Abstract
This paper aims to highlight the importance of exercise in patients with rheumatoid arthritis (RA) and to demonstrate the multitude of beneficial effects that properly designed exercise training has in this population. RA is a chronic, systemic, autoimmune disease characterised by decrements to joint health including joint pain and inflammation, fatigue, increased incidence and progression of cardiovascular disease, and accelerated loss of muscle mass, that is, “rheumatoid cachexia”. These factors contribute to functional limitation, disability, comorbidities, and reduced quality of life. Exercise training for RA patients has been shown to be efficacious in reversing cachexia and substantially improving function without exacerbating disease activity and is likely to reduce cardiovascular risk. Thus, all RA patients should be encouraged to include aerobic and resistance exercise training as part of routine care. Understanding the perceptions of RA patients and health professionals to exercise is key to patients initiating and adhering to effective exercise training.
1. Background
Rheumatoid arthritis (RA) is a chronic, systemic, autoimmune disease, and the most common form of chronic joint inflammation, affecting 0.5–1% of the UK population. RA is most prevalent in individuals aged 40 years or older with the risk of developing RA being up to 5 times higher in women [1]. As a consequence of their disease RA patients typically suffer severe joint pain, reduced muscle strength, and impaired physical function [2]. Although outcomes of the disease have improved with modern approaches to drug treatment, using agents such as methotrexate and biologics, the disease is still a progressive one with long-term joint damage and disability the expectation rather than the rule.
A major feature of the disease is severe inflammation of the synovium where there is a 3–100 times elevation of proinflammatory cytokines such as tumour necrosis factor alpha (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and C-reactive protein (CRP) [3]. The course of RA is typically one of exacerbations and remissions but, even during inactive phases of the disease, systemic levels of cytokines remain dysregulated when compared to those without rheumatoid arthritis [4]. RA also results in downregulation of anabolic factors for muscle, for example, muscle levels of insulin-like growth factor I (IGF-1) [5]. The circulating levels of cytokines reflect disease activity and level of inflammation present and also may play a significant role in the systemic effects of the disease, such as vascular disease [4] and rheumatoid cachexia [6].
In addition to the articular features of the disease, RA is associated with increased morbidity and mortality from cardiovascular disease (CVD) [7, 8]. The relative risk of myocardial infarction is estimated to be double in women with RA relative to those without [8], and CVD events typically occur a decade earlier and to a greater extent in patients with RA relative to healthy controls; sometimes even before the fulfilment of all criteria of RA [9]. A recent meta-analysis of 24 studies, comprising 111,758 patients with 22,927 cardiovascular events, reported a 50% increased risk of CVD deaths in patients with RA compared with the general population [10]. This increase in CVD in RA patients appears to be independent of traditional cardiovascular risk factors [11]. Given that chronic low-grade inflammation is thought to play an important role in the underlying cause of CVD, atherosclerosis [12], it seems reasonable to hypothesize that systemic inflammation contributes to elevated CVD in persons with RA [9].
Most RA patients also suffer from an accelerated loss of muscle mass, a condition known as “rheumatoid cachexia”. This loss contributes to disability and has a significant impact on an individuals' quality of life [13]. Rheumatoid cachexia has been reported in two thirds of all RA patients, including patients with stable RA [5, 14]. Roubenoff and colleagues [6] proposed that rheumatoid cachexia is caused by the cytokine-driven (principally TNF-α) hypermetabolism and protein degradation. However, poor nutrition [15] and low physical activity levels [16] are also believed to contribute.
Low physical activity is an important and reversible characteristic of RA. It has been demonstrated that RA patients do less exercise than their healthy counterparts; more than 80% of RA patients are physically inactive in some countries [17], whilst in the UK it is believed that approximately 68% of RA patients are physically inactive [17]. The extreme physical inactivity of RA patients' becomes a vicious circle in terms of health and disease progression. Thus it has become apparent that encouraging physical activity is an important and essential part of the overall treatment of RA.
The purpose of this paper is to highlight the importance of exercise in patients with RA and to demonstrate the multitude of beneficial effects that a properly designed exercise intervention has in this population. In order to present this aim, this paper has been organised into separate sections. Firstly, a brief explanation of the background of RA and the benefits of exercise in the general population is presented. Secondly, the benefits of exercise in RA are highlighted, focusing on the areas of cardiovascular disease, musculoskeletal and joint health, and overall function. Thirdly, the perceptions of RA patients regarding exercise are discussed and finally exercise prescription for RA is reviewed. This expert review has been derived from a combination of systematic reviews and other research papers focusing on randomised controlled trials, published guidelines, the recent literature, and also making use of our own specialised experience. It is not within the scope of this review to discuss the benefits of standard low-intensity physiotherapy techniques such as range of motion, stretching, and/or specific joint strengthening. The review, however, does encompass a range of physical activity and physical exercise. We broadly define physical activity as any bodily movement produced by skeletal muscles resulting in energy expenditure above resting levels and physical exercise (“exercise” or “exercise training”) to be a subset of leisure time physical activity that pertains to planned, structured, and repetitive bodily movements, aimed at improving or maintaining fitness, physical performance, or health [18]. We have based our definition of functional ability from the disablement process in RA as described by Escalante and Del Rincon (2002) of pathology, impairment, functional limitation, and disability [19].
Overview of the Benefits of Exercise in the General Population: Older Adults —
It is widely acknowledged that regular exercise/physical activity provides multiple health benefits for the general population and patients with chronic diseases. This includes improvements in cardiovascular health and reducing the risk of coronary artery disease, stroke, and type 2 diabetes by attenuating hypertension and dyslipidemia, improving insulin sensitivity and reducing adiposity [20]; reducing the risk of colon and breast cancers [21]; increasing muscle strength and mechanical properties and bone mineral density [22, 23]; improving balance and reducing the incidence of falls [24]; facilitating psychological well-being [25]. By engaging in recommended exercises older adults can help reduce the risk of chronic disease (e.g., of developing CVD by about 30%–50% [26]), premature mortality, functional limitation, and disability [27].
Basic recommendations from the American College of Sports Medicine (ACSM) suggest for health benefit that every adult should accumulate at least 30 minutes of moderate-intensity physical activity on most days of the week. ACSM have issued a separate set of guidelines for older adults, that is, men and women aged 65 years and above and adults aged 50–64 years with clinically significant chronic conditions such as RA. These guidelines are similar with additional importance stressed on muscle strengthening exercises and exercises to improve balance and flexibility [27].
2. Benefits of Exercise in RA
Apart from the general effects of exercise previously mentioned in the general population, exercise has been shown to have specific health benefits in people with RA. In fact, as evident from past research, including findings from randomised controlled trials [5, 28–41], exercise is considered to be fundamentally beneficial for RA patients. The reported benefits of properly designed physical exercise programs include improved cardiorespiratory fitness and cardiovascular health, increased muscle mass, reduced adiposity (including attenuated trunk fat), improved strength, and physical functioning, all achieved without exacerbation of disease activity or joint damage. Furthermore, when comparing the effectiveness of high and low intensity exercise training in stable RA, it is found that the former was more effective in increasing aerobic capacity, muscle strength, joint mobility, and physical function with no detrimental effect on disease activity in patients with controlled [5, 36] and active RA [37].
2.1. Cardiovascular Disease and Exercise
A goal for any RA treatment regime should be to reduce cardiovascular comorbidity, in line with the overall aim of prolonging and improving quality of life. The benefits of physical activity, exercise training, and cardiorespiratory fitness in primary and secondary cardiovascular disease prevention are well established [42, 43]. Low aerobic fitness is strongly associated with all-cause and cardiovascular disease mortality in apparently healthy men and women, those with comorbid conditions (obesity, hypertension, and type 2 diabetes mellitus) and those with known coronary artery disease [44].
In general, patients with RA are less physically active and have aerobic capacities, the measure of cardiorespiratory fitness, 20 to 30% lower than age-matched healthy controls [45, 46]. Furthermore, in a cross-sectional study of 65 RA patients (43 females), Metsios et al. [47] observed that physically inactive RA patients had a significantly worse cardiovascular risk factor profile (higher systolic blood pressure and elevated total cholesterol, and low-density lipoprotein levels) when compared with physically active RA patients.
Exercise training and increased physical activity reduces cardiovascular events in the general population. Meta-analyses of exercise-based cardiac rehabilitation estimate a reduction in mortality of around 20 to 30% [48]. Given that the main cause of reduced life expectancy in persons with RA is CVD related, the probable cardioprotective benefit of exercise training and regular physical activity to RA patients cannot be ignored. To date, however, most studies of the beneficial effects of exercise training in RA have focused on improvements in functional ability and other RA-related disease outcomes. In a recent Cochrane review, moderate evidence for a positive effect of short-term dynamic exercise on aerobic capacity in RA patients was found [49]. It is worth noting, however, that none of the 8 studies reviewed reported any other cardiovascular risk factors. A wider review of 40 studies of exercise in RA [50] observed that none investigated exercise interventions in relation to CVD in RA. Clearly, future studies are required to specifically investigate the effect of exercise training and cardiorespiratory fitness on CVD risk in RA.
