Abstract
Endoscopic ultrasound (EUS) has recently evolved through technological improvement of equipment, with a major clinical impact in digestive and mediastinal diseases. State-of-the-art EUS equipment now includes real-time sono-elastography, which might be useful for a better characterization of lesions and increased accuracy of differential diagnosis (for e.g. lymph nodes or focal pancreatic lesions). Contrast-enhanced EUS imaging is also available, and is already being used for the differential diagnosis of focal pancreatic masses. The recent development of low mechanical index contrast harmonic EUS imaging offers hope for improved diagnosis, staging and monitoring of anti-angiogenic treatment. Tridimensional EUS (3D-EUS) techniques can be applied to enhance the spatial understanding of EUS anatomy, especially for improved staging of tumors, obtained through a better assessment of the relationship with major surrounding vessels. Despite the progress gained through all these imaging techniques, they cannot replace cytological or histological diagnosis. However, real-time optical histological diagnosis can be achieved through the use of single-fiber confocal laser endomicroscopy techniques placed under real-time EUS-guidance through a 22G needle. Last, but not least, EUS-assisted natural orifice transluminal endoscopic surgery (NOTES) procedures offer a whole new area of imaging applications, used either for combination of NOTES peritoneoscopy and intraperitoneal EUS, but also for access of retroperitoneal organs through posterior EUS guidance.
Keywords: Endoscopic ultrasound, Real-time sono-elastography, Contrast-enhancement, Tridimensional (3D), Hybrid imaging, Endoscopic ultrasound-guided fine needle aspiration
INTRODUCTION
Endoscopic ultrasound (EUS) has evolved in recent years into a technique with a major clinical impact in digestive and mediastinal diseases. Thus, EUS determines a change in the diagnosis in approximately a quarter of patients, as well as a change in management in half of the patients examined[1]. A major step in the development of the EUS imaging techniques (Table 1) was represented by the appearance of electronic linear EUS scopes, which allowed a significant improvement in image quality, as well as the development of several EUS-guided or EUS-assisted procedures, which start with the real-time targeted placement of a fine-needle aspiration needle under direct imaging by ultrasound guidance[2].
Table 1.
Comparative assessment of new endoscopic ultrasound imaging techniques
| EUS technique | Advantage | Disadvantage | Cost | Invasiveness |
| Real-time sono-elastography | Improved diagnosis of focal pancreatic masses | Assessment in large RCTs needed | Average | Minimal |
| Contrast-enhanced EUS | Improved diagnosis and staging of focal pancreatic masses | Assessment in large RCTs needed | Average | Minimal |
| 3D-EUS | Moderate improvement of staging in pancreatic area | Limited assessment in clinical applications | Average | Minimal |
| Optical diagnosis | Improvement of real-time diagnosis | Limited assessment in clinical applications | High | Average |
| EUS-NOTES | Improvement of therapeutic options | Limited assessment in clinical applications | High | High |
EUS: Endoscopic ultrasound; RCTs: Randomized clinical trials; 3D-EUS: Tridimensional EUS; NOTES: Natural orifice transluminal endoscopic surgery.
REAL-TIME SONO-ELASTOGRAPHY
Technique
Real-time sono-elastography (RTSE) represents a technique which allows the calculation and visualization of tissue strain and hardness based on the average tissue strain in a selected region of interested[3]. The technique allows the real-time visualization of the calculated strain values (Figure 1A), displayed in a transparent layout over the gray-scale images, in a similar fashion with color Doppler imaging[4]. Several generations of software led to the improvement of image quality, reduced artifacts, but more important to the possibility of averaging through several cycles and calculation of semi-quantitative values of tissue strain inside a defined region of interest (for e.g. a lymph node or a focal pancreatic mass). By obtaining average hue histogram values inside a region of interest, the system displays the average strain inside a defined region of interest, as a semi-quantitative value that estimates tissue elasticity at that level.
Figure 1.
