Abstract
This study challenged to produce phosphate-based glasses (PBG) for the treatment of osseous defects. The glasses contained, among other components, 40 mol% CaO and 1–5 mol% TiO2. The mechanical performance and in vitro biocompatibility using both human osteosarcoma and primary osteoblasts were carried out. Incorporation of TiO2 into PBG had no significant effect on strength and modulus. These glasses encouraged attachment and maintained high viability of osteosarcoma cells similar to the positive control surface. Cells grown directly (on glasses) or indirectly (in the presence of glass extracts) showed similar proliferation pattern to the positive control cells with no significant effect of TiO2 detected. Increasing TiO2 content, however, has a profound effect on cytoskeleton organization and spreading and maturation of primary osteoblasts. It is believed that TiO2 might have acted as a chemical cue-modulating cells response, and hence the substrates supported maturation/mineralization of the primary osteoblasts.
1. Introduction
Research on materials used for hard/soft tissue regeneration has focused on those that are degradable and capable of stimulating new tissue formation. In this context, phosphate-based glasses (PBG) offer good candidates. They can be prepared in different forms: blocks, rods, powder, fibres, or microtubes according to the intended application [1–4]. For instance, bulk glasses have been proven to enhance new bone formation [3] and to reduce the number of Staphylococcus aureus and Methicillin resistant staphylococcus aureus (MRSA) that are normally associated with bone infection in an in vitro model designed for that purpose [5]. Glass fibres were also shown to allow for the formation of prototypic muscle fibre [6] and to reduce the number of Staphylococcus epidermidis that are mostly associated with biomaterials-related infection [2].
These glasses are also of great interest for dental applications. They demonstrated better sealing ability than the commercially available gutta percha filling material when incorporated as powder into polycaprolactone matrix and used as root canal filling material [7]. Furthermore, they can be potentially used for other applications such as augmentation of alveolar ridge, treatment of peri-implantitis or infection associated with dry socket that occasionally occurs after tooth extraction, and cell-delivery vehicle to inaccessible areas (e.g., advanced periodontitis) [8].
Currently, for the treatment of osseous defects, atrophic alveolar ridge as an example, autograft represents the gold standard modality for such application, but the material supply is often a problem [9]. Guided bone regeneration technique is another approach; nevertheless, providing enough space for bone regeneration is always a problem. Bioactive glasses as PerioGlas and Biogran that can be easily molded and packed into the bony defect are also used; however, the compromise between the particle sizes and degradation to produce particles favourable for osteogenesis without inducing inflammation is always a dilemma [10].
Phosphate glasses with large particle sizes and even a combination of different particles sizes and compositions can be used as an alternative. Under such circumstances, it is feasible to make the highly degrading particles close to the bony wall of the defect to provide room for formation of new bone and also to allow for the growth of vascularisation that represents a common problem with alloplastic materials [11]. The center of the defect, however, could be filled with particles having a relatively low degradation to provide some support for the newly formed bone.
Our previous work showed that inclusion of TiO2 (1–5 mol%) into glasses with 30 mol% CaO, 20 mol% Na2O, and 50% mol P2O5 improved the direct cell response, that is, when cells directly seeded on the material surface [3]. In another study, it was also reported that the extract of PBG containing 40 mol% CaO enhanced cell growth and antigen expression of bone cells, that is, indirect cell contact [12]. Having PBG combining good cellular response at both direct and indirect contact level, and hence better clinical efficacy, is the main aim of the present study.
Therefore, the scope of this study is to produce glasses having both 40 mol% CaO and 0–5 mol% TiO2 and to assess their potential application for the treatment of a variety of osseous defects in oral and maxillofacial regions, including ridge augmentation, sinus elevation, extraction sites, cystectomies and apicoectomies, and periodontal and peri-implant defects via: (a) evaluation of their mechanical properties represented in biaxial flexural, three-point bending, and characteristic strength as well as Young's modulus and (b) determination of biological properties of these glasses by assessing their effect on osteoblast-like cells behaviour (viability, attachment, proliferation, and differentiation) and on human primary osteoblasts (cytoskeleton organization, cell spreading, and maturation). These glasses are designed to be soluble, thus allow any defective tissues to grow within and occupy the space voided as they degrade. Moreover, the released ions will markedly influence cell attachment, spreading, proliferation, differentiation, and, therefore, function.
2. Experimental
2.1. Glass Preparation
Glass rods of 15 mm diameter were prepared using NaH2PO4, CaCO3, P2O5, and TiO2 (BDH, Poole, UK, all chemicals were >98% purity) as precursors by the conventional melt quenching process. These glasses contain 0, 1, 3, and 5 mol% TiO2 and they are encoded as CNP, CNPT1, CNPT3, and CNPT5, respectively. More information on glass composition and preparation was previously explained [13]. Each rod was then sectioned into approximately 1 mm thick discs using a Testbourne diamond saw with methanol as a coolant/lubricant.
2.2. Preparation of the Specimens
Discs from each composition were subjected to a series of grinding steps on one side using a series of waterproof silicon carbide papers: P no. 120 for 30 seconds at 300 RPM to flatten the surface, then P no. 500, 1000, and 2400, respectively, for 1 min at 500 RPM to smoothen the surfaces, and finally P no. 4000 for 2 min to get a smooth mirror-finish surface on a Struers Rotopol-11 (Struers, UK). These discs were used for biaxial flexural test and biological assessment.