Summary of CV Health and RA —
(i) RA patients have an increased CV risk factor profile; (ii) RA patients have been shown to be less active and have poor aerobic fitness; (iii) the relationships between physical activity, aerobic fitness, and CV risk in RA patients requires more research; (iv) reducing CV risk through exercise could have an enormous impact in patients with RA.
2.2. Musculoskeletal Health and Exercise
2.2.1. Rheumatoid Cachexia and Skeletal Muscle Function
As mentioned previously, approximately two thirds of RA patients suffer from cachexia (i.e., significant muscle wasting) [5, 14]. “Rheumatoid cachexia” is defined as a loss of body cell mass which predominates in skeletal muscle. Unlike the cachexia associated with conditions such as HIV-AIDS, cancers, COPD, and frail old age, rheumatoid cachexia is usually characterised by stable bodyweight as the decrease in muscle mass is masked by a concomitant increase in fat mass [51]. These detrimental changes in body composition not only causes muscle weakness and increased disability, but also contribute to fatigue and augmented risk of diabetes and CVD [5, 6, 47]. It has been proposed [6] that cachexia occurs in RA due to the excess production of proinflammatory cytokines, principally TNF-α, which is catabolic and thought to alter the balance between protein degradation and protein synthesis in RA. However, it is unlikely that this is the only cause as specifically blocking TNF-α has proved unsuccessful in reversing muscle loss in previously untreated RA patients [52]. Thus the precise mechanism by which rheumatoid cachexia occurs is not known but reduced insulin action, muscle IGF-I levels, testosterone, and low habitual physical activity are likely to be contributing mediators [5, 53, 54]. Furthermore, the use of high-dose steroid therapy to control disease activity can exacerbate muscle atrophy in RA [55]. In addition, the symptoms of the disease, for example, pain and fatigue, also result in RA patients being less physically active; decreasing physical activity then becomes part of the viscous circle of further decreasing muscle mass and has detrimental effects on other aspects of skeletal muscle health [56].
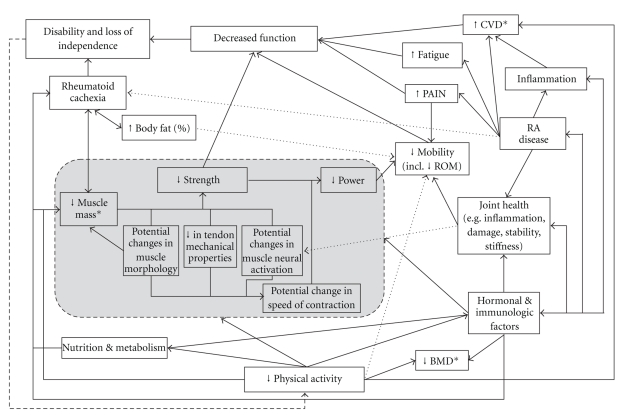
Loss of strength, of up to 70%, is a common finding in RA patients in comparison to healthy counterparts [57]. Loss of muscle mass is the main contributor to loss of muscle strength; however, it is not the only factor responsible [23, 58]. With RA, the loss of muscle mass, decreased physical activity, and immunologic factors may combine with alterations in skeletal muscle properties that could result in decreased muscle strength. Lower strength and power then lead on to functional limitation in RA. A summary of these pertinent factors and how they are interlinked with other RA disease-related factors that result in functional limitation are shown in Figure 1. Although there was a suggestion that RA patients have a lower activation capacity [59], recent studies have shown that in stable RA quadriceps muscle recruitment, strength, and other skeletal muscle properties are not compromised [60, 61]. However, a case study in active RA indicates that these parameters might be negatively affected during increased disease activity and especially in the presence of an effusion, which adversely affects mechanical joint and muscle function. Quadriceps wasting, as well as a dramatic loss of force production, which was not due to pain or impaired muscle quality, was observed [62]. This needs to be further investigated in larger studies with active RA. If muscle physiological properties are impaired during times of disease flare, it is likely that this would impact on the length of recovery time needed after flare. This would thereby further emphasise the importance of early and persistent exercise training in these patients and early treatment of joint effusions to avoid possible reflex inhibition and altered joint geometry caused by the effusion that may interfere with exercise training.
Figure 1.
A summary of the influence of skeletal muscle properties on the factors affecting functional limitation, disability and loss of independence in RA. Note: not all of the skeletal muscle properties have been routinely demonstrated with RA (e.g., [60, 61]). BMD: bone mineral density, CVD: cardiovascular disease, ROM: range of motion. *Factors that are adversely affected by medications.
The impaired physical function that is characteristic of RA is strongly correlated with the diminished muscle mass [13], but to date there is no standard treatment for rheumatoid cachexia.
High intensity resistance exercise has been shown to safely reverse cachexia in patients with RA and, as a consequence of this restoration of muscle mass, to substantially improve physical function and reduce disability in RA patients [5, 28, 63, 64]. For example, a 24-week high-intensity progressive resistance training (PRT) program produced significant increases in lean body mass, reduced fat mass (notably trunk adiposity), and substantial improvements in muscle strength and physical function in RA patients [5]. It is notable that the low-intensity range of movement exercises performed by an age-, sex- and disease-matched group of patients as the control condition elicited no changes in body composition or physical function. This investigation also revealed increases in previously diminished muscle levels of IGF-I- and IGF-binding protein-3 following PRT suggesting a probable contributing mechanism for rheumatoid cachexia. Other exercise training programs have also been suggested to induce an anti-inflammatory effect, specifically relating to TNF-α production [57]. However, immune function (including TNF-α and IL-6) was unaltered following 12 weeks of high-intensity PRT [65].
In terms of the magnitude of hypertrophic and strengthening effects of PRT observed in RA patients [5, 63, 64] these are similar to those reported for healthy middle-aged or older subjects (e.g., [23, 57, 66–68]). The study by Hakkinen and colleagues [64] in fact provides a direct comparison of training response. They identified almost identical body composition changes (increased thigh muscle cross-section and reduced thigh fat thickness) and comparable strength increases in female RA patients and age-matched healthy women following completion of the same resistance exercise program. Furthermore, a range of skeletal muscle parameters (force, muscle architecture, coactivation of antagonist muscles, contractile properties, etc.) were observed to be no different between well-controlled RA and their healthy counterparts, resulting in similar muscle quality (muscle force per size) between the groups, even in cachetic RA patients [60, 61]. Consequently it is now clear that patients with RA are not resistant to the anabolic effects of exercise as previously suggested [69]. These findings are important to health professionals and those involved in prescribing exercise for people with RA as rheumatoid muscle should respond to exercise training in a similar way to that of muscle in healthy individuals. In fact now much research is promoting the fact that there are more detrimental effects if exercise is not undertaken [70].
As high-intensity PRT performed by RA patients, with both newly diagnosed and long-standing disease, has proved to be efficacious in increasing muscle mass, strength, and improving physical function, whilst being well tolerated and safe, it is advocated that such programs are included in disease management to counteract the effects rheumatoid cachexia [5, 56, 57, 71, 72]. PRT can also benefit other health aspects, for example, improving coordination and balance which RA can detrimentally affect. It is also important to maintain normal muscle strength in order to stabilise the knee joint, preventing joint angulation, and later osteoarthritis [73]. Further health benefits are detailed below.
Summary of Rheumatoid Cachexia and Musculoskeletal Health and Exercise Types for Treatment —
(i) At least 50% RA patients suffer loss of lean mass; (ii) intensive progressive resistance training can increase lean mass, reduce fat mass, increase strength and improve function; (iii) PRT is the most effective exercise to improve skeletal muscle size and strength; (iv) PRT, even performed at high intensity, is safe in RA.
2.2.2. Bone Mineral Density
In people with RA, not only does the typically sedentary lifestyle put them at greater risk of lower bone mineral density (BMD), but the disease itself (systemic inflammatory activity and high-dose oral steroid medication when used as part of RA treatment) results in radiological changes including bone loss (especially peripherally) [74, 75]. Lower BMD has been shown to occur at the femoral neck, distal forearm, and hip, but not the spine, in RA when compared with controls [74, 76]. Lower BMD in RA is found in patients on glucocorticoid treatment, and those with lower strength (handgrip and quadriceps) and physical capacity [74, 77, 78]. Thus highlighting how physical activity that involves muscle strengthening may assist in mitigating the bone loss in people with RA.
Loss of BMD with age is difficult to mitigate and requires long-term weight “loading” on bone (either by repetitive weight-bearing and/or strengthening exercises) [78]. Several studies have reported no change in BMD with exercise training programmes in people with RA. However most of these investigations have either been too short in duration to detect changes, have featured low participant numbers, or did not include sufficient weight loading stimulus [56, 79]. The Rheumatoid Arthritis Patients in Training program (RAPIT) study observed a reduced rate of BMD loss in the hip, but not the spine, during 2 years of high-intensity weight-bearing exercise training [77]. This mitigation of BMD loss was associated with increases in both muscle strength and aerobic fitness. The authors concluded that there is an essential role for the combination of high-intensity, weight- and impact-bearing exercises in improving bone mineral density in RA patients [77].
