Abstract
Positive modulation of a patient’s immune system to produce antitumor immunity is an attractive strategy that may improve the dismal outcomes typically associated with non-small-cell lung cancer (NSCLC). Using methods that either augment specific antitumor immunity or positively influence the patient’s immune system to allow the de novo generation of immunity to encompass current strategies used in recent clinical trials of NSCLC. Encouraging results of Phase II trials in antigen-specific immunotherapy have led to three subsequent Phase III trials, which are currently enrolling. Results of these trials will improve our understanding of the role that immunotherapy plays in the treatment of NSCLC. Successful application of a humoral vaccine in Cuba led to its approval for the treatment of advanced NSCLC patients in that country. To date, trials involving nonspecific immunotherapeutic interventions have failed to improve outcomes in NSCLC and may indicate a need to combine them with antigen-specific vaccines. Although these trials will greatly advance our knowledge of NSCLC immunotherapy, we believe truly efficacious immunotherapy may only result from implementation of strategies to both augment antitumor immunity and counteract tumor-mediated immunosuppression.
Keywords: allogenic tumor cell vaccines, CpG oligonucleotides, EGF, immunotherapy, lung cancer vaccine, MAGE-A3, MUC1, ONTAK
Non-small-cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality in the USA and, despite recent advances in therapy, most patients only have palliative therapeutic options as they typically present with late-stage disease [101]. Development of strategies to induce antitumor immunity represents an active area of research for the development of novel immunotherapeutic modalities for NSCLC. To date, clinical trials of lung cancer immunotherapy have, unfortunately, had limited success. Recent US FDA approval of immunotherapy for the treatment of prostate cancer [1] and reports on the beneficial effects of a human papilloma virus vaccine for the treatment of vulvar intraepithelial neoplasia [2] provide the encouraging proof-of-concept needed to continue development of immunotherapeutic strategies for NSCLC.
This article discusses two types of NSCLC immunotherapy clinical trial, which can be divided into separate categories based on whether the experimental modality aims to create specific antitumor immunity or positively influence the immune system to allow de novo generation of antitumor immunity. Antigen-specific immunotherapy uses vaccines to induce specific antitumor immunity against relevant tumor-associated antigens incorporated within the vaccine formulation. Immune-system modulators include immunologically active agents that influence the patient’s immune system to allow recognition of the tumor as foreign and create immunity de novo to eradicate the cancer. Encouraging results of early studies have led to multiple Phase III trials, completed or currently enrolling, to test new immunotherapeutic approaches for the treatment of NSCLC.
Antigen-specific immunotherapy
Antigen-specific immunotherapy vaccines within this category incorporate relevant antigens to induce antitumor immunity to specific proteins co-expressed by cancer cells. Most of the targeted antigens are self proteins with the potential to cause autoimmunity; however, this harmful prospect has only been seen in select melanoma trials using peptide-based vaccines combined with the antagonistic anti-cytotoxic T-lymphocyte antigen (CTLA)-4 antibody [3,4]. Interestingly, the combination of the anti-CTLA-4 antibody with antigen-specific immunotherapy in melanoma did not worsen autoimmunity or improve the clinical effect. This suggests that the application of the anti-CTLA-4 antibody nonspecifically emancipates all self-reactive cytotoxic T lymphocytes, inducing the widespread autoimmunity seen in those trials. This is a fundamentally different scenario from antigen-specific immunotherapy where the induced immunity will be confined to tumor-associated antigens and may explain why autoimmunity has not been seen in these trials. In addition, it invokes the theory that immunogenic tumors, such as melanomas, will more likely benefit from the removal of cancer-induced suppression of pre-existing antitumor immunity, whereas less immunogenic tumors, such as NSCLC, will require active immunization in order to produce an effective immune response [5].