For three-point bending test, however, each glass specimen was fabricated into bars of approximately 2 mm thickness, 4-5 mm width, and approximately 14 mm in length. Each specimen was ground to the required size from a disc shape using a Struers Rotopol 11 and then subsequently ground on one side as described above. The edges of the polished side were also bevelled to eliminate edges flaws.
2.3. Mechanical Characterisation
The mechanical characterisation was performed on glasses with 0 and 5 mol% TiO2.
2.3.1. Biaxial Flexural Tests
At least 25 specimens of each group were subjected to a biaxial flexural strength test using a Zwick HC10 servo hydraulic Testing Frame (Zwick Ltd.). Each disc was placed on a supporting circle with a diameter of 12 mm. The polished surface of the specimen was the tension side, while the unpolished surface was loaded with a point load at a crosshead speed of 1 mm/min and with a 1 kN load cell until failure occurred. The load to failure (N) of each specimen was recorded and the biaxial flexural strength (MPa) was calculated using
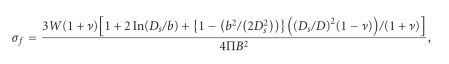 |
(1) |
where σf is the strength (MPa), W the fracture load (N), Ds the diameter of the support circle (12 mm), b the diameter of the areas with uniform load ( = 2B/3) (mm), B the thickness of the disc (mm), and D is the diameter of disc (mm). For v, the Poisson's ratio, a value of 0.25 was used, as recommended with ISO 6872 [14].
2.3.2. Three-Point Bending Test
Three-point bend test as carried out on 8 specimens from each group in order to determine Young's Modulus for these glasses, as the biaxial flexure standard test model (ball on ring) did not provide the necessary formula to be able to obtain this value. These tests were also carried out on a Zwick HC10 testing frame and loaded at a crosshead speed of 1 mm/min with a 1 kN load cell. The testing jig was composed of three beams, two of which act as support beams that are 12.48 mm apart, and the third acting as the loading beam. As stated in the previous section, tests were carried out with the polished side in tension. The bending strength and Young's modulus were calculated from the following (2), respectively,
| (2) |
where σb is the bending strength (MPa), P is the load at failure (N), L is the support span (mm), b is the width of the specimen (mm), d is the thickness of the specimen (mm), Eb is Young's modulus (GPa), and m is the gradient of the elastic region of the load-displacement curve.
2.3.3. Weibull Modulus
Biaxial flexural strength data were analysed using independent t-test at a significant level of P < .05. In addition, the biaxial flexural strength data was also analysed statistically with the Weibull distribution using the WinSMITH Weibull 0.2 software program. Software was used to rank the biaxial flexural strength data in an ascending order. The Weibull modulus was calculated using
| (3) |
where PF(σc) is the probability of failure, σc the fracture strength, σ0 the characteristic strength (PF(σc) = 63.2%), and m is the Weibull modulus. Accordingly, plotting In [In 1/(1 − Pf)] against In σ will provide a slop with the value of the Weibull modulus.
2.4. In Vitro Biological Assessment Using Human Osteosarcoma Cells
2.4.1. Culture
Glass discs were sterilised by dry heating at 180°C for three hours, and pretreated by incubation in a growth medium (Dulbecco's Modified Eagles Medium (DMEM, Gibco), 10% fetal calf serum (FCS), and 1% penicillin and streptomycin (P/S) solution (Gibco)) for 24 hours at 37°C humified atmosphere incubator of 5% CO2 in air. Cells were cultured at a density of 3 × 104 cells/disc, and the growth medium was changed every three days. Thermanox was used as a positive control.
2.4.2. Viability
After 1, 3, and 7 days of culture, samples were stained for 1 hour with a standard growth medium containing 1 μl/ml live/dead staining (calcein AM/propidium iodide). Then, the cell viability was assessed in three dimensions using confocal microscopy (Bio-Rad, USA), and the samples were scanned using a 20x lens. Projection images were created by superimposing the z-stack images that were captured throughout the construct thickness using ImageJ software (National Institute of Health).
2.4.3. Attachment
After 1 day of culture, samples were overnight fixed with 3% glutraldehyde in 0.1 M sodium cacodylate buffer (Agar Scientific Ltd., Essex, UK) at 4°C. Samples were then dehydrated in graded alcohol, critically dried in hexamethyldisilazane (HMDS, Taab Laboratories Ltd., Berkshire, UK) for 1 min, and finally left to air dry. The dried samples were then mounted on aluminum stub, sputter coated with gold-palladium alloy, and viewed using a scanning electron microscope (JSM 5410LV, JEOL, USA).
2.4.4. Proliferation
The proliferation of cells grown directly on the surface of glass discs (for up to 7 days) or in the presence of glass extract (for up to 5 days) was conducted using Alamar Blue assay [3]. The absorbance of each sample was measured at 530 nm (A530) and 590 nm (A590) using a Fluroskan Ascent plate reader (Labsystems, Helsinki, Finland). The glass extract was prepared by incubating the PBG discs into 3 ml the growth medium for 24 h, and then the medium was used as nutrient for cells.
2.5. In Vitro Biological Assessment Using Human Primary Osteoblasts
2.5.1. Culture
In these experiments, human primary osteoblasts cells (Promocell, UK) were seeded on the samples with density 104 cells/disc and incubated with growth medium (Modified Eagles Medium (α-MEM Gibco), 10% FCS, and 1% P/S). The media was changed every three days.