Summary of Exercise Types for Bone Health —
(i) Load-bearing exercise, PRT and/or weight bearing, is required to increase BMD; (ii) combination of PRT and weight/impact-bearing exercises may be required to improve BMD.
2.3. Joint Health and Exercise
The role of exercise in promoting the joint health of a person with RA is of great importance, especially as this is the most pronounced and invariant element of the RA disease pathology [80]. The health of the joint involves a combination of factors, as detailed below.
Tendons are extensible structures that transmit forces from muscle to bone and reversibly deform under mechanical loads, with stiffer tendons providing more efficient force production. RA causes synovial inflammation of tendon sheaths, leading to synovial hypertrophy and sometimes infiltration of synovial tissue within the tendon. The raised circulating inflammatory cytokines also affect collagen, leading to damage and disorganisation of the tendon structure. In addition, tendons gradually lose their elasticity and stiffness as they age and in persons who do not engage regularly in physical activities or following disuse [23, 81, 82]. Only recently have tendon properties been investigated in RA, with tendon stiffness in stable established RA being lower than that of matched healthy controls (manuscript in preparation). In the case study example described above [62], lower patella tendon stiffness that was observed only in the effused knee during the acute phase was found in both knees 1 year later, despite maintenance of regular physical activity. Local effects of the joint effusion are likely to be responsible for the acute decrease in tendon stiffness whilst the systemic inflammatory processes of RA could be responsible for the long-term effects. Tendon stiffness can be increased, however, following strength training in older people [23] and with endurance training [83]. Potential beneficial exercise training effects in tendons of RA patients are to date unknown and warrant further investigation.
The ligament forms another essential component of the joint, with the main function being to passively stabilise and guide the joint through its normal range of motion [84]. Similar to the research surrounding tendons and the effects of exercise, it is known that exercise strengthens ligaments and that even relatively short periods of immobilisation weakens them [85, 86]. Thus, it may be suggested that regular physical activity for the RA patient is essential in order to maintain normal ligament and, consequently, overall joint health and function.
The primary function of cartilage within the synovial joint is to protect the bone from damage by helping to minimise friction between adjacent bones during movement [87]. It is known that periods of compression and decompression, which can be achieved through the mechanical forces and regular cyclic loading of an exercise bout, are required to prevent cartilage tissue from becoming fragile and dysfunctional [88–90]. Furthermore, it is known that cartilage responds in a site-specific way to this loading [88].
For many years, intensive dynamic and weight-bearing exercises were considered inappropriate for people with RA due to concerns that such activities may exacerbate disease [36]. Furthermore, research has revealed that patients are concerned about whether such exercise can cause damage to the structure of the joint [91]. Research by de Jong and colleagues [28, 77, 92, 93] has shed light on this area of concern. They investigated the effects of a high-intensity exercise program in the RAPIT study. This involved biweekly participation in a 1.25-hour exercise session including aerobic, muscle strengthening, joint mobility, and an impact-delivering “sport” or “game” sessions for 2 years. When compared to patients receiving usual care, it was concluded that exercise did not cause an increase in the rate of damage to either large [28] or the small joints of the hands and feet [77]. Although initially there was a suspicion that those large joints which were badly damaged prior to the start of the study deteriorated more rapidly in the exercise group than controls [93], results from a follow-up study led the authors to retract this conclusion [92]. At 18 months following the cessation of the exercise program, there was no significant difference in the rate of damage of the large joints between those patients available at follow-up who were still exercising and those who had discontinued exercise [92]. Another finding from the RAPIT study indicates that there was no significant change in cartilage oligomeric matrix protein (COMP) level, a measure of cartilage damage, in patients after 3 months of exercising [94].
Range of movement and flexibility are also improved as a result of exercise, reducing movement limitation [95]. For example, Van Den Ende et al. [37] found that joint mobility increased as a result of a short-term intensive exercise programme in RA patients with active disease. Joint proprioception has also been reported to improve after physical activity and deteriorate after immobilisation or joint disease [96, 97]. Whilst yet to be determined in the RA population [98], elderly people who regularly practiced tai chi showed better proprioception at the ankle and knee joints than sedentary controls [99]. It may also be that joint lubrication is enhanced as a result of physical activity, further acting to promote the health of the RA joint. More specifically, after resting for long periods, synovial fluid is squeezed out from between the two surfaces of joint, resulting in contact between the areas of cartilage. When movement is resumed, the mechanism of fluid film lubrication is reactivated [100].
A study by Lynberg et al. [39] is typical of findings that PRT does not exacerbate joint inflammation (synovitis, joint swelling, joint tenderness, periarticular tenderness, and range of motion were all clinically assessed). Furthermore, in patients with moderate disease activity a reduction in the number of clinically active joints after vigorous exercise has sometimes been observed [36, 101].
RA is also characterised by an increase in blood flow (synovial hyperaemia) and vascularisation of the synovium [102, 103]. Whilst the links between this process and joint destruction are poorly understood, it is thought that proliferation of the joint synovium and the action of cytokines such IL-1 and TNF-α act to break down the superficial layers of joint cartilage [104]. This matrix degeneration potentially leads to joint failure, functional limitation, and disability [105]. However, some evidence suggests that intermittent cycles of raised intra-articular pressure during dynamic exercise might increase synovial blood flow, suggesting a beneficial effect of dynamic exercises in joint inflammation [106]. Using a quantitative method, ultrasonography, recent research has suggested no acute effect of handgrip exercise on synovial hyperaemia of the wrist joint in RA patients [107]. In summary, adequate strength and endurance of the muscles alongside tone and elasticity of the connective tissues promotes optimal joint stability, alignment and attenuation of impact and compressive forces [89, 108].
Summary of Exercise Types for Joint Health —
(i) Resistance training increases tendon stiffness and strengthens connective tissue; (ii) cyclic loading (e.g., walking, cycling, strength endurance exercises) enhances cartilage integrity and joint lubrication; (iii) mobility exercises increase range of motion.
2.4. Improving Overall Function
Patients with rheumatoid arthritis usually suffer from disability, severe pain, joint stiffness, and fatigue which impair physical function [109]. Even after controlling for the disease with development of powerful disease-modifying antirheumatic drugs (DMARDs), patients still suffer from functional limitation, often leading to work disability [110]. However, exercise has been shown to significantly improve some or all of these symptoms, especially function as well as psychological well-being [5, 56, 63, 111, 112]. For example, a two-year strength training program resulted in improvements in subjective patient assessments of disability by the Health Assessment Questionnaire (HAQ) [45]. Similarly, Marcora and colleagues [63] found a significant inverse correlation between increases in leg lean mass following 12 weeks PRT and the perceived difficulty in performing activities of daily living (ADLs). However, this beneficial effect on subjective measures of function is not universal. For example, an intensive PRT program failed to improve modified HAQ scores in a group of RA patients despite significant improvements in muscle mass and strength [5]. It was concluded that patients involved in this program had relatively low disability and that the modified HAQ was not sensitive to change in a low disability group.
Another factor common in RA that limits overall function is fatigue. Fatigue is frequently experienced in RA with 42% of RA patients experiencing severe fatigue [113]. Often patients report fatigue as one of the most annoying symptoms [114]. However, an internationally accepted definition of fatigue in RA currently does not exist, and its aetiology still remains a mystery. Fatigue can be described as a subjective experience, a feeling of “extreme, persistent tiredness, weakness or exhaustion which can be both mental and physical” [115]. Identifying ways to reduce fatigue and improving overall quality of life are very important. So far few methods have shown to be effective, however, recent research suggests that fatigue can be reduced by performing exercise [116]. A systematic review which explored the effectiveness of nonpharmacological interventions for fatigue [112] also concluded that both aerobic and resistance exercise interventions reduce RA fatigue.
Summary: It Is Important to Note the Following —
(i) Exercise can reduce pain, morning stiffness, and even reduce fatigue in RA; (ii) exercise can improve functional ability and psychological well-being; (iii) exercise has not been shown to exacerbate disease activity.
3. Perceptions of RA Patients Regarding Exercise
Whilst there are numerous reasons why exercise is considered to be of fundamental benefit, it is apparent that the RA population is less physically active than the general population. Therefore, it is important for those involved in the care of RA patients to be aware of factors that may positively and negatively affect the uptake of and compliance to an exercise prescription.
The perceptions of people with RA may provide reasoning for the lower physical activity levels of RA patients when compared to the general population [17]. Thus, understanding the perceptions of RA patients regarding exercise is salient to the role of the health professional [91].
