Abstract
AIM: To investigate if non-alcoholic fatty liver disease (NAFLD) is an early mediator for prediction of metabolic syndrome, and if liver B-ultrasound can be used for its diagnosis.
METHODS: We classified 861 obese children (6-16 years old) into three subgroups: group 0 (normal liver in ultrasound and normal transaminases); group 1 (fatty liver in ultrasound and normal transaminases); and group 2 (fatty liver in ultrasound and elevated transaminases). We measured the body mass index, waist and hip circumference, blood pressure, fasting blood glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), whole-body insulin sensitivity index (WBISI), lipid profile and transaminases in all the participants. The risk of developing metabolic syndrome (MS) was assessed according to the degree of liver fatty infiltration based on the B-ultrasound examination.
RESULTS: Among the 861 obese children, 587 (68.18%) were classified as having NAFLD, and 221 (25.67%) as having MS. The prevalence of MS in NAFLD children (groups 1 and 2) was 37.64% (221/587), which was much higher than that in non-NAFLD group (group 0, 12.04%) (P < 0.01). There were significantly higher incidences concerning every component of MS in group 2 compared with group 0 (P < 0.05). The incidence of NAFLD in MS patients was 84.61% (187/221), which was significantly higher than that of hypertension (57.46%, 127/221) and glucose metabolic anomalies (22.62%, 50/221), and almost equal to the prevalence of dyslipidemia (89.14%, 197/221). Based on the B-ultrasound scales, the presence of moderate and severe liver fatty infiltration carried a high risk of hypertension [odds ratio (OR): 2.18, 95% confidence interval (95% CI): 1.27-3.75], dyslipidemia (OR: 7.99, 95% CI: 4.34-14.73), impaired fasting glucose (OR: 3.65, 95% CI: 1.04-12.85), and whole MS (OR: 3.77; 95% CI: 1.90-7.47, P < 0.01). The state of insulin resistance (calculated by HOMA-IR and WBISI) deteriorated as the degree of fatty infiltration increased.
CONCLUSION: NAFLD is not only a liver disease, but also an early mediator that reflects metabolic disorder, and liver B-ultrasound can be a useful tool for MS screening.
Keywords: Childhood obesity, Non-alcoholic fatty liver disease, Metabolic syndrome, Liver B ultrasonography
INTRODUCTION
There is a growing concern for non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome (MS) in obese children[1-3]. NAFLD is a clinicopathological syndrome that ranges from simple steatosis to steatohepatitis, fibrosis or cirrhosis of the liver[4]. It is associated with dyslipidemia, obesity, and insulin resistance, which are the main features of MS. NAFLD and MS often are seen in the same individual[5-8], whereas insulin resistance probably is a key event that links them together. The mortality of patients with NAFLD has increased significantly among the general population, and cardiovascular risk competes with liver-related risk in dictating the final outcome[9,10]. Several prospective studies in adults have demonstrated that NAFLD is associated with an increased incidence of MS and type 2 diabetes mellitus in elderly patients, and independent patients with obesity[11-14]. Thus, NAFLD may be not only a liver disease, but also an early mediator of type 2 diabetes mellitus and MS in adults. However, the impact of NAFLD on MS among the young population is still not clear. The debate remains as to whether NAFLD should be included as one of the components of the MS. This study aimed to assess idiopathic NAFLD among Chinese obese children, to verify the prevalence of MS in NAFLD patients based on B-ultrasound scan, and to investigate whether NAFLD is associated with MS and MS profile, and whether it is an important risk factor for MS as well.
MATERIALS AND METHODS
Study design and population
A total of 861 obese children and adolescents, aged between 6 and 16 years, who were referred to our endocrinology department with the complaint of obesity from January 2004 to September 2009, were enrolled in this study. Based on an accepted criteria for obesity diagnosis in Chinese children[15], all the participants had a body mass index (BMI) that was above the 95th percentile for their age and sex, based on the national reference data in 2004[16]. Exclusion criteria were the known presence of endocrine metabolic or kidney diseases, and the use of medication that altered blood pressure, liver function, and glucose or lipid metabolism. The demographic distribution of subjects is displayed in Table 1. Participants underwent a routine clinical examination, including physical examination, biochemical tests, oral glucose tolerance test (OGTT) and liver ultrasonographic scanning. There was no difference in age and Tanner stages between boys and girls. However, significant differences existed in clinical, anthropometric and laboratory data in relation to sex (Table 1).
Table 1.