Prior the test, the sterilised samples were washed with Hepes Saline (HS, Sigma Aldrich) and ultrapure sterilized water. Tests were conducted in triplicate. Thermanox was used as positive controls. Samples for the experiment were not preincubated in the media to avoid binding of the protein and other media components to the surface, which may result in some differences in cells response [15].
2.5.2. Cytoskeleton and Spreading
Cytoskeletons were assessed for cells on the samples after 3 days in culture (early time point). The cells were fixed in 4% formaldehyde/PBS, with 1% sucrose at 37°C for 15 min and then washed in PBS. Following this, the cells were permeabilised in a perm buffer (10.3 g sucrose, 0.292 g NaCl, 0.06 g MgCl2, 0.476 g Hepes buffer, and 0.5 ml Triton X, in 100 ml distilled water, pH 7.2) at 4°C for 5 min and subsequently incubated in 1% BSA/PBS at 37°C for 5 min. The cells were stained simultaneously with rhodamine phalloidin (1 : 100 in 1% BSA/PBS, Molecular Probes, OR, USA) and anti-β-tubulin primary antibody (1 : 100 in 1% BSA/PBS; tub 2.1 monoclonal antihuman raised in mouse, (IgG1) Sigma, UK) for 1 h at 37°C. Next the samples were next washed in 0.5% Tween 20/PBS (5 min × 3) and a secondary antibody (1 : 50 in 1% BSA/PBS monoclonal horse antimouse (IgG), Vector Laboratories, UK) was added for 1 h at 37°C. The samples were then washed in Tween 20/PBS, and FITC conjugated streptavidin was added (1 : 50 in 1% BSA/PBS, Vector Laboratories, UK) for 30 min at 4°C. Samples were mounted in Vectorshield fluorescent DAPI mountant and viewed using fluorescence.
Cells spreading, area of cells contact, on the glass samples was evaluated using ImageJ software (Wayne Rasband, National Institute of Mental Health, Bethesda, Maryland, USA). On the actin images the contour of the individual cells was obtained and subtracted from the background. The calculated total area of cells on each sample was presented as a percentage of the sample surface area.
2.5.3. Maturation-Osteocalcin and Osteopontin Expression
Cells were incubated for 21 days as a late time point. At this time, the cells typically prepare to mineralize (if osteoblast differentiation is supported) by secreting bone-specific extracellular matrix proteins (e.g., osteocalcin and osteopontin) just prior to bone formation. Here, both proteins (osteocalcin and osteopontin) were stained (immunofluorescence) after 21 days in culture as previously described [16].
2.6. Statistical Analysis
Statistical analysis was applied using a one way ANOVA test for the biological assessment study while independent t-test for the mechanical study using SPSS for windows (release 12, SPSS UK Ltd., UK). One way ANOVA was then followed by Dunnett (2-sided) t-test that treated Thermanox as a control and compared all other groups against it. The mean difference was considered to be significant at the 0.5 level and 95% confidence interval.
3. Results
3.1. Mechanical Properties
3.1.1. Biaxial Flexural Tests
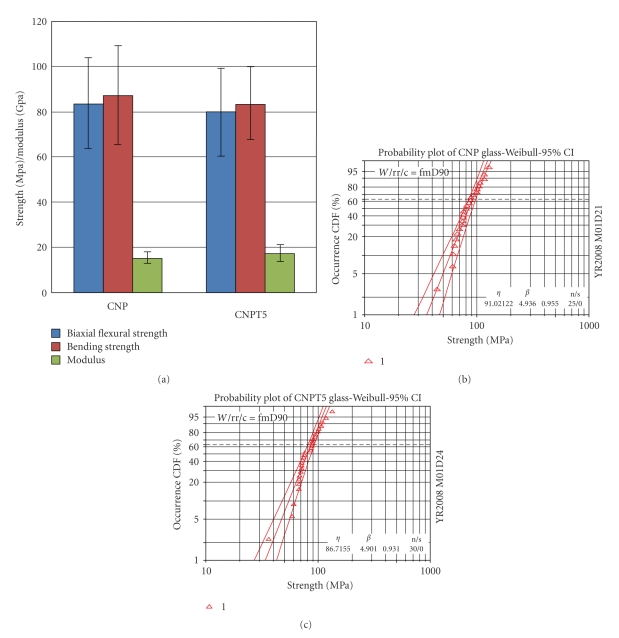
The mean biaxial flexural strength was 79.5 ± 18.5 and 83.5 ± 19.4 MPa for CNPT5 and CNP glass, respectively, Figure 1(a). The independent t-test showed no statistical significant difference between these values.
Figure 1.
(a) Biaxial flexural, bending strength (MPa), and modulus (GPa) of CNP and CNPT5 glasses. (b) Weibull distribution function failure probability versus biaxial flexure strength for CNP glass. (c) Weibull distribution function failure probability versus biaxial flexure strength for CNPT5 glasses. Incorporation of 5 mol% TiO2 has no significant effect on the studied mechanical properties.
3.1.2. Three-Point Bending Test
The bending strength values were 83.7 ± 16.1 and 87.4 ± 22.1 MPa for CNPT5 and CNP, respectively. The independent t-test showed no statistical difference between these sets of results. This can also be said when comparing the biaxial flexure values against the bending strength values for each glass composition, Figure 1(a).