The Obstacles to Action study (New Zealand) [117] investigated factors influencing exercise participation for individuals with self-reported arthritis who were defined as “nonexercisers”, “insufficiently active”, and “regular exercisers”. Their qualitative analysis of focus group discussions revealed that active people with arthritis believed more strongly in the benefits of physical activity, reported significantly higher levels of encouragement from others, and had greater overall levels of self-efficacy when compared with the less active participants. Arthritis, fatigue, and discomfort were ranked by both groups as the top three barriers. However, the active participants reported significantly lower impact scores for these barriers than the inactive group, and these findings persisted after adjusting for occupational status, body mass index, and comorbidities [117].
Other barriers suggested to affect the successful uptake of exercise recommendations in arthritis patients have also been revealed. Physical barriers have included pain, fatigue, and physical capabilities, alongside the additional complications of further comorbidities. Psychological aspects such as a lack of enjoyment, motivation, and confidence have been identified as negative influences. However, receiving assistance from instructors and the opportunity for social interaction have been highlighted as factors encouraging patients to exercise. Especially prevalent in those on a limited income, environmental barriers such as cost and a lack of adequate insurance have also been revealed as barriers among nonexercisers. It has also become clear that a lack of transportation can be a major hindrance. Time constraints brought about by lifestyle and other commitments is a factor common to both the general and patient population, often further compounded by the distance necessary to travel to an exercise facility [40, 91, 117–120]. It is also important to consider patient perceptions and potential barriers when promoting the maintenance of an exercise program. For example, working towards strengthening patient beliefs that they are able to continue exercise outside of the healthcare environment may be valuable [121].
As previously identified in OA patients [122], worry that exercise may have detrimental effects on joint health was also present in RA patients. Additionally, these patients had specific apprehensions regarding the effects of impact and repetitive exercises [91]. Joint pain has also been highlighted as a definitive barrier and has also been perceived as a prominent factor in determining the patients' exercise behaviour [120, 123]. In contrast, however, qualitative research suggests that patients feel that their joints benefit from exercise, with quotes indicating that joints are “lubricated” as a result of movement and patients feel more agile [124]. Similar perceptions indicating feelings of reduced pain have also been established [120]. However, evidence suggests that whilst patients with arthritis believe exercise to be an important factor in treatment, uncertainty about which exercises to do, and how to do them without causing harm, deters many patient from exercising at all [125]. Within the Obstacles to Action study [117] “insufficient advice from a healthcare provider” was a theme for the insufficiently active individuals, with queries relating to the type, frequency, and intensity of appropriate exercise.
Due to their condition RA patients are in frequent contact with their health professionals and this contact influences their perceptions about the role of exercise as part of their treatment. Moreover, because patients are constantly making decisions about treatment due to the fluctuating nature of RA, it is important for patients to understand how to modify their exercise according to their symptoms [126, 127]. The perceptions and behaviour of the rheumatologist is an important consideration when working towards a successful exercise prescription. Research by Iversen et al. [126] found that discussions about exercise were four times more likely to occur when the rheumatologist initiated exercise discussion, with discussions strongly impacting on the likelihood that a patient received an exercise prescription. Furthermore, although high-intensity exercise is now considered to provide the greatest benefit, the outcome expectations of patients, rheumatologists, and physiotherapists for high intensity exercise have been found to be significantly less positive than those for a conventional exercise program [128], with rheumatologists reporting their most negative attitudes towards aerobic exercise [127]. It is also interesting to note that, when examining the predictors of exercise behaviour in RA patients 6 months following a visit with their rheumatologist, Iversen et al. [126] found that if a patient's rheumatologist was currently performing aerobic exercise, the patient was more likely to be engaged in exercise.
A further issue relating to the health professional is their own assertion and certainty when prescribing exercise to those with RA. In the study by Iversen et al. [126], only 51% of rheumatologists reported they felt confident that they knew when exercises were appropriate for their patients with RA. Correspondingly, recent research has revealed that patients perceive uncertainties within the health profession regarding the impact of exercise on pain and joint health. In particular, this was in relation to whether the sensation of exercise discomfort or pain equated to actual joint damage and the effects of different types of exercise on the health of their joints [91]. These concerns pose a further challenge to RA patients [38, 45, 93, 129, 130].
Despite these reservations, patients have demonstrated an awareness of the advantages of exercise in terms of improving strength, mobility, and function and reducing pain [91]. However, due to the effects of RA and considering the aforementioned issues, if the perception of exercise as a positive feature of RA treatment is to supersede the apparent negative connotations, continual emphasis of the benefits of exercise in this population is of great importance [40, 77, 131]. This also means that clear exercise guidelines and prescription is necessary to attend to the fact that RA patients are currently faced with ambiguous and incomplete information. In addition to the pivotal role of the rheumatologist in influencing exercise prescription [126, 127], these recommendations are also relevant to the other health professionals involved in the treatment and care of RA patients (i.e., nurse specialists, physiotherapists, and occupational therapists) and significant others such as the patient's family and friends.
Key Recommendations for Health Professionals and Significant Others in the Improvement of Patient Perceptions Regarding Exercise —
(i) Impart better advice regarding the effects and benefits of exercise; (ii) clarify specific exercise recommendations; (iii) consider methods of overcoming individual barriers to exercise.
4. Exercise Prescription for RA
The benefits of dynamic exercise in improving outcomes for patients with RA were highlighted following a systematic review by Van Den Ende et al. (1998) [72]. However, this early meta-analysis [72] was limited to six studies. In the intervening decade, numerous studies of varying quality have investigated the effects of aerobic and/or muscle strengthening exercise training programs for RA patients. This growing body of evidence, which is the subject of a number of systematic reviews [49, 50, 132–135], strongly suggests that exercise is effective in management of patients with RA, and does not induce adverse effects. Current guidelines now advise that exercise is beneficial for most individuals with RA (e.g., NICE guidelines, 2009). However, whilst the exercise benefits for RA patients are widely recognized, further studies are required to investigate the most effective exercise prescription (intensity, frequency, duration, and mode), the optimum modes of exercise delivery, and how adherence to training can be facilitated. A summary of exercise types and recommendations for individuals with RA based on current evidence is depicted in Table 1. Typically exercise interventions have focused on effects of aerobic training, strength training and a combination of aerobic training and strength training.
Table 1.
Summary of general exercise guidelines for RA. This information is derived from ACSM exercise management guidelines [140] and the research literature.
| Benefit | Type of Exercise | How best to Achieve |
|---|---|---|
| Improve CV health | Cycling | 60–80% HR max |
| Walking | 30–60 mins/session | |
| Swimming | 3–5 days/week | |
| Dance | Increase duration, then intensity over time | |
| Increase muscle mass & strength | 60–80% 1RM | |
| Free weights | 8–10 exercises (large muscle groups) | |
| Weight machines | 8–12 reps/exercise | |
| Therabands | 2-3 sets | |
| 2-3 days/week | ||
| Increase intensity over time | ||
| Increase ROM & flexibility for enhanced joint health | Stretching | 10–15 minutes |
| Tai Chi exercises | 2 days/week | |
| Yoga/Pilates | ||
| Improve balance* | One leg stance | On a regular basis |
| Stability ball | ||
| Strengthening core muscles | ||
*The effects of balance training alone in RA patients to enhance functional capacity through increased proprioception and coordination and to reduce the risk of falls have yet to be conducted [98]. Thus the effectiveness and safety of balance training are unclear.
4.1. Aerobic Training
The aerobic activities most often included in exercise interventions are walking, running, cycling, exercise in water, and aerobic dance. Walking is a good mode of exercise as it is inexpensive, requires no special skills, is safe, and can be performed both indoors and outdoors. Regular brisk walking, even in short bouts, improves aerobic fitness and reduces aspects of CVD risk in healthy adults [136]. Cycling is also an excellent mode of aerobic activity that works the large muscle groups of the lower extremity. Cycling, in line with the guidelines in Table 1, improves aerobic capacity, muscle strength, and joint mobility (e.g., by 17%, 17%, and 16%, resp., [36]) with no exacerbation of disease activity. Water-based exercise has also been studied in RA. Hydrotherapy (the use of water) has been shown to be very effective for RA sufferers. As little as two 30-minute sessions for 4 weeks have been shown to significantly reduce joint tenderness, improve knee range of movement, and improve emotional and psychological well-being [137]. Dancing is another form of aerobic exercise which has reported improvements in aerobic power and resulted in positive changes in depression, anxiety, and fatigue, with no deterioration in disease activity in RA patients [41]. See Table 1 for aerobic exercise types and recommendations for individuals with RA.
4.2. Resistance Training
With a loss in muscle mass, and subsequent functional limitation and burgeoning disability a characteristic of the disease, RA patients should be encouraged to perform exercises which elicit muscle hypertrophy and strengthening. Several studies have demonstrated the beneficial effects for RA patients of performing muscle strengthening exercises, in particular PRT. These improvements include increases in muscle mass, reduction in fat mass, and substantial improvements in physical function [5, 63, 64]. Exercises that involve the large muscle groups of the upper and lower extremities as well as hand strengthening exercises have been shown to be effective [5, 63, 111]. The effects of a two-year dynamic strength training program in early RA patients [45] found significant improvements in muscle strength (19–59%) along with reductions in systemic inflammation, pain, morning stiffness, and disease activity. These findings suggest that long-term dynamic strength training can significantly improve the physical well-being of RA patients without exacerbating disease activity. Muscle strength gains from PRT programmes can also be maintained over several years of continued training at sufficient intensity [56, 92]. These examples have assisted in recommendations for strength training being developed, a summary of which is presented in Table 1.