Characteristics of 861 obese patients
| Girls (n = 263) | Boys (n = 598) | |
| Age (yr) | 10.53 ± 2.26 | 10.81 ± 1.97 |
| Tanner stage (T1/T2-4) | 126/137 | 287/311 |
| BMI (kg/m2)a | 27.68 ± 4.24 | 28.38 ± 3.47 |
| BMI Z-scorea | 4.31 ± 1.58 | 3.05 ± 1.16 |
| WHR (waist/hip ratio)a | 0.93 ± 0.07 | 0.96 ± 0.05 |
| Waist (cm)a | 86.53 ± 11.83 | 90.91 ± 10.25 |
| Systolic pressure (mmHg)a | 114.47 ± 13.50 | 117.33 ± 12.55 |
| Diastolic pressure (mmHg) | 67.85 ± 9.32 | 68.99 ± 9.00 |
| Cholesterol (mmol/L) | 4.48 ± 1.01 | 4.43 ± 0.93 |
| Triglycerides (mmol/L) | 1.79 (0.19-5.17) | 1.77 (0.17-10.78) |
| HDL | 1.23 ± 0.28 | 1.26 ± 0.34 |
| LDLa | 2.69 ± 0.73 | 2.51 ± 0.68 |
| AIa | 2.30 ± 0.83 | 2.11 ± 0.74 |
| Uric acid (μmol/L) | 377.0 (203.2-705.4) | 383.0 (198.2-708.5) |
| Fasting glucose (mmol/L) | 4.87 ± 0.58 | 4.90 ± 0.57 |
| 120-min glucose (mmol/L)a | 6.31 ± 1.52 | 6.01 ± 1.44 |
| HbA1c (%) | 5.74 ± 0.57 | 5.69 ± 0.52 |
| Fasting insulin (mIU/L)a | 17.9 (0.3-303.0) | 15.3 (0.3-219.0) |
| HOMA-IRa | 3.77 (0.07-78.11) | 3.33 (0.07-51.59) |
| WBISIa | 2.59 (0.39-11.90) | 3.37 (0.52-24.19) |
| ALT (U/L)a | 37.5 (8.0-283.0) | 29.0 (4.0-561.0) |
P < 0.05 boys vs girls. Data are expressed as mean ± SD or median (range). BMI: Body mass index; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; AI: Atherogenic index; HOMA-IR: Homeostasis model assessment of insulin resistance; WBISI: Whole-body insulin sensitivity index; ALT: Alanine aminotransferase.
The protocol was approved by the Medical Ethics Committee of The Children’s Hospital of Zhejiang University School of Medicine. Written informed consent from parents (or guardians) and children (where appropriate) were obtained.
Definition of disease, syndrome and disorders
NAFLD: NAFLD was defined according to the revised definition and treatment guidelines for NAFLD by the Chinese Hepatology Association in February 2006[17], and was diagnosed by means of a protocol using clinical, laboratory and ultrasound examinations in combination. In this study, NAFLD was diagnosed as a diffusely echogenic change on liver B-ultrasonography (fatty infiltration in liver), with or without elevated serum aminotransferase levels and other factors that can cause liver fatty infiltration or aminotransferase elevation, such as hepatitis virus infection, drug-induced injury, and other metabolic diseases, such as Wilson’s disease, were excluded. There was no history of current or past alcohol drinking. Two subgroups were classified in NAFLD obese children: group 1 (fatty liver in ultrasound and normal transaminases) and group 2 (fatty liver in ultrasound and elevated transaminases). Group 0 was diagnosed as obese children without liver disorder (normal liver in ultrasound and normal transaminases).
MS: The diagnosis criteria have been described in our previous study[18], which followed the suggestions of the Chinese Diabetes Association and the definition modified from the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATPIII). MS was diagnosed if patients met three or more of the following criteria for age and sex: (1) central obesity; (2) hyperglycemia (fasting glucose ≥ 6.1 mmol/L, or random glucose ≥ 7.8 mmol/L) or impaired glucose tolerance (IGT) or type 2 diabetes; (3) systolic or diastolic blood pressure above the 95th percentile for age and sex; and (4) hypertriglyceridemia (triglyceride concentration > 1.7 mmol/L) or low high-density lipoprotein-cholesterol (HDL-C) (< 1.03 mmol/L). In this study, high blood pressure was based on the percentile data of the 7th edition of Practical Pediatric Text Book by Zhu Fu-Tang[15]. IGT was defined as a glucose level > 7.8 mmol/L but < 11.1 mmol/L at 2 h. Type 2 diabetes was diagnosed according to the criteria of American Diabetes Association in 1997 and World health Organization in 1999.
Laboratory assessment
Height was measured without socks and shoes, and weight was measured in children who were wearing only underclothing. Waist was measured at the midpoint between the lower border of the rib cage and the iliac crest. Hip circumference was determined at the widest circle of the bottom. Pubertal development stages were assessed using Tanner stage criteria. Blood pressure was taken twice using the right arm, with the subject in a quiet sitting position, and the average level was recorded.
