the regeneration of a wide spectrum of tissues, including skin, blood vessel, muscle, adipose, tendon/ligament, cartilage, bone, tooth, and nerve tissues. Indeed, many previous studies have evaluated the administration of FGFs directly to the sites of wounds, similar to that of other growth factors. However, when free FGF solutions are injected in vivo, they rapidly lose their biological functional activity, primarily due to diffusional loss and/or enzymatic inactivation/degradation [7–9]. Therefore, to gain satisfactory performance, a large amount of FGFs with a continuous dose for the determined period is required.
Conversely, when FGFs are adsorbed onto or encapsulated within materials, their degradation risk can be largely protected while securing the biological activity. Therefore, to make full use of the FGFs, it is essential to develop appropriate materials and substrates to contain and deliver them to defective regions, after which allowing their release at a controllable and sustainable rate. A wide range of biomaterials including synthetic and natural polymers and even tissue matrices have been studied as candidate materials to carry FGFs and elicit their therapeutic efficacy in vitro and/or in vivo.
While their functional activity has been demonstrated in different types of cells in vitro and/or target tissues in vivo, there have been relatively few, if any, reports addressing the delivery mechanism. In this part, we review the applications of FGFs in concert with medical materials for tissue regeneration. Specific targets include skin, cartilage, bone, blood vessel, muscle, tendon/ligament, and nerve. The materials are developed to specifically conjugate with FGFs or encapsulate within the structure and are engineered in the form of hydrogels or porous scaffolds or nano- and microparticulates.
5.1. Porous Scaffolds
Generally, the most common scaffold materials range from polymers (synthetic or natural) and ceramics to their composites, which can be chosen depending on the target tissues of concern. Many natural polymers such as collagen, alginate, fibrin, silk, chitosan, and glycosaminoglycans (GAGs) are biologically well defined, tissue compatible, and degradable; therefore, they are regarded as feasible materials for the intake of growth factors within the structures [62]. Such polymers are easily engineered into porous scaffolds by dissolution in water-based solutions and subsequent freeze-drying [63, 64]. Because they dissolve well in water, the incorporation of FGFs is possible during the fabrication step. When incorporated within the structure, growth factors are released through the scaffold when they come into contact with the fluid. In most cases, bulk diffusion is the dominant method of releasing growth factors, which is led by water permeation and can be accelerated by collapse of the polymer network.
Due to their comprising a class of proteins or polysaccharides, natural polymers contain a large number of ionic groups. Apart from chitosan, which is highly positively charged, all of the other proteins mentioned above preserve a large number of negatively charged groups [65]. Therefore, depending on their charge characteristics (basic or acidic), FGFs can form charge-charge interactions with natural polymers. In such cases, the FGF-incorporated scaffolds show sustained and long-term delivery of FGFs if their structure is maintained without collapse [65, 66]. More specific biochemical interactions between the FGFs and natural proteins are favored in maintenance of the stability of FGFs, which can be exploited by utilizing some binding sites of FGFs, such as the heparin binding site [8, 67, 68]. Collagen scaffolds mixed with heparin hold FGF2 within the structure better than scaffolds without heparin, which is beneficial for long-term FGF delivery [68]. Hydrogel-type scaffolds that absorb a high level of water within their pore structure and are mechanically meta-stable gel matrices will be discussed separately in the following section.
Synthetic polymers primarily those that are degradable, such as poly(lactic acid), poly(glycolic acid), poly(caprolactone), and their copolymers have also been well developed into porous scaffolds by many possible processing routes [69]. Unlike natural polymers, this class of polymers can be dissolved in nonaqueous organic solvents that would otherwise melt at elevated temperatures, and most growth factors, including FGFs, are very susceptible to degradation when incorporated during the scaffold processing stages. Therefore, surface modification of the scaffolds is recommended to carry the growth factors. Through chemical treatment of the surface of synthetic polymers such as amination or carboxylation, growth factors can be covalently coupled via the formation of an amide linkage [70, 71]. However, in such cases, growth factors are only on the scaffold surface, which limits the continual biological action of FGFs and long-term targeting while influencing the initial adhesive reactions of cells.
Apart from the polymer groups, bioceramics are promising candidate materials for grafting defects of hard tissues, such as bones and teeth [72–79]. Many publications and clinical trials have already demonstrated the high performance of some bioceramics including calcium phosphates and bioactive glasses/glass ceramics [73, 77, 79]. Therefore, for hard tissue regeneration, there is a potential need to use bioceramics in concert with growth factors including FGFs, leading to some pioneering studies on the incorporation of FGFs within bone grafts [80, 81]. However, most currently applicable bioceramics can only be obtained following heat treatment (generally above a thousand degree), which limits the direct loading of growth factors within the scaffolds. Nevertheless, some unique properties such as granular morphology (grain boundaries), surface charges, crystallography, and micropores are known to significantly alter the adsorption of proteins and their release behaviors [82–85], which suggests the possible manipulation of FGFs on bioceramic scaffolds that is different from the case in polymers.
One promising form of bioceramics is self-setting or hardening cements, such as calcium phosphate cements that quickly harden when they come in contact with water-based solutions and are easily moldable and applicable in an injectable type [78, 115]. While their applications in hard tissue reconstruction have a long history, the need for using this class of material for drug delivery including growth factors has emerged recently. FGF-incorporating cements that target bones and teeth are considered to hold great promise; however, additional studies evaluating their use are necessary [116].
5.2. Hydrogels
Natural polymers such as collagen, gelatin, fibrin, and glycosaminoglycans are the most commonly used hydrogels for tissue regeneration and drug delivery. These hydrogels largely mimic the native extracellular matrices (ECMs) of tissues, and cells recognize the hydrogel molecules in a manner similar to the recognition that occurs under in vivo conditions [117]. When FGFs are loaded within the hydrogels of natural polymers, they play a role in controlling cell processes, such as cell division, migration, and differentiation. Because the growth factors easily bind to ECM components such as heparan sulfate proteoglycans and fibronectin [118, 119], these compounds are often combined with hydrogel matrices. The bound growth factors are secured within the hydrogel matrices until released by an enzymatic reaction or hydrolytic cleavage.
Collagen hydrogels form a fibrillar network, which is also degradable enzymatically through the action of collagenase. The binding of growth factors to the collagen fibrillar network is largely noncovalent. However, recent studies have reported the recombinant fusion of growth factors into well-known collagen binding sequences such as the sequence from collagenase, von Willerbrand factor, or fibronectin [120]. Gelatin is a denatured form of collagen that has been investigated as a good candidate carrier of FGFs [121]. Depending on the charge characteristics (acidic or basic) and biodegradability of gelatin, the release profile of FGFs can vary greatly. In the case of FGF2, acidic gelatin hydrogel with low water content was better at stimulating angiogenesis [122]. Fibrin hydrogel can be formed through the spontaneous polymerization of fibrinogen in the presence of thrombin protease. Thus, growth factors can be incorporated into the fibrin network during the coagulation [123]. The FGF2 noncovalently bound to the fibrin network was able to provide growth factor-specific bioactivity, such as enhancing endothelial cell proliferation [124]. GAGs are strongly anionic polymer hydrogels that can absorb a large amount of water while preserving good mechanical integrity. Similar to collagen, GAGs bind growth factors noncovalently but very stably and then further release them through the enzymatic cleavage reaction [125]. The structure of GAGs is often modified to provide sites of covalent binding for biomecules, such as adhesive proteins and growth factors.