4.3. Combination of Aerobic and Strength Exercises
The optimum exercise program for RA patients would include both aerobic and resistance training. With poor cardiovascular health being the main cause of death in RA and with RA patients tending to have poor cardiorespiratory fitness, the requirement of aerobic exercise as part of treatment is crucial. Whilst the addition of strengthening exercises helps to mitigate rheumatoid cachexia and other musculoskeletal and joint health issues, and induces substantial improvements in physical function and the ability to perform ADLs. Both types of exercises may need to be required for maintaining BMD [77].
4.4. General Exercise Guidelines
Exercise programs for RA patients should be initially supervised by an experienced exercise professional so that the program can be tailored to individual aspirations and adapted to the disease activity, joint defects, and symptoms of patients [93]. Following on from moderate to high intensity PRT or combined programs, RA patients have been shown to have high adherence rates to exercise in “real life” situation that help maintain improvements [56, 92]. Although, continuation of both high-intensity and high-frequency sessions may be required for maintenance of training gains in aerobic fitness, muscle strength, and functional ability [92], but evidence is still required regarding the minimum maintenance regimen. Home-based exercise programs have also been investigated and have been shown to improve quality of life and functional status [138]. However, due to the difficulties in ensuring that exercise of sufficient intensity is performed these exercises often fail to elicit significant increases in muscle strength or aerobic fitness. Although the minimal exercise dose for functional improvements and health maintenance is unknown, even regular training performed once weekly has been shown to improve function assessed subjectively by HAQ scores and health status [139].
As many RA patients have below average physical capacity, exercise training should be initiated at a lower intensity. Evidence of exercise prescription in RA patients with severe disability (Functional classes III and IV) is still lacking [57, 93]. Even so, strengthening exercises are recommended for all stages of RA [134]. Exercise programs, even over long periods and at high intensities, have been found to be safe as well as effective [92]. However, little is known as to whether exercise, particularly strength training, should be continued through inflammatory “flares” and further research should be conducted on the effects of exercise on joints that are already severely damaged. For continued training adaptation (i.e., increased fitness) a progression of the exercise dose (i.e., duration and/or intensity) is required.
Unfortunately, studies have also shown that most of the beneficial muscle adaptations are also lost after cessation of the exercise training [56]. Thus, as with healthy individuals, the beneficial effects of exercise (PRT, aerobic, mobility) are lost if training is discontinued.
5. Conclusions
The importance for the inclusion of exercise training in the treatment of RA is now clear and proven. Exercise in general seems to improve overall function in RA without any proven detrimental effects to disease activity. Thus all RA patients should be encouraged to include some form of aerobic and resistance exercise training as part of their routine care. More research is still required on the optimal dose and types of exercises, especially when combining types, as well as how best to incorporate exercise into the lives of RA patients across the variable course of the disease.
References
- 1.Brosseau L, Wells GA, Tugwell P, et al. Ottawa panel evidence-based clinical practice guidelines for therapeutic exercises in the management of rheumatoid arthritis in adults. Physical Therapy. 2004;84(10):934–972. [PubMed] [Google Scholar]
- 2.Ekdahl C, Broman G. Muscle strength, endurance, and aerobic capacity in rheumatoid arthritis: a comparative study with healthy subjects. Annals of the Rheumatic Diseases. 1992;51(1):35–40. doi: 10.1136/ard.51.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes IB. Rheumatoid arthritis: from bench to bedside. Rheumatic Disease Clinics of North America. 2001;27(2):373–387. doi: 10.1016/s0889-857x(05)70207-6. [DOI] [PubMed] [Google Scholar]
- 4.Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how "high-grade" systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108(24):2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 5.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Care and Research. 2009;61(12):1726–1734. doi: 10.1002/art.24891. [DOI] [PubMed] [Google Scholar]
- 6.Roubenoff R, Roubenoff RA, Cannon JG, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. Journal of Clinical Investigation. 1994;93(6):2379–2386. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitas GD, Erb N. Tackling ischaemic heart disease in rheumatoid arthritis. Rheumatology. 2003;42(5):607–613. doi: 10.1093/rheumatology/keg175. [DOI] [PubMed] [Google Scholar]
- 8.Solomon DHM, Karlson EW, Rimm EBS, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 9.Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis and Rheumatism. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 10.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Care and Research. 2008;59(12):1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 11.Del Rincon I, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis and Rheumatism. 2001;44(12):2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 12.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 13.Giles JT, Bartlett SJ, Andersen RE, Fontaine KR, Bathon JM. Association of body composition with disability in rheumatoid arthritis: impact of appendicular fat and lean tissue mass. Arthritis Care and Research. 2008;59(10):1407–1415. doi: 10.1002/art.24109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsmith J, Abad L, Kehayias J, Roubenoff R. Tumor necrosis factor-alpha production is associated with less body cell mass in women with rheumatoid arthritis. Journal of Rheumatology. 2004;31(1):23–29. [PubMed] [Google Scholar]
- 15.Akner G, Cederholm T. Treatment of protein-energy malnutrition in chronic nonmalignant disorders. American Journal of Clinical Nutrition. 2001;74(1):6–24. doi: 10.1093/ajcn/74.1.6. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R, Walsmith J, Lundgren N, Snydman L, Dolnikowski GJ, Roberts S. Low physical activity reduces total energy expenditure in women with rheumatoid arthritis: implications for dietary intake recommendations. American Journal of Clinical Nutrition. 2002;76(4):774–779. doi: 10.1093/ajcn/76.4.774. [DOI] [PubMed] [Google Scholar]
- 17.Sokka T, Häkkinen A. Poor physical fitness and performance as predictors of mortality in normal populations and patients with rheumatic and other diseases. Clinical and Experimental Rheumatology. 2008;26(5):S14–S20. [PubMed] [Google Scholar]
- 18.Bouchard C, Blair S, Haskell W, editors. Physical Activity and Health. Champaign, Ill, USA: Human Kinetics; 2007. [Google Scholar]
- 19.Escalante A, Del Rincon I. The disablement process in rheumatoid arthritis. Arthritis and Rheumatism. 2002;47(3):333–342. doi: 10.1002/art.10418. [DOI] [PubMed] [Google Scholar]
- 20.Brukner PD, Brown WJ. Is exercise good for you? Medical Journal of Australia. 2005;183(10):538–541. doi: 10.5694/j.1326-5377.2005.tb07159.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. Journal of the National Cancer Institute. 1994;86(18):1403–1408. doi: 10.1093/jnci/86.18.1403. [DOI] [PubMed] [Google Scholar]
- 22.Bassey EJ, Ramsdale SJ. Increase in femoral bone density in young women following high-impact exercise. Osteoporosis International. 1994;4(2):72–75. doi: 10.1007/BF01623226. [DOI] [PubMed] [Google Scholar]
- 23.Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise in humans. Experimental Physiology. 2006;91(3):483–498. doi: 10.1113/expphysiol.2005.032896. [DOI] [PubMed] [Google Scholar]
- 24.Rao SS. Prevention of falls in older patients. American Family Physician. 2005;72(1):81–94. [PubMed] [Google Scholar]
- 25.Ross CE, Hayes D. Exercise and psychologic well-being in the community. American Journal of Epidemiology. 1988;127(4):762–771. doi: 10.1093/oxfordjournals.aje.a114857. [DOI] [PubMed] [Google Scholar]
- 26.Bauman AE. Updating the evidence that physical activity is good for health: an epidemiological review. Journal of Science and Medicine in Sport. 2004;7(1):6–19. doi: 10.1016/s1440-2440(04)80273-1. [DOI] [PubMed] [Google Scholar]
- 27.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 28.De Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis and Rheumatism. 2003;48(9):2415–2424. doi: 10.1002/art.11216. [DOI] [PubMed] [Google Scholar]
