Abstract
The fate of intraperitoneally injected or implanted male rat bone marrow-derived stromal cells inside female sibling host animals was traced using Y-chromosome-sensitive PCR. When injected intraperitoneally, Y-chromosome-positive cells were found in all studied organs: heart muscle, lung, thymus, liver, spleen, kidney, skin, and femoral bone marrow with a few exceptions regardless of whether they had gone through osteogenic differentiation or not. In the implant experiments, expanded donor cells were seeded on poly(lactide-co-glycolide) scaffolds and grown under three different conditions (no additives, in osteogenic media for one or two weeks) prior to implantation into corticomedullar femoral defects. Although the impact of osteogenic in vitro cell differentiation on cell migration was more obvious in the implantation experiments than in the intraperitoneal experiments, the donor cells stay alive when injected intraperitoneally or grown in an implant and migrate inside the host. However, when the implants contained bioactive glass, no signs of Y-chromosomal DNA were observed in all studied organs including the implants indicating that the cells had been eliminated.
1. Introduction
Tissue engineering is a relatively new field of medicine that has been hoped to provide assistance in treating failing tissues and organs [1]. Although many of the practical issues are still unsolved, using the patient's own stem cells in combination with suitable tissue engineering scaffolds [2] removes all the immunological complications of using allografts and can, at least in theory, provide an unlimited source of, for example, bone tissue [2, 3].
Evidence that bone marrow contains cells that can differentiate into mesenchymal cells started with the work of Friedenstein and coworkers [4, 5]. These bone marrow-derived stromal cells (BMSCs) were later shown to have bone forming capabilities when grown under certain conditions [6] as well as chondrogenic and adipogenic properties [7].
BMSCs, including human, have been shown to retain their activity after intraperitoneal injection [8]. There are also compelling indications that injected BMSCs can circulate in the body and home in on target tissues [8, 9]. In this study, we aimed to test whether the degree of osteogenic in vitro differentiation of the bone marrow-derived donor cells affected the fate of the cells when infused into the peritoneal cavity of rats.
Less is known about what happens to the donor cells inside the host body when implanted into bone defects. Bone is a highly vascular tissue [10], and therefore it is extremely plausible that implanted cells leave the defect area and migrate to other organs of the host. While this might cause unique risks for tissue engineering, it also creates unique opportunities for stem cell therapy, and for this reason we traced the implanted cells to find out if they migrated to other organs outside the defect area.
Poly(dl-lactide-co-glycolide) (PLGA) was chosen as the scaffold material for the seeding of cells. PLGA is a porous biodegradable copolymer based on synthetic aliphatic polyesters and is used in several medical applications [11]. It can be synthesized to achieve specific physical and chemical characteristics. PLGA has also been shown to be a suitable material for seeding cells [10]. As the material characteristics can be further improved by composite preparation with bioceramics [12], we also tested the impact of bioactive glass (BAG) in the PLGA scaffolds on the behavior of the donor cells inside the host animals.
2. Materials and Methods
2.1. Study Design
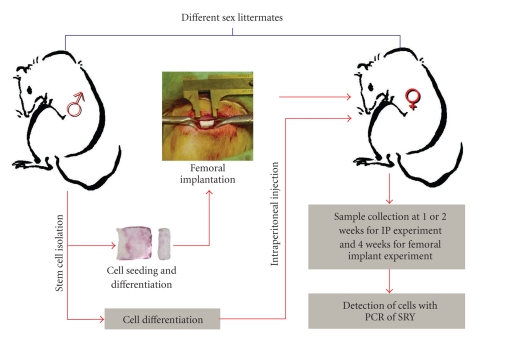
The fate of implanted BMSCs was studied by isolating cells from male rat's femurs and tibias, implanting these cells into their female siblings by intraperitoneal injection or femoral implantation within porous PLGA scaffolds, and detecting them from isolated host tissues with Y-chromosome-sensitive PCR (Figure 1).
Figure 1.