Synthetic hydrogel polymers can be formed with various compositions, including poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and poly(hydroxyethyl methacrylate) (PHEMA) [126]. During preparation of the synthetic hydrogels, various target binding domains or cleavage domains such as MMP-cleavable sequences and growth factor binding ligands can be introduced, and these ultimately play a role in covalently linking growth factors such as FGFs and further allowing cleavage of the network to release FGFs [127]. When compared to the natural polymer hydrogels, which have limited properties to control, the development of synthetic polymer hydrogels to retain the functional and some cell/tissue responsive properties are now increasing rapidly. Indeed, cell responsive functional groups can be tuned to deliver specific molecules including FGFs, and the mechanical and degradation properties can be manipulated to be responsive to surrounding conditions, such as pH, temperature, light, and mechanical load [7, 128]. As one example, a PEG hydrogel system was engineered to be crosslinked through a photoreaction, during which time physical properties such as the degree of swelling could be modulated [7]. The release of FGF2 from the hydrogel was highly dependent on the crosslinking density, which was mediated by the swelling ability.
5.3. Nano- and Micro-Particulates
When compared to the structured porous scaffolds or hydrogels, particulate forms with sizes at the nano- (tens to hundreds of nanometers) and micron-scale (a few micrometers) have gained interest for specialized delivery of growth factors, requiring a system to be delivered through the blood stream and oral administration, and within intracellular compartments [129–132]. Many different formulations of natural and synthetic polymeric materials for this purpose have been reported, and these primarily include liposomes, micelles, dendrimers, microspheres, nanospheres, and nanoshells [132]. The common process in production of those particulates requires the formation of droplets within solutions that were conditioned to disperse individual particles while preserving the morphological (spherical) integrity. The size and composition of the particulates are easily controllable, which ultimately determines the release profile of growth factors.
As described in the previous section, natural polymers such as gelatin and collagen are possible candidates for in situ encapsulation of FGFs during the formation of particulates, where the charge interaction of natural polymers with FGFs must be considered. Similarly, synthetic polymers can be exploited in the form of nanoshells (or nanocapsules), in which hydrophobic polymers comprise an outer shell that surrounds an inner water-based portion that contains hydrophilic growth factors [89, 133]. Depending on the polymer composition and outer shell thickness, the release profile of growth factors can be controlled. Due to their sizes being far greater or less than one hundred nanometers, the nanoparticulates are commonly used as gene delivery vehicles [134]. DNA designed to encode FGFs can be encapsulated within the nanocapsules to form a complex and then further transfected to the target cells to elicit the biological functionality of FGFs [135].
When compared to the polymeric compositions, inorganic particulates for growth factor delivery have been relatively less studied. Recent attention has been given to the mesoporous silica nanoparticles, which have a large amount of mesopores with sizes of about 2–10 nm [136, 137]. Growth factors can be entrapped within the mesopores of the particles and then delivered into the target tissues or cells. One of the widely available inorganic materials for gene delivery is calcium phosphate (CaP) nanoparticles because the calcium ion can easily bind with negatively charged nucleic acids, which then forms a CaP-DNA complex. Intracellular delivery of genes that encode FGFs can be implemented using inorganic nanoparticulate delivery systems [138, 139].
While nano- and microparticulates are easily manipulated to take up growth factors and can be safely implemented into defect sites, the scaffolds and hydrogels can provide 3D matrices for cells to adhere, migrate, populate, and differentiate. Therefore, particulates carrying growth factors are often embedded within the matrices to act as cell scaffolding and produce therapeutic effects [139, 140], which is an effective method of fully utilizing the roles and functions of FGFs in cells and tissues for regenerative therapy and tissue engineering.
6. Tissue-Specific Applications
6.1. Skin
FGFs have the biological activity of stimulating the proliferation of fibroblasts and angiogenesis, which facilitates potential use in skin wound healing. Both FGF1 and FGF2 are known to be highly released by damaged endothelial cells and macrophages at wound sites, and if FGF2 activity is blocked, wound angiogenesis is almost completely impaired [141]. FGF2 is also known to induce scar-free healing [142]. FGF7 and FGF10 play a role in stimulation of the migration and proliferation of keratinocytes [143].
However, owing to the short half-like of free FGFs, the use of delivery systems has been proposed. Among the FGFs, application studies of wound healing and skin regeneration have primarily been conducted on FGF2. Because acidic gelatin is highly negatively charged, it can hold FGF2 well by forming an ionic complex with gelatin. The 2 mg freeze-dried gelatin microspheres were soaked in 20 μl aqueous solution of FGF2 (10 mg/ml with an isoelectric point 9.6). While the free-FGF2 administered in vivo (10, 50, and 100 μg) to guinea pigs with full-thickness skin defect could not induce sufficient dermal wound healing, gelatin microspheres incorporated with FGF2 greatly accelerated dermal tissue regeneration [86]. Using a synthetic hydrogel of chitosan, which is photocrosslinkable, FGF2-incorporation and sustained release were also implemented [87]. The FGF2-incorporated hydrogel was photocrosslinked and injected into the skin wound of healing-impaired diabetic (db/db) mice. The involvement of FGF2 within chitosan hydrogel was shown to greatly improve wound closure in terms of granulation tissue formation, capillary formation, and epithelialization of the wounds.
6.2. Blood Vessels
The angiogenesis and blood vessel formation induced by the treatment of FGFs have been relatively well studied. The most commonly used carrier systems for FGFs are natural polymers including gelatin, which can be prepared to have different charge statuses (negative for acidic and positive for basic gelatin) [122]. Tabata et al. prepared gelatin hydrogels with different water contents and charge statuses and then incorporated them with FGF2. When the complex was implanted subcutaneously into mice, the most significant neovascularization was induced in the FGF2-incorporating acidic gelatin with a low water content. This was largely due to the effect of released FGF2, which was manipulated to be sustained and controlled from the gelatin hydrogel.
A sustained release of FGF2 and its stimulation of human umbilical vein endothelial cell (HUVEC) growth were reported using the heparinized collagen matrix [68], where the heparin was first immobilized to collagen, and FGF2 was then bound to the heparinized collagen because the FGF2 has a heparin-binding domain. The heparin was also immobilized onto synthetic polymer PLGA scaffold to allow a high affinity to FGF2. The FGF2 loading was greatly enhanced, and the release from the heparinized-PLGA was controllable. When subcutaneously implanted in vivo, the FGF2-loaded scaffold effectively induced blood vessel formation [88].
Synthetic polymer PLGA microspheres were also used to incorporate FGF2 and its sustained-release, which was then combined with alginate porous scaffold [89]. When implanted in the rat peritoneum, the FGF2-PLGA administered group showed accelerated vascularization, with a 4-fold increase in penetrating capillaries when compared to the FGF2-free group occurring. This was mainly attributed to the sustained release of FGF2 from the microcarriers.
6.3. Muscles
Muscle regeneration has also been greatly controlled by the FGFs which are abundant in regenerating areas of muscles [144, 145]. FGF6 is of particular interest because it is muscle specific and highly upregulated during muscle regeneration. FGF2 was demonstrated to promote the recruitment of skeletal muscle satellite cells using a single myofiber culture model [146]. Doukas et al. used a gene delivery system that encodes FGF2 and FGF6 for the repair of skeletal muscle [147]. Specifically, plasmid and adenovirus vectors were immobilized in a collagen-gelatin mixture that was then delivered to muscle wounds. They found early muscle angiogenic response and subsequent arteriogenesis, and muscle repair was also greatly enhanced showing regenerating myotubes with specific markers expressions. Although there have been some controversies regarding the critical role of FGFs in muscle repair [148], it is largely accepted that their roles in muscle regeneration are closely related to the revascularization process [149].