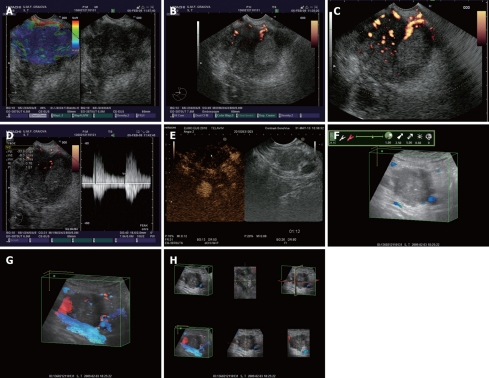
Pancreatic carcinoma at the level of pancreatic head depicted by different endoscopic ultrasound imaging techniques. A: Real-time elastography showing an in-homogenous hard mass; B: Power Doppler endoscopic ultrasound (EUS) without contrast-enhancement; C: Power Doppler EUS after contrast-enhancement with Sono-Vue, showing a hypovascular mass; D: Pulsed Doppler (triplex mode) after contrast-enhancement, with high resistivity and pulsatility indexes of intratumoral arteries; E: Contrast-enhanced low-mechanical index EUS harmonic imaging, showing a hypovascular appearance in the late (venous) phase; F: Tridimensional EUS showing an enhanced image in the opacity mode; G: Transparency mode obtained after contrast-enhancement with Sono-Vue; H: Multiview tridimensional (3D)-EUS display of the pancreatic tumor.
Applications
Several applications were described for RTSE, as a technique that offers additional information as compared with gray-scale EUS images[5]. The technique allows the selection of the most probable lymph nodes to be malignant, as well as the identification of lymph nodes that are most probable to be benign[5-7]. This was suggested to be helpful for the selection and guidance of EUS-guided fine needle aspiration (FNA) for staging purposes in lung cancer or other digestive and mediastinal cancers (including esophageal, gastric or pancreatic cancer). EUS elastography was also reported to be useful for the differentiation of focal pancreatic masses, especially in pseudotumoral chronic pancreatitis and pancreatic cancer, in the presence of negative (false-negative) EUS-guided FNA and a strong suspicion of pancreatic cancer[8-11]. The results of initial studies were recently validated in two multicentre studies[12,13]. Both studies indicated similar values for sensitivity, specificity, negative predictive value, positive predictive value and overall accuracy (92.6% vs 92.3%, 71.7% vs 80%, 76% vs 77.4%, 90.9% vs 93.3% and 87.4% vs 89.2%, respectively). It was thus suggested that the overall accuracy of 85-90% of EUS elastography might change current clinical decision making algorithms for the patients with focal pancreatic masses, especially in false-negative cases of EUS-FNA, when the suspicion of pancreatic cancer is still strong[12]. This warrants a more aggressive approach in negative EUS-FNA cases where EUS elastography suggests a hard mass, with the patients referred directly to surgery or to repeat EUS-guided FNA.
The method was also tested in initial feasibility studies in diffuse pancreatic diseases like early chronic pancreatitis or autoimmune pancreatitis[14,15].
Future usage of RTSE as a technique that simulates virtual palpation might include distant transmission of information and simulation of tele-palpation by using haptic devices and systems. This could lead to a better educational tool in order to simulate intra-operative palpation, and could also help provide guidance for remote surgical laparoscopic and robotic techniques.
CONTRAST-ENHANCEMENT
Technique
The development and subsequent approval of blood-pool contrast agents was a major step forward for the development of contrast specific ultrasound techniques[16]. Several contrast agents are clinically available, including Albunex, Levovist and Echovist (first generation), as well as SonoVue, Sonazoid and Optison (second generation), etc. All of them are quite safe, without severe complications or long-lasting side-effects. The usage of second-generation microbubble contrast agents further improved the diagnostic capabilities, through a strong increase in ultrasound backscatter and enhancement of echogenicity during the dynamic assessment of small volume and slow velocity blood flow. The advantage of second generation microbubble contrast agents is that they are able to pass through the lungs, thus remaining confined to the intravascular space for a longer time. Also, because of the low solubility they are more stable with favorable resonance at low acoustic pressures, hence longer specific imaging in real-time.