Flow chart illustrating the different phases and the setup of the implant and intraperitoneal experiments.
2.2. Experimental Animals
For the intraperitoneal experiment, 18 female Sprague Dawley rats weighing about 200 g were used. For the implantation experiments, 15 one-year-old female Sprague Dawley rats weighing at least 300 g were used. The rats were housed individually in their cages with free access to food pellets and drinking water. The animal facilities are managed according to the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and inspected for GLP Compliance. The study design was approved by the Animals Ethical Committee of the State Provincial Office of Southern Finland.
2.3. Preparation of Porous PLGA and PLGA/BAG Composites
PLGA 90/10 and PLGA/BAG 90/10 (20 wt-%) composites were made by solvent casting using dichloromethane (DCM) as a solvent. Polar composites were made as follows. A uniform layer of BAG (20 wt%) was applied on the bottom of a Teflon-coated form. PLGA/DCM solution (25 w/v% (g/ml)) was poured onto the BAG layer. The solvent was first slowly evaporated (4°C; 1 week) after which the forms were placed in a fume cupboard at room temperature (48 h). A similar process was used for the preparation of composites with a homogenous structure. PLGA/DCM solution (25 w/v%) containing 20 wt% BAG was poured into a Teflon-coated form and stirred until most of the solvent was evaporated. The remaining solvent was first slowly evaporated (4°C; 3 days) after which the composites were placed in a fume cupboard at room temperature (48 h). The prepared films were stored in a desiccator until further use. Films were processed into porous scaffolds by gas foaming with high-pressure CO2 in an autoclave (50 bars) for 1 h after which the gas was rapidly released from the vessel (5 s). The porous composite films were stored under nitrogen in a desiccator (RT) prior to use. This method has been described in detail in previous work [13].
2.4. Cell Isolation and Culture
For cell isolation, two eight-week-old male rats were killed by cervical dislocation under CO2 anesthesia. The femurs and tibias were dissected and sterilized by dipping into 70% ethanol and put into sterile phosphate buffered saline (PBS). In sterile conditions, both ends of the bone were cut off and the cells were flushed out with alpha-minimum essential medium (α-MEM) containing antibiotics using a syringe and an 18 G needle. The cell suspension was centrifuged and resuspended in α-MEM containing antibiotics (100 units/ml penicillin and 100 g/ml streptomycin) and 20% fetal calf serum (FCS).
Marrow stromal cells can be isolated from other cells in the marrow by their tendency to adhere to plastic surfaces. After 2 days, the floating cells were removed and the adherent cells were allowed to proliferate for another 5 days in a humidified incubator at 37°C and 5% CO2. The medium was changed twice a week.
2.5. Intraperitoneal Experiment
After the cell culture expansion period of 7 days, the cells were trypsinized and cultured according to three different protocols (106 cells/culturing condition). The cells were grown in proliferation medium (α-MEM with 100 units/ml penicillin and 100 g/ml streptomycin and 20% FCS) for two weeks, in proliferation medium for one week and then given osteogenic medium (α-MEM, antibiotics, 20% FCS, 50 g/ml ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone) during the second week, or in osteogenic medium for two weeks with dexamethasone present only during the first week. Dexamethasone was omitted the second week as this synthetic glucocorticoid has been reported to inhibit preostoblastic cells into terminal osteoblasts [14, 15].
Trypsinized expanded or differentiated BMSCs (Table 1) were suspended into Hanks-buffer (100000 cells/ml) and 1 ml of the cell suspension was injected intraperitoneally into the right lower abdomen of 18 female siblings of the cell donor rats. The rats were killed 1 or 2 weeks after injection and the samples of lung, spleen, liver, thymus, and bone marrow from the femur were collected for DNA extraction.
Table 1.