6.4. Adipose Tissues
The roles of FGF2 in adipose regeneration are closely associated with angiogenesis. Kawaguchi et al. reported the induction of de novo adipogenesis in mouse subcutis in response to the injection of FGF2-Matrigel mixture [91], which is associated with vascular formation. Additionally, matrigel matrix containing gelatin microspheres incorporated with FGF2 was used for the controlled release of FGF2 and induction of adipogenesis [92]. At 6 weeks after subcutaneous implantation in mice, the FGF2-incorporating group showed significantly higher formation of adipose tissue accompanied with angiogenesis when compared to that treated with free FGF2. The adipogenesis was dose dependent (0.01, 0.1, and 1 μg FGF2) and the best result was obtained in the 0.1 μg FGF2-incorporated group, suggesting that the use of polymer carrier incorporating an appropriate level of FGF2 provides a tool for adipose tissue engineering. Gelatin microspheres incorporating FGF2 were also used in other studies of adipose tissue regeneration [93, 94]. Kimura et al. showed that FGF2-incorporated gelatin microspheres enabled preadipocytes to differentiate adipose tissue formation [93]. They further showed that collagen scaffold containing gelatin-FGF2 microspheres stimulated adipogenesis in a rabbit fat defect in response to the treatment of FGF2-complex scaffold [94].
6.5. Tendon/Ligament
Following injury (~during one week) of tendons and ligaments, the level of FGF2 and its receptors has been shown to increase in vivo, with FGF2 playing a significant role in the recruitment of progenitor cell differentiation and the repair process [150, 151]. For applications of human bone marrow stem cells (hBMSCs) in the repair of tendons and ligaments, the effects of FGF2 on their proliferation and differentiation were investigated [3]. Specifically, when the FGF2 concentration was low (3 ng/ml), the action was positive in terms of triggering both cell proliferation and expression of genes related to tendon and ligament tissue. However, treatment with a high dose (30 ng/ml) did not show any beneficial effects on the hBMSCs. Targeting anterior cruciate ligament (ACL), FGF2 was incorporated into a gelatin hydrogel and then mixed with PLA woven fabric [95]. Following implantation of FGF2-combined materials wrapped with a collagen membrane in the tibia and femur of rabbit, ACL and bone were both regenerated with enhanced mechanical strength.
One recent study showed the possibility of tendon tissue engineering using BMSCs and a delivery system encapsulating FGF2 within electrospun nanofiber that was combined with silk microfiber fabric [96]. The behavior of BMSCs, including their proliferation and tendogenic differentiation, was significantly promoted upon FGF2-loaded fibrous scaffolds.
6.6. Cartilage
While the differentiated traits of chondrocytes are generally lost during expansion in monolayer culture, chondrocytes treated with 5 ng/ml FGF2 within the poly(glycolic acid) fibrous scaffold and further expanded (up to 2000 fold) were shown to redifferentiate to recover the chondrocytes phenotypes [152]. When FGF2 was impregnated within collagen sponge (soaked in 80 μg/ml PBS for 24 h at 4°C) and implanted subcutaneously in nude mice, the cartilage regeneration was remarkably accelerated with cartilage tissues that were immature at 2 weeks and almost mature at 4 weeks [97]. With regard to the mesenchymal stem cells (MSCs), FGF2 have been shown to modulate cell growth and maintain the undifferentiated state of stem cells, facilitating the expansion of stem cells [98]. Moreover, its regulation of chondrogenic differentiation of bone marrow stem cells (BMSCs) has been demonstrated [153]. Chiou et al. also reported that the treatment of FGF2 in either BMSCs or adipose stem cells (ASCs) significantly enhanced chondrogenesis [154], where cell proliferation was shown to increase dose dependently. Specifically, they found that FGF2 at lower concentration (10 ng/ml) enhanced chondrogenic differentiation, while the effect was negated at higher concentration (50 ng/ml), suggesting that the use of FGF2 at an appropriate dose is beneficial for cartilage repair [154].
To induce slow release of FGF2 from the scaffold, FGF2 was impregnated within gelatin microspheres [99]. While free FGF2 showed a rapid in vivo clearance (~3% remained after 24 h), the level of gelatin-impregnated FGF2 remained approximately 44% and 18% at days 3 and 14, respectively, suggesting that the gelatin held the FGF2 effectively. The FGF2-containing gelatin was preadministrated in an ear-shaped polymer scaffold that also contained chondrocytes for 1 week. When the tissue-engineered construct was implanted subcutaneously in mice, significantly improved chondrogenic and neovascularization traits were observed, suggesting the importance of sustained FGF2 release in cartilage tissue engineering. To augment the repair of tracheal stenosis, an animal study in rabbit was also conducted using biopolymer scaffold-chondrocytes containing FGF2 [100]. The results demonstrated greatly enhanced chondrogenesis with cartilage accumulation in the engineered tracheal wall at three months after implantation. The combinatory approach of FGF2 with polymer scaffold was also conducted by Ma et al., who coated the PLA scaffold surface with collagen and FGF2 [101]. To accomplish this, the PLA surface was first chemically treated to covalently graft collagen molecules and then physically coated with collagen solution containing FGF2 [101]. The chondrocytes showed significantly improved proliferation on the FGF2-implemented scaffold.
6.7. Bones
Possible effects of FGF2 on osteogenesis and bone regeneration have also been reported. Lisignoli et al. cultured BMSCs derived from rats within a hyaluronate-based polymer scaffold with or without FGF2 [102]. They found that the presence of FGF2 strongly enhanced the expression of osteogenic markers and mineralization, demonstrating a possible role in bone regeneration. Tabata et al. studied the role of FGF2 in a rabbit skull defect model [103]. Gelatin hydrogel was also used to incorporate FGF2 (100 μg) to form a polyionic complex and function as an effective carrier. At 12 weeks, the implants showed dramatic improvement in defect closure, bone mineral density, and bone regeneration in groups treated with varying doses (2 to 200 μg) of FGF2-gelatin when compared to an untreated group.
The improvement of bone cell proliferation and differentiation by the FGF2 was also observed in an experiment using hydroxyapatite (HA) porous granules incorporating FGF2 [104]. The FGF2 administrated at 0.25 μM to 100 mg of HA granules was shown to remain at approximately 80% after release for 3 days. Specifically, the osteoblastic differentiation of cells (MC3T3-E1) such as alkaline phosphatase (ALP) activity and mRNA levels of bone-related genes (osteocalcin, collagen I and ALP) were shown to be significantly up-regulated by the FGF2 treatment, suggesting that HA also may preserve the biological activity of FGF2. A recent study also showed that the in vivo bone formation in rats greatly improved in response to the use of an appropriate carrier of FGF2 [105]. Collagen was made into a hybrid membrane with nanobioactive glass, which was impregnated with FGF2 (100 μg) and then implanted within a rat calvarium defect. The nanobioactive glass-amended hybrid scaffold greatly enhanced the defect closure and bone formation, and the FGF2-treated group displayed further improvements, showing the synergistic effect of FGF2 with a bioactive inorganic component.
The use of FGF2 in dental implants has also been shown to influence the formation of bone around Ti-based metals. FGF2 suspended in Matrigel was administrated to the surface of Ti and then implanted in ovariectomized rats [106]. The Matrigel used was shown to prolong the life span of FGF2 upon sustained release for up to 21 days in vitro. The implant samples after three months were found to induce great enhancement in new-bone formation (2-fold) and mechanical stability (3-fold) on the Matrigel-FGF2-treated group when compared to groups treated with FGF2 or Matrigel alone.
The treatment of osteochondral complex tissue was also investigated in vivo using a composite scaffold made of HA/collagen incorporating FGF2 [107]. Either 0.5 μg (50 μl from 10 μg/ml) or 5 μg (50 μl from 100 μg/ml) FGF2 was impregnated within the HA/collagen scaffold and then implanted into the osteochondral defect in a rabbit femoral trochlear groove of the knee. During the periods of implantation through 3 to 24 weeks, the 0.5 μg FGF2-treated scaffold group displayed greatly enhanced bone regeneration and satisfactory cartilage regeneration, suggesting that the HA/collagen composite is a good candidate for delivering FGF2 during the regeneration of osteochondral defect.
