Abstract
Epidermodysplasia verruciformis (EV) type human papillomavirus (HPV) DNAs have been detected by PCR in squamous cell carcinomas (SCCs) from both organ transplant recipients (OTR) and immunocompetent (IC) individuals. Their role in the development of skin cancer remains unclear, and previous studies have not addressed whether the viruses are transcriptionally active. Here we have used in situ hybridisation (ISH) to investigate the transcriptional activity and DNA localisation of these viruses. EV-HPV gene transcripts were demonstrated in 4 of 11 (36%) OTR SCC, 1 of 2 (50%) IC SCC and 1 of 5 (20%) OTR warts positive by PCR. Where detected, viral DNAs co-localised with E2/E4 early region gene transcripts in the middle or upper epidermal layers. Non-EV cutaneous HPV gene transcripts were demonstrated in 1 of 5 (20%) OTR SCC and 4 of 10 (40%) OTR warts. Where mixed infection was present by PCR, transcripts for both types were detected in 2 of 6 (33%) cases. Our results provide evidence of EV-HPV gene expression in SCCs. Although only a proportion of tumours were positive, the similarly low transcriptional activity in viral warts suggests that this may be an underestimate. These observations, together with emerging epidemiological and functional data, provide further reason to focus on the contribution of EV-HPV types to the pathogenesis of cutaneous SCC.
Keywords: Human papillomavirus, squamous cell carcinoma, immunosuppression, organ transplantation
Introduction
Human papillomaviruses (HPVs) are highly species-specific DNA tumour viruses whose life cycle is inseparably linked to differentiation processes in pluristratified epithelia (Stanley et al, 1994). These viruses achieve efficient dissemination by infecting cutaneous and mucosal epithelia, stimulating proliferation, and replicating in terminally differentiated keratinocytes to cause benign warts. The association of certain mucosal HPVs, in particular types 16, 18, 31 and 33, with the genesis of anogenital carcinomas is well described, and over 95% of carcinomas of the cervix are positive for HPV DNA (Bosch et al, 2002). Viral genome integration and consequent over-expression of the E6 and E7 oncoproteins is associated with the inhibition of apoptosis via p53 dependent and independent mechanisms (reviewed in Thomas et al, 1999).
An association of cutaneous HPV types 5 and 8 with warty lesions and squamous cell carcinomas (SCC) occurring in the sun exposed skin of patients with the rare inherited condition epidermodysplasia verruciformis (EV) led to proposals that these viruses, designated EV types, may also be oncogenic (Majewski and Jablonska, 1995). EV-HPV gene transcription has been demonstrated in several skin carcinomas from EV patients (Orth, 1987; Yutsudo and Hakura, 1987; Yutsudo et al, 1994) suggesting that the virus is biologically active in these lesions. However, the mechanisms by which EV types might contribute to the development of cutaneous SCC remain unclear. Unlike oncogenic mucosal HPVs, EV-HPV DNAs persist extrachromosomally in cancers, although there have been rare reports of integrated viral DNAs within metastatic EV tumours (Yabe et al, 1989). In addition, EV-associated E6 proteins are unable to abrogate apoptosis via the degradation of p53 (Elbel et al, 1997). Instead, they may inhibit apoptosis by abrogating the function of BAK protein, a Bcl-2 family member expressed in keratinocytes (Jackson et al, 2000).
PCR-based epidemiological studies have identified HPV DNA in over 80% of immunosuppressed and 30% of immunocompetent SCCs; EV-HPV types are consistently over-represented and multiple infections are frequently present in immunosuppressed tumours (Harwood and Proby, 2002; Pfister, 2003). EV-HPVs are frequently found in normal skin, the likely reservoir being hair follicles, probably within the stem cell niche (Boxman et al, 1997; Boxman et al, 2000; Antonsson et al, 2000). The presence of EV-HPV types in normal skin and hair follicles has been linked in separate studies to an increased risk of premalignant actinic keratoses (Boxman et al, 2001) and invasive SCC (Struijk et al, 2003; Harwood et al, 2004). Seroepidemiological studies have also shown an association between prevalence of EV-HPV infection and SCC risk (Bouwes Bavinck et al, 2000; Feltkamp et al, 2003; Masini et al, 2003). These data imply that EV-HPVs may play a role in the pathogenesis of SCC. Despite considerable epidemiological data suggesting a causal association, there are no studies addressing whether EV viruses detected in these circumstances are localised to malignant cells, nor whether they are transcriptionally active, both of which would support a carcinogenic role. In order to examine this, we have used in situ hybridisation (ISH) to establish the pattern of viral gene transcription and DNA localisation in benign and malignant OTR and IC lesions which were positive by PCR for EV and non-EV cutaneous HPV DNAs.
Results
Viral gene transcription and DNA localisation in benign warts
Viral gene expression was first examined in 4 benign cutaneous warts from IC individuals. These lesions were all positive by degenerate nested PCR for non-EV cutaneous HPV type 2. As expected from a previous study of similar lesions (Peh et al, 2002) transcripts of both early and late viral genes were detected (Table 1), thereby confirming the sensitivity of RNA-ISH for detecting HPV2 mRNAs. DNA-ISH demonstrated the presence of detectable HPV2 DNA levels in all 4 samples (Table 1).
Table 1.