Subjects underwent routine biochemical evaluation in the morning before 09:00 h after an overnight fast for at least 8 h. Fasting glucose, insulin, lipids total triglyceride (TG), total cholesterol (TCHO), HDL-C and low-density lipoprotein-cholesterol (LDL-C), liver function [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] and uric acid were detected. Hepatitis serial serological tests (antibodies for hepatitis A-E) and glucose and insulin levels were also recorded during a standard (75 g) OGTT. Blood glucose was determined using a glucose oxidase method (North Biotechnology Company, Beijing, China) with intra-assay and inter-assay coefficient of variation (CVs) of 2.1% and 4.4%. Insulin serum levels were determined by radioimmunoassay (North Biotechnology Company) with intra-assay and inter-assay CVs of 6.4% and 9.7%, respectively. The serum concentrations of TCHO, TG, HDL-C, LDL-C, ALT, AST and uric acid were measured by routine enzymatic methods in our clinical laboratory.
The homeostasis model assessment of insulin resistance (HOMA-IR), based on serum fasting glucose and insulin levels, was used to measure insulin resistance. The whole body insulin sensitive index (WBISI) and the ratio of early insulin increment to early glucose increment (I30-0/G30-0) following oral glucose loading (75 g) were also obtained. HOMA-IR = [FIN (mU/L) × FBG (mg/dL)]/22.5; WBISI = 10000/[FIN (mU/L) × FBG (mg/dL) × average insulin (mU/L) × average glucose (mg/dL)]1/2; BMI = weight (kg)/[height (m)]2 (FIN: Free insulin; FBG: Free blood glucose).
Liver ultrasound examination
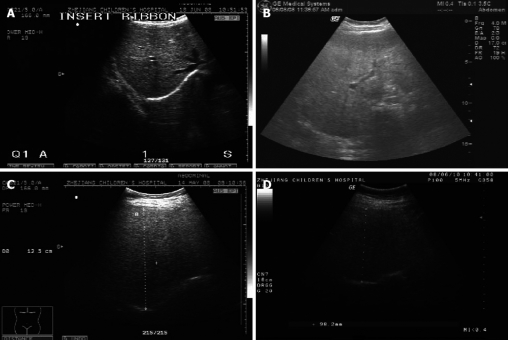
Liver ultrasound examination was carried out by one specialist who was unaware of the aims of the study and blinded to laboratory values on the same equipment (GE, LOGIC 500), using a convex 3.5-5.0 MHz probe. Sagittal hepatic sections that encompassed longitudinal images of the right liver lobe and the ipsilateral kidney were obtained. Liver-kidney contrast with two other well-known ultrasonographical findings of fatty liver, vascular blurring and deep attenuation, enabled us to grade fatty change semi-quantitatively. Fatty infiltration was graded semi-quantitatively into four classes[19,20]: no steatosis (class 0), mild steatosis (class 1), moderate steatosis (class 2) and severe steatosis (class 3) (Figure 1).
Figure 1.
Liver B-ultrasound scans show the panel of four different classes of liver steatosis. A: Class 0: not observed; B: Class 1: mild, liver-kidney contrast without vascular blurring and deep attenuation; C: Class 2: moderate, liver-kidney contrast with vascular blurring, but no deep attenuation; D: Class 3: severe, combination of liver-kidney contrast with vascular blurring and deep attenuation.
Statistical analysis
Data were collected using an MS-Excel spreadsheet. Data were analyzed using the JMP Statistical Discovery Software version 7.0 (SAS Institute, Cary, NC, USA). Group comparisons for continuous data were performed using t tests for independent means or one-way analysis of variance. A non-parametric test was used to evaluate the significance of abnormally distributed data. For categorical data, we employed the χ2 test, Fisher’s exact test, or binomial test of proportions. Multivariate logistic regression models were used to adjust for covariate effects on the odds ratio (OR). Statistical significance was set at P < 0.05.
RESULTS
Prevalence of NAFLD and MS in Chinese obese children
Of 861 obese children, 587 (68.18%) were diagnosed as having NAFLD, and 221 (25.67%) as having MS. The prevalences of hypertension, dyslipidemia, hyperuricacidemia, impaired fasting glucose, IGT and diabetes were 37.28% (321), 42.04% (362), 28.69% (247), 6.38%(55), 8.01% (69) and 1.39% (12), respectively. Moreover, the incidence of NAFLD in MS patients reached 84.61% (187/221), which was significantly higher than that of hypertension (57.46%, 127/221) and glucose metabolic anomalies (22.62%, 50/221), but almost equal to the prevalence of dyslipidemia (89.14%, 197/221).
NAFLD is associated with MS and its components among obese children
To investigate whether liver disorders are associated with MS, participants were divided into three subgroups: group 0 (normal liver in ultrasound and normal transaminases); group 1 (fatty liver in ultrasound and normal transaminases); and group 2 (fatty liver ultrasound and elevated transaminases). Table 2 indicates that sex, age and Tanner stage were comparable among the three subgroups. Nevertheless, the occurrence rates of MS increased with deterioration of liver from 12.04% in group 0 to 29.36% in group 1, and 39.74% in group 2 (P < 0.05). The prevalence of hypertension, dyslipidemia, IGT and diabetes in group 2 was significantly higher than that in group 0 (P < 0.05). Further investigation indicated that the prevalence of dyslipidemia and IGT increased steadily from group 0 to group 1 and group 2 (15.32% to 46.78% to 76.82%; 1.82% to 6.88% to 13.24%, P < 0.05). In addition, the levels of triglycerides, cholesterol, ALT and uric acid in group 2 were significantly higher than those in group 1 and group 0 (P < 0.05) (Table 3). It was indicated that the NAFLD was closely associated with progression of MS and its components in these obese children.