Initial applications used spectral (pulsed) Doppler, color or power Doppler imaging, with contrast agents used as vascular signal enhancers[17]. Contrast agents can thus rescue non-diagnostic Doppler examinations by increasing the intensity of weak flow signals to detectable levels. The appearance of contrast specific ultrasound modes further allowed the cancellation of tissue signals and utilization of the non-linear response of microbubbles (especially the second generation harmonic). The development of low-mechanical index techniques consequently led to a significant improvement consisting of visualization of the dynamic enhancement pattern in real-time. The main advantage is the absence of motion artifacts caused by cardiac or respiratory movements, including also flash and blooming (overpainting) artifacts.
Conventional imaging applications
One specific use of contrast-enhancement techniques in EUS was to detect low-velocity, low-volume flow of pancreatic tumors, with emphasis on the differential diagnosis between focal pancreatitis and pancreatic cancer[18-23]. An initial feasibility study in a pig model showed that the use of contrast agents is possible during EUS, leading to improved visualisation of the splanchnic vasculature[18]. Several studies further showed that contrast-enhanced power Doppler EUS can be successfully used for the differential diagnosis of chronic pseudotumoral pancreatitis and pancreatic cancer, with a sensitivity and specificity higher than 90%, in the presence of hypovascular malignant tumors[19-23]. An initial study that used Optison in combination with power Doppler contrast-enhanced EUS showed a sensitivity, specificity, positive predictive value, negative predictive value and overall accuracy of 94%, 100%, 100%, 88% and 96%, respectively[19]. Another study used different criteria for benign and malignant lesions, after contrast-enhancement with Sono-Vue and power Doppler EUS examinations, in combination with pulsed (spectral) Doppler. Malignant lesions were defined by the presence of irregular arterial vessels over a short distance and no detectable venous vessels inside the lesion, while benign lesions included regular appearance of vessels over a distance of at least 20 mm after injection of SonoVue and detection of both arterial and venous vessels[20]. By using this methodology, the sensitivity and specificity for the detection of pancreatic cancer were 91.1% and 93.3%, with an overall accuracy of 91.9%. Two other studies confirmed these results, although they used Levovist, a first generation contrast agent, in combination with color or power Doppler[21,22]. By using power Doppler vascularity index values calculated by special software (Figure 1B-D), a recent study also depicted values of sensitivity and specificity of 90.5% and 90.1% for the characterization and differentiation of focal pancreatic masses after contrast-enhancement with Sono-Vue[23].
Harmonic imaging applications
Contrast harmonic imaging based on the second harmonic, in combination with microbubble specific software, allows an improved visualization of vascular and parenchymal phases, in a similar approach with computer tomography (CT) or magnetic resonance (MR) techniques. However, contrast harmonic imaging has several advantages and differences as compared with contrast-enhanced CT or MR, due to the different pharmacokinetics and containment inside the intra-vascular space of the ultrasound contrast agents[16]. The most important advantage is that contrast-enhancement patterns can be followed in real-time, with a very good temporal resolution (Figure 1E), while the administration can be easily repeated. Because the technology has recently become available for use during EUS examinations, a few studies have already assessed the value of contrast-enhanced harmonic EUS, based on second-generation contrast agents (mostly Sono-Vue)[24,25]. An initial feasibility study showed that harmonic EUS with low mechanical index can be used for the differential diagnosis of pancreatic cancer and chronic pancreatitis[24]. By using a mechanical index of 0.4 in conjunction with harmonic EUS with a low mechanical index, both real-time visualization of finely branching vessels of the pancreas, as well as intermittent parenchymal perfusion images could be obtained[25]. The method showed irregular “network like” structures inside the pancreatic carcinoma masses, with hypovascular heterogenous perfusion images in the intermittent mode. This contrasted with focal masses in chronic pancreatitis that were homogenous iso- or hyper-vascular, thus allowing a correct differential diagnosis.