HPV Gene Expression in viral warts
| Warta | Patientb | HPV typec | K10 riboprobed | HPV riboprobes testede | Positive riboprobes | DNA probesf |
|---|---|---|---|---|---|---|
| 1 | IC | 2 | + | 2E1, 2 E2, 2E4, 2E6, 2E7, 2L1, 2L2 | All | 2/57 (+) |
| 2 | IC | 2 | + | 2E1, 2 E2, 2E4, 2E6, 2E7, 2L1, 2L2 | All | 2/57 (+) |
| 3 | IC | 2 | + | 2E1, 2 E2, 2E4, 2E6, 2E7, 2L1, 2L2 | All | 2/57 (+) |
| 4 | IC | 2 | + | 2E1, 2 E2, 2E4, 2E6, 2E7, 2L1, 2L2 | All | 2/57 (+) |
| 5 | OTR | 57 | + | 2 E2, 2E4, 2E6, 2E7, 2L1, 2L2 | All | 2/57 (+) |
| 6 | OTR | 3, 37 | + | 3E1, 3E4, 3E7, 3L1 | None | 3/10 (−) |
| 7 | OTR | 10, 23, 57 | + | 2 E2, 2E4, 2L1, 2L2, 23E4, 23L1 | None | 2/57 (+), 3/10 (−) |
| 8 | OTR | 2, 11, 28, 41, RTRx5 |
+ | 2 E2, 2E4, 2L1, 2L2, 6E4, 3E4 | gFaint 2E4, faint 2E2 | 2/57 (+), 3/10 (+), 6/11 (−) |
| 9 | OTR | 7, 20, 27 | + | 20E4 | None | NT |
| 10 | OTR | 11, 20 | + | 20E4, 6E4 | None | 6/11 (−), 20 (−) |
| 11 | OTR | 23, 27 | − | 23E4, 23L1 | None | NT |
| 12 | OTR | 1, 27, 77, 23- related |
+ | 77E7 | None | NT |
| 13 | OTR | 2, 20 | + | 2E4, 2E6, 2L2, 20E4 | None | NT |
| 14 A | OTR | 20, 57 | + | 20E4, 20E7, 2E4, 2L2 | 20E4, 2E4 | 2/57 (+), 20 (−) |
| 14 B | OTR | 3, 57 | + | 3E4, 2E4 | None | 2/57 (+), 3/10 (+) |
| 14 C | OTR | 3, 57 | + | 3E4, 2E2, 2E4, 2L2 | None | 2/57 (+), 3/10 (+) |
| 15 | OTR | 1, 27, 77 | + | 77E7 | None | 2/57 (+) |
| 16 | OTR | 27, 77 | + | 2E4, 77E7 | Faint 77E7 | 2/57 (+) |
| 17 | OTR | 24, 27, 77, RTRx9 |
+ | 77E7 | None | NT |
Sections from three separate areas of wart 14 were examined
IC, immunocompetent; OTR, organ transplant recipient
HPV DNA detected by PCR typing. RTRx5 and RTRx9 sequences have not been fully characterised but belong to the EV-HPV superfamily. In addition one novel HPV sequence with closest homology to EV-HPV type 23 was detected.
Riboprobes to keratin 10 were used to confirm the presence of mRNA. K10-negative samples were excluded from further analysis.
HPV2 riboprobes were used to detect transcripts from types within the A4 subfamily (HPVs 2, 27, 57); HPV3 riboprobes were used to detect transcripts from highly homologous HPV type 28; HPV6 riboprobes were used to detect transcripts from highly homologous HPV type 11
DNA probe mixtures were used as follows: 2/57 to detect types 2, 27, 57; 3/10 to detect types 3, 10, 28; 6/11 to detect types 6 and 11. Results obtained with the probes are listed in parentheses. NT, no DNA probes were tested
Results were described as faint where it could not be excluded that staining was due to high background rather than low levels of specific signal
PCR typing revealed that all 51 OTR warts contained DNAs from fully characterised HPV types; thirteen of these were selected for investigation of viral DNA and transcripts on the basis of the availability of the appropriate HPV clones as well as sufficient lesional tissue. These 13 samples harboured several different cutaneous types from both EV (HPVs 20, 23, 24, 37 together with partially characterised sequences RTRx5 and x9) and non-EV (HPVs 1, 2, 3, 7, 10, 27, 28, 41, 57, 77) subfamilies (Table 1) as well as, less commonly, benign mucosal types (HPVs 6, 11). DNA-ISH demonstrated the presence of detectable levels of viral DNA in 6 of 7 warts (86%) tested with non-EV cutaneous DNA probes (Table 1). However, both samples tested with probes to EV-HPV type 20 proved to be negative, as did 2 samples tested for mucosal types. In all cases viral DNA was localised in the middle or upper epidermis (Figures 1 and 2).
Figure 1.
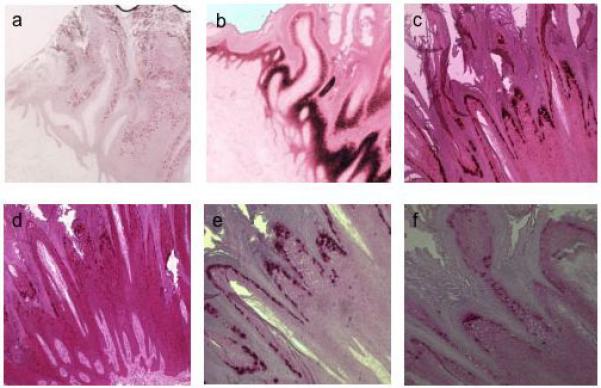
In situ hybridisation of viral wart 5. a, DNA probe for HPV2; antisense riboprobes for: b, keratin 10; c, HPV2 L1; d, HPV2 E4; e, HPV2 L2; f, HPV2 E7.
Figure 2.
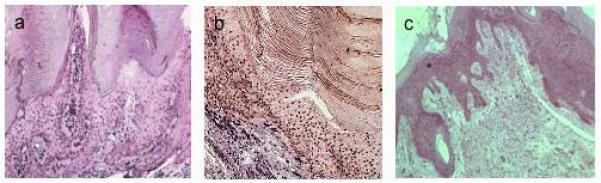
In situ hybridisation of viral wart 8. DNA probes for: a, HPV2; b, HPV3; c, antisense riboprobe for HPV2 E4.