Table 2.
Comparison of prevalence of metabolic syndrome and its components among three groups
| Group 0 (n = 274) |
NAFLD |
||
| Group 1 (n = 436) | Group 2 (n = 151) | ||
| Age (yr) | 10.36 ± 2.15 | 10.59 ± 1.85 | 10.40 ± 2.28 |
| Sex (M/F) | 187/87 | 305/131 | 106/45 |
| Tanner stage (T1/T2–4) | 130/144 | 209/227 | 74/77 |
| BMI | 26.88 ± 3.19 | 28.78 ± 3.96a | 28.85 ± 3.69a |
| Hypertension | 68 (24.82) | 180 (41.28)a | 73 (48.34)a |
| Dyslipidemia | 42 (15.32) | 204 (46.78)a | 116 (76.82)ac |
| Impaired fasting glucose | 5 (1.82) | 30 (6.88) | 20 (13.24)ac |
| IGT | 13 (4.74) | 38 (8.72) | 18 (11.92)a |
| Diabetes | 0 (0.00) | 5 (1.14) | 7 (4.63)ac |
| MS | 33 (12.04) | 128 (29.36)a | 60 (39.74)ac |
P < 0.05 vs group 0;
P < 0.05, group 1 vs group 2. Data are expressed as percentage, mean ± SD. NAFLD: Non-alcoholic fatty liver disease; BMI: Body mass index; IGT: Impaired glucose tolerance; MS: Metabolic syndrome.
Table 3.
Clinical and laboratory data of non-alcoholic fatty liver disease compared with obese children without liver disorder
| Group 0 (n = 274) |
NAFLD |
||
| Group 1 (n = 436) | Group 2 (n = 151) | ||
| Age (yr) | 10.36 ± 2.15 | 10.59 ± 1.85 | 10.40 ± 2.28 |
| BMI (kg/m2) | 26.58 ± 3.19 | 28.85 ± 3.69a | 28.78 ± 3.96a |
| BMI Z-score | 3.17 ± 1.26 | 3.33 ± 1.37 | 3.93 ± 1.60ac |
| Waist circumference (cm) | 85.63 ± 10.17 | 91.58 ± 11.24a | 92.2 ± 9.31a |
| Father BMI (kg/m2) | 25.12 ± 3.68 | 25.68 ± 5.78 | 25.55 ± 3.75 |
| Mother BMI (kg/m2) | 22.83 ± 3.14 | 23.46 ± 3.61a | 23.64 ± 3.38a |
| Systolic blood pressure (mmHg) | 113.62 ± 11.89 | 118.30 ± 13.37a | 118.03 ± 12.73a |
| Diastolic blood pressure (mmHg) | 67.80 ± 9.02 | 69.00 ± 9.38 | 69.43 ± 8.67a |
| ALT (U/L) | 24 (8-74) | 29 (4-75)a | 51.5 (76-561)ac |
| Uric acid (μmol/L) | 352.9 (186.5-603.20) | 365.9 (168.10-708.50)a | 412.5 (7.38-771.30)ac |
| Triglycerides (mmol/L) | 1.4 (0.19-6.76) | 1.42 (0.26-5.90) | 1.77 (0.28-8.64)ac |
| Cholesterol (mmol/L) | 4.37 ± 0.99 | 4.34 ± 0.87 | 4.71 ± 0.99ac |
| HDL | 1.29 ± 0.39 | 1.23 ± 0.28 | 1.22 ± 0.28 |
| LDL | 2.52 ± 0.66 | 2.54 ± 0.68 | 2.68 ± 0.78 |
| AI | 2.07 ± 0.73 | 2.15 ± 0.70 | 2.31 ± 0.92a |
| Fasting blood glucose (mmol/L) | 4.82 ± 0.52 | 4.88 ± 0.69 | 4.95 ± 0.55a |
| 30-min OGTT glucose (mmol/L) | 7.54 ± 1.17 | 7.73 ± 1.24 | 7.69 ± 1.21 |
| 120-min OGTT glucose (mmol/L) | 5.66 ± 1.36 | 6.31 ± 1.42a | 6.22 ± 1.63a |
| HbA1c (%) | 5.70 ± 0.50 | 5.71 ± 0.53 | 5.65 ± 0.62 |
| Fasting insulin (mIU/L) | 12.7 (0.3-187.0) | 17.0 (0.3-219.0)a | 18.2 (2.1-303.0)a |
| 30-min OGTT insulin (mIU/L) | 85.3 (1.8-400.0) | 112.0 (0.5-400.0)a | 106.2 (1.6-400.0)a |
| 120-min OGTT insulin (mIU/L) | 36.3 (3.2-400.0) | 63.6 (1.7-386.0)a | 69.8 (7.0-400.0)a |
| HOMA-IR | 2.61 (0.07-41.56) | 3.71 (0.08-51.59)a | 3.95 (0.48-78.11)a |
| WBISI | 4.0 (0.6-16.4) | 2.8 (0.9-24.2)a | 2.6 (0.4-16.7)a |
P < 0.05 vs group 0;
P < 0.05, group 1 vs group 2. Data are expressed as mean ± SD or median (range). NAFLD: Non-alcoholic fatty liver disease; BMI: Body mass index; ALT: Alanine aminotransferase; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; AI: Atherogenic index; OGTT: Oral glucose tolerance test; HOMA-IR: Homeostasis model assessment of insulin resistance; WBISI: Whole-body insulin sensitivity index.