The use of microbubble contrast agents has already been recommended for the monitoring of the response to anti-angiogenic treatment, because the conventional criteria [Response Evaluation Criteria in Solid Tumors (RECIST) and World Health Organization size criteria] do not show changes in the tumor parenchymal perfusion, hence they cannot predict response in the presence of tumor necrosis without volume changes[16]. Thus, a clear correlation has been proven between histological intratumoral microvessel density, vascular endothelial growth factor (VEGF) and microvessels visualized by contrast-enhanced ultrasound in pancreatic ductal carcinoma[26]. By using a similar approach, contrast-enhanced EUS might prove very useful for the real-time monitoring of the efficacy of antiangiogenic treatments. Contrast-enhancd harmonic EUS would certainly have the advantage of an improved resolution and decreased artifacts induced by bowel air and obesity.
Targeted imaging and targeted treatment
Microbubbles used as ultrasound contrast agents can be targeted in vivo to specific endothelial cell surface receptors[27]. Thus, different ligands can be conjugated to the outer surface of microbubbles and directed selectively towards endothelial cells. Microbubbles can be linked with monoclonal antibodies directed against VEGF receptor 2 (VEGFR2), thus allowing the binding to tumor-associated epithelium in vivo[28]. This allows in vivo quantification of VEGFR2 expression in tumor vessels, permitting both the selection of antiangiogenic drugs (e.g. bevacizumab which blocks specifically the VEGF-VEGFR2 pathway), as well as monitoring of treatment response. Multitarget quantification and visualization of targeted contrast-enhanced ultrasound microbubbles conjugated with either VEGFR2 and/or α(v)β(3) integrin were tested in initial experimental designs[29]. Targeted treatment during targeted contrast-enhanced ultrasound has been recently proven interesting due to the enhanced cellular uptake of drugs and genes in the presence of ultrasound and especially contrast-enhanced ultrasound, a process called sonoporation[30]. New microbubbles incorporating chemotherapeutic or gene vectors can be delivered at a cellular level through the formation of transient porosities in the cell membrane.
3D-EUS
Tridimensional EUS was recently reviewed in a separate paper[31]. The method enhances the spatial understanding of EUS anatomy, especially for the pancreatico-biliary area. The method can better depict the relationship with major surrounding vessels, consequently improving staging and resectability, mainly for pancreatic tumors (Figure 1F-H). Contrast-enhanced 3D-EUS can also be performed, allowing a better calculation of the vascular index that might offer important prognostic information, linked with the status before and after antiangiogenic treatment.
REAL-TIME OPTICAL DIAGNOSIS
Real-time optical pathological diagnosis might be achieved based on recent advances in single fiber-based optical techniques, the best example being confocal laser endomicroscopy[32]. Miniaturization of a confocal laser endomicroscopy miniprobe allowed the EUS-guided placement of a miniprobe through a 22G needle, inside different organs/lesions located in the vicinity of the digestive tract, e.g. pancreas, spleen, adrenal, liver, etc. The method has been shown to be feasible, yielding high quality confocal laser endoscopy images, equivalent of real-time histopathology images.
EUS-NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY
The combination of EUS and natural orifice transluminal endoscopic surgery (NOTES) has already been described as a combination of NOTES peritoneoscopy and intraperitoneal EUS through transgastric and transcolonic approaches[33]. Thus, intraperitoneal EUS is considered safe and feasible, allowing adequate visualization of 4 sections of liver. Although objective landmarks for EUS were absent, intraperitoneal EUS could replace laparoscopic US, while NOTES peritoneoscopy can successfully replace laparoscopy. EUS-guided NOTES procedures were proven to be useful in a comparative sequential study which assessed mediastinoscopy/thoracoscopy, gastrojejunostomy and adrenalectomy[34]. EUS-guided access was useful mainly to obtain initial access or to identify structures in difficult areas, especially in the mediastinal or retroperitoneal regions. Furthermore, both an anterior and a posterior approach of the pancreas are possible through EUS-NOTES procedures, indicating a possible role for these combined techniques[35]. The aim was to improve pancreatic cancer staging of borderline cases and minimal invasive therapy of pancreatic diseases. Peritoneoscopy based on a EUS-assisted anterior transgastric approach, as well as EUS-guided posterior transgastric access to the pancreas, were both shown to be possible in this small non-survival animal study. Different therapeutic procedures like gastro-jejunostomies and cholecysto-gastrostomies were also shown to be possible after initial EUS-assisted procedures. Future survival studies with randomized design should establish clearly the clinical role of these procedures.