Expression of the positive control keratin 10 mRNA was detected in all lesions with the exception of wart 11, which was excluded from further analysis. Viral gene expression was demonstrated in 1 of 5 samples (20%) tested with EV-HPV antisense riboprobes and 4 of 10 samples (40%) tested with non-EV cutaneous antisense riboprobes, although in 2 of these 4 samples only faint signal was observed. Only 1 of 3 samples tested with both EV and non-EV riboprobes showed evidence of co-expression (HPV types 2 and 20, sample 14A, Table 1). No staining was observed using sense probes to the same transcripts, thereby confirming that the signal represented genuine RNA-RNA hybrids. Transcripts of both early and late viral genes were detected (Table 1, Figure 1). Viral gene expression was greatest in the middle to upper epidermal layers, mirroring the pattern of viral DNA localisation.
In one OTR wart (sample 14, Table 1), 3 separate sections of the lesions were available for analysis. HPV57 was present by PCR in all 3 sections, but sections B and C additionally harboured HPV3 whereas HPV20 was detected in section A. Viral gene expression was detectable only in section A, consistent with spatial variation in both HPV types present and their expression within a single lesion. Multiple sections of other warts and SCCs were not examined in this manner, but it is plausible that such variation may also have been present.
Viral gene transcription and DNA localisation in SCC
HPV DNAs were identified by PCR in 63 of 84 (75%) OTR SCCs and 6 of 17 (35%) IC SCCs; of these, 51 SCCs in total contained DNAs from fully characterised HPV types. From these samples 3 IC and 15 OTR lesions were selected for inclusion in the study on the basis of the availability of the appropriate HPV clones as well as sufficient lesional tissue. No other specific selection criteria were used. The majority of samples (16 of 18, 89%) harboured EV-HPV DNAs (types 5, 8, 14, 15, 19, 20, 21, 23 together with the partially characterised sequences Z959969 and PSOX-1 and two novel EV-HPV sequences), although only one sample (SCC 4; Figure 3) demonstrated features suggestive of a possible viral cytopathic effect (Majewski and Jablonska, 1995). The remainder contained only cutaneous non-EV types (HPVs 2, 3, 10, 27, 77). Seven OTR SCCs were co-infected with both EV and non-EV types. Three samples failed to demonstrate mRNA transcripts of the positive control keratin 10 and were excluded from further analysis. Of the remaining samples, viral gene expression was demonstrated in 1 of 2 (50%) IC and 4 of 11 (36%) OTR samples tested with EV-HPV antisense riboprobes and 1 of 5 (20%) OTR samples tested with non-EV antisense riboprobes. Only 1 of the 3 samples tested with both EV and non-EV riboprobes showed evidence of co-expression (HPV types 3/10 and 15, SCC18, Table 2 and Figure 4). Furthermore, in this sample only one of the two HPV types, HPV15, demonstrated strong signal, although the formation of mismatched hybrids between the HPV3 riboprobe and HPV10 mRNA means that the level of HPV10 gene expression is likely to have been underestimated.
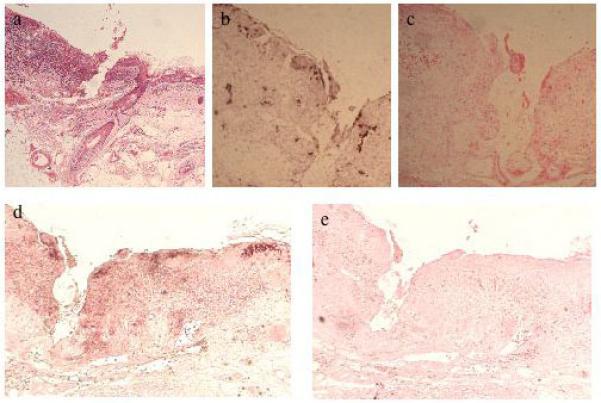
Figure 3.
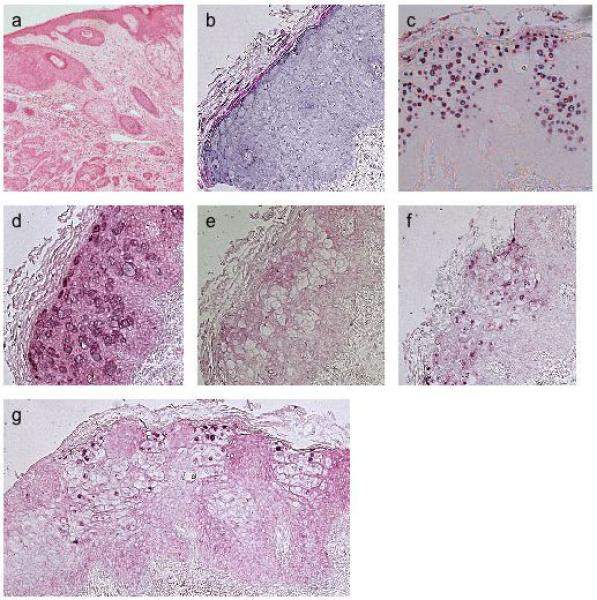
In situ hybridisation of SCC4. H&E staining of sections: a, at low magnification showing carcinoma in situ and invasive tumour; b, at high magnification showing an in situ portion of this SCC which was contiguous with the invasive component shown in (a). Some of the cells have enlarged and pale-staining cytoplasm which might be keeping with a viral cytopathic effect; ISH shows the carcinoma in situ component using: c, DNA probe for HPV20; antisense (d) and sense (e) riboprobes for HPV20 E4; antisense riboprobes for f, HPV20 E7; g, HPV20 E6.
Table 2.