Young's modulus (Eb) data obtained from the load deflection curve of the three-point bending test gave values of 17.4 ± 3.8 and 15.3 ± 3.6 GPa for CNPT5 and CNP, respectively, Figure 1(a). Independent t-test indicated that there was no statistical significant difference between these sets of result also.
3.1.3. Weibull Modulus
The characteristic strength (σ) of CNPT5 glass was 86.7 MPa which is lower than that observed for CNP glasses (91.0 MPa). The 95% confidence intervals of characteristic strength identified no significant difference between CNPT5 and CNP glasses, where the confidence intervals overlapped for both compositions (from 81.26 to 92.5 MPa for CNPT5 and from 84.8 to 97.7 MPa for CNP glasses). Furthermore, Weibull modulus for CNPT5 (4.9) showed no difference to that of CNP glasses (4.9), but the 95% confidence intervals for Weibull modulus also identified no significant difference between these two glasses (the 95% confidence interval ranged from 4.0 to 6.0 for CNPT5 while from 3.9 to 6.3 for CNP glasses), Figures 1(b) and 1(c).
3.2. In Vitro Biological Assessment Using Human Osteosarcoma Cells
3.2.1. Viability
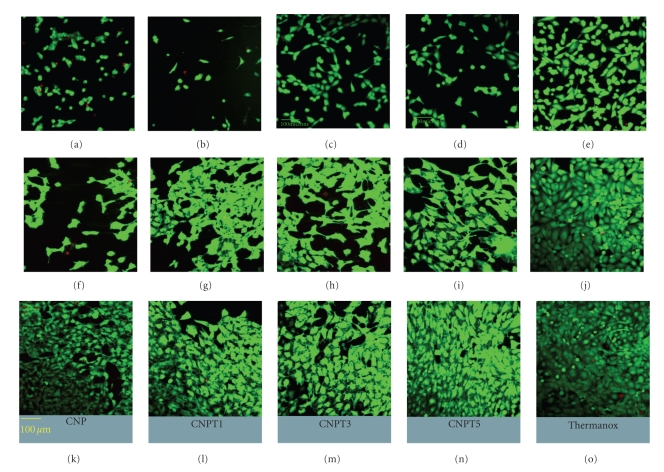
After 1 day of culture, live cells were detected on the surfaces of all glass discs, but not as confluent as on Thermanox positive control, Figures 2(a), 2(b), 2(c), 2(d), and 2(e). After 3 days of culture, there was an increase in the density of HOS cells growing on the surface of all tested glass compositions compared to day 1 of culture except CNP sample, Figures 2(f), 2(g), 2(h), 2(i), and 2(j). At Day 7, however, all glass surfaces supported high cell viability as well as the positive control so that the cells covered all studied surfaces, Figures 2(k), 2(l), 2(m), 2(n), and 2(o).
Figure 2.
Confocal laser scanning microscopy images showing human osteosarcoma cells growing on the surface of CNP, CNPT1, CNPT3, and CNPT5 compared with Thermanox at Day 1 (a–e), Day 3 (f–j), and Day 7 (k–o), respectively. Live cells appear green while the dead cells look red. Cells viability increased with time and the differences between samples disappeared by Day 7.
3.2.2. Attachment
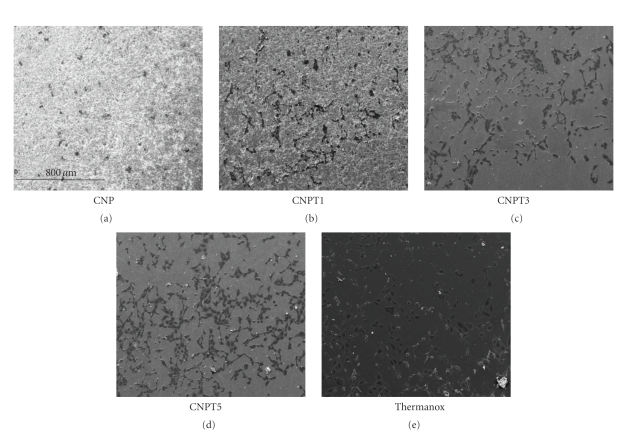
After 1 day of culture, HOS cells presented with well spread morphology on all tested glass compositions in a manner comparable to the positive control cells. Those grown on the surface of CNP and CNPT1 showed lower density than the positive control cells. Cells growing on CNPT3 and CNPT5 glass discs, however, showed similar density to the positive control cells, Figure 3.
Figure 3.
Scanning electron microscopy images showing human osteosarcoma cells growing on the surface of CNP, CNPT1, CNPT3, and CNPT5 compared with Thermanox after 1 day of culture. All surfaces supported cells attachment, but the higher the TiO2 content the greater the cell density.
3.2.3. Proliferation
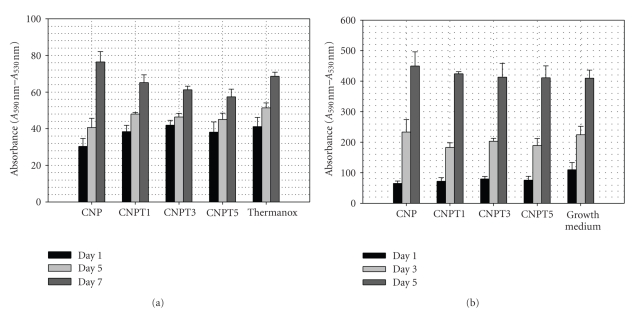
At Day 1 of culture, all tested glass compositions except CNP showed no significant (P ≥ .05) differences in cell number compared with the positive control surface. CNP sample, however, supported significantly (P < .05) lower cell numbers than other tested samples. As it can be clearly seen and as a function of time, the number of cells growing on the surfaces of all tested glass compositions showed an increase compared to those at Day 1 of culture. This increase was highly significant between either Days 1 or 5 and 7 of culture, but not between Days 1 and 5 of culture, Figure 4(a).