Differentiation protocols used in the intraperitoneal experiment.
| Intraperitoneal | MSC differentiation | Implantation time |
|---|---|---|
| Group I, IV | Expansion culture only | |
| Group II, V | I week in α-MEM + 1 week in osteogenic media | I–III: one week, IV–VI: two weeks |
| Group III, VI | 2 weeks in osteogenic media, 2nd week w/o dexa |
2.6. Cell Seeding and Femoral Implantation Experiments
After the cell culture expansion period of 7 days, two separate implant experiments were conducted (Table 2). Prior to cell seeding, the polymer discs, 10 mm in diameter and 2.5 mm in thickness were sterilized in 70% ethanol for 1 h and then washed twice with sterile PBS. The discs were then incubated over night in α-MEM without serum at 37°C and put into 24 well plates.
Table 2.
Differentiation protocols and materials used in the implant experiments.
| Implant I | Material | MSC differentiation |
|---|---|---|
| Group I | PLGA | 2 weeks in plain medium |
| Group II | PLGA | 1 week in α-MEM + 1 week in osteogenic media |
| Group III | PLGA | 2 weeks in osteogenic media, 2nd week w/o dexa |
| Implant II | ||
| Group I | PLGA | 2 weeks in osteogenic media, 2nd week w/o dexa |
| Group II | PLGA + BAG | 2 weeks in osteogenic media, 2nd week w/o dexa |
To study the impact of in vitro cell differentiation on migration in the host animal (implant experiment I), 50000 expanded cells were seeded on 90/10 PLGA implants without BAG. After seeding, three different protocols were used for cell proliferation and differentiation. In group I, the seeded cells were kept in proliferation medium, α-MEM with 100 units/ml penicillin and 100 g/ml streptomycin and 20% FCS for two weeks. In group II, the cells were grown in proliferation medium for one week and then given osteogenic medium (α-MEM, antibiotics, 20% FCS, 50 g/ml ascorbic acid, 10 mM β-glycerophosphate and 10 nM dexamethasone) during the second week. In group III, the cells were grown in osteogenic medium for two weeks with dexamethasone present only during the first week.
After two weeks, the seeded implants were either stained for alkaline phosphatase using p-nitrophenol as a substrate (Sigma; Kit 86-r) or implanted into rat femoral bone defects as described below.
To study the effect of BAG on donor cell viability and migration in the host animal (implant experiment II), 50000 expanded cells in a small volume (~50 μl) were seeded on 90/10 PLGA with and without 20% w-% BAG. After 3 h incubation at 37°C, 1 ml of osteogenic growth medium (dexamethasone present only the first week) was added and the cells were grown on both implant types for two weeks in a humidified incubator at 37°C and 5% CO2.
2.7. Surgical Procedure for Femoral Implantation Experiments
General anesthesia was induced by subcutaneous injection of 0, 15–0, 2 ml/100 g rat weight Hypnorm (Jansen Pharmaceuticals, Belgium): Dormicum (Roche, Switzerland): sterile water (1 : 1 : 2). Bone defects of 2.4 × 3 × 8 mm were made with a dental drill (rose bur) in the anterolateral part of subtrochanteric femur [16]. Each femoral implant (2.5 × 3 × 8 mm) was inserted into the left or right femur of the rats. The polar glass composite material was inserted with the ceramic side to the dorsolateral side. After the operation, 100 μl of buprenorphin (Temgesic, Leiras Finland) was injected subcutaneously to relieve postoperative pain.
A slower release rate of the donor cells was expected, therefore, a four-week observation point was selected. The animals were killed by cervical dislocation under CO2 anesthesia and the implants and the organ samples (i.e., opposite femur and pieces of the spleen, liver, kidney, lung, heart, thymus and the skin from the defect area, and blood) were collected for DNA analyses. The sample from the heart was taken from the apex in order to get as little blood as possible with the sample.