NALFD is accompanied by insulin resistance in obese children
Insulin resistance is a key event that causes MS in both adults and children[21]. To investigate whether NAFLD is associated with insulin resistance, we performed OGTT and insulin releasing test in all the participants. As for the results of blood glucose, fasting blood glucose and 120-min OGTT glucose levels in group 2 were significantly higher than those in group 0 (P < 0.05). The blood level of insulin, including fasting insulin, 30-min OGTT insulin and 120-min OGTT insulin, were significantly increased in groups 1 and 2 as compared with those in group 0 (P < 0.05). The HOMA-IR and WBISI reflected insulin resistance and sensitivity respectively. Our analysis indicated that HOMA-IR was elevated significantly in groups 1 and 2, whereas WBISI decreased significantly as compared with that of group 0 (P < 0.05) (Table 3). This finding revealed that NAFLD was significantly associated with insulin resistance among these obese children.
MS is associated with liver steatosis found by ultrasound examination
Fatty infiltration is another indicator that reflects liver damage, which can be easily detected by ultrasound examination. Based on B-ultrasound examination, 861 obese children were classified into class 0 (274 cases without steatosis), class 1 (105 cases with mild steatosis), and classes 2-3 (482 cases with moderate and severe steatosis). It was indicated that the relative risk of MS increased to 3.10 [95% confidence interval (95% CI): 1.20-8.00] in class 1 and 3.77 (95% CI: 1.90-7.47) in classes 2-3 (P < 0.01) (Table 4). Based on the B-ultrasound scales, the presence of moderate and severe liver fatty infiltration carried a high risk of developing hypertension (OR: 2.18, 95% CI: 1.27-3.75), dyslipidemia (OR: 7.99, 95% CI: 4.34-14.73), impaired fasting glucose (OR: 3.65, 95% CI: 1.04-12.85), and hyperuricacidemia (OR: 3.76, 95% CI: 2.03-6.96) (Table 4, Figures 2, 3 and 4). We also determined whether HOMA-IR and WBISI were associated with the degree of fatty liver. It was revealed that the HOMA-IR increased significantly from 2.64 in class 0 to 3.57 in classes 2-3 , but the WBISI decreased from 3.97 in class 0 to 2.67 in classes 2-3 among the obese subjects. All these findings indicated that the scale of fatty infiltration in liver was closely related to MS and insulin resistance among Chinese obese children.
Table 4.
Metabolic syndrome in obese patients diagnosed by B-ultrasound
| Scale (0) (n = 274) | Scale (1) (n = 105) | Scale (2-3) (n = 482) | P | |
| Hypertension | ||||
| No | 206 | 54 | 280 | |
| Yes | 68 | 51 | 202 | < 0.05 |
| OR (95% CI) | 1.0 | 2.87 (1.29-6.37) | 2.18 (1.27-3.75) | |
| Dyslipidemia | ||||
| No | 232 | 57 | 210 | |
| Yes | 42 | 48 | 272 | < 0.01 |
| OR (95% CI) | 1.0 | 5.21 (2.23-12.18) | 7.99 (4.34-14.73) | |
| Impaired fasting glucose | ||||
| No | 269 | 102 | 435 | |
| Yes | 5 | 3 | 47 | < 0.05 |
| OR (95% CI) | 1.0 | 1.0 (0.1-9.94) | 3.65 (1.04-12.85) | |
| Impaired glucose tolerance | ||||
| No | 261 | 99 | 432 | |
| Yes | 13 | 6 | 50 | > 0.05 |
| OR (95% CI) | 1.0 | 1.53 (0.27-8.74) | 2.9 (0.95-8.87) | |
| Hyperuricacidemia | ||||
| No | 232 | 81 | 301 | |
| Yes | 42 | 24 | 181 | < 0.01 |
| OR (95% CI) | 1.0 | 1.83 (0.71-4.74) | 3.76 (2.03-6.96) | |
| MS | ||||
| No | 241 | 75 | 324 | |
| Yes | 33 | 30 | 158 | < 0.01 |
| OR (95% CI) | 1.0 | 3.10 (1.20-8.00) | 3.77 (1.90-7.47) | |
MS: Metabolic syndrome; OR: Odds ratio; CI: Confidence interval.