Figure 4.
(a) Alamar blue assay results show the proliferation of human osteosarcoma cells growing on the surface of CNP, CNPT1, CNPT3, and CNPT5 in comparison with positive control cells. Errors bars represent the average absorbance considered from three replicate specimens (±SD). (b) Alamar blue assay results show the proliferation of human osteosarcoma cells growing in the presence of extracts of CNP, CNPT1, CNPT3, and CNPT5 in comparison with positive control cells. Errors bars represent the average absorbance considered from three replicate specimens (±SD).
Regarding the compositional differences, there were no significant differences in cell number growing on CNPT1, CNPT3, and CNPT5 and positive control at Days 1 and 5. At Day 7, however, there was significantly lower cell number on CNPT3 and CNPT5 than the positive control. CNPT1, however, showed no significant differences from the positive control surface. Cells growing on CNP showed significantly lower number than the positive control cells at Days 1 and 5 but not at Day 7.
A similar trend of increasing cell number with time was also seen when cells grown in the presence of the glass extract. Moreover, the cells managed to grow at similar rate to the positive control cells cultured in normal growth medium at different time points, Figure 4(b).
3.3. In Vitro Biological Assessment Using Human Primary Osteoblasts
3.3.1. Cytoskeleton and Spreading
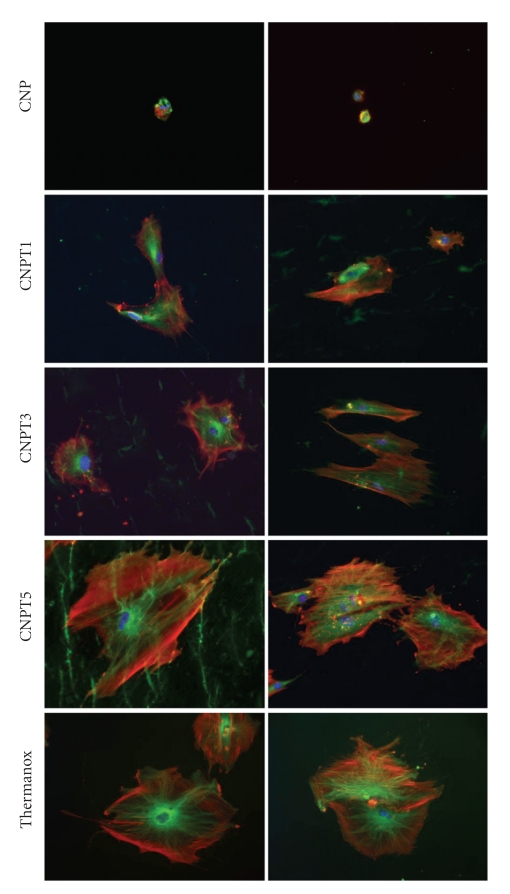
Assessment of the cytoskeleton after 3 days in culture revealed significant differences between the glass samples depending on TiO2 content. In general, it was observed that with the increase of TiO2 content, the organization of the cytoskeleton also increased. Three main proteins were assessed in this study; actin, which is a major structural protein, gives information on the general condition of the cells ability to adhere and spread [17]; tubulin is another structural protein and is important in cell metabolism as vesicles are moved in and out of the cell (endocytosis and exocytosis) along the tubulin microtubules [17, 18]. These proteins are important in the investigation of cell signalling, proliferation, and differentiation.
The number of the cells growth on CNP was clearly limited and the cells were very small; their cytoskeletons were not developed, Figure 5. Improvement of the cytoskeleton organization was observed for those glasses containing TiO2. Cells grown on CNPT and CNPT3 samples were quite well developed, with pronounced stress (actin) fibres and well-organized tubulin network radiating from the centre of the cells, but the cytoskeleton structures was less developed than those observed for control samples. Cells grown on CNPT5, however, had large size, high spread, very well-developed actin and tublin networks, and was comparable to those gown on positive control samples, Figure 5.
Figure 5.
Immunofluorescence images show human primary osteoblasts grown on the surface of CNP, CNPT1, CNPT3, and CNPT5 and stained with actin (red), tubulin (green—left column), and nuclei (blue) in comparison with those grown on Thermanox after 3 days in culture. Improved cytoskeleton organization of cells was observed with increasing TiO2 content in the substrate.
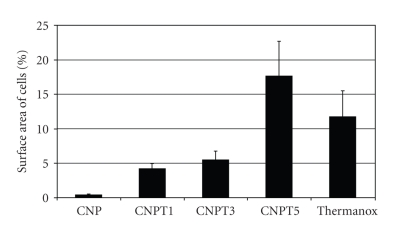
Analysis of the surface area of cells grown on each sample, as a measure of cell spreading, showed that with increasing TiO2 content, the surface area increased. The calculated surface area for cells grown on CNP was far below 1%, which demonstrated that cells did not spread well on those samples. The surface area recorded for cells grown on CNPT5 was higher than that for positive control cells, Figure 6.
Figure 6.