2.8. DNA Isolation
For DNA isolation, a 3 × 3 × 3 mm sample of tissue was cut into smaller pieces and then digested for 16–24 h at 55°C with Proteinase K (Finnzymes, Finland). The DNA was extracted using a phenol-chloroform extraction protocol, where the DNA was serially extracted from phenol, phenol-chloroform 1 : 1, and chloroform [17]. Between the extractions, the samples were centrifuged (13200 rpm, 4 min) and the upper liquid phase was moved to a new tube. The extraction product was finally treated with 30% PEG 6000/1.8 M NaCl and incubated on ice for 2 hours. The isolated DNA was then centrifuged (13200 rpm, 10 min) and washed twice with 70% ethanol and finally suspended into TE-buffer. The concentration of the isolated DNA was measured spectrophotometrically.
2.9. Detection Y-Chromosomal Marker by PCR
The isolated tissue DNA was analyzed for the sex determination region on the Y-chromosome (SRY) with PCR. The primers (forward 5′-GGCTTCAAAGTAGATTAGTTGGG-3′ and reverse 5′-ATGCATTCATGGGGCGCTTGAC-3′) were designed according to the DNA sequence for the Rat SRY 5′ flanking region (GenBank AJ_222688). Polymerase chain reaction conditions were 94°C for 5 min followed by 30 cycles of 94°C for 1 min, 61°C for 1 min, and 72°C for 1 min.
The final 392 bp PCR products were analyzed with electrophoresis in 1% agarose gels containing ethidium bromide.
3. Results
3.1. Intraperitoneal Experiment
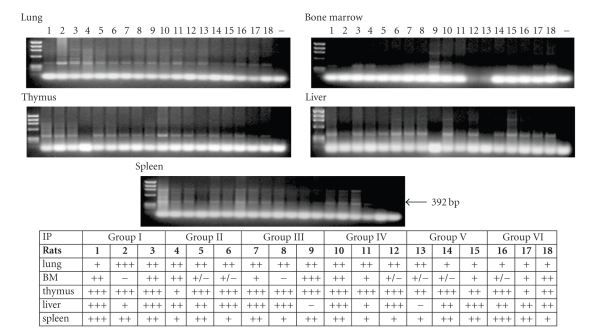
BMSCs grown in expansion or in osteogenic media were used in the intraperitoneal experiment (Table 1). After 1 and 2 weeks, samples from the lung, bone marrow, thymus, liver, and spleen were collected and analyzed for their contents of transplanted male cells by PCR of the sex determination region on the Y-chromosome (SRY). Regardless of cell culturing protocols, all 18 animals that received BMSCs injected into the peritoneal cavity were clearly positive for the Y-chromosome in the lung, spleen, and thymus samples. The liver was positive for most animals, in groups III and V, there was one negative animal, respectively, and the bone marrow was also positive with some variation in the band intensity for all animals except one in groups I and III (Figure 2).
Figure 2.
Presence of Y-chromosomal donor DNA in host tissues after intraperitoneal transplantation. − = negative control. In the table, + indicates faint bands, ++ distinct bands, +/+/+ strong bands, +/− traces of, and − no bands of Y-chromosomal DNA.
3.2. Implant Experiments
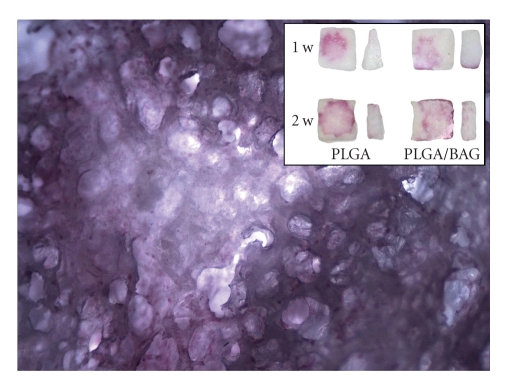
Light microscopy examination showed the porous structure of the scaffold, the proliferation of seeded cells, and the ALP staining that is characteristic for osteoblastic cells (Figure 3). The presence of BAG in the scaffolds did not change the morphology of the implanted cells.
Figure 3.
ALP-stained BMSCs in 90/10 PLGA scaffold with 20 w-% BAG after 2 weeks in osteogenic media (closeups 4 x magnification stereomicroscopy image). Insert shows ALP-stained BMSCs in PLGA and PLGA/BAG scaffolds after one and two weeks in osteogenic media.