- 29.Baillet A, Payraud E, Niderprim VA, et al. A dynamic exercise programme to improve patients’ disability in rheumatoid arthritis: a prospective randomized controlled trial. Rheumatology. 2009;48(4):410–415. doi: 10.1093/rheumatology/ken511. [DOI] [PubMed] [Google Scholar]
- 30.Bilberg A, Ahlmen M, Mannerkorpi K. Moderately intensive exercise in a temperate pool for patients with rheumatoid arthritis: a randomized controlled study. Rheumatology. 2005;44(4):502–508. doi: 10.1093/rheumatology/keh528. [DOI] [PubMed] [Google Scholar]
- 31.Harkcom TM, Lampman RM, Banwell BF, Castor CW. Therapeutic value of graded aerobic exercise training in rheumatoid arthritis. Arthritis and Rheumatism. 1985;28(1):32–39. doi: 10.1002/art.1780280106. [DOI] [PubMed] [Google Scholar]
- 32.Melikoglu MA, Karatay S, Senel K, Akcay F. Association between dynamic exercise therapy and IGF-1 and IGFBP-3 concentrations in the patients with rheumatoid arthritis. Rheumatology International. 2006;26(4):309–313. doi: 10.1007/s00296-005-0605-y. [DOI] [PubMed] [Google Scholar]
- 33.Nordemar R. Physical training in rheumatoid arthritis: a controlled long-term study. II. Functional capacity and general attitudes. Scandinavian Journal of Rheumatology. 1981;10(1):25–30. [PubMed] [Google Scholar]
- 34.Van Den Berg MH, Ronday HK, Peeters AJ, et al. Using internet technology to deliver a home-based physical activity intervention for patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Care and Research. 2006;55(6):935–945. doi: 10.1002/art.22339. [DOI] [PubMed] [Google Scholar]
- 35.Westby MD, Wade JP, Rangno KK, Berkowitz J. A randomized controlled trial to evaluate the effectiveness of an exercise program in women with rheumatoid arthritis taking low dose prednisone. Journal of Rheumatology. 2000;27(7):1674–1680. [PubMed] [Google Scholar]
- 36.Van Den Ende CHM, Hazes JMW, Le Cessie S, et al. Comparison of high and low intensity training in well controlled rheumatoid arthritis. Results of a randomised clinical trial. Annals of the Rheumatic Diseases. 1996;55(11):798–805. doi: 10.1136/ard.55.11.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Den Ende CHM, Breedveld FC, Le Cessie S, Dijkmans BAC, De Mug AW, Hazes JMW. Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Annals of the Rheumatic Diseases. 2000;59(8):615–621. doi: 10.1136/ard.59.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen TM, Hansen G, Langgaard AM, Rasmussen JO. Longterm physical training in rheumatoid arthritis. A randomized trial with different training programs and blinded observers. Scandinavian Journal of Rheumatology. 1993;22(3):107–112. doi: 10.3109/03009749309099253. [DOI] [PubMed] [Google Scholar]
- 39.Lyngberg KK, Ramsing BU, Nawrocki A, Harreby M, Danneskiold-Samsøe B. Safe and effective isokinetic knee extension training in rheumatoid arthritis. Arthritis and Rheumatism. 1994;37(5):623–628. doi: 10.1002/art.1780370504. [DOI] [PubMed] [Google Scholar]
- 40.Neuberger GB, Aaronson LS, Gajewski B, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Care and Research. 2007;57(6):943–952. doi: 10.1002/art.22903. [DOI] [PubMed] [Google Scholar]
- 41.Noreau L, Martineau H, Roy L, Belzile M. Effects of a modified dance-based exercise on cardiorespiratory fitness, psychological state and health status of persons with rheumatoid arthritis. American Journal of Physical Medicine and Rehabilitation. 1995;74(1):19–27. doi: 10.1097/00002060-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clinic Proceedings. 2009;84(4):373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 44.Franklin BA, McCullough PA. Cardiorespiratory fitness: an independent and additive marker of risk stratification and health outcomes. Mayo Clinic Proceedings. 2009;84(9):776–779. doi: 10.4065/84.9.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Häkkinen A, Sokka T, Kotaniemi A, Hannonen P. A randomized two-year study of the effects of dynamic strength training on muscle strength, disease activity, functional capacity, and bone mineral density in early rheumatoid arthritis. Arthritis and Rheumatism. 2001;44(3):515–522. doi: 10.1002/1529-0131(200103)44:3<515::AID-ANR98>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Minor MA, Hewett JE. Physical fitness and work capacity in women with rheumatoid arthritis. Arthritis Care and Research. 1995;8(3):146–154. doi: 10.1002/art.1790080306. [DOI] [PubMed] [Google Scholar]
- 47.Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, et al. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. European Journal of Cardiovascular Prevention and Rehabilitation. 2009;16(2):188–194. doi: 10.1097/HJR.0b013e3283271ceb. [DOI] [PubMed] [Google Scholar]
- 48.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. American Journal of Medicine. 2004;116(10):682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, Schoones J, Van den Ende EC. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2009;(4, article no. CD006853) doi: 10.1002/14651858.CD006853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metsios GS, Stavropoulos-Kalinoglou A, Sandoo A, et al. Vascular function and inflammation in rheumatoid arthritis: the role of physical activity. Open Cardiovascular Medicine Journal. 2010;4:89–96. doi: 10.2174/1874192401004020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roubenoff R, Roubenoff RA, Ward LM, Holland SM, Hellmann DB. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. Journal of Rheumatology. 1992;19(10):1505–1510. [PubMed] [Google Scholar]
- 52.Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. American Journal of Clinical Nutrition. 2006;84(6):1463–1472. doi: 10.1093/ajcn/84.6.1463. [DOI] [PubMed] [Google Scholar]
- 53.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. International Journal of Cardiology. 2002;85(1):89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 54.Cutolo M. Androgens in rheumatoid arthritis: when are they effectors? Arthritis Research & Therapy. 2009;11(5):p. 126. doi: 10.1186/ar2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibson JNA, Poyser NL, Morrison WL, Scrimgeour CM, Rennie MJ. Muscle protein synthesis in patients with rheumatoid arthritis: effect of chronic corticosteroid therapy on prostaglandin F(2α) availability. European Journal of Clinical Investigation. 1991;21(4):406–412. doi: 10.1111/j.1365-2362.1991.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 56.Häkkinen A. Effectiveness and safety of strength training in rheumatoid arthritis. Current Opinion in Rheumatology. 2004;16(2):132–137. doi: 10.1097/00002281-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scandinavian Journal of Medicine and Science in Sports. 2006;16(supplement 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 58.Degens H, Erskine RM, Morse CI. Disproportionate changes in skeletal muscle strength and size with resistance training and ageing. Journal of Musculoskeletal Neuronal Interactions. 2009;9(3):123–129. [PubMed] [Google Scholar]
- 59.Bearne LM, Scott DL, Hurley MV. Exercise can reverse quadriceps sensorimotor dysfunction that is associated with rheumatoid arthritis without exacerbating disease activity. Rheumatology. 2002;41(2):157–166. doi: 10.1093/rheumatology/41.2.157. [DOI] [PubMed] [Google Scholar]
- 60.Matschke V, Murphy P, Lemmey AB, Maddison P, Thom JM. Skeletal muscle properties in rheumatoid arthritis patients. Medicine and Science in Sports and Exercise. 2010;42:2149–2155. doi: 10.1249/MSS.0b013e3181e304c3. [DOI] [PubMed] [Google Scholar]
- 61.Matschke V, Murphy P, Lemmey AB, Maddison PJ, Thom JM. Muscle quality, architecture, and activation in cachectic patients with rheumatoid arthritis. Journal of Rheumatology. 2010;37(2):282–284. doi: 10.3899/jrheum.090584. [DOI] [PubMed] [Google Scholar]
- 62.Matschke V, Thom J, Lemmey A, Maddison P, Jones J. Inflammatory joint effusionalters the properties of the tendon-muscle complex in rheumatoid arthritis: a case study. Arthritis Care and Research. In press. [Google Scholar]
- 63.Marcora SM, Lemmey AB, Maddison PJ. Can progressive resistance training reverse cachexia in patients with rheumatoid arthritis? Results of a pilot study. Journal of Rheumatology. 2005;32(6):1031–1039. [PubMed] [Google Scholar]
- 64.Häkkinen A, Pakarinen A, Hannonen P, et al. Effects of prolonged combined strength and endurance training on physical fitness, body composition and serum hormones in women with rheumatoid arthritis and in healthy controls. Clinical and Experimental Rheumatology. 2005;23(4):505–512. [PubMed] [Google Scholar]