While there has been some consensus regarding improvement of the proliferative potential of osteoblasts, some adverse effects of FGF2 on the osteogenic differentiation and mineralization have also been identified. Bosetti et al. investigated the influence of different FGFs (FGF2, FGF4, and FGF6) on the behavior of human primary osteoblasts [155]. They demonstrated that all FGFs treated in culture medium at 0.7 μM induced osteoblast proliferation but inhibited ALP activity and mineralization. However, when Vitamin D was co-administrated with FGFs, the ALP and mineralization were greatly enhanced, suggesting the combinatory use of FGFs with other mineralizing agents for bone induction and bone tissue engineering. Coadministration of FGF2 with melatonin to the Ti implants showed promotion of osseointegration during 4 weeks of implantation in rat tibia [108].
Apart from the use of FGF2, which has been the most widely studied, FGF1 was also shown to stimulate angiogenesis as well as osteogenesis in vitro [81]. FGF1 was entrapped within the HA-fibrin composite scaffold, which was further implanted in rats subcutaneously. At 2 and 4 weeks postimplantation, the FGF1-containing scaffolds was found to stimulate angiogenesis with blood vessel formation and the expression of osteogenic markers (osteopontin and osteocalcin), suggesting that HA-fibrin scaffold incorporating FGF1 is effective for bone repair.
6.8. Dental Tissues
Many dental applications of FGFs are found in periodontal regeneration [100–103]. Additionally, periodontal ligament tissue has been shown to be regenerated by the action of FGF2 contained in a gelatin carrier [109]. Tan et al. reported that a periodontal defect in dogs could be significantly regenerated by treatment with MSCs that were transfected with FGF2 [156]. At eight weeks after implantation in nonhuman primates, the FGF2-gelatin group showed significant regeneration of periodontal tissues in a dose-dependent manner [157]. Shirakata et al. demonstrated that treatment with FGF2 induced the promotion of periodontal healing in two-wall intrabony defects in dogs, suggesting that they were a possible candidate for replacement of the established benchmarks enamel matrix derivative or platelet-derived growth factor combined tricalcium phosphate [110].
6.9. Nerves
As one of the neurotrophic factors, FGFs have been shown to enhance the in vitro survival and neurite extension of various types of neurons as well as in vivo wound healing and neuronal functions.
FGF2 is known to exist at the blood-brain barrier in matrix-bound and soluble form, and it is produced by astrocytes and has autocrine effects on astrocytes proliferation and stellation [158]. When compared to the soluble form, FGF2 bound to matrices has improved half life [159], and its effects on astrocytes can be potentiated [160, 161]. Delgado-Rivera et al. covalently linked FGF2 onto a nanofiber polymer scaffold and observed significant autocrine expression of FGF2 and the modulation of astrocytes-neuron interactions, suggesting the utility of the system in nerve injury and disease [5].
For the repair of spinal cord injury (SCI), Marquet et al. developed a porous PLA modified with copolymer and combined with fibrin glue containing FGF2 and found that the scaffold allowed cell migration and angiogenesis in the transected spinal cord of rats, suggesting the potential for the use of the system for SCI, although more clear examination and functional studies must be conducted to confirm these findings [112]. Due to the difficulty of the proteins in penetrating the blood-spinal cord barrier, a local delivery system consisting of poly(ethylene glycol)-modified FGF2 and using an intrathecal delivery has recently been developed by Kang et al. [113]. The in vivo distribution of FGF2 in the spinal cord tissue was greatly enhanced. Baumann et al. also developed a polymer nanocomposite hydrogel FGF1 that has also been shown to have neuroprotective functions in the repair of SCI. Tsai et al. demonstrated that rat SCI treated with FGF1 showed significant functional recovery [162]. They also used a synthetic polymer and hydrogel of fibrin or collagen to carry growth factors such as FGF1 and NT-3, and showed that FGF1 involvement significantly improved the axonal regeneration of vestibular neurons [111].
For the peripheral nerve regeneration, FGF2 was loaded within a polymer tube channel and implanted in the 15 mm gap of the sciatic nerve [90]. The results revealed the in vitro sustained release of FGF2 with biological activity, and in vivo regeneration of nerve cables bridging nerve stumps. Similar to other tissue applications, anionic gelatin was also used to embed FGF2 and induce sustainable delivery to the intratemporal facial nerve. The results at six weeks after implantation demonstrated that facial nerve functions such as facial movements, electrophysiology and histological morphology, were greatly improved [114].
7. Concluding Remarks
Fibroblast growth factors (FGFs) that signal through FGF receptors (FGFRs) regulate a wide range of biological functions, including cell proliferation, survival, migration, and differentiation. Among the signal pathways, RAS/MAP kinase is known to be predominant in the case of FGFs. While the biological functions of FGFs are largely implicated in many types of cells in vitro through this signaling pathway, the maintenance of stability and half-life in vivo should be considered. Biomaterial-based systems, including delivery carriers of FGFs and scaffolds of stem cells regulated by the FGFs functions, have recently been potentially developed and shown to have many good results in vivo. Future clinical applications of FGFs in the regeneration of tissues, including skin, muscle, tendon/ligament, bone, tooth, and nerve tissues will be realized when their biological functions are maximized by the appropriate use of biomaterials and stem cells.
Table 3.
Tissue applications of fibroblast growth factors.
| Target tissue | Subfamily of FGF | Materials/carriers | In vivo/in vitro | Animal/cell | Functions/effects | Ref |
|---|---|---|---|---|---|---|
| Skin | FGF2 | Gelatin microsphere | In vivo | Guinea pig | Wound healing | [86] |
| FGF2 | Chitosan hydrogel | In vivo | Mouse | Wound healing | [87] | |
| Vessels | FGF2 | Gelatin hydrogel | In vivo | Mouse | Vascularization | [88] |
| FGF2 | Heparinized collagen | In vitro | Endothelial cell | Cell growth | [68] | |
| FGF2 | Heparinized PLGA scaffold | In vivo/In vitro | Mouse | Vascularization | [88] | |
| FGF2 | PLGA microsphere-alginate porous scaffold | In vivo | Rat | Capillary penetration, vascularization | [89] | |
| Muscle | FGF2 | PLGA nanoparticle | In vivo/In vitro | Mouse | Arteriogenesis | [90] |
| Adipose | FGF2 | Matrigel | In vivo | Mouse | Adipogenesis | [91] |
| FGF2 | Matrigel-gelatin microspheres | In vivo | Mouse | Adipogenesis | [92] | |
| FGF2 | Gelatin microsphere-collagen scaffold | In vivo | Mouse/rabbit | Adipose regeneration | [93, 94] | |
| Tendon/ Ligament | FGF2 | Gelatin-PLA scaffold | In vivo | Rabbit | ACL and bone regeneration | [95] |
| FGF2 | Silk/PLGA scaffold | In vitro | BMSCs | Proliferation, differentiation | [96] | |
| Cartilage | FGF2 | PGA scaffold | In vitro | Chondrocyte | Dedifferentiation | [97] |
| FGF2 | Collagen sponge | In vivo | Mouse | Cartilage regeneration | [98] | |
| FGF2 | Gelatin microsphere-polymer scaffold | In vivo/In vitro | Mouse | Chondrogenesis, vascularization | [99] | |
| FGF2 | Collagen-PGLA-PLCL scaffold | In vivo | Chondrocyte | Tracheal regeneration | [100] | |
| FGF2 | Collagen-PLLA scaffold | In vitro | Chondrocyte | Proliferation | [101] | |
| Bone | FGF1 | Hydroxyapatite-fibrin scaffold | In vivo | Rat | Osteogenic markers, bone regeneration | [81] |
| FGF2 | Hyaluronate scaffold | In vitro | BMSCs | Osteogenic markers, mineralization | [102] | |
| FGF2 | Gelatin hydrogel | In vivo | Rabbit | Mineralization, bone regeneration | [103] | |
| FGF2 | Hydroxyapatite porous granules | In vitro | MC3T3-E1 | Cell proliferation, osteoblast differentiation | [104] | |
| FGF2 | Collagen-bioactive glass | In vivo | Rat | Bone regeneration | [105] | |
| FGF2 | Ti based metals-matrigel | In vivo/In vitro | Rat | Bone regeneration | [106] | |
| FGF2 | Hydroxyapatite/collagen scaffold | In vivo | Rabbit | Bone regeneration, cartilage regeneration | [107] | |