PCR analyses of the first implant experiment (see, Table 2), where the impact of in vitro cell differentiation on migration in the host animal was studied, revealed Y-chromosome-positive signals in the spleen, heart, skin taken from the wound area, and liver of all 9 animals (Figure 4). The kidneys were negative for donor cells in all rats. In group I (cells grown in proliferation medium only), 2/3 animals showed positive lung samples, and 1/3 was positive when the cells were grown in osteogenic media for two weeks before implantation (group III). None of the lungs were positive when the cells were left in proliferation medium for one week and then cultured in osteogenic medium for an additional week (group II), but strong positive signals were detected in the thymuses of these animals. Also the other animals with negative lungs, except one in group III, showed strong positive Y-chromosome signals in the thymus and/or in the blood.
Figure 4.
Presence of Y-chromosomal donor DNA in host tissues four weeks after femoral implantation. In the implant panel, I, II, and III are samples of the nonimplanted cell-seeded polymer. + = positive control; − = negative control. In the table, + indicates faint bands, ++ distinct bands, +/+/+ strong bands, +/− traces of, and − no bands of Y-chromosomal DNA.
Positive signals of the Y-chromosome were detected in all 9 implant samples. Faint signals were observed in the bone marrow from the femur opposite the implant except for one animal in group I.
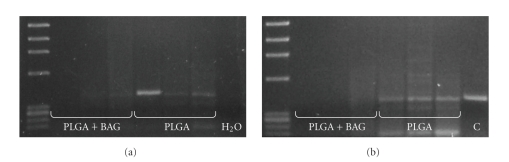
As differentiated cells with osteoblastic morphology were less prone to get stuck in the lungs, the cell seeding protocol used for group III was chosen for the second implant experiment (Table 2). In this experiment, the effect of BAG on donor cell viability and migration in the host animal was studied as above. Positive signals for the Y-chromosome were only found in the 90/10 PLGA implants without BAG (Figure 5(a)) four weeks after operation. In addition, the animals that had implants containing no BAG showed distinct SRY bands in the bone marrow of the femur opposite the operated one, whereas the bone marrow of the animals with PLGA/BAG implants was negative (Figure 5(b)). This was the case for the other tissues analyzed as well (not shown).
Figure 5.
Presence of donor cells four weeks after implantation. No signals of Y-chromosomal DNA were detected in (a) the implants containing BAG and (b) no donor cells escaped from the PGLA/BAG implants were found in the opposite femoral bone marrow. H2O = negative control; C = positive control.
4. Discussion
The capacity of BMSCs to mobilize into peripheral blood in response to trauma, such as burns and skeletal muscle injuries, has been well documented [18, 19]. It is also known that a small amount of BMSCs continually circulate in the bloodstream [20].
Many studies have shown that BMSCs migrate inside the host when injected systemically [21, 22] or intraperitoneally [8, 23] and that they have the ability to engraft to sites of tissue injury and inflammation [24]. Intravenously injected BMSCs travel to the right side of the heart and through there to the pulmonary artery and are mostly trapped in the microvasculature of the lung because of their size and the adhesion potential. BMSCs injected into the artery pass through the tissue that the artery oxygenates and then go into the pulmonary circulation and seem to have the same fate of accumulating in the lungs from where they unlikely migrate to the injury site [22]. Despite a dynamic in vivo distribution of undifferentiated BMSCs after intraperitoneal cavity infusion in rats, considerable amount of the cells were found lodging in the lungs as well two days after injection [23]. This is not surprising as intraperitoneally injected cells travel through the lymphatic circulation into the left subclavian vein and to the right side of the heart.
Here we studied the fate of injected and implanted undifferentiated and osteogenically differentiated BMSCs inside the host. We used the widely accepted method of detecting Y-chromosome specific sequences by amplifying with PCR [25, 26]. Female tissues are negative for the Y-chromosome except if they have given birth to male offspring, when some fetal cells can hide in the female organs [27]. In our study, the recipient rats had not been pregnant.