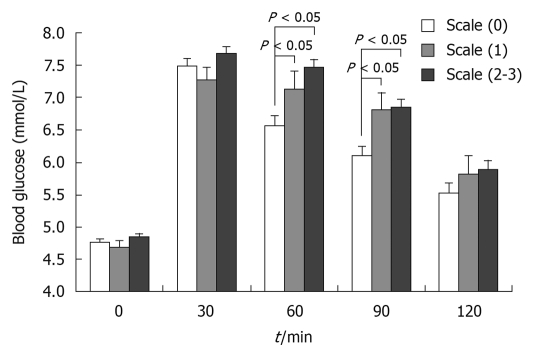
Figure 2.
Oral glucose tolerance test in obese patients based on liver B-ultrasound gradings. When patients were stratified according to the presence of liver fatty infiltration based on the B-ultrasound scans, their glucose levels at 60 min and 90 min after tolerance were significantly higher in classes 1-3 (P < 0.05) than in class 0, but there was no difference between class 1 and classes 2-3 (P > 0.05).
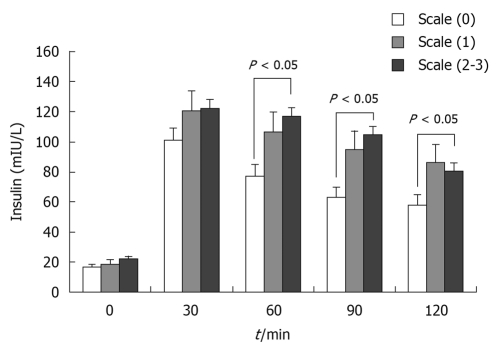
Figure 3.
Insulin releasing test in obese patients based on liver B-ultrasound gradings. There was no difference in fasting insulin among the three groups, but 60, 90 and 120 min insulin levels were markedly increased in the class 2-3 groups compared with class 0 (P < 0.05).
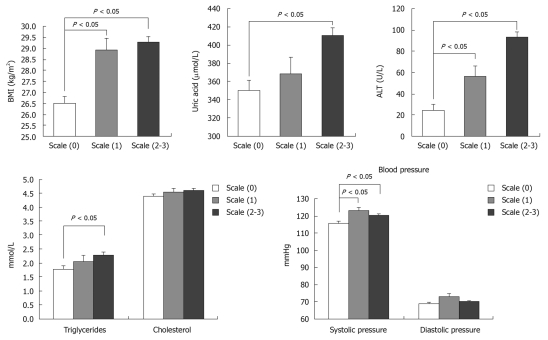
Figure 4.
Clinical features of the metabolic syndrome in obese patients based on liver B-ultrasound gradings. Concerning the individual metabolic component, body mass index (BMI), alanine aminotransferase (ALT) and systolic pressure were significantly higher in class 1 and classes 2-3 compared with class 0 (P < 0.05), but there was no difference between class 1 and classes 2-3 (P > 0.05). The levels of uric acid and triglyceride were only markedly increased in classes 2-3 compared with class 0 (P < 0.05). There was no difference in cholesterol levels among the three groups (P > 0.05).
DISCUSSION
The prevalence of both NAFLD and MS was higher in this study at 68.18% and 25.67%, respectively, than that of 9.6% and 4.2% in the general pediatric population[22]. NAFLD is regarded as an increasing clinical problem in children and adolescents, and accounts for the vast majority of cases with elevated serum liver enzymes[23]. Moreover, NAFLD is known to be related to the factors that predict the development of coronary heart disease, such as dyslipidemia, central obesity and MS.
Apart from that, NAFLD has been shown increasingly and more convincingly to be an important component of MS. In this study, we demonstrated that the prevalence of MS was three times higher in NAFLD obese children than in those without liver disorders (39.74% vs 12.04%, P < 0.05). The incidence of each component of MS was also significantly higher in NAFLD subjects (P < 0.05). Moreover, the presence of moderate and severe liver fatty infiltration confirmed by B-ultrasonography carried a high risk of development of MS, which highlights that NAFLD is closely associated with features of MS. In contrast, the incidence of NAFLD in MS patients reached 84.61%, which was significantly higher than that of hypertension (57.46%) and glucose metabolic anomalies (22.62%), and almost equal to the prevalence of dyslipidemia (89.14%). This indicates that NAFLD might be an early stage mediator for prediction of MS.