- 65.Rall LC, Roubenoff R, Cannon JG, Abad LW, Dinarello CA, Meydani SN. Effects of progressive resistance training on immune response in aging and chronic inflammation. Medicine and Science in Sports and Exercise. 1996;28(11):1356–1365. doi: 10.1097/00005768-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. Journal of Applied Physiology. 1991;71(2):644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 67.Morse CI, Thom JM, Mian OS, Birch KM, Narici MV. Gastrocnemius specific force is increased in elderly males following a 12-month physical training programme. European Journal of Applied Physiology. 2007;100(5):563–570. doi: 10.1007/s00421-006-0246-1. [DOI] [PubMed] [Google Scholar]
- 68.Nichols JF, Omizo DK, Peterson KK, Nelson KP. Efficacy of heavy-resistance training for active women over sixty: muscular strength, body composition, and program adherence. Journal of the American Geriatrics Society. 1993;41(3):205–210. doi: 10.1111/j.1532-5415.1993.tb06692.x. [DOI] [PubMed] [Google Scholar]
- 69.Rall LC, Meydani SN, Kehayias JJ, Dawson-Hughes B, Roubenoff R. The effect of progressive resistance training in rheumatoid arthritis: increased strength without changes in energy balance or body composition. Arthritis and Rheumatism. 1996;39(3):415–426. doi: 10.1002/art.1780390309. [DOI] [PubMed] [Google Scholar]
- 70.Plasqui G. The role of physical activity in rheumatoid arthritis. Physiology and Behavior. 2008;94(2):270–275. doi: 10.1016/j.physbeh.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Flint-Wagner HG, Lisse J, Lohman TG, et al. Assessment of a sixteen-week training program on strength, pain, and function in rheumatoid arthritis patients. Journal of Clinical Rheumatology. 2009;15(4):165–171. doi: 10.1097/RHU.0b013e318190f95f. [DOI] [PubMed] [Google Scholar]
- 72.Van Den Ende CHM, Vliet Vlieland TPM, Munneke M, Hazes JMW. Dynamic exercise therapy in rheumatoid arthritis: a systematic review. British Journal of Rheumatology. 1998;37(6):677–687. doi: 10.1093/rheumatology/37.6.677. [DOI] [PubMed] [Google Scholar]
- 73.Peyron JG. Review of the main epidemiologic-etiologic evidence that implies mechanical forces as factors in osteoarthritis. Engineering in Medicine. 1986;15(2):77–79. doi: 10.1243/emed_jour_1986_015_022_02. [DOI] [PubMed] [Google Scholar]
- 74.Franck H, Gottwalt J. Peripheral bone density in patients with rheumatoid arthritis. Clinical Rheumatology. 2009;28(10):1141–1145. doi: 10.1007/s10067-009-1211-2. [DOI] [PubMed] [Google Scholar]
- 75.Sinigaglia L, Varenna M, Girasole G, Bianchi G. Epidemiology of osteoporosis in rheumatic diseases. Rheumatic Disease Clinics of North America. 2006;32(4):631–658. doi: 10.1016/j.rdc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Madsen OR, Egsmose C, Hansen B, Sorensen OH. Soft tissue composition, quadriceps strength, bone quality and bone mass in rheumatoid arthritis. Clinical and Experimental Rheumatology. 1998;16(1):27–32. [PubMed] [Google Scholar]
- 77.De Jong Z, Munneke M, Jansen LM, et al. Differences between participants and nonparticipants in an exercise trial for adults with rheumatoid arthritis. Arthritis Care and Research. 2004;51(4):593–600. doi: 10.1002/art.20531. [DOI] [PubMed] [Google Scholar]
- 78.Madsen OR, Sorensen OH, Egsmose C. Bone quality and bone mass as assessed by quantitative ultrasound and dual energy x ray absorptiometry in women with rheumatoid arthritis: relationship with quadriceps strength. Annals of the Rheumatic Diseases. 2002;61(4):325–329. doi: 10.1136/ard.61.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Jong Z, Munneke M, Lems WF, et al. Slowing of bone loss in patients with rheumatoid arthritis by long-term high-intensity exercise: results of a randomized, controlled trial. Arthritis and Rheumatism. 2004;50(4):1066–1076. doi: 10.1002/art.20117. [DOI] [PubMed] [Google Scholar]
- 80.Maini R, Feldmann M. Rheumatoid arthritis; immunopathgenesis of rheumatoid arthritis. In: Isenberg D, Maddison P, Woo P, Glass D, Breedveld F, editors. Oxford Textbook of Rheumatology. Oxford, UK: Oxford University Press; 2004. p. 677. [Google Scholar]
- 81.Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. Journal of Applied Physiology. 2006;100(6):2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- 82.Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. Journal of Applied Physiology. 2005;98(6):2278–2286. doi: 10.1152/japplphysiol.01266.2004. [DOI] [PubMed] [Google Scholar]
- 83.Buchanan CI, Marsh RL. Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comparative Biochemistry and Physiology. 2002;133(4):1101–1107. doi: 10.1016/s1095-6433(02)00139-3. [DOI] [PubMed] [Google Scholar]
- 84.Frank CB. Ligament structure, physiology and function. Journal of Musculoskeletal Neuronal Interactions. 2004;4(2):199–201. [PubMed] [Google Scholar]
- 85.Benjamin M, Ralphs JR. Tendons in health and disease. Manual Therapy. 1996;1(4):186–191. doi: 10.1054/math.1996.0267. [DOI] [PubMed] [Google Scholar]
- 86.Benjamin M, Ralphs JR. Tendons and ligaments—an overview. Histology and Histopathology. 1997;12(4):1135–1144. [PubMed] [Google Scholar]
- 87.Milner CE. Funtional Anatomy for Sport and Exercise; Quick Reference. Routledge, UK: Oxon; 2008. [Google Scholar]
- 88.Arokoski JPA, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scandinavian Journal of Medicine and Science in Sports. 2000;10(4):186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 89.Minor MA. Physical activity and management of arthritis. Annals of Behavioral Medicine. 1991;13(3):117–124. [Google Scholar]
- 90.Palmoski MJ, Brandt KD. Running inhibits the reversal of atrophic changes in canine knee cartilage after removal of a leg cast. Arthritis and Rheumatism. 1981;24(11):1329–1337. doi: 10.1002/art.1780241101. [DOI] [PubMed] [Google Scholar]
- 91.Law R-J, Breslin A, Oliver EJ, et al. Perceptions of the effects of exercise on joint health in rheumatoid arthritis patients. Rheumatology. 2010;49(12):2444–2451. doi: 10.1093/rheumatology/keq299. [DOI] [PubMed] [Google Scholar]
- 92.de Jong Z, Munneke M, Kroon HM, et al. Long-term follow-up of a high-intensity exercise program in patients with rheumatoid arthritis. Clinical Rheumatology. 2009;28(6):663–671. doi: 10.1007/s10067-009-1125-z. [DOI] [PubMed] [Google Scholar]
- 93.De Jong Z, Vliet Vlieland TPM. Safety of exercise in patients with rheumatoid arthritis. Current Opinion in Rheumatology. 2005;17(2):177–182. doi: 10.1097/01.bor.0000151400.33899.88. [DOI] [PubMed] [Google Scholar]
- 94.de Jong Z, Munneke M, Vilim V, et al. Value of serum cartilage oligomeric matrix protein as a prognostic marker of large-joint damage in rheumatoid arthritis—data from the RAPIT study. Rheumatology. 2008;47(6):868–871. doi: 10.1093/rheumatology/ken052. [DOI] [PubMed] [Google Scholar]
- 95.Fentem PH. Benefits of exercise in health and disease. British Medical Journal. 1994;308(6939):1291–1295. doi: 10.1136/bmj.308.6939.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hurley MV, Scott DL. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. British Journal of Rheumatology. 1998;37(11):1181–1187. doi: 10.1093/rheumatology/37.11.1181. [DOI] [PubMed] [Google Scholar]
- 97.Sharma L, Pai YC. Impaired proprioception and osteoarthritis. Current Opinion in Rheumatology. 1997;9(3):253–258. doi: 10.1097/00002281-199705000-00013. [DOI] [PubMed] [Google Scholar]
- 98.Silva KN, Mizusaki Imoto A, Almeida GJ, Atallah AN, Peccin MS, Fernandes Moça Trevisani V. Balance training (proprioceptive training) for patients with rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2010;5, article no. CD007648 doi: 10.1002/14651858.CD007648.pub2. [DOI] [PubMed] [Google Scholar]
- 99.Xu D, Hong Y, Li J, Chan K. Effect of tai chi exercise on proprioception of ankle and knee joints in old people. British Journal of Sports Medicine. 2004;38(1):50–54. doi: 10.1136/bjsm.2002.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scholes S, Bull A, Unsworth A, Amis A. Biomechanics of articulations and derangments in disease. In: Isenberg D, Maddison PJ, Woo P, Glass D, Breedveld F, editors. Oxford Textbook of Rheumatology. Oxford, UK: Oxford University Press; 2004. pp. 379–386. [Google Scholar]
- 101.Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis and Rheumatism. 1989;32(11):1396–1405. doi: 10.1002/anr.1780321108. [DOI] [PubMed] [Google Scholar]
- 102.Carotti M, Salaffi F, Manganelli P, Salera D, Simonetti B, Grassi W. Power Doppler sonography in the assessment of synovial tissue of the knee joint in rheumatoid arthritis: a preliminary experience. Annals of the Rheumatic Diseases. 2002;61(10):877–882. doi: 10.1136/ard.61.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rooney M, Condell D, Quinlan W, et al. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis and Rheumatism. 1988;31(8):956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- 104.Rannou F, François M, Corvol MT, Berenbaum F. Cartilage breakdown in rheumatoid arthritis. Joint Bone Spine. 2006;73(1):29–36. doi: 10.1016/j.jbspin.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 105.Anandarajah AP, Schwarz EM. Dynamic exercises in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2004;63(11):1359–1361. doi: 10.1136/ard.2004.020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.James MJ, Cleland LG, Gaffney RD, Proudman SM, Chatterton BE. Effect of exercise on 99mtc-dtpa clearance from knees with effusions. Journal of Rheumatology. 1994;21(3):501–504. [PubMed] [Google Scholar]