Intraperitoneally infused BMSCs, regardless of the differentiation stage, were found in lungs at both observation time points, that is, after one and two weeks. When released from the bone implants, less cells were trapped in the lungs, especially differentiated cells, which are smaller in size than the undifferentiated cells. The peritoneum is highly vascularized and therefore it is apparent that more cells get access to the lymphatics and blood circulation simultaneously, which increases the risk of getting trapped in the small capillaries of the lungs. The slower release rate of cells escaping the implants results in fewer cells migrating at the same time, which probably results in less lung entrapment compared to intraperitoneally injected cells.
Positive signals were found in the spleens of all animals, both in the intraperitoneally injection experiments and in the implant study without BAG. In the intraperitoneal injection study, the livers of two animals did not give positive signals for Y-chromosomal cells (in groups II and V), whereas all implanted animals had donor cells in the liver. In previous reports [19], high numbers of transplanted cells have been observed in the liver and spleen. Rombouts and Ploemacher [28] showed that there was loss of homing ability of transplanted BMSCs in total body irradiated animals to both bone marrow and spleen following culture of the cells. However, in their study only small numbers (100 and 1000 cells) of cells were transplanted and this probably made the detection of labeled cells difficult. Following injection of larger amounts (4 × 106) of BMSCs, both the liver and the spleen contained histologically detectable cells one week after transplantation [22, 29]. Our results support the theory that the liver and spleen might serve as a secondary obstacle for the circulating BMSCs that have passed the lung barrier. There is, however, great controversy whether these findings are due to homing of the cells to liver and spleen or whether they are trapped within these organs, which belong to the reticuloendothelial system [21, 23, 29] that functions as a part of the immune system to remove cell debris from the blood stream.
Several studies report that systematically administered BMSCs are capable of homing to the bone marrow [30, 31] and the thymus [32, 33]. Both are organs of lymphopoiesis, especially in the generation of T lymphocytes. These cells are formed in the bone marrow and migrate to the thymus to undergo maturation. Although thymus is the primary organ where T cell maturation happens, it has been shown that in the absence of the thymus, the bone marrow provides appropriate support for T cell maturation [34]. In this study, BMSCs migrated preferentially to the thymus instead of the bone marrow after intraperitoneal injection and femoral implantation. Bone marrow-derived cells have been shown to express chemokine receptors that promote migration to the thymus [33] and to the bone marrow [29]. As the transplanted cell circulates around the host body passing both tissues countless times, it can be speculated that the transplanted BMSCs express receptors that promote active migration to the thymus in preference to bone marrow. These chemokine receptors might also be involved in homing of BMSCs to other lymphatic organs in general, including the spleen [33]. What is interesting is that even osteogenically differentiated cells seem to have the capacity to home into both the thymus and the bone marrow in line with the findings of Thalmeier et al. [35], who showed that bone marrow-derived stromal cells expressing osteogenic markers could be detected in several recipient organs including the thymus and bone marrow.
Three additional tissues, that is, heart, skin, and blood, were analyzed from the animals of the bone implant experiment. Strong signals were detected in all skin samples taken from the wound area, indicating that the donor cells engraft into the injured skin. It remains to be studied whether these cells participate in the wound healing process. Our results show that seeded cells that escape from the PLGA implants, most probably due to degradation of the scaffold, were also capable to engraft into the heart as all animals showed clearly detectable signs of donor cells in the heart samples. Interactions between coronary artery epithelium and BMSCs have been shown in ischemic hearts and in animals pretreated with inflammatory cytokines [36], and intravenous BMSC injection has been shown to increase left ventricular ejection fraction of infarcted pig hearts [37]. On the other hand, intravenously infused BMSCs do not home to intact myocardium [21]. What the reason is for the cells in this study to be found in uninjured heart tissue remains unclear.