HPV Gene Expression in SCC
| SCC | Patienta | HPV typeb | K 10 riboprobec | HPV riboprobes testedd | Positive riboprobes | DNA probes e |
|---|---|---|---|---|---|---|
| 1 | IC | 10 | − | 3E4 | None | NT |
| 2 | IC | 5, 20, 12- related |
+ | 5E4, 5E6, 5E7, 5L1, 20E4, 20E6, 20E7, 20L1 |
5E4, 20E4 | 5(−), 20(+) |
| 3 | IC | 5, 21 | + | 5E4, 5E7, 5L1 | None | 5(−) |
| 4 | OTR | 3, 20, 23 | + | 20E4, 20E6, 20E7, 20L1, 3E1, 3E4, 3E7, 3L1, 23E1, 23E4, 23E7, 23L1 |
20E4, 20E6, 20E7 f | 20 (+), 3/10 (−) |
| 5 | OTR | 5 | + | 5E4 | None | 5 (−) |
| 6 | OTR | 5, 27, 77 | − | 77E7, 5E4 | None | NT |
| 7 | OTR | 14, 77 | − | 77E7 | None | NT |
| 8 | OTR | 10, 23 | + | 23E1, 23E4, 23E7, 23L1 | None | 3/10 (−) |
| 9 | OTR | 19, 20 | + | 20E4, 20E6, 20E7, 20L1, 5E4, 2E4, 3E4 g | 20E4 | 20(−) |
| 10 | OTR | 14, Z95969 | + | 14E4, 14E7, 14L1 | None | 14(−) |
| 11 | OTR | 2, 3 | + | 2E4, 2E7, 3E4, 3E7 | None | NT |
| 12 | OTR | 20 | + | 20E4, 20E6, 20E7, 20L1 | None | 20(−) |
| 13 | OTR | 3, 14 | + | 3E4, 3E7, 14E4, 14E7 | None | NT |
| 14 | OTR | 3, Z95969 | + | 3E4, 3E7 | None | 3/10 (−) |
| 15 | OTR | 5, 36-related | + | 5E4, 5E6, 5E7, 5L1 | 5E4 | 5(+) |
| 16 | OTR | 8, 20 | + | 20E4, 20E6, 20E7, 20L1 | None | 20(−) |
| 17 | OTR | 5, PSOX1 | + | 5E4, 5E6, 5E7, 5L1 | None | 5(−) |
| 18 | OTR | 10, 15 | + | 3E4, 3E7, 15E4, 15E7, 15L1 | hFaint 3E4, 15E4 | 3/10 (−), 15(−) |
IC, immunocompetent; OTR, organ transplant recipient
HPV DNAs detected by PCR typing. Z95969 and PSOX1 sequences have not been fully characterised but belong to the EV-HPV superfamily. In addition two novel HPV sequences with closest homology to EV-HPV types 12 and 36 were detected.
Probes to keratin 10 were used to confirm the presence of mRNA. K10-negative samples were excluded from further analysis.
HPV3 probes were used to detect transcripts from highly homologous HPV type 10
Results obtained with the listed DNA probes are in parentheses. NT, no DNA probes were tested
Strong staining was obtained in the carcinoma in situ component but not invasive region of the lesion
Sample 9 was examined with probes to HPV types 2, 3 and 5 to examine cross-reactivity between EV-HPV types 5 and 20 and between HPV5 and non-EV cutaneous types 2 and 3
Results were described as faint where it could not be excluded that staining was due to high background rather than low levels of specific signal
Figure 4.
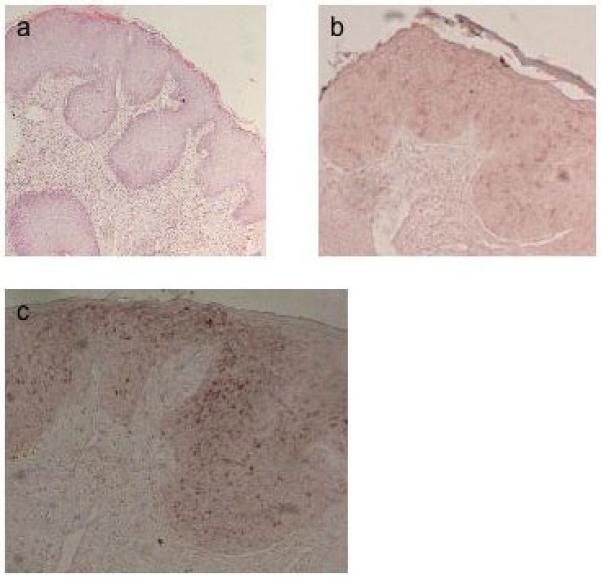
In situ hybridisation of SCC18. a, H&E staining of section; antisense riboprobes for b, HPV3 E4; c, HPV15 E4.
The specificity of all positive results was confirmed by hybridisation with the corresponding sense probes on paired sections, which in all cases failed to produce a signal. As an additional control for specificity, as well as to confirm the results obtained in SCC2, where transcripts homologous to both HPV5 and HPV20 E4 riboprobes were detected (Figure 5), the HPV20-positive SCC9 sample was examined with additional riboprobes to the E4 region of HPV types 2, 3 and 5. As expected, only the 20E4 probe yielded a positive signal, thereby demonstrating that the stringency of the hybridisation protocol did not permit cross-reactivity between EV types 5 and 20 which share 65% DNA sequence homology over the region targeted by the probes (GenBank database).
Figure 5.

In situ hybridisation of SCC2. a, H&E staining of section; b, antisense riboprobe for HPV20 E4; antisense (c) and sense (d) riboprobes for HPV5 E4
Irrespective of HPV type, the highest levels of viral transcripts were detected in the mid to upper epidermal layers, with those homologous to the E4 riboprobe present in the greatest abundance. These transcripts were designated E2/E4 since it could not be excluded that some of these transcripts might encode the overlapping E2 gene product rather than E4. In 4 of the 5 positive SCCs, transcripts were detected throughout the lesion with only moderate variation in the level of gene expression in different areas. In SCC4, however, considerable variability in gene expression was observed and only the carcinoma in situ component demonstrated abundant viral mRNAs (Figure 3). Viral DNAs were detected by DNA-ISH in 2 of 9 (22%) OTR and 1 of 2 (50%) IC SCCs tested with EV-HPV probes (Table 2). In these positive samples, HPV DNA was localised in the mid to upper epidermal layers concomitant with E2/E4 gene transcripts (Figures 3, 6). All of the 4 samples hybridised with the mixed probe to non-EV cutaneous types 3 and 10 were negative.