- 107.Ellegaard K, Torp-Pedersen S, Henriksen M, Lund H, Danneskiold-Samsøe B, Bliddal H. Influence of recent exercise and skin temperature on ultrasound doppler measurements in patients with rheumatoid arthritis—an intervention study. Rheumatology. 2009;48(12):1520–1523. doi: 10.1093/rheumatology/kep294. [DOI] [PubMed] [Google Scholar]
- 108.Bland JH. Joint, muscle and cartilage physiology as related to exercise. Application and practical management in rheumatic disease. Arthritis and Rheumatism. 1988;1(2):99–108. [Google Scholar]
- 109.Young A, Stokes M, Iles JF. Effects of joint pathology on muscle. Clinical Orthopaedics and Related Research. 1987;219:21–27. [PubMed] [Google Scholar]
- 110.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis and Rheumatism. 1999;42(10):2220–2230. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 111.Brorsson S, Hilliges M, Sollerman C, Nilsdotter A. A six-week hand exercise programme improves strength and hand function in patients with rheumatoid arthritis. Journal of Rehabilitation Medicine. 2009;41(5):338–342. doi: 10.2340/16501977-0334. [DOI] [PubMed] [Google Scholar]
- 112.Neill J, Belan I, Ried K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosus: a systematic review. Journal of Advanced Nursing. 2006;56(6):617–635. doi: 10.1111/j.1365-2648.2006.04054.x. [DOI] [PubMed] [Google Scholar]
- 113.van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology. 2010;49(7):1294–1302. doi: 10.1093/rheumatology/keq043. [DOI] [PubMed] [Google Scholar]
- 114.Hewlett S, Carr M, Ryan S, et al. Outcomes generated by patients with rheumatoid arthritis: how important are they? Musculoskeletal Care. 2005;3:131–142. doi: 10.1002/msc.3. [DOI] [PubMed] [Google Scholar]
- 115.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. Journal of Psychosomatic Research. 2004;56(2):157–170. doi: 10.1016/S0022-3999(03)00371-4. [DOI] [PubMed] [Google Scholar]
- 116.Neuberger GB, Press AN, Lindsley HB, et al. Effects of exercise on fatigue, aerobic fitness, and disease activity measures in persons with rheumatoid arthritis. Research in Nursing and Health. 1997;20(3):195–204. doi: 10.1002/(sici)1098-240x(199706)20:3<195::aid-nur3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 117.Hutton I, Gamble G, McLean G, Butcher H, Gow P, Dalbeth N. What is associated with being active in arthritis? Analysis from the obstacles to action study. Internal Medicine Journal. 2010;40(7):512–520. doi: 10.1111/j.1445-5994.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- 118.Gyurcsik NC, Brawley LR, Spink KS, Brittain DR, Fuller DL, Chad K. Physical activity in women with arthritis: examining perceived barriers and self-regulatory efficacy to cope. Arthritis Care and Research. 2009;61(8):1087–1094. doi: 10.1002/art.24697. [DOI] [PubMed] [Google Scholar]
- 119.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Preventive Medicine. 2004;39(5):1056–1061. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Wilcox S, Der Ananian C, Abbott J, et al. Perceived exercise barriers, enablers, and benefits among exercising and nonexercising adults with arthritis: results from a qualitative study. Arthritis Care and Research. 2006;55(4):616–627. doi: 10.1002/art.22098. [DOI] [PubMed] [Google Scholar]
- 121.Swärdh E, Biguet G, Opava CH. Views on exercise maintenance: variations among patients with rheumatoid arthritis. Physical Therapy. 2008;88(9):1049–1060. doi: 10.2522/ptj.20070178. [DOI] [PubMed] [Google Scholar]
- 122.Hendry M, Williams NH, Markland D, Wilkinson C, Maddison P. Why should we exercise when our knees hurt? A qualitative study of primary care patients with osteoarthritis of the knee. Family Practice. 2006;23(5):558–567. doi: 10.1093/fampra/cml022. [DOI] [PubMed] [Google Scholar]
- 123.Der Ananian C, Wilcox S, Saunders R, Watkins K, Evans A. Factors that influence exercise among adults with arthritis in three activity levels. Preventing Chronic Disease. 2006;3(3):1–16. [PMC free article] [PubMed] [Google Scholar]
- 124.Kamwendo K, Askenbom M, Wahlgren C. Physical activity in the life of the patient with rheumatoid arthritis. Physiotherapy Research International. 1999;4(4):278–292. doi: 10.1002/pri.174. [DOI] [PubMed] [Google Scholar]
- 125.Lambert BL, Butin DN, Moran D, et al. Arthritis care: comparison of physicians’ and patients’ views. Seminars in Arthritis and Rheumatism. 2000;30(2):100–110. doi: 10.1053/sarh.2000.9203. [DOI] [PubMed] [Google Scholar]
- 126.Iversen MD, Fossel AH, Ayers K, Palmsten A, Wang HW, Daltroy LH. Predictors of exercise behavior in patients with rheumatoid arthritis 6 months following a visit with their rheumatologist. Physical Therapy. 2004;84(8):706–716. [PubMed] [Google Scholar]
- 127.Iversen MD, Fossel AH, Daltroy LH. Rheumatologist-patient communication about exercise and physical therapy in the management of rheumatoid arthritis. Arthritis Care and Research. 1999;12(3):180–192. doi: 10.1002/1529-0131(199906)12:3<180::aid-art5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 128.Munneke M, De Jong Z, Zwinderman AH, et al. High intensity exercise or conventional exercises for patients with rheumatoid arthritis? Outcome expectations of patients, rheumatologists, and physiotherapists. Annals of the Rheumatic Diseases. 2004;63(7):804–808. doi: 10.1136/ard.2003.011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Jong Z, Munneke M, Zwinderman AH, et al. Long term high intensity exercise and damage of small joints in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2004;63(11):1399–1405. doi: 10.1136/ard.2003.015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Häkkinen A, Hannonen P, Nyman K, Lyyski T, Häkkinen K. Effects of concurrent strength and endurance training in women with early or longstanding rheumatoid arthritis: comparison with healthy subjects. Arthritis Care and Research. 2003;49(6):789–797. doi: 10.1002/art.11466. [DOI] [PubMed] [Google Scholar]
- 131.Gecht MR, Connell KJ, Sinacore JM, Prohaska TR. A survey of exercise beliefs and exercise habits among people with arthritis. Arthritis Care and Research. 1996;9(2):82–88. doi: 10.1002/1529-0131(199604)9:2<82::aid-anr1790090203>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 132.Baillet A, Zeboulon N, Gossec L, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthritis Care and Research. 2010;62(7):984–992. doi: 10.1002/acr.20146. [DOI] [PubMed] [Google Scholar]
- 133.Cairns AP, McVeigh JG. A systematic review of the effects of dynamic exercise in rheumatoid arthritis. Rheumatology International. 2009;30(2):147–158. doi: 10.1007/s00296-009-1090-5. [DOI] [PubMed] [Google Scholar]
- 134.Forestier R, Andre-Vert J, Guillez P, et al. Non-drug treatment (excluding surgery) in rheumatoid arthritis: clinical practice guidelines. Joint Bone Spine. 2009;76(6):691–698. doi: 10.1016/j.jbspin.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 135.Stenstrom CH, Minor MA. Evidence for the benefit of aerobic and strengthening exercise in rheumatoid arthritis. Arthritis Care and Research. 2003;49(3):428–434. doi: 10.1002/art.11051. [DOI] [PubMed] [Google Scholar]
- 136.Murphy M, Nevill A, Neville C, Biddle S, Hardman A. Accumulating brisk walking for fitness, cardiovascular risk, and psychological health. Medicine and Science in Sports and Exercise. 2002;34(9):1468–1474. doi: 10.1097/00005768-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 137.Hall J, Skevington SM, Maddisson PJ, Chapman K. A randomised and controlled trial of hydrotherapy in rheumatoid arthritis. Arthritis and Rheumatism . 1996;9:206–215. doi: 10.1002/1529-0131(199606)9:3<206::aid-anr1790090309>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 138.Karatepe AG, Gunaydin R, Turkmen G, Kaya T. Effects of home-based exercise program on the functional status and the quality of life in patients with rheumatoid arthritis: 1 year follow-up study. Rheumatology International. 2011;31:171–176. doi: 10.1007/s00296-009-1242-7. [DOI] [PubMed] [Google Scholar]
- 139.Linde L, Sorensen J, Ostergaard M, et al. What factors influence the health status of patients with rheumatoid arthritis measured by the SF-12v2 health survey and the health assessment questionnaire? Journal of Rheumatology. 2009;36(10):2183–2189. doi: 10.3899/jrheum.090134. [DOI] [PubMed] [Google Scholar]
- 140.Durstine JL, Moore GE, Painter PL, Roberts SO, editors. ACSM's Exercise Managment for Persons with Chronic Diseases and Disabilities. 3rd edition. Champaign, Ill, USA: Human Kinetics; 2003. [Google Scholar]