| FGF2 | Ti implant-melatonin | In vivo | Rat | Osseointegration | [108] | |
| Dental | FGF2 | Gelatin microsphere | In vivo | Dog | Periodontal regeneration | [109] |
| FGF2 | Tricalcium phosphate | In vivo | Dog | Alveolar tissue regeneration | [110] | |
| Nerve | FGF1 | pHEMA-MMA | In vivo | Rat | Axonal regeneration | [111] |
| FGF2 | Polyamide nanofiber scaffold | In vitro | Astrocyte | Neurite outgrowth | [5] | |
| FGF2 | Porous PLA scaffold | In vivo | Rat | Cell migration, angiogenesis | [112] | |
| FGF2 | Polyethylene glycol | In vivo | Rat | Spinal cord injury repair | [113] | |
| FGF2 | Polymer tube channel | In vivo/In vitro | Rat | Peripheral nerve regeneration | [90] | |
| FGF2 | Gelatin hydrogel | In vivo | Guinea pig | Facial nerve functions | [114] | |
Acknowledgments
This work was supported by Priority Research Centers Program (Grant no. 2009-0093829) and WCU (World Class University) program (Grant no. R31-10069) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Moya ML, Cheng M-H, Huang J-J, et al. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31(10):2816–2826. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankemeier S, Keus M, Zeichen J, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Engineering. 2005;11(1-2):41–49. doi: 10.1089/ten.2005.11.41. [DOI] [PubMed] [Google Scholar]
- 4.Masukawa H, Miura Y, Sato I, Oiso Y, Suzuki A. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. Journal of Cellular Biochemistry. 2001;83(1):121–128. doi: 10.1002/jcb.1203. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Rivera R, Harris SL, Ahmed I, et al. Increased FGF-2 secretion and ability to support neurite outgrowth by astrocytes cultured on polyamide nanofibrillar matrices. Matrix Biology. 2009;28(3):137–147. doi: 10.1016/j.matbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Rophael JA, Craft RO, Palmer JA, et al. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. American Journal of Pathology. 2007;171(6):2048–2057. doi: 10.2353/ajpath.2007.070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreopoulos FM, Persaud I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials. 2006;27(11):2468–2476. doi: 10.1016/j.biomaterials.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Cai S, Liu Y, Xiao ZS, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Freudenberg U, Hermann A, Welzel PB, et al. A star-PEG-heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials. 2009;30(28):5049–5060. doi: 10.1016/j.biomaterials.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biology. 2001;2(3, article no. 3005) doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katoh M, Katoh M. FGF signaling network in the gastrointestinal tract (review) International journal of oncology. 2006;29(1):163–168. [PubMed] [Google Scholar]
- 12.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nature Reviews Cancer. 2010;10(2):116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 13.Itoh N. The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biological and Pharmaceutical Bulletin. 2007;30(10):1819–1825. doi: 10.1248/bpb.30.1819. [DOI] [PubMed] [Google Scholar]
- 14.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine and Growth Factor Reviews. 2005;16(2):139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Jaye M, Schlessinger J, Dionne CA. Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochimica et Biophysica Acta. 1992;1135(2):185–199. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- 16.Tekin M, Hişmi BÖ, Fitoz S, et al. Homozygous mutations in fibroblast growth factor 3 are associated with a new form of syndromic deafness characterized by inner ear agenesis, microtia, and microdontia. American Journal of Human Genetics. 2007;80(2):338–344. doi: 10.1086/510920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falardeau J, Chung WCJ, Beenken A, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. Journal of Clinical Investigation. 2008;118(8):2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milunsky JM, Zhao G, Maher TA, Colby R, Everman DB. LADD syndrome is caused by FGF10 mutations. Clinical Genetics. 2006;69(4):349–354. doi: 10.1111/j.1399-0004.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 19.White KE, Evans WE, O’Riordan JLH, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nature Genetics. 2000;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 20.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 21.Pearson G, Robinson F, Gibson TB, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 22.Shao D, Lazar MA. Modulating nuclear receptor function: may the phos be with you. Journal of Clinical Investigation. 1999;103(12):1617–1618. doi: 10.1172/JCI7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong A, Lamothe B, Lee A, Schlessinger J, Lax I. FRS2α attenuates FGF receptor signaling by Grb2-mediated recruitment of the ubiquitin ligase Cbl. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(10):6684–6689. doi: 10.1073/pnas.052138899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lax I, Wong A, Lamothe B, et al. The docking protein FRS2α controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Molecular Cell. 2002;10(4):709–719. doi: 10.1016/s1097-2765(02)00689-5. [DOI] [PubMed] [Google Scholar]
- 25.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine and Growth Factor Reviews. 2005;16(2):233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Lamothe B, Yamada M, Schaeper U, Birchmeier W, Lax I, Schlessinger J. The docking protein Gab1 is an essential component of an indirect mechanism for fibroblast growth factor stimulation of the phosphatidylinositol 3-kinase/Akt antiapoptotic pathway. Molecular and Cellular Biology. 2004;24(13):5657–5666. doi: 10.1128/MCB.24.13.5657-5666.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böttcher RT, Pollet N, Delius H, Niehrs C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nature Cell Biology. 2003;6(1):38–44. doi: 10.1038/ncb1082. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi M, Honegger AM, Rotin D, et al. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (flg) is a binding site for the SH2 domain of phospholipase C-γ1. Molecular and Cellular Biology. 1991;11(10):5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters KG, Marie J, Wilson E, et al. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992;358(6388):678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- 30.Spivak-Kroizman T, Mohammadi M, Hu P, Jaye M, Schlessinger J, Lax I. Point mutation in the fibroblast growth factor receptor eliminates phosphatidylinositol hydrolysis without affecting neuronal differentiation of PC12 cells. Journal of Biological Chemistry. 1994;269(20):14419–14423. [PubMed] [Google Scholar]
- 31.Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. Journal of Neuroscience. 2000;20(6):2238–2246. doi: 10.1523/JNEUROSCI.20-06-02238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacher B, Peecher P, Von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97(7):645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 33.Unger EF, Goncalves L, Epstein SE, et al. Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. American Journal of Cardiology. 2000;85(12):1414–1419. doi: 10.1016/s0002-9149(00)00787-6. [DOI] [PubMed] [Google Scholar]
- 34.Laham RJ, Rezaee M, Post M, et al. Intracoronary and intravenous administration of basic fibroblast growth factor: myocardial and tissue distribution. Drug Metabolism and Disposition. 1999;27(7):821–826. [PubMed] [Google Scholar]
- 35.Figg WD, Dahut W, Duray P, et al. A randomized Phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clinical Cancer Research. 2001;7(7):1888–1893. [PubMed] [Google Scholar]