Figure 6.
In situ hybridisation of SCC15. a, H&E staining of section; DNA probes for: b, HPV5, c, negative control; antisense (d) and sense (e) riboprobes for HPV5 E4.
Discussion
This is the first report of ISH used to localise EV-HPV DNA in warts and SCCs of both OTR and IC patients. We have demonstrated gene transcripts of both EV and non-EV HPVs in a proportion of lesions. EV-HPV transcripts were detected in 4 of 11 (36%) SCCs and 1 of 5 (20%) OTR warts. The apparently low transcriptional activity in warts is likely to reflect the sensitivity of the technique, which suggests that the presence of transcripts in fewer than half of the OTR-associated SCCs may similarly be an underestimate. Taken together, these data suggest that EV-HPV DNA is present and transcriptionally active in some SCCs outside the context of epidermodysplasia verruciformis.
Sensitivity/methodological limitations of ISH techniques
DNA- and RNA-ISH techniques were initially optimised in IC HPV2-positive warts. Viral DNA localised to lesional keratinocytes and all 4 samples were positive with the seven HPV2 riboprobes tested (L1, L2, E1, E2, E4, E6, E7), demonstrating, as would be expected in warts, a high level of viral activity. DNA-ISH was positive for the majority of OTR warts (6 of 8, 75%) with transcription demonstrated in only 4 of 12 (33%) lesions. Neither the EV- nor the mucosal HPV-harbouring warts tested were positive by DNA-ISH for those types, while EV-HPV transcription was demonstrated in 20% (1 of 5). Overall, therefore, DNA-ISH was positive in 83% (10/12) and RNA-ISH in 50% (8/16) of warts. EV-HPV gene transcripts were demonstrated in 5 of 13 (38%) SCCs, but in the majority only E2/E4 mRNAs were detectable.
Apparent discrepancies between DNA and RNA ISH data may be explained in several ways: (i) higher viral gene expression in IC compared with OTR lesions, or higher viral gene expression in HPV2-associated lesions compared with those infected with other types; (ii) varying sensitivities of the techniques used for detection of the different HPVs e.g. if RNA:RNA hybrids formed between HPV2 riboprobes and transcripts have higher melting temperatures than hybrids formed by EV HPVs and by other non-EV types, transcripts would be more easily detected in HPV2-positive lesions than others. Certainly, HPV2 riboprobes may prove relatively insensitive at detecting HPV2-related types 27 and 57 due to formation of mismatched RNA:RNA hybrids with reduced melting temperatures; (iii) lower EV viral copy number compared with non-EV copy number (and/or increased sensitivity of the EV-PCR detection methodology), such that the levels of viral mRNAs other than E2/E4, if present, were below the detection threshold of this technique; (iv) relative selectivity of transcripts sought in OTR compared with IC lesions owing to a high frequency of mixed infection, particularly with partially characterised and novel EV types for which no probes are available; (v) regional variation in levels of gene transcription within lesions (as in wart 14), or a combination of these factors.
The apparently lower DNA and RNA ISH expression in SCCs compared with rates of HPV DNA PCR positivity may also be partly explained by recent data suggesting that such HPV positivity in tumour biopsies may reflect surface contamination. Forslund and colleagues found that only 6 of 18 (33%) of PCR positive SCCs remained positive after tape-stripping of the lesional surface (Forslund et al, 2004), a rate remarkably similar to our observation that HPV transcripts were detectable in only 38% of HPV positive SCCs. Our DNA and RNA ISH data may therefore be a more accurate reflection of true HPV positivity in these tumours and a means of identifying those tumours in which PCR positivity is more likely to be biologically relevant.
Patterns of gene expression in benign and malignant lesions
Although EV-HPV mRNAs were detected in fewer warts than SCCs (20% versus 38% respectively), we observed a comparable pattern of viral transcription in benign and malignant lesions. Overall, EV-HPV transcripts were detected more frequently in SCCs than warts whereas the incidence of non-EV cutaneous HPV transcripts was higher in warts. Indeed, the data obtained in the two types of lesion were approximately inverted: 38% of SCC (4 of 11 OTR and 1 of 2 IC samples) displayed EV and 20% (1 of 5 OTR SCC) non-EV transcripts compared with 20% (1 of 5 OTR warts) and 40% (4 of 10 OTR warts) respectively of warts.
A relatively high frequency of lesions harboured mixed infection by PCR: 9 of 13 (69%) OTR warts, 7 of 15 (47%) OTR SCC but none of 3 IC SCC were positive for both EV and non-EV HPV DNAs, in line with our previous findings (Harwood et al, 1999; Harwood et al, 2000). Despite this, only 1 of 3 of warts and 1 of 3 of SCC tested displayed transcripts from both EV and non-EV types, and in the case of the SCC sample strong signal was observed for the EV type alone.
Our transcriptional data does not prove that HPV replication is occurring in SCCs, although it does provide some supportive evidence for this possibility. In this respect, our findings of abundant E4 expression are relevant. E4 is now thought to encode a late protein whose expression occurs concomitantly with viral DNA replication (Rogel-Gaillard et al, 1992; Doorbar et al, 1997). In agreement with this, we found co-localisation of E2/E4 mRNAs with viral DNA in the 3 SCC samples positive for EV DNA-ISH (Figure 3, 6).