To date, the biological mechanisms that are involved in the higher risk of developing metabolic disorders in patients with NAFLD are not fully understood. Insulin resistance seems to be the key event. Nevertheless, most obese patients with NAFLD had hyperinsulinemia and higher insulin resistance compared with those without liver disorders, as calculated by fasting insulin, 30 min and 120 min insulin after glucose loading, HOMA-IR and WBISI. In contrast, patients with NAFLD were more obese and exhibited higher insulin resistance and more marked metabolic complications than those with simple obesity. Fatty liver itself is an insulin resistance status[24], and because hepatic fat accumulation can lead to hepatic insulin resistance, the latter may occur before any alternation in peripheral insulin action or peripheral insulin resistance. Moreover, insulin resistance may cause abnormalities of lipid storage and lipolysis in insulin-sensitive tissues, which may induce increased fatty acid flux from adipose tissue to the liver and result in steatosis[25]. Insulin resistance may also cause lipid peroxidation, which in turn, activates inflammatory cytokines and promotes the progression of innocent steatosis to non-alcoholic steatohepatitis and liver fibrosis[26]. The impairment in fat and glucose metabolism when insulin resistance occurs, can lead to similar biochemical and clinical abnormalities in patients with NAFLD, and sooner or later it will inevitably develop to systemic MS.
An accurate fatty liver diagnosis and staging of non-alcoholic steatohepatitis requires liver biopsy. However, liver biopsy is not performed often in patients, especially in children, with no significant or trivial liver diseases. Most of the patients with liver steatosis can be well-managed without a need for liver biopsy. In our study, steatosis was assessed by liver ultrasonography with a sensitivity of 83% and a specificity of 100% in comparison with the gold standard of histological diagnosis. Therefore, liver ultrasonography is being strongly suggested as a non-invasive study of NAFLD[18,27,28]. It was particularly interesting to find that both the prevalence of MS and every component of MS increased as the liver ultrasonographic grade deteriorated. Moreover, liver fat is highly significantly associated with all components of MS. Compared with abdominal and overall obesity, fatty liver has the highest frequency of clustering, greater specificity, higher positive predictive value and the most attributable risk for detecting risk factors of cardiovascular disease, type 2 diabetes and MS[29,30]. Thus, fatty liver seems to be an early mediator for prediction of other metabolic disorders. Since B ultrasound scan can be easily and widely applied in hospitals, this simple and effective scanning technique may provide a new method of MS screening in the future.
All findings in our study have stimulated interest in the possible role of NAFLD in the development of metabolic complications. Their coexistence in the same individual increases the likelihood of having more severe dysmetabolic status. Several studies in adults also have demonstrated that NAFLD is the primary hepatic complication of obesity and insulin resistance, and may be considered the early hepatic manifestation of MS. Early treatment, such as lifestyle or diet modification, aerobic exercise, or medication (metformin or vitamin E), for NAFLD may not only improve the prognosis of liver disease, but also reduce the occurrence of underlying metabolic and vascular complications. Furthermore, it is also implied that earlier adjustment to mobilizing fat out of the liver might reduce the risks of MS.
COMMENTS
Background
There is a growing concern for non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome (MS) in obese children. NAFLD and MS often are seen in the same individual, and insulin resistance is a probable key event that links them together. NAFLD may not only be a liver disease, but also an early mediator of type 2 diabetes mellitus and MS in adults. However, the impact of NAFLD on MS among the young population is still not clear.
Research frontiers
NAFLD is now considered a metabolic pathway to advanced liver disease, cirrhosis and hepatocellular carcinoma. Type 2 diabetes mellitus, obesity and dyslipidemia are the principal factors associated with NAFLD, which is now considered the hepatic expression of MS. The risk of liver disease associated with the classical features of MS in children is still unclear. We still need to clarify the mechanisms that are responsible for liver disease progression from pure fatty liver to steatohepatitis and to cirrhosis, and the reasons why only a few NAFLD cases progress to terminal liver failure while others (the majority) will have a cardiovascular outcome.
Innovations and breakthroughs
An accurate fatty liver diagnosis and staging of non-alcoholic steatohepatitis require liver biopsy. However, liver biopsy is not performed often in patients, especially children, with no significant, or trivial liver diseases. Most of the patients with liver steatosis can be well-managed without a need for liver biopsy. In this study, steatosis was assessed by liver ultrasonography, which showed that both the prevalence of MS and every component of MS increased as the liver ultrasonographic grade deteriorated. Liver fat was highly significantly associated with all components of MS.
Applications
Since B ultrasound scan can be easily and widely applied in hospitals, this simple and effective scanning technique may provide a new method of MS screening in the future in the general population.
Peer review
The authors addressed an important subject and described a population of patients referred for obesity with respect to the presence of sonographic evidence for NAFLD, and their metabolic profiles with respect to the insulin axis.
Acknowledgments
We are grateful to Dr. Li-Qin Chen and Zhong-Sheng Yu for their assay expertise. We sincerely thank the parents and children for participating in these studies. We thank the nursing staff of our department for their dedicated care of these young patients during the collection and evaluation of blood samples.