Spreading on surface of human primary osteoblasts grown on the surface of CNP, CNPT1, CNPT3, CNPT5, and Thermanox after 3 days of culture.
3.3.2. Maturation-Osteocalcin and Osteopontin Expression
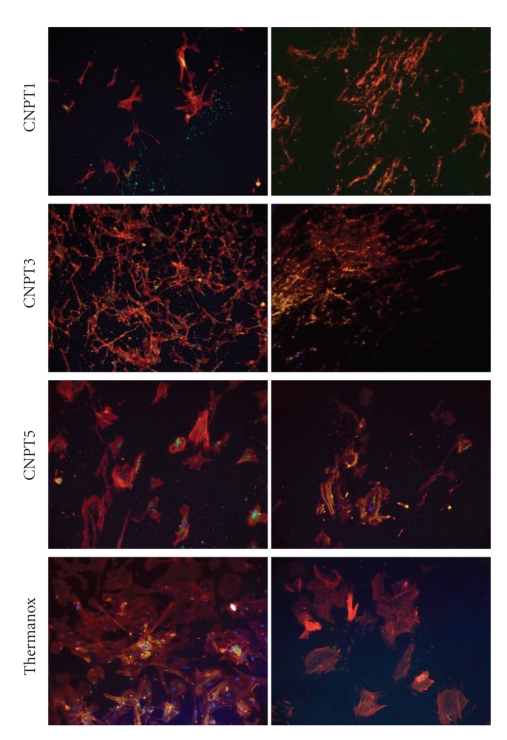
Assessment of functional osteoblast marker proteins after 21 days in culture revealed differences between the samples depending on TiO2 content, Figure 6. Positive expression of osteocalcin (OC) was only evidenced for cells grown on CNPT3 and CNPT5 samples. Osteopontin (OPN), however, was evidenced for cells growing on all studied samples except CNP samples that showed no viable cells on their surfaces at this time point, Figure 7.
Figure 7.
Immunofluorescence images show human primary osteoblasts grown on the surface of CNPT1, CNPT3, and CNPT5 and stained for OC and OPN in comparison with those grown on Thermanox after 21 days in culture. OC was only detected for CNPT3, CNPT5, and Thermanox while OPN was detected for all studied samples.
Moreover, there was a major difference in the morphology of cells grown on different samples. Cells grown on CNPT1 and CNPT3 were typically small, but the density was reasonably high. Those grown on CNPT5, however, had well developed structure and clear and well-organized morphology, which was similar to the positive control cells.
4. Discussion
Bone regeneration research has mostly focused on degradable materials that are capable of stimulating bone regrowth. PBG are controllably degradable and can be prepared in different forms and compositions to support bone regrowth [3]. They might therefore be a good candidate for bone regeneration. Highly degradable (dynamic) surfaces, however, would not allow for maintained cells attachment. The degradation products could also adversely affect cells function, for example, antigen expression [12]. The main challenge was producing glasses that endorse cells to form new tissues, continuously replacing these degraded glasses. They also release degradation products (extracts) that are vital for the function of those cells in their vicinity (indirect contact). It was hypothesised that combination of TiO2 (1–5 mol%) that proved to significantly improve direct cell growth on PBG surfaces, with the highest possible CaO (40 mol%), known to produce tolerable extracts [12], could improve the cellular response at both direct and indirect contact level. Hence, better clinical efficacy would be expected. Additionally, it might improve PBG mechanical properties and allow for easy PBG shaping at chair side time. The aim of this study was, therefore, to test the mechanical and biological performance of these glasses.
Knowledge of the mechanical properties is a crucial requirement for evaluating the possibility of using these glasses in biomedical applications. A previous study examined the effect of altering the TiO2 content on numerous physical properties relating to the 50 P2O5–40 CaO–10 Na2O system. The findings showed that as a result of varying the Ti content by a few percent, there were significant differences in the physical properties, and the data seemed to exhibit a good correlation [13]. In order to examine whether this is reflected in the mechanical behaviour, this study was carried out using CNPT5 and CNP. By comparing the results of these two compositions, we can make a reasonable assumption as to whether or not further results will exhibit a correlation.
As the results indicated, incorporation of TiO2 had no effect on the biaxial flexural strength or the bending strength. Moreover, the addition of TiO2 did not significantly (P ≥ .05) enhance the modulus when compared with the un-doped glasses. As reported in the literature, the yield strength and modulus of natural cortical bone vary from 104–121 MPa [19] and 1–20 GPa, respectively [20]. Therefore, according to the results obtained in this study, the strength as well as the modulus profiles for cortical bone can be matched. Moreover, the bending strength of TiO2-doped glass developed in this study is higher than those recorded for Bioglass (40–60 MPa), while its Young's modulus is lower (30–35 GPa). A previous study commented that structural changes which occur as result of TiO2 addition greatly influence the degradation properties and are attributed to the increase in crosslink density and strengthening of the phosphate network. Incorporation of 5 mol% TiO2 reduces the degradation rate by two orders of magnitude from 0.017 to 0.0008 mg/mm2/h [13]. These structural changes, however significantly they are improving the chemical stability of TiO2 doped glasses, have not shown to improve their bulk mechanical properties, as stipulated above. Moreover, the Weibull distribution plots indicate no significant changes in the mode or type of failure for these glasses. It is also interesting to note that the bend strength values from the two different testing modes show no statistically significant difference, indicating the applicability of the biaxial flexure test as a reliable method for determining bend strength in brittle materials.