Implications of these data for the role of HPV in cutaneous carcinogenesis
Recent functional studies from our laboratory and others have shown that E6 and E7 proteins of NMSC-associated HPV types display in vitro transforming activity (Jackson et al, 2000; Jackson et al, 2002; Caldeira et al, 2003; Giampieri and Storey, 2004; Giampieri et al, 2004). Together with recent epidemiological data (Bouwes Bavinck et al, 2000; Feltkamp et al, 2003; Masini et al, 2003; Struijk et al, 2003; Harwood et al, 2004), these studies support a role for EV-HPVs in skin carcinogenesis. However, until now it has not been clear whether the identification of HPV DNAs in these lesions represents active infection or merely reflects the ability of sensitive PCR techniques to detect low copy number latent infection.
In our series, EV-HPV E2/E4 transcripts were detected in 1 of 2 (50%) IC and 4 of 11 (36%) OTR SCCs, suggesting that the presence of HPV represents active rather than latent or ‘passenger’ status. E6 and E7 transcripts were found in only 1 OTR SCC, whereas in anogenital cancer continued expression of E6 and E7 is required for maintenance of the malignant phenotype (Bosch et al, 2002). However, there is no current evidence that the same is true in HPV-positive SCCs. The apparent absence of E6 and E7 transcripts in these lesions could alternatively reflect the sensitivity of our ISH technique as E6 and E7 mRNAs may prove difficult to detect even in anogenital tumours (Bohm et al, 1993). Finally, some authors have proposed a ‘hit-and-run’ mechanism of action for HPV in skin carcinogenesis in which HPV is required for initiation but not progression of tumorigenesis, such that persistent viral presence and expression of E6 and E7 would not be required (reviewed in Pfister 2003).
In summary, this study has provided evidence of localisation of HPV DNA to malignant keratinocytes in SCCs as well as EV-HPV gene transcription in almost 40% of tumours, although methodological limitations of the techniques used suggest that this may be an underestimate. Taken together with recent epidemiological and functional findings, these data provide further reason to focus on EV-HPVs in the pathogenesis of cutaneous SCC.
Material and Methods
HPV typing
DNA was extracted from wart and SCC biopsies using QIAamp DNA Mini kits (QIAGEN, Crawley, U.K) and HPV typing undertaken using a panel of seven nested primers and one single round primer pair as previously described (Harwood et al, 1999). In total 55 (4 IC, 51 OTR) wart biopsies and 101 SCCs (17 IC, 84 OTR) were HPV typed. Only samples containing HPV DNAs from one or more fully characterised types where cloned HPVs were available were selected (Tables 1-2). Ethical approval for this investigation was obtained from East London and City Health Authority local ethics committee and the study was conducted according to the Declaration of Helsinki Principles.
Riboprobe constructs and labelling of probes
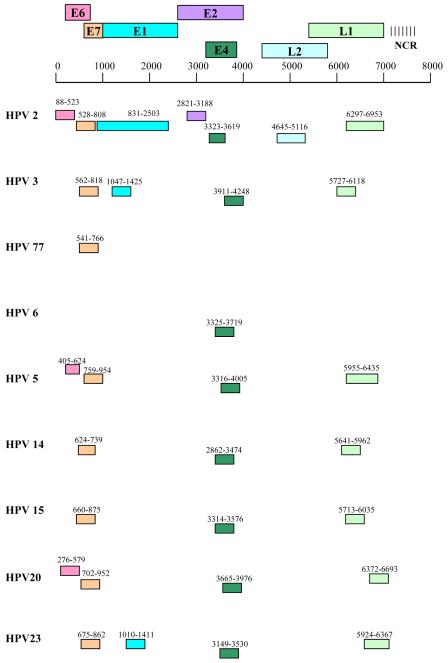
A non-radioactive RNA-ISH protocol was carried out to study HPV gene expression. A human cytokeratin 10 riboprobe construct (kind gift from Dr P. Bowden, UWCM, Wales) was used as a positive control for the presence of genomic mRNA. HPV riboprobe constructs were made by cloning DNA sequences from the relevant viral genes into the polylinker site of pGEM 3Z or 4Z vectors in the appropriate orientation to obtain sense and antisense transcripts from the SP6 and T7 promoters respectively. Riboprobe constructs were restricted to the most frequently detected HPV types, not including partially-characterised or novel HPV sequences. For HPV77 a single E7 riboprobe construct was made as only a partial genomic clone was available. The location of the riboprobes of various types is shown in Figure 7. Digoxigenin-labelled riboprobes were generated by the in vitro transcription of linearised template DNA in the presence of digoxigenin-UTP using a DIG RNA labelling kit (Roche, Mannheim, Germany). A series of 10-fold dilutions of each riboprobe was spotted onto nitrocellulose membrane and labelling efficiency determined by immunological detection using a DIG nucleic acid detection kit (Roche).
Figure 7.
Schematic representation of the location of subgenomic HPV riboprobes in the HPV genome. The start and end nucleotide positions are shown for the various HPV types.
RNA-ISH
ISH was performed on paraffin-embedded tissue sections of 5 microns. Sections were dewaxed in xylene, rehydrated through an alcohol series, fixed in 4% paraformaldehyde and partially digested with 25 mg/ml Proteinase K for 10 minutes at 37°C. Sections were next re-fixed in 4% paraformaldehyde, treated with 0.25% acetic anhydride in 100mM triethanolamine for 10 minutes at ambient temperature and dehydrated through an alcohol series prior to incubation with 1 μl of appropriate riboprobe in 10 μl hybridisation buffer (10 x SSC, 500mM Tris pH7.6, 50mM Na2HPO4, 50mM NaH2PO4, 0.1% Ficoll, 0.1% polyvinyl pyrrolidone) at 37°C overnight. Sections were then washed at low stringency (2 x SSC / 0.1% SDS) at 55°C for 10 minutes, treated with 2 μg/ml RNAse in 5 x SSC at 37°C for 10 minutes to remove unbound probe and washed at high stringency (0.1 x SSC / 0.04 % SDS) at 65°C for 2 × 20 minutes. Subsequently, sections were incubated with anti-digoxigenin alkaline phosphatase followed by the colour change reagent 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium salt (both from Roche) according to manufacturer’s instructions. Sections were counter-stained with aqueous eosin, mounted in Faramount aqueous mounting medium (Dako Ltd., Ely, U.K.) and results visualized by light microscopy. In developing the ISH protocol outlined above, several variables including proteinase K digestion conditions, post-hybridisation washes and methodology associated with visualisation of bound probe were optimised on IC wart samples to obtain the best signal to background ratio.