Footnotes
Supported by Zhejiang Provincial Natural Science Foundation, No. Y2080047; funds for Zhejiang Major Medical and Health Science and Technology Program and funds from Ministry of Health, No. WKJ2008-2-026; Major Special Zhejiang Provincial Science and Technology Fund, No. 2008c03002-1; and the National Key Technology R&D Program of China, No. 2009BAI80B01
Peer reviewer: Michael L Schilsky, MD, Associate Professor of Medicine and Surgery, Yale University School of Medicine, 333 Cedar Street, LMP 1080, New Haven, CT 06520, United States
S- Editor Sun H L- Editor Kerr C E- Editor Lin YP
References
- 1.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008;32:381–387. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 2.Calcaterra V, Klersy C, Muratori T, Telli S, Caramagna C, Scaglia F, Cisternino M, Larizza D. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clin Endocrinol (Oxf) 2008;68:868–872. doi: 10.1111/j.1365-2265.2007.03115.x. [DOI] [PubMed] [Google Scholar]
- 3.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 4.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 5.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 6.Marion AW, Baker AJ, Dhawan A. Fatty liver disease in children. Arch Dis Child. 2004;89:648–652. doi: 10.1136/adc.2003.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Fiorello S, Cavallo MG, Zalunardo B, Lirussi F, Alessandri C, et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578–1582. doi: 10.1210/jc.2004-1024. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 9.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoliquido A, Di Campli C, Miele L, Gabrieli ML, Forgione A, Zocco MA, Lupascu A, Di Giorgio A, Flore R, Pola P, et al. Hepatic steatosis and vascular disease. Eur Rev Med Pharmacol Sci. 2005;9:269–271. [PubMed] [Google Scholar]
- 11.Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861–867. doi: 10.1038/ajg.2009.67. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Misawa K, Kawata S. 1. Fatty liver and non-alcoholic steatohepatitis. Intern Med. 2007;46:101–103. doi: 10.2169/internalmedicine.46.1784. [DOI] [PubMed] [Google Scholar]
- 13.Marchesini G, Babini M. Nonalcoholic fatty liver disease and the metabolic syndrome. Minerva Cardioangiol. 2006;54:229–239. [PubMed] [Google Scholar]
- 14.Fan JG. Impact of non-alcoholic fatty liver disease on accelerated metabolic complications. J Dig Dis. 2008;9:63–67. doi: 10.1111/j.1751-2980.2008.00323.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu YM, Jiang ZF. Practical Pediatric Textbook of Fu-Tang Zhu. 7th ed. Beijing: People’s Hygiene Publishing; pp. 27–30, 553, 1414, 2687. [Google Scholar]
- 16.Chinese Obese Problem Work Team. BMI classification standard of Chinese school-age child and adolescents for overweight and obesity. Zhonghua Liuxingbingxue Zazhi. 2004;25:97–102. [Google Scholar]
- 17.Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association. Guidelines for diagnosis and treatment of nonalcoholic fatty liver disease. Zhonghua Ganzangbing Zazhi. 2006;14:161–163. [PubMed] [Google Scholar]
- 18.Fu JF, Liang L, Zou CC, Hong F, Wang CL, Wang XM, Zhao ZY. Prevalence of the metabolic syndrome in Zhejiang Chinese obese children and adolescents and the effect of metformin combined with lifestyle intervention. Int J Obes (Lond) 2007;31:15–22. doi: 10.1038/sj.ijo.0803453. [DOI] [PubMed] [Google Scholar]
- 19.Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996;24:25–29. doi: 10.1002/(SICI)1097-0096(199601)24:1<25::AID-JCU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J Exp Med. 1983;139:43–50. doi: 10.1620/tjem.139.43. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher EJ, LeRoith D, Karnieli E. The metabolic syndrome--from insulin resistance to obesity and diabetes. Endocrinol Metab Clin North Am. 2008;37:559–579, vii. doi: 10.1016/j.ecl.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Bitsori M, Kafatos A. Dysmetabolic syndrome in childhood and adolescence. Acta Paediatr. 2005;94:995–1005. doi: 10.1111/j.1651-2227.2005.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 24.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 25.Lonardo A, Lombardini S, Scaglioni F, Carulli L, Ricchi M, Ganazzi D, Adinolfi LE, Ruggiero G, Carulli N, Loria P. Hepatic steatosis and insulin resistance: does etiology make a difference? J Hepatol. 2006;44:190–196. doi: 10.1016/j.jhep.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury J, Sanyal AJ. Insulin resistance in NASH. Front Biosci. 2005;10:1520–1533. doi: 10.2741/1636. [DOI] [PubMed] [Google Scholar]
- 27.Graif M, Yanuka M, Baraz M, Blank A, Moshkovitz M, Kessler A, Gilat T, Weiss J, Walach E, Amazeen P, et al. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Invest Radiol. 2000;35:319–324. doi: 10.1097/00004424-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 29.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24:1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 30.Lidofsky SD. Nonalcoholic fatty liver disease: diagnosis and relation to metabolic syndrome and approach to treatment. Curr Diab Rep. 2008;8:25–30. doi: 10.1007/s11892-008-0006-1. [DOI] [PubMed] [Google Scholar]