CONCLUSION
EUS reached maturity as an imaging technique, as compared with the initial description in 1980. With a superior resolution as compared with cross-sectional imaging and with the addition of recent techniques like real-time sono-elastography, contrast-enhancement and 3D reconstructions, EUS seems likely to represent the technique of choice for early diagnosis, staging and stratification of prognosis. EUS-guided FNA or EUS-assisted procedures are also considered procedures of choice for the pathological confirmation of advanced cases, as well as for targeted treatment procedures. All of these procedures lead to a significant clinical impact of EUS, especially due to the improved clinical decision making algorithms, which nowadays incorporate routine EUS-guided or EUS-assisted procedures. The transition of these procedures to real-time optical diagnosis might offer additional value, allowing the immediate initiation of minimal invasive therapeutic procedures. Also, the appearance of combined EUS-NOTES procedures might enhance the safety and success of recent NOTES applications.
Footnotes
Supported by “Utility of EUS and OCT for the minimal invasive evaluation of tumour neo-angiogenesis in the patients with digestive cancers” financed by the Romanian Ministry of Education and Research - National University Research Council (UEFISCSU - Ideas Program), No. 239/2007
Peer reviewer: Richard Hu, MD, MSc, Division of Gastroenterology, Department of Medicine, Olive view-UCLA Medical Center, 14445 Olive View Drive, Los Angeles, CA 91342, United States
S- Editor Wang YR L- Editor O'Neill M E- Editor Lin YP
References
- 1.Chong AK, Caddy GR, Desmond PV, Chen RY. Prospective study of the clinical impact of EUS. Gastrointest Endosc. 2005;62:399–405. doi: 10.1016/s0016-5107(05)01631-7. [DOI] [PubMed] [Google Scholar]
- 2.Bhutani MS. Interventional endoscopic ultrasonography: state of the art at the new millenium. Endoscopy. 2000;32:62–71. doi: 10.1055/s-2000-139. [DOI] [PubMed] [Google Scholar]
- 3.Frey H. [Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity] Radiologe. 2003;43:850–855. doi: 10.1007/s00117-003-0943-2. [DOI] [PubMed] [Google Scholar]
- 4.Saftoiu A, Vilman P. Endoscopic ultrasound elastography-- a new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161–165. [PubMed] [Google Scholar]
- 5.Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344–348. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 6.Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952–957. doi: 10.1055/s-2007-966946. [DOI] [PubMed] [Google Scholar]
- 7.Săftoiu A, Vilmann P, Ciurea T, Popescu GL, Iordache A, Hassan H, Gorunescu F, Iordache S. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291–300. doi: 10.1016/j.gie.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–978. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 9.Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–1094. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Hirche TO, Ignee A, Barreiros AP, Schreiber-Dietrich D, Jungblut S, Ott M, Hirche H, Dietrich CF. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–917. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101–1108. doi: 10.1016/j.gie.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Săftoiu A, Vilmann P, Gorunescu F, Will U, Giovannini M, Janssen J, Iglesias-Garcia J, Arcidiacono P, Hocke M, Mckay C, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of chronic pancreatitis and pancreatic cancer: A multicentric study. Gut. 2009;58(Suppl II):OP257. [Google Scholar]
- 13.Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587–1593. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirooka Y, Itoh A, Kawashima H, Hara K, Kanamori A, Uchida H, Goto J, Nonogaki K, Matsumoto Y, Ohmiya N, et al. Preliminary results in the diagnosis of the early stage chronic pancreatitis using EUS-elastography. Gastrointest Endosc. 2006;63:AB258. [Google Scholar]