Since constructs were not available for all HPV types, HPV2 riboprobes were used to detect transcripts from related types 27 and 57 which share greater than 80% DNA sequence homology across the regions targeted by the probes (GenBank database, National Centre for Biotechnology Information, National Institutes of Health, Bethesda MD). Similarly, HPV3 riboprobes were used to detect transcripts from the closely related HPVs 10 and 28 (approximately 77% DNA sequence homology; GenBank database) and HPV6 riboprobes used to detect HPV11 transcripts (greater than 80% homology over the targeted region of the E4 gene; GenBank database). Whenever possible samples were tested with a complete panel of the relevant riboprobes but in cases of limited tissue availability a subset, which always included the E4 riboprobe, was used. This was due to the observation that E4 transcripts were consistently present in greatest abundance, irrespective of HPV type. In order to exclude non-specific binding, all sections were tested with both sense and antisense probes to each viral gene of interest.
DNA probes and ISH
DNA probes were made by the restriction enzyme digestion of plasmids containing the complete genomes of HPV types 2, 3, 5 6, 10, 11, 20 or 57. Linear HPV DNA was separated from plasmid DNA by agarose gel electrophoresis, purified using the QIAquick gel extraction kit (QIAGEN) and labelled using a DIG-DNA labelling kit (Roche) according to the manufacturer’s instructions. Linearised plasmid DNA was labelled to make negative control probes. Labelling efficiency was determined as described for riboprobes. DNA-ISH was performed on paraffin-embedded sections as described for RNA-ISH with a few modifications. Specifically, after rehydration through an alcohol series, sections were incubated in 0.02M HCl for 10 minutes, followed by treatment with 3% H2O2 in PBS for 10 minutes and 0.01% Triton X-100 in PBS for 3 minutes instead of fixation with 4% paraformaldehyde. Proteinase K digestion was performed for 10 minutes using 500 μg/ml enzyme followed by two 5 minute washes in PBS containing 2 mg/ml glycine. Sections were then treated in 20% acetic acid on ice for 15 seconds, fixed in 4% paraformaldehyde and dehydrated through an alcohol series as for RNA-ISH. Hybridisation mixtures for each section comprising 1 μl probe and 9 μl hybridisation buffer were boiled for 1 minute to denature probes prior to addition to sections. Sections were then incubated at 80°C for 10 minutes prior to overnight incubation at 30°C. Post-hybridisation washes were performed for 10 minutes each in: 2 x SSC at 50°C, 0.2 x SSC at ambient temperature and at 42°C and 0.1 x SSC at ambient temperature. Signal was amplified using the TSA Plus DNP-AP system (Perkin Elmer, Boston, MA) according to the manufacturer’s instructions. Sections were incubated with colour change reagent and visualised as for RNA-ISH. Since constructs were not available for all HPV types, DNA probe mixtures were used as followed: HPV2/57 to detect closely related type 27; 3/10 to detect closely related type 28. As for RNA-ISH, the above protocol was developed by optimising conditions using IC wart samples.
Acknowledgements
We are grateful to E-M de Villiers for providing plasmid clones for HPV types 2, 3, 5, 6, 11, 57 and 77 and to G. Orth for providing plasmid clones for HPVs 10, 14, 15, 20 and 23. We are also indebted to Dr Rino Cerio for dermatopathological advice. TS was supported by a Joint Research Board grant. LB was supported by the British Skin Foundation. KJP, CAH and CMP are supported by Cancer Research-UK.
Abbreviations
- EV
epidermodysplasia verruciformis
- HPV
human papillomavirus
- IC
immunocompetent
- ISH
in situ hybridisation
- OTR
organ transplant recipient
- PCR
polymerase chain reaction
- SCC
squamous cell carcinoma
References
- Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. 2000;74:11636–11641. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm S, Wilczynski SP, Pfister H, Iftner T. The predominant mRNA class in HPV16-infected genital neoplasias does not encode the E6 or the E7 protein. Int J Cancer. 1993;55:791–798. doi: 10.1002/ijc.2910550517. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Lorincz A, Munoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Path. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Stark S, Petridis AK, et al. The presence of antibodies against virus-like particles of epidermodysplasia verruciformis-associated human papillomavirus type 8 in patients with actinic keratoses. Br J Dermatol. 2000;142:103–109. doi: 10.1046/j.1365-2133.2000.03248.x. [DOI] [PubMed] [Google Scholar]
- Boxman IL, Berkhout RJ, Mulder LH, Wolkers MC, Bouwes Bavinck JN, Vermeer BJ, ter Schegget J. Detection of EV-HPV DNA in plucked hairs from renal transplant recipients and healthy volunteers. J Invest Dermatol. 1997;108:712–715. doi: 10.1111/1523-1747.ep12292090. [DOI] [PubMed] [Google Scholar]
- Boxman IL, Russell A, Mulder LH, Bouwes Bavinck JN, ter Schegget J, Green A. Case-control study in a subtropical Australian population to assess the relation between non-melanoma skin cancer and epidermodysplasia verruciformis human papillomavirus DNA in plucked eyebrow hairs. Int J Cancer. 2000;86:118–121. doi: 10.1002/(sici)1097-0215(20000401)86:1<118::aid-ijc18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Boxman IL, Russell A, Mulder LH, Bouwes Bavinck JN, ter Schegget J, Green A. Association between epidermodysplasia verruciformis-associated human papillomavirus DNA in plucked eyebrow hair and solar keratoses. J Invest Dermatol. 2001;117:1108–1112. doi: 10.1046/j.1523-1747.2001.01499.x. [DOI] [PubMed] [Google Scholar]