- 36.Eisen T, Boshoff C, Mak I, et al. Continuous low dose Thalidomide: a phase II study in advanced melanoma, renal cell, ovarian and breast cancer. British Journal of Cancer. 2000;82(4):812–817. doi: 10.1054/bjoc.1999.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry TD, Grines CL, Watkins MW, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. Journal of the American College of Cardiology. 2007;50(11):1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Hébert JM, Rosenquist T, Götz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78(6):1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 39.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. New England Journal of Medicine. 2004;351(25):2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 40.Moore EE, Bendele AM, Thompson DL, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis and Cartilage. 2005;13(7):623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Nicholes K, Guillet S, Tomlinson E, et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. American Journal of Pathology. 2002;160(6):2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. Journal of Clinical Investigation. 2005;115(1):102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 44.Aono Y, Simada T, et al. The neutralization of FGF-23 ameliorates hypophosphatemia and rickets in Hyp mice. Journal of Bone and Mineral Research. 2003;18(2):p. S16. [Google Scholar]
- 45.Hutley L, Shurety W, Newell F, et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53(12):3097–3106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 46.Dignass AU, Tsunekawa S, Podolsky DK. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology. 1994;106(5):1254–1262. doi: 10.1016/0016-5085(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 47.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy M, Drago J, Bartlett PF. Fibroblast growth factor stimulates the proliferation and differentiation of neural precursor cells in vitro. Journal of Neuroscience Research. 1990;25(4):463–475. doi: 10.1002/jnr.490250404. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka S, Kunath T, Hadjantonakis A-K, Nagy A, Rossant J. Promotion to trophoblast stem cell proliferation by FGF4. Science. 1998;282(5396):2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 50.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 51.Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126(16):3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- 52.Ohbayashi N, Shibayama M, Kurotaki Y, et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes and Development. 2002;16(7):870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor V, Zgraggen C, Naef R, Suter U. Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: immunohistochemistry and antibody perturbation. Journal of Neuroscience Research. 2000;62(1):40–55. doi: 10.1002/1097-4547(20001001)62:1<40::AID-JNR5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Webb SE, Lee KKH, Tang MK, Ede DA. Fibroblast growth factors 2 and 4 stimulate migration of mouse embryonic limb myogenic cells. Developmental Dynamics. 1997;209(2):206–216. doi: 10.1002/(SICI)1097-0177(199706)209:2<206::AID-AJA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 55.Tsuboi R, Sato C, Kurita Y, Ron D, Rubin JS, Ogawa H. Keratinocyte growth factor (FGF-7) stimulates migration and plasminogen activator activity of normal human keratinocytes. Journal of Investigative Dermatology. 1993;101(1):49–53. doi: 10.1111/1523-1747.ep12358892. [DOI] [PubMed] [Google Scholar]
- 56.Kubota Y, Ito K. Chemotactic migration of mesencephalic neural crest cells in the mouse. Developmental Dynamics. 2000;217(2):170–179. doi: 10.1002/(SICI)1097-0177(200002)217:2<170::AID-DVDY4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 57.Werner S, Weinberg W, Liao X, et al. Targeted expression of a dominant-negative FGF receptor mutant in the epidermis of transgenic mice reveals a role of FGF in keratinocyte organization and differentiation. EMBO Journal. 1993;12(7):2635–2643. doi: 10.1002/j.1460-2075.1993.tb05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 59.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Medicine. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 60.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine and Growth Factor Reviews. 2005;16(2):159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends in Molecular Medicine. 2002;8(10):483–489. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- 62.Kimura Y. Biodegradable polymers. In: Tsuruta T, editor. Biomedical Applications of Polymeric Materials. Boca Raton, Fla, USA: CRC Press; 1993. pp. 163–189. [Google Scholar]
- 63.Chen G, Ushida T, Tateishi T. Development of biodegradable porous scaffolds for tissue engineering. Materials Science and Engineering C. 2001;17(1-2):63–69. [Google Scholar]
- 64.Mano JF, Silva GA, Azevedo HS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. Journal of the Royal Society Interface. 2007;4(17):999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced Drug Delivery Reviews. 2007;59(4-5):207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Tan H, Gong Y, Lao L, Mao Z, Gao C. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. Journal of Materials Science: Materials in Medicine. 2007;18(10):1961–1968. doi: 10.1007/s10856-007-3095-5. [DOI] [PubMed] [Google Scholar]
- 67.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 68.Wissink MJB, Beernink R, Poot AA, et al. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. Journal of Controlled Release. 2000;64(1–3):103–114. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 69.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 70.Penn LS, Wang H. Chemical modification of polymer surfaces: a review. Polymers for Advanced Technologies. 1994;5(12):809–817. [Google Scholar]
- 71.Bramfeldt H, Sarazin P, Vermette P. Smooth muscle cell adhesion in surface-modified three-dimensional copolymer scaffolds prepared from co-continuous blends. Journal of Biomedical Materials Research A. 2009;91(1):305–315. doi: 10.1002/jbm.a.32244. [DOI] [PubMed] [Google Scholar]
- 72.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Composites Science and Technology. 2001;61(9):1189–1224. [Google Scholar]
- 73.Hench LL. Bioceramics. Journal of the American Ceramic Society. 1998;81(7):1705–1727. [Google Scholar]
- 74.Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D. Orthopaedic applications of bone graft & graft substitutes: a review. Indian Journal of Medical Research. 2010;132(7):15–30. [PubMed] [Google Scholar]
- 75.Ikada Y. Challenges in tissue engineering. Journal of the Royal Society Interface. 2006;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akkus O, Pujol J, Qi G. Bioactive sol-gel foams for tissue repair. Journal of Biomedical Materials Research. 2002;59(2):340–348. doi: 10.1002/jbm.1250. [DOI] [PubMed] [Google Scholar]
- 77.Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12(2):155–163. doi: 10.1016/0142-9612(91)90194-f. [DOI] [PubMed] [Google Scholar]
- 78.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clinical Orthopaedics and Related Research. 1981;157:259–278. [PubMed] [Google Scholar]
- 79.Best SM, Porter AE, Thian ES, Huang J. Bioceramics: past, present and for the future. Journal of the European Ceramic Society. 2008;28(7):1319–1327. [Google Scholar]
- 80.Komaki H, Tanaka T, Chazono M, Kikuchi T. Repair of segmental bone defects in rabbit tibiae using a complex of β-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials. 2006;27(29):5118–5126. doi: 10.1016/j.biomaterials.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 81.Kelpke SS, Zinn KR, Rue LW, Thompson JA. Site-specific delivery of acidic fibroblast growth factor stimulates angiogenic and osteogenic responses in vivo. Journal of Biomedical Materials Research - Part A. 2004;71(2):316–325. doi: 10.1002/jbm.a.30163. [DOI] [PubMed] [Google Scholar]
- 82.Noshi T, Yoshikawa T, Ikeuchi M, et al. Enhancement of the in vivo osteogenic potential of marrow/hydroxyapatite composites by bovine bone morphogenetic protein. Journal of Biomedical Materials Research. 2000;52(4):621–630. doi: 10.1002/1097-4636(20001215)52:4<621::aid-jbm6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 83.Vallet-Regí M, Balas F, Colilla M, Manzano M. Bone-regenerative bioceramic implants with drug and protein controlled delivery capability. Progress in Solid State Chemistry. 2008;36(3):163–191. [Google Scholar]
- 84.Ijntema K, Heuvelsland WJM, Dirix CAMC, Sam AP. Hydroxyapatite microcarriers for biocontrolled release of protein drugs. International Journal of Pharmaceutics. 1994;112(3):215–224. [Google Scholar]
- 85.Matsumoto T, Okazaki M, Inoue M, et al. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials. 2004;25(17):3807–3812. doi: 10.1016/j.biomaterials.2003.10.081. [DOI] [PubMed] [Google Scholar]