- 15.Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–720. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 16.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D'Onofrio M, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 17.Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ. 2001;322:1222–1225. doi: 10.1136/bmj.322.7296.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhutani MS, Hoffman BJ, van Velse A, Hawes RH. Contrast-enhanced endoscopic ultrasonography with galactose microparticles: SHU508 A (Levovist) Endoscopy. 1997;29:635–639. doi: 10.1055/s-2007-1004270. [DOI] [PubMed] [Google Scholar]
- 19.Becker D, Strobel D, Bernatik T, Hahn EG. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784–789. doi: 10.1067/mge.2001.115007. [DOI] [PubMed] [Google Scholar]
- 20.Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–250. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–597.e1. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008;34:525–532. doi: 10.1016/j.ultrasmedbio.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Săftoiu A, Iordache S, Gheonea DI, Filip M, Ioncica AM, Popescu GL, Iordache AL, Ciurea T. Contrast-enhanced power doppler endoscopic ultrasound used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2009;69:AB332. [Google Scholar]
- 24.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: a new technique. Z Gastroenterol. 2005;43:1219–1223. doi: 10.1055/s-2005-858662. [DOI] [PubMed] [Google Scholar]
- 25.Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–150. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Ohshima T, Yamaguchi T, Ishihara T, Yoshikawa M, Kobayashi A, Sakaue N, Baba T, Yamada S, Saisho H. Evaluation of blood flow in pancreatic ductal carcinoma using contrast-enhanced, wide-band Doppler ultrasonography: correlation with tumor characteristics and vascular endothelial growth factor. Pancreas. 2004;28:335–343. doi: 10.1097/00006676-200404000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Kruskal JB. Can contrast-enhanced US with targeted microbubbles monitor the response to antiangiogenic therapies? Radiology. 2008;246:339–340. doi: 10.1148/radiol.2462071720. [DOI] [PubMed] [Google Scholar]
- 28.Willmann JK, Paulmurugan R, Chen K, Gheysens O, Rodriguez-Porcel M, Lutz AM, Chen IY, Chen X, Gambhir SS. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246:508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmowski M, Huppert J, Ladewig G, Hauff P, Reinhardt M, Mueller MM, Woenne EC, Jenne JW, Maurer M, Kauffmann GW, et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol Cancer Ther. 2008;7:101–109. doi: 10.1158/1535-7163.MCT-07-0409. [DOI] [PubMed] [Google Scholar]
- 30.Postema M, Gilja OH. Ultrasound-directed drug delivery. Curr Pharm Biotechnol. 2007;8:355–361. doi: 10.2174/138920107783018453. [DOI] [PubMed] [Google Scholar]
- 31.Saftoiu A, Gheonea DI. Tridimensional (3D) endoscopic ultrasound - a pictorial review. J Gastrointestin Liver Dis. 2009;18:501–505. [PubMed] [Google Scholar]
- 32.Becker V, Von Delius S, Voermans RP, Fockens P, Woodward TA, Raimondo M, Wallace MB, Meining A. Puncture-needle guided in vivo histology of intra-abdominal organs using probe-based confocal laser endomicroscopy (pCLE) in a porcine model. Gastrointest Endosc. 2009;69:AB166. doi: 10.1016/j.gie.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Feasibility of transgastric and transcolonic natural orifice transluminal endoscopic surgery peritoneoscopy combined with intraperitoneal EUS. Gastrointest Endosc. 2009;69:e61–e67. doi: 10.1016/j.gie.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 34.Fritscher-Ravens A, Ghanbari A, Cuming T, Kahle E, Niemann H, Koehler P, Patel K. Comparative study of NOTES alone vs. EUS-guided NOTES procedures. Endoscopy. 2008;40:925–930. doi: 10.1055/s-2008-1077732. [DOI] [PubMed] [Google Scholar]
- 35.Saftoiu A, Vilmann P, Surlin V, Fleming JB, Uthamanthil RK, Rimbas M, Singh H, Bektas M, Bhutani MS. Feasibility study of EUS-NOTES as a novel approach for pancreatic cancer staging and therapy: an international collaborative study. Gastrointest Endosc. 2010;71:AB222–AB223. doi: 10.5754/hge11774. [DOI] [PubMed] [Google Scholar]