The positive signals detected in the blood of several animals in our implant experiment support the idea that BMSCs can reach almost any organ via the bloodstream. It is therefore not surprising to find donor BMSCs in a variety of the host's organs, providing that these donor cells have stayed alive, which seems to be the case for the cells in our experiments.
Bioactive glass, however, affected the outcome of the seeded cells, but we can only speculate their fate. As no traces of male cells were detected in these implants, it can be assumed that all cells had escaped, “dead or alive”. Furthermore, as no donor cells were observed in any of the other tested tissues, the cells seemed to have been destroyed before the tissue samples were taken for DNA analyses four weeks after implantation or more unlikely accumulated in different organs than the ones tested. This negative effect of BAG on BMSCs in vivo could possibly be due to high local alkalinity of dissolving bioactive glass [38].
5. Conclusions
In this study, we showed that in vitro cultured BMSCs can be transplanted successfully intraperitoneally and in bone implants and are found in a wide variety of organs after transplantation. Femoral implantation experiments showed that donor cells with osteoblastic morphology passed the lung barrier and did not lose the ability to engraft the bone marrow of the host animal. In contrast, BAG in the PLGA seemed to have a negative effect on the migration of implanted cells. Further studies are needed to conclude whether there is permanent engraftment of BMSCs in different organs and if there is, then what their contribution to the tissue architecture is.
Acknowledgments
The authors would like to thank Toini Tolvanen for her help with the histological stainings, and TEKES, The National Technology Agency of Finland, for financial support. T. Wilson and C. Stark are members of TGBS, National Graduate School of Muscoskeletal Disorders and Biomaterials, Finland. Timothy Wilson and Christoffer Stark are contributed equally to this work.
References
- 1.Nerem RM. Tissue engineering: the hope, the hype, and the future. Tissue Engineering. 2006;12(5):1143–1150. doi: 10.1089/ten.2006.12.1143. [DOI] [PubMed] [Google Scholar]
- 2.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromolecular Bioscience. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Experimental Hematology. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Gorskaja UF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 6.Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell and Tissue Research. 1988;254(2):317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 7.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.Gordon D, Pavlovska G, Glover CP, Uney JB, Wraith D, Scolding NJ. Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neuroscience Letters. 2008;448(1):71–73. doi: 10.1016/j.neulet.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovascular Research. 2003;58(2):390–398. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- 10.Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. Journal of Biomedical Materials Research. 2000;51(3):376–382. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 12.Lu HH, Tang A, Oh SC, Spalazzi JP, Dionisio K. Compositional effects on the formation of a calcium phosphate layer and the response of osteoblast-like cells on polymer-bioactive glass composites. Biomaterials. 2005;26(32):6323–6334. doi: 10.1016/j.biomaterials.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Orava E, Korventausta J, Rosenberg M, Jokinen M, Rosling A. In vitro degradation of porous poly(dl-lactide-co-glycolide) (PLGA)/bioactive glass composite foams with a polar structure. Polymer Degradation and Stability. 2007;92(1):14–23. [Google Scholar]
- 14.Ogston N, Harrison AJ, Cheung HFJ, Ashton BA, Hampson G. Dexamethasone and retinoic acid differentially regulate growth and differentiation in an immortalised human clonal bone marrow stromal cell line with osteoblastic characteristics. Steroids. 2002;67(11):895–906. doi: 10.1016/s0039-128x(02)00054-5. [DOI] [PubMed] [Google Scholar]
- 15.Shao J, Xu X, Li G, et al. Inhibitory effects of pharmacological doses of dexamethasone on mineralization of mesenchymal progenitor cells in vitro. Pharmazie. 2009;64(10):674–679. [PubMed] [Google Scholar]