- 86.Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21(5):489–499. doi: 10.1016/s0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 87.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24(13):2339–2349. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 88.Yoon JJ, Chung HJ, Lee HJ, Park TG. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. Journal of Biomedical Materials Research A. 2006;79(4):934–942. doi: 10.1002/jbm.a.30843. [DOI] [PubMed] [Google Scholar]
- 89.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. Journal of Biomedical Materials Research A. 2003;65(4):489–497. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 90.Aebischer P, Salessiotis AN, Winn SR. Basic fibroblast growth factor released from synthetic guidance channels facilitates peripheral nerve regeneration across long nerve gaps. Journal of Neuroscience Research. 1989;23(3):282–289. doi: 10.1002/jnr.490230306. [DOI] [PubMed] [Google Scholar]
- 91.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tabata Y, Miyao M, Inamoto T, et al. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Engineering. 2000;6(3):279–289. doi: 10.1089/10763270050044452. [DOI] [PubMed] [Google Scholar]
- 93.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24(14):2513–2521. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 94.Kimura Y, Tsuji W, Yamashiro H, Toi M, Inamoto T, Tabata Y. In situ adipogenesis in fat tissue augmented by collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Journal of Tissue Engineering and Regenerative Medicine. 2010;4(1):55–61. doi: 10.1002/term.218. [DOI] [PubMed] [Google Scholar]
- 95.Kimura Y, Hokugo A, Takamoto T, Tabata Y, Kurosawa H. Regeneration of anterior cruciate ligament by biodegradable scaffold combined with local controlled release of basic fibroblast growth factor and collagen wrapping. Tissue Engineering C. 2008;14(1):47–57. doi: 10.1089/tec.2007.0286. [DOI] [PubMed] [Google Scholar]
- 96.Sahoo S, Toh SL, Goh JCH. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31(11):2990–2998. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Fujisato T, Sajiki T, Qiang L, Ikada Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials. 1996;17(2):155–162. doi: 10.1016/0142-9612(96)85760-7. [DOI] [PubMed] [Google Scholar]
- 98.Fujita M, Kinoshita Y, Sato E, et al. Proliferation and differentiation of rat bone marrow stromal cells on poly(glycolic acid)-collagen sponge. Tissue Engineering. 2005;11(9-10):1346–1355. doi: 10.1089/ten.2005.11.1346. [DOI] [PubMed] [Google Scholar]
- 99.Isogai N, Morotomi T, Hayakawa S, et al. Combined chondrocyte-copolymer implantation with slow release of basic fibroblast growth factor for tissue engineering an auricular cartilage construct. Journal of Biomedical Materials Research A. 2005;74(3):408–418. doi: 10.1002/jbm.a.30343. [DOI] [PubMed] [Google Scholar]
- 100.Komura M, Komura H, Kanamori Y, et al. An animal model study for tissue-engineered trachea fabricated from a biodegradable scaffold using chondrocytes to augment repair of tracheal stenosis. Journal of Pediatric Surgery. 2008;43(12):2141–2146. doi: 10.1016/j.jpedsurg.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 101.Ma Z, Gao C, Gong Y, Shen J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials. 2005;26(11):1253–1259. doi: 10.1016/j.biomaterials.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 102.Lisignoli G, Zini N, Remiddi G, et al. Basic fibroblast growth factor enhances in vitro mineralization of rat bone marrow stromal cells grown on non-woven hyaluronic acid based polymer scaffold. Biomaterials. 2001;22(15):2095–2105. doi: 10.1016/s0142-9612(00)00398-7. [DOI] [PubMed] [Google Scholar]
- 103.Tabata Y, Nagano A, Muniruzzaman MD, Ikada Y. In vitro sorption and desorption of basic fibroblast growth factor from biodegradable hydrogels. Biomaterials. 1998;19(19):1781–1789. doi: 10.1016/s0142-9612(98)00089-1. [DOI] [PubMed] [Google Scholar]
- 104.Jeong I, Yu H-S, Kim M-K, Jang J-H, Kim H-W. FGF2-adsorbed macroporous hydroxyapatite bone granules stimulate in vitro osteoblastic gene expression and differentiation. Journal of Materials Science: Materials in Medicine. 2010;21(4):1335–1342. doi: 10.1007/s10856-009-3971-2. [DOI] [PubMed] [Google Scholar]
- 105.Hong KS, Kim E-C, Bang S-H, et al. Bone regeneration by bioactive hybrid membrane containing FGF2 within rat calvarium. Journal of Biomedical Materials Research A. 2010;94(4):1187–1194. doi: 10.1002/jbm.a.32799. [DOI] [PubMed] [Google Scholar]
- 106.Gao Y, Zhu S, Luo E, Li J, Feng G, Hu J. Basic fibroblast growth factor suspended in Matrigel improves titanium implant fixation in ovariectomized rats. Journal of Controlled Release. 2009;139(1):15–21. doi: 10.1016/j.jconrel.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 107.Maehara H, Sotome S, Yoshii T, et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2) Journal of Orthopaedic Research. 2010;28(5):677–686. doi: 10.1002/jor.21032. [DOI] [PubMed] [Google Scholar]
- 108.Takechi M, Tatehara S, Satomura K, Fujisawa K, Nagayama M. Effect of FGF-2 and melatonin on implant bone healing: a histomorphometric study. Journal of Materials Science: Materials in Medicine. 2008;19(8):2949–2952. doi: 10.1007/s10856-008-3416-3. [DOI] [PubMed] [Google Scholar]
- 109.Murakami S, Takayama S, Ikezawa K, et al. Regeneration of periodontal tissues by basic fibroblast growth factor. Journal of Periodontal Research. 1999;34(7):425–430. doi: 10.1111/j.1600-0765.1999.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 110.Shirakata Y, Taniyama K, Yoshimoto T, et al. Regenerative effect of basic fibroblast growth factor on periodontal healing in two-wall intrabony defects in dogs. Journal of Clinical Periodontology. 2010;37(4):374–381. doi: 10.1111/j.1600-051X.2010.01539.x. [DOI] [PubMed] [Google Scholar]
- 111.Tsai EC, Dalton PD, Shoichet MS, Tator CH. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials. 2006;27(3):519–533. doi: 10.1016/j.biomaterials.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 112.Maquet V, Martin D, Scholtes F, et al. Poly(D,L-lactide) foams modified by poly(ethylene oxide)-block-poly(D,L-lactide) copolymers and a-FGF: in vitro and in vivo evaluation for spinal cord regeneration. Biomaterials. 2001;22(10):1137–1146. doi: 10.1016/s0142-9612(00)00357-4. [DOI] [PubMed] [Google Scholar]
- 113.Kang CE, Tator CH, Shoichet MS. Poly(ethylene glycol) modification enhances penetration of fibroblast growth factor 2 to injured spinal cord tissue from an intrathecal delivery system. Journal of Controlled Release. 2010;144(1):25–31. doi: 10.1016/j.jconrel.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 114.Komobuchi H, Hato N, Teraoka M, et al. Basic fibroblast growth factor combined with biodegradable hydrogel promotes healing of facial nerve after compression injury: an experimental study. Acta Oto-Laryngologica. 2010;130(1):173–178. doi: 10.3109/00016480902896139. [DOI] [PubMed] [Google Scholar]
- 115.Rajzer I, Castaño O, Engel E, Planell JA. Injectable and fast resorbable calcium phosphate cement for body-setting bone grafts. Journal of Materials Science: Materials in Medicine. 2010;21(7):2049–2056. doi: 10.1007/s10856-010-4078-5. [DOI] [PubMed] [Google Scholar]
- 116.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. Journal of Controlled Release. 2006;113(2):102–110. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 117.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chemical Reviews. 2001;101(7):1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 118.Lindahl U, Li JP. Interactions between heparan sulfate and proteins-design and functional implications. International review of cell and molecular biology. 2009;276:105–159. doi: 10.1016/S1937-6448(09)76003-4. [DOI] [PubMed] [Google Scholar]
- 119.Wijelath ES, Rahman S, Namekata M, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circulation Research. 2006;99(8):853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin H, Chen B, Sun W, Zhao W, Zhao Y, Dai J. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials. 2006;27(33):5708–5714. doi: 10.1016/j.biomaterials.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 121.Midy V, Rey C, Bres E, Dard M. Basic fibroblast growth factor adsorption and release properties of calcium phosphate. Journal of Biomedical Materials Research. 1998;41(3):405–411. doi: 10.1002/(sici)1097-4636(19980905)41:3<405::aid-jbm10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 122.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20(22):2169–2175. doi: 10.1016/s0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 123.Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thrombosis and Haemostasis. 2003;89(3):573–582. [PubMed] [Google Scholar]