- Caldeira S, Zehbe I, Accardi R, et al. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol. 2003;77:2195–2206. doi: 10.1128/JVI.77.3.2195-2206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J, Foo C, Coleman N, et al. Characterisation of events during the late stage of HPV 16 infection in vivo using high affinity fabs to E4. Virology. 1997;238:40–52. doi: 10.1006/viro.1997.8768. [DOI] [PubMed] [Google Scholar]
- Elbel M, Carl S, Spaderna S, Iftner T. A comparative analysis of the interactions of the E6 proteins from cutaneous and genital papillomaviruses with p53 and E6AP in correlation to their transforming potential. Virology. 1997;239:1322–1349. doi: 10.1006/viro.1997.8860. [DOI] [PubMed] [Google Scholar]
- Feltkamp MCW, Broer R, di Summa FM, et al. Seroreactivity to epidermodysplasia verruciformis-related human papillomavirus types is associated with nonmelanoma skin cancer. Cancer Research. 2003;63:2695–2700. [PubMed] [Google Scholar]
- Forslund O, Lindelof B, Hradil E, et al. High prevalence of cutaneous human papillomavirus DNA on the top of skin tumours but not in “stripped” biopsies from the same tumours. J Invest Dermatol. 2004;123:388–394. doi: 10.1111/j.0022-202X.2004.23205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri S, Garcia-Escudero R, Green J, Storey A. Human papillomavirus type 77 E6 protein selectively inhibits p53-dependent transcription of proapoptotic genes following UV-B irradiation. Oncogene. 2004;23:5864–5870. doi: 10.1038/sj.onc.1207711. [DOI] [PubMed] [Google Scholar]
- Giampieri S, Storey A. Repair of UV-induced thymine dimers is compromised in cells expressing the E6 protein from human papillomaviruses types 5 and 18. Br J Cancer. 2004;90:2203–2209. doi: 10.1038/sj.bjc.6601829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood CA, Proby CM. Human papillomaviruses and non-melanoma skin cancer. Curr Opin Infect Dis. 2002;15:101–114. doi: 10.1097/00001432-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Harwood CA, Spink PJ, Surentheran T, et al. Degenerate and nested PCR: a highly sensitive and specific method for detection of human papillomavirus infection in cutaneous warts. J Clin Microbiol. 1999;37:3545–3555. doi: 10.1128/jcm.37.11.3545-3555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby CM. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–297. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Harwood CA, Surentheran T, Sasieni P, et al. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br J Derm. 2004;150:949–957. doi: 10.1111/j.1365-2133.2004.05847.x. [DOI] [PubMed] [Google Scholar]
- Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065–3073. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Ghali L, Harwood C, Storey A. Reduced apoptotic levels in squamous but not basal cell carcinomas correlates with detection of cutaneous human papillomavirus. Br J Cancer. 2002;87:319–323. doi: 10.1038/sj.bjc.6600431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S, Jablonska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312–1318. [PubMed] [Google Scholar]
- Masini C, Fuchs PG, Gabrielli F, et al. Evidence for association of human papillomavirus infection and cutaneous squamous cell carcinoma in immunocompetent individuals. Arch Dermatol. 2003;139:890–4. doi: 10.1001/archderm.139.7.890. [DOI] [PubMed] [Google Scholar]
- Orth G. Epidermodysplasia verruciformis. In: Salzman NP, Howley PM, editors. The Papovaviridae. Vol. 2. The Papillomaviruses. Plenum; New York, NY: 1987. pp. 199–243. [Google Scholar]
- Peh W, Middleton K, Christensen N, et al. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J Virol. 2002;76:10401–10416. doi: 10.1128/JVI.76.20.10401-10416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. Human papillomavirus and skin cancer. J Natl Cancer Inst Monogr. 2003;31:52–6. doi: 10.1093/oxfordjournals.jncimonographs.a003483. [DOI] [PubMed] [Google Scholar]
- Rogel-Gaillard C, Breitburd F, Orth G. Human papillomavirus type 1 E4 proteins differing by their N-terminal ends have distinct cellular locations when transiently expressed in vitro. J Virol. 1992;66:816–823. doi: 10.1128/jvi.66.2.816-823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M, Coleman N, Chambers M. The host response to lesions induced by human papillomavirus. Ciba Found Symp. 1994;187:21–32. [PubMed] [Google Scholar]
- Struijk L, Bouwes Bavinck JN, Wanningen P, van der Meijden E, Westendorp RG, ter Schegget J, Feltkamp MC. Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J Invest Dermatol. 2003;121:1531–1535. doi: 10.1046/j.1523-1747.2003.12632.x. [DOI] [PubMed] [Google Scholar]
- Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- Yabe Y, Tanimura Y, Sakai A, Hitsumoto T, Nohara N. Molecular characteristics and physical state of human papillomavirus DNA change with progressing malignancy: studies in a patient with epidermodysplasia verruciformis. Int J Cancer. 1989;43:1022–1028. doi: 10.1002/ijc.2910430611. [DOI] [PubMed] [Google Scholar]
- Yutsudo M, Hakura A. Human papillomavirus type 17 transcripts expressed in skin carcinoma tissue of a patient with epidermodysplasia verruciformis. Int J Cancer. 1987;39:586–589. doi: 10.1002/ijc.2910390507. [DOI] [PubMed] [Google Scholar]
- Yutsudo M, Tanigaki T, Kanda R, et al. Involvement of human papillomavirus type 20 in epidermodysplasia verruciformis skin carcinogenesis. J Clin Microbiol. 1994;32:1076–1078. doi: 10.1128/jcm.32.4.1076-1078.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]