The limitation of this finite repertoire of antigen specificity is the possibility that the tumor cells may evade the immune system if they lose or mutate the targeted proteins. This may be obviated if such an intense immune reaction is created that induces diversification of immunity through the production of de novo immune responses to antigens released from the destruction of the tumor cells in a manner known as epitope spreading [6]. With the success of antigen-specific immunotherapy in mouse models of cancer, it is not surprising that, to date, some of the most promising results are found using this strategy. The Stimulating Targeted Antigenic Responses in Phase II clinical trials demonstrating benign safety profiles have been translated into three enrolling Phase III clinical trials and one approved therapy in Cuba [7–10].
MAGE-A3 as Adjuvant Non-Small-Cell Lung Cancer Immunotherapy trial
The MAGE-A3 as Adjuvant Non-Small-Cell Lung Cancer Immunotherapy (MAGRIT) trial (NCT00480025) is an enrolling Phase III study of early-stage NSCLC patients, who are to be treated with a vaccine targeting the MAGE-A3 protein [102], which is overexpressed on approximately half of NSCLC tumors [11]. Dependent on stage, these patients have a 21–53% relapse rate despite attempting curative resection [12,13], indicating the presence of postoperative micrometastases, necessitating the use of adjuvant chemotherapy to improve outcomes [14]. Unfortunately, current adjuvant strategies confer a success rate of less than 10%, leaving a high risk of relapse in this population. As the strength of the immune system lies in its specificity and surveillance properties, it appears ideally suited for eradicating residual disease. Therefore, enhancement of the antitumor immunity in this setting seems optimal as it enjoys the advantage of low tumor burden and, generally, an advantageous patient performance status given the early stage of disease. Encouraging results demonstrating an improved disease-free interval in the Phase II trial and experience with a similar vaccine in melanoma [15] resulted in the ongoing Phase III trial.
In the Phase II trial of NSCLC [8], 182 patients with MAGE-A3-positive stage Ib and II NSCLC after curative resection were randomized in a 2:1 vaccine versus placebo schedule, using a similar vaccine that was previously shown to induce humoral and cellular immune responses in melanoma patients [15]. The vaccine consists of the full-length MAGE-A3 protein complexed to a fragment of the Haemophilus influenzae protein D and administered with the adjuvants saponin and monophosphoryl lipid A [16]. Patients were administered with the vaccine or placebo intramuscularly every 3 weeks for five doses and then every 3 months for 2 years. Patients in the treatment arm had a hazard ratio for disease-free interval of 0.73 (p = 0.11) in favor of vaccination, and the vaccine was well tolerated with predominantly grade I and II constitutional symptoms and injection-site reactions. These results prompted the initiation of the Phase III clinical trial in early-stage NSCLC patients, which was started in 2007.
With a targeted enrollment of approximately 2300 individuals, inclusion criteria was expanded to include stage IIIA patients in addition to stage IB and II patients with tumors expressing MAGE-A3. The vaccine’s adjuvant was augmented through the addition of the Toll-like receptor (TLR)9 agonist, CpG 7909 [17], which is known to enhance immunity in human trials of hepatitis B virus vaccination [18]. The stratification approach initially separates patients using four cycles of a platinum-based chemotherapy before randomizing these patients into receiving 13 intramuscular injections of the placebo or MAGE-A3 vaccine over 27 months. Disease-free survival is the primary end point, with secondary end points including overall survival, lung cancer-specific survival, seropositivity of MAGE-A3 and the H. influenzae protein D, and safety parameters. This trial has a projected primary completion date of October 2015.
Advantages of this study include the use of a known tumor antigen with strong immunologic data [15] that, given its identity as a cancer testes antigen, should have limited potential for autoimmunity [19]. The early stage of the patients in this trial is worth noting owing to the greatly reduced tumor burden after surgery, and expected superior performance status and immune status compared with patients with advanced disease. Given these factors, this trial may have the greatest potential to demonstrate a positive impact on survival, especially when considering that the immune system’s role in surveillance is one of its strengths. In addition, the use of the stratification scheme to separate patients with and without adjuvant chemotherapy should both reduce confounding variables and possibly provide insight into the role chemotherapy may play on the health of the immune system as it pertains to vaccines.