- 124.Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin∗. Journal of Biological Chemistry. 1998;273(13):7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- 125.Cai S, Liu Y, Xiao ZS, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 126.Hoffman AS. Hydrogels for biomedical applications. Advanced Drug Delivery Reviews. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 127.Patterson J, Martino MM, Hubbell JA. Biomimetic materials in tissue engineering. Materials Today. 2010;13(1-2):14–22. [Google Scholar]
- 128.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews. 2001;53(3):321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 129.Anitua E, Sánchez M, Orive G, Andia I. Delivering growth factors for therapeutics. Trends in Pharmacological Sciences. 2008;29(1):37–41. doi: 10.1016/j.tips.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 130.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharmaceutical Research. 2003;20(8):1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 131.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharmaceutical Research. 2000;17(5):497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 132.Zhang S, Uludağ H. Nanoparticulate systems for growth factor delivery. Pharmaceutical Research. 2009;26(7):1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 133.Lee JS, Go DH, Bae JW, Lee SJ, Park KD. Heparin conjugated polymeric micelle for long-term delivery of basic fibroblast growth factor. Journal of Controlled Release. 2007;117(2):204–209. doi: 10.1016/j.jconrel.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 134.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced Drug Delivery Reviews. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 135.Ochiya T, Nagahara S, Sano A, Itoh H, Terada M. Biomaterials for gene delivery: atelocollagen-mediated controlled release of molecular medicines. Current gene therapy. 2001;1(1):31–52. doi: 10.2174/1566523013348887. [DOI] [PubMed] [Google Scholar]
- 136.Slowing II, Vivero-Escoto JL, Wu C-W, Lin VS-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Advanced Drug Delivery Reviews. 2008;60(11):1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 137.Slowing II, Trewyn BG, Giri S, Lin VS-Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Advanced Functional Materials. 2007;17(8):1225–1236. [Google Scholar]
- 138.Roy I, Mitra S, Maitra A, Mozumdar S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. International Journal of Pharmaceutics. 2003;250(1):25–33. doi: 10.1016/s0378-5173(02)00452-0. [DOI] [PubMed] [Google Scholar]
- 139.Komura M, Komura H, Kanamori Y, et al. An animal model study for tissue-engineered trachea fabricated from a biodegradable scaffold using chondrocytes to augment repair of tracheal stenosis. Journal of Pediatric Surgery. 2008;43(12):2141–2146. doi: 10.1016/j.jpedsurg.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 140.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Engineering. 2006;12(6):1475–1487. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 141.Broadley KN, Aquino AM, Hicks B, et al. The diabetic rat as an impaired wound healing model: stimulatory effects of transforming growth factor-beta and basic fibroblast growth factor. Biotechnology therapeutics. 1989;1(1):55–68. [PubMed] [Google Scholar]
- 142.Spyrou GE, Naylor IL. The effect of basic fibroblast growth factor on scarring. British Journal of Plastic Surgery. 2002;55(4):275–282. doi: 10.1054/bjps.2002.3831. [DOI] [PubMed] [Google Scholar]
- 143.Tagashira S, Harada H, Katsumata T, Itoh N, Nakatsuka M. Cloning of mouse FGF10 and up-regulation of its gene expression during wound healing. Gene. 1997;197(1-2):399–404. doi: 10.1016/s0378-1119(97)00187-x. [DOI] [PubMed] [Google Scholar]
- 144.Guthridge M, Wilson M, Cowling J, Bertolini J, Hearn MTW. The role of basic fibroblast growth factor in skeletal muscle regeneration. Growth Factors. 1992;6(1):53–63. doi: 10.3109/08977199209008871. [DOI] [PubMed] [Google Scholar]
- 145.Lefaucheur JP, Sebille A. Basic fibroblast growth factor promotes in vivo muscle regeneration in murine muscular dystrophy. Neuroscience Letters. 1995;202(1-2):121–124. doi: 10.1016/0304-3940(95)12223-0. [DOI] [PubMed] [Google Scholar]
- 146.Yablonka-Reuveni Z, Seger R, Rivera AJ. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. Journal of Histochemistry and Cytochemistry. 1999;47(1):23–42. doi: 10.1177/002215549904700104. [DOI] [PubMed] [Google Scholar]
- 147.Doukas J, Blease K, Craig D, et al. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Molecular Therapy. 2002;5(5):517–527. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- 148.Mitchell CA, McGeachie JK, Grounds MD. The exogenous administration of basic fibroblast growth factor to regenerating skeletal muscle in mice does not enhance the process of regeneration. Growth Factors. 1996;13(1-2):37–55. doi: 10.3109/08977199609034565. [DOI] [PubMed] [Google Scholar]
- 149.Lefaucheur J-P, Gjata B, Lafont H, Sebille A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-β1. Journal of Neuroimmunology. 1996;70(1):37–44. doi: 10.1016/s0165-5728(96)00099-9. [DOI] [PubMed] [Google Scholar]
- 150.Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lineaweaver WC. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. Journal of Hand Surgery. 1998;23(6):1052–1058. doi: 10.1016/S0363-5023(98)80015-4. [DOI] [PubMed] [Google Scholar]
- 151.Cool SM, Snyman CP, Nurcombe V, Forwood M. Temporal expression of fibroblast growth factor receptors during primary ligament repair. Knee Surgery, Sports Traumatology, Arthroscopy. 2004;12(5):490–496. doi: 10.1007/s00167-003-0444-x. [DOI] [PubMed] [Google Scholar]
- 152.Martin I, Vunjak-Novakovic G, Yang J, Langer R, Freed LE. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three- dimensional cartilaginous tissue. Experimental Cell Research. 1999;253(2):681–688. doi: 10.1006/excr.1999.4708. [DOI] [PubMed] [Google Scholar]
- 153.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. Journal of Cellular Physiology. 2005;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 154.Chiou M, Xu Y, Longaker MT. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochemical and Biophysical Research Communications. 2006;343(2):644–652. doi: 10.1016/j.bbrc.2006.02.171. [DOI] [PubMed] [Google Scholar]
- 155.Bosetti M, Boccafoschi F, Leigheb M, Cannas MF. Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomolecular Engineering. 2007;24(6):613–618. doi: 10.1016/j.bioeng.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 156.Tan Z, Zhao Q, Gong P, et al. Research on promoting periodontal regeneration with human basic fibroblast growth factor-modified bone marrow mesenchymal stromal cell gene therapy. Cytotherapy. 2009;11(3):317–325. doi: 10.1080/14653240902824757. [DOI] [PubMed] [Google Scholar]
- 157.Takayama S, Murakami S, Shimabukuro Y, Kitamura M, Okada H. Periodontal regeneration by FGF-2 (bFGF) in primate models. Journal of Dental Research. 2001;80(12):2075–2079. doi: 10.1177/00220345010800121001. [DOI] [PubMed] [Google Scholar]
- 158.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 159.Benoit DSW, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomaterialia. 2005;1(4):461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 160.Gomez-Pinilla F, Vu L, Cotman CW. Regulation of astrocyte proliferation by FGF-2 and heparan sulfate in vivo. Journal of Neuroscience. 1995;15(3):2021–2029. doi: 10.1523/JNEUROSCI.15-03-02021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gómez-Pinilla F, Miller S, Choi J, Cotman CW. Heparan sulfate potentiates the autocrine action of basic fibroblast growth factor in astrocytes: an in vivo and in vitro study. Neuroscience. 1996;76(1):137–145. doi: 10.1016/s0306-4522(96)00327-2. [DOI] [PubMed] [Google Scholar]
- 162.Tsai M-C, Shen L-F, Kuo H-S, Cheng H, Chak K-F. Involvement of acidic fibroblast growth factor in spinal cord injury repair processes revealed by a proteomics approach. Molecular and Cellular Proteomics. 2008;7(9):1668–1687. doi: 10.1074/mcp.M800076-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]