- 16.Ekholm E, Tommila M, Forsback A-P, et al. Hydroxyapatite coating of cellulose sponge does not improve its osteogenic potency in rat bone. Acta Biomaterialia. 2005;1(5):535–544. doi: 10.1016/j.actbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Russel D. Molecular Cloning: A Laboratory Manual. Woodbury, NY, USA: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 18.Mansilla E, Marín GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplantation Proceedings. 2006;38(3):967–969. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Ramírez M, Lucia A, Gómez-Gallego F, et al. Mobilisation of mesenchymal cells into blood in response to skeletal muscle injury. British Journal of Sports Medicine. 2006;40(8):719–722. doi: 10.1136/bjsm.2006.028639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. International Journal of Biochemistry and Cell Biology. 2004;36(4):585–597. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 22.Hara M, Murakami T, Kobayashi E. In vivo bioimaging using photogenic rats: fate of injected bone marrow-derived mesenchymal stromal cells. Journal of Autoimmunity. 2008;30(3):163–171. doi: 10.1016/j.jaut.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 24.Pereira RF, Halford KW, O’Hara MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suttorp M, Sprenger C, Dreger P, et al. Amplification of a Y-chromosomal DNA sequence by the polymerase chain reaction for documentation of residual recipient cells in small samples from bone marrow, peripheral blood and cerebrospinal fluid after bone marrow transplantation. Electrophoresis. 1993;14(3):174–178. doi: 10.1002/elps.1150140129. [DOI] [PubMed] [Google Scholar]
- 26.Tashiro H, Fukuda Y, Kimura A, et al. Monitoring for rejection and engraftment by means of the polymerase chain reaction after rat orthotopic liver transplantation. Transplantation. 1994;58(6):745–748. [PubMed] [Google Scholar]
- 27.Huu SN, Dubernard G, Aractingi S, Khosrotehrani K. Feto-maternal cell trafficking: a transfer of pregnancy associated progenitor cells. Stem Cell Reviews. 2006;2(2):111–116. doi: 10.1007/s12015-006-0017-8. [DOI] [PubMed] [Google Scholar]
- 28.Rombouts WJC, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 29.Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 30.Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78(4):503–508. doi: 10.1097/01.tp.0000128334.93343.b3. [DOI] [PubMed] [Google Scholar]
- 31.Erices AA, Allers CI, Conget PA, Rojas CV, Minguell JJ. Human cord blood-derived mesenchymal stem cells home and survive in the marrow of immunodeficient mice after systemic infusion. Cell Transplantation. 2003;12(6):555–561. doi: 10.3727/000000003108747154. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Hisha H, Inaba M, et al. Evidence for migration of donor bone marrow stromal cells into recipient thymus after bone marrow transplantation plus bone grafts: a role of stromal cells in positive selection. Experimental Hematology. 2000;28(8):950–960. doi: 10.1016/s0301-472x(00)00483-5. [DOI] [PubMed] [Google Scholar]
- 33.Von Lüttichau I, Notohamiprodjo M, Wechselberger A, et al. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells and Development. 2005;14(3):329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
- 34.Dejbakhsh-Jones S, Jerabek L, Weissman IL, Strober S. Extrathymic maturation of αβ T cells from hemopoietic stem cells. Journal of Immunology. 1995;155(7):3338–3344. [PubMed] [Google Scholar]
- 35.Thalmeier K, Meissner P, Moosmann S, Sagebiel S, Wiest I, Huss R. Mesenchymal differentiation and organ distribution of established human stromal cell lines in NOD/SCID mice. Acta Haematologica. 2001;105(3):159–165. doi: 10.1159/000046559. [DOI] [PubMed] [Google Scholar]
- 36.Segers VFM, Van Riet I, Andries LJ, et al. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. American Journal of Physiology. 2006;290(4):H1370–H1377. doi: 10.1152/ajpheart.00523.2005. [DOI] [PubMed] [Google Scholar]
- 37.Price MJ, Chou C-C, Frantzen M, et al. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. International Journal of Cardiology. 2006;111(2):231–239. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 38.Sepulveda P, Jones JR, Hench LL. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. Journal of Biomedical Materials Research. 2002;61(2):301–311. doi: 10.1002/jbm.10207. [DOI] [PubMed] [Google Scholar]