In assessing the potential of a new material for biomedical application, biocompatibility is one of the most important concerns of many researchers [21]. Generally, in vitro cell culture is commonly used as a simple screening method to experiment the cell-material interaction. The success of this material initially depends on its ability to encourage cells to attach and adhere to its surface. These two protein-dependant processes determine the next cellular events such as proliferation and differentiation [22]. For bone regeneration, cell lines, as a representative of osteoblastic behaviour, were commonly employed, taking into account that they showed different differentiation behaviours from the primary cells [23]. Therefore, in this study, osteoblasts cell lines were used to initially assess the glass biocompatibility, while human primary osteoblasts were used to assess cytoskeleton organisation and differentiation.
Bone formation processes usually involve cell proliferation, extracellular matrix production, maturation, and then mineralisation. During the first two stages, the cells increase in number and produce extracellular matrix proteins as type I collagen or fibronectin. After the downregulation of proliferation, proteins associated with the osteoblastic phenotype can be detected. At the beginning of mineralisation, cells tend to produce alkaline phosphatase (ALP) as an early mineralisation marker and proteins such as sialoprotein, osteopontin (OP), and osteocalcin (OC) as late mineralisation markers [24].
The findings from both cell viability and attachment studies suggested that inclusion of 40 mol% CaO into the glass produced an improvement in osteosarcoma cells attachment, viability and proliferation when compared with our previous work carried on glasses having 30 mol% CaO [25, 26]. This means that inclusion of high CaO improved direct cell response. More interestingly, the osteosarcoma cells grown in the presence of the glass extracts as if in the standard growth medium. This confirms the hypothesis that using high CaO and very small TiO2 content did improve not only the biological response at the direct contact level but also in the surrounding vicinity of the glass structure.
The glasses used in this study were shown to be more hydrophilic than hydroxyapatite since the water contact angle reported for hydroxyapatite was 48° [27] while about 11° for these glasses [13]. These contact angles are also lower than other titanium phosphate glasses prepared by Navarro et al. (CA ~ 30°) [22]. As well known, the cell response to a material is affected by the surface properties such roughness, wettability, or surface free energy which are of paramount importance as they directly affect cell adhesion and differentiation. The hydrophillicity of these glasses could be accounted for the good cellular response. The absence of clear differences between the tested compositions with osteosarcoma cells can be also referred to the absence of any significant differences in surface properties, as roughness (all samples are prepared to the same mirror finish appearance) and contact angles.
Analysis of primary osteoblast cytoskeleton organization and area of contact with the underlying substrates, on the other hand, indicated that increasing TiO2 content was vital to improve cells behaviour on the glass surfaces. Both the cytoskeleton organization and surface area were the greatest on glass samples with the highest TiO2 content and cells showed better developed structures than observed on positive control samples. It is believed that TiO2, which in concomitant with high biocompatibility, might have acted as a chemical cue modulating cells response. It can be speculated this was also due to change to the degradation rate or due to some differences in protein bonding to the substrate which is particularly important in the early days experiments. On the other hand, the least promising results were observed for the samples without TiO2. Cells on those samples were undeveloped, and their sizes, shapes, and structures indicated that this substrate did not support their growth. This may be associated with the greatest rate of the samples degradation (1-2 orders of magnitude higher than those doped with TiO2 according to the amount of TiO2 incorporated into the glass [13]) which might have caused cells detachment. It is also possible that the amount of released element was too high for the cells which impaired their growth. These findings were also confirmed by osteocalcin and osteopontin expression study.
5. Conclusion
Compared to the previous work with 30 mol% CaO [25], glasses with 40 mol% CaO showed improved osteosarcoma cells attachment, viability, and proliferation. The extracts from glasses containing 40 mol% CaO and/or TiO2 supported normal growth of osteosarcoma cells as the normal growth medium. The effect of TiO2 as a modifying oxide into 40 mol% CaO containing glasses was not significant on mechanical strength and modulus. It, however, had had significant on primary osteoblast cells development, cytoskeleton organisation, area of spreading on the substrate, and maturation/mineralization process. This effect was more pronounced with increasing TiO2 content.
Acknowledgments
The authors would like to acknowledge the EPSRC for providing the funding to conduct this study and Andrew Heart from the Centre for Cell Engineering of the University of Glasgow for help with cell culture experiments. This work was supported in part by WCU Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (no. R31-10069).
References
- 1.Abou Neel EA, Ahmed I, Blaker JJ, et al. Effect of iron on the surface, degradation and ion release properties of phosphate-based glass fibres. Acta Biomaterialia. 2005;1(5):553–563. doi: 10.1016/j.actbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Abou Neel EA, Ahmed I, Pratten J, Nazhat SN, Knowles JC. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials. 2005;26(15):2247–2254. doi: 10.1016/j.biomaterials.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Abou Neel EA, Mizoguchi T, Ito M, Bitar M, Salih V, Knowles JC. In vitro bioactivity and gene expression by cells cultured on titanium dioxide doped phosphate-based glasses. Biomaterials. 2007;28(19):2967–2977. doi: 10.1016/j.biomaterials.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Abou Neel EA, Young AM, Nazhat SN, Knowles JC. A facile synthesis route to prepare microtubes from phosphate glass fibres. Advanced Materials. 2007;19(19):2856–2862. [Google Scholar]
- 5.Valappil SP, Pickup DM, Carroll DL, et al. Effect of silver content on the structure and antibacterial activity of silver-doped phosphate-based glasses. Antimicrobial Agents and Chemotherapy. 2007;51(12):4453–4461. doi: 10.1128/AAC.00605-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah R, Sinanan ACM, Knowles JC, Hunt NP, Lewis MP. Craniofacial muscle engineering using a 3-dimensional phosphate glass fibre construct. Biomaterials. 2005;26(13):1497–1505. doi: 10.1016/j.biomaterials.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 7.Alani A, Knowles JC, Chrzanowski W, Ng Y-L, Gulabivala K. Ion release characteristics, precipitate formation and sealing ability of a phosphate glass-polycaprolactone-based composite for use as a root canal obturation material. Dental Materials. 2009;25(3):400–410. doi: 10.1016/j.dental.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Abou Neel EA, Knowles JC. Phosphate-based glasses for biomedical applications. In: Di Silvio L, editor. Cellular Response to Biomaterials. chapter 7. 2009. p. 177. [Google Scholar]
- 9.Pikos MA. Mandibular block autografts for alveolar ridge augmentation. Atlas of the Oral and Maxillofacial Surgery Clinics of North America. 2005;13(2):91–107. doi: 10.1016/j.cxom.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Nasr HF, Aichelmann-Reidy ME, Yukna RA. Bone and bone substitutes. Periodontology 2000. 1999;19(1):74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 11.Schimming R, Schmelzeisen R. Tissue-engineered bone for maxillary sinus augmentation. Journal of Oral and Maxillofacial Surgery. 2004;62(6):724–729. doi: 10.1016/j.joms.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Salih V, Franks K, James M, Hastings GW, Knowles JC, Olsen I. Development of soluble glasses for biomedical use—part II: the biological response of human osteoblast cell lines to phosphate-based soluble glasses. Journal of Materials Science. 2000;11(10):615–620. doi: 10.1023/a:1008901612674. [DOI] [PubMed] [Google Scholar]
- 13.Abou Neel EA, Chrzanowski W, Valappil SP, et al. Doping of a high calcium oxide metaphosphate glass with titanium dioxide. Journal of Non-Crystalline Solids. 2009;355(16-17):991–1000. [Google Scholar]
- 14. ISO 6872:2008-Dental ceramics.
- 15.Chrzanowski W, Abou Neel EA, Armitage DA, Zhao X, Knowles JC, Salih V. In vitro studies on the influence of surface modification of Ni-Ti alloy on human bone cells. Journal of Biomedical Materials Research. Part A. 2010;93(4):1596–1608. doi: 10.1002/jbm.a.32646. [DOI] [PubMed] [Google Scholar]
- 16.Dalby MJ, Gadegaard N, Tare R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Materials. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 17.Curtis A, Riehle M. Tissue engineering: the biophysical background. Physics in Medicine and Biology. 2001;46(4):R47–465. doi: 10.1088/0031-9155/46/4/201. [DOI] [PubMed] [Google Scholar]
- 18.Dalby MJ. Topographically induced direct cell mechanotransduction. Medical Engineering and Physics. 2005;27(9):730–742. doi: 10.1016/j.medengphy.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Burstein AH, Reilly DT, Martens M. Aging of bone tissue: mechanical properties. Journal of Bone and Joint Surgery. Series A. 1976;58(1):82–86. [PubMed] [Google Scholar]
- 20.Rho J-Y, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Medical Engineering and Physics. 1998;20(2):92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 21.Lopes MA, Knowles JC, Kuru L, Santos JD, Monteiro FJ, Olsen I. Flow cytometry for assessing biocompatibility. Journal of Biomedical Materials Research. 1998;41(4):649–656. doi: 10.1002/(sici)1097-4636(19980915)41:4<649::aid-jbm17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Navarro M, Engel E, Planell JA, Amaral I, Barbosa M, Ginebra MP. Surface characterization and cell response of a PLA/CaP glass biodegradable composite material. Journal of Biomedical Materials Research. Part A. 2008;85(2):477–486. doi: 10.1002/jbm.a.31546. [DOI] [PubMed] [Google Scholar]
- 23.Simon M, Lagneau C, Moreno J, Lissac M, Dalard F, Grosgogeat B. Corrosion resistance and biocompatibility of a new porous surface for titanium implants. European Journal of Oral Sciences. 2005;113(6):537–545. doi: 10.1111/j.1600-0722.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 24.Min K-S, Kwon Y-Y, Lee H-J, et al. Effects of proinflammatory cytokines on the expression of mineralization markers and heme oxygenase-1 in human pulp cells. Journal of Endodontics. 2006;32(1):39–43. doi: 10.1016/j.joen.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Abou Neel EA, Knowles JC. Physical and biocompatibility studies of novel titanium dioxide doped phosphate-based glasses for bone tissue engineering applications. Journal of Materials Science. 2008;19(1):377–386. doi: 10.1007/s10856-007-3079-5. [DOI] [PubMed] [Google Scholar]
- 26.Abou Neel EA, Chrzanowski W, Knowles JC. Effect of increasing titanium dioxide content on bulk and surface properties of phosphate-based glasses. Acta Biomaterialia. 2008;4(3):523–534. doi: 10.1016/j.actbio.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Lopes MA, Monteiro FJ, Santos JD, Serro AP, Saramago B. Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. Journal of Biomedical Materials Research. 1999;45(4):370–375. doi: 10.1002/(sici)1097-4636(19990615)45:4<370::aid-jbm12>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]