Stimulating Targeted Antigenic Responses To NSCLC trial
The Stimulating Targeted Antigenic Responses To NSCLC (START) trial (NCT00409188) is a randomized, placebo-controlled trial of unresectable stage III NSCLC patients with a targeted enrollment of 1300 patients who have completed at least two cycles of chemotherapy [103]. The L-BLP25 vaccine used in these studies incorporates known immunogenic sequences [20] from the tumor antigen, MUC1, which has a wealth of data to support its role as a tumor antigen [21]. The current clinical trial was initiated after subset analysis of stage IIIB patients from the Phase II trial revealed a promising trend towards increased median survival in vaccinated patients who completed chemotherapy and radiotherapy, and had a high chance of relapse.
The Phase II trial enrolled 171 randomized patients to vaccine versus best supportive care, where patients in the experimental arm were immunized with the L-BLP25 vaccine [8]. The vaccine consists of the core lipopeptides from MUC1 with the approved human adjuvant, monophosphoryl lipid A, which has immunology and safety data in human studies [22]. After 3 days of low-dose cyclophosphamide administration with proven positive immunomodulatory effects on suppressive regulatory T cells [23], patients received eight weekly vaccinations followed by booster immunizations every 6 weeks. When including all participants, patients in the vaccinated arm had a median survival of 17.4 months compared with 13.0 months in the best supportive care group (p = 0.11). Subset analysis of patients with stage IIIB disease revealed an improvement in the median survival to 30.6 versus 13.3 months (p = 0.07), respectively. Adverse events attributable to the vaccine arm included nausea to cyclophosphamide, and grade I skin reactions in half of the patients and one grade II skin reaction. The benign safety profile of the vaccine and strong trend towards improved survival prompted the resultant Phase III trial.
In a placebo-controlled trial involving patients with unresectable stage III NSCLC, patients were randomized in a 2:1 fashion to receive the L-BLP25 vaccine versus placebo. Patients in the vaccination arm receive low-dose cyclophosphamide 3 days prior to the initial vaccine followed by seven weekly injections of the vaccine. The study’s primary outcome is overall survival with secondary end points being time to symptom progression, time to progression, 1-, 2- and 3-year survival and safety. There is an estimated primary completion date of December 2010.
This trial is important for the use of an immunogenic fragment of the tumor antigen, MUC1, with a wealth of data to support it as a tumor antigen in both mouse and previous human experiments [21]. Perhaps the most interesting intervention in this trial is the use of cyclophosphamide pretreatment to attempt to reduce the presence and function of regulatory T cells that appear to correlate with lung cancer and disease progression [24]. Results of this trial should help delineate if single-antigen vaccination with inclusion of an intervention to decrease cancer-induced immunosuppression alters survival in the advanced-stage disease population.
Survival; Tumor-free, Overall; & Progression-free trial
The Survival; Tumor-free, Overall; and Progression-free (STOP) trial (NCT00676507) incorporates the vaccine belagenpumatucel-L, which consists of four human NSCLC cell lines, each transfected with the antisense gene for TGF-β2, which decreases the expression of this immunosuppressive cytokine [104]. Use of the four NSCLC cell lines obviates the need to a priori identify tumor antigens and, thus, the necessity to include multiple antigens to predictably cover all NSCLC patients. Use of allogenic cell lines in this vaccine followed very promising results of the first allogenic whole-tumor cell vaccine for lung cancer, where a B7-transfected lung adenocarcinoma cell line vaccine induced clinical benefit in 33% of patients with metastatic NSCLC [25]. Using the belagenpumatucel-L vaccine in a Phase II trial, prolonged 1- and 2-year survival correlated with vaccine dose and provided encouraging data to support the ongoing Phase III trial.
In the dose-escalation Phase II trial, stage II, III and IV NSCLC patients were randomized into three cohorts to receive one of three concentrations of vaccine every 1–2 months for a total of 16 immunizations [9]. Although the median survival of 440 days is difficult to interpret given the inclusion of patients at a wide range of stages, there was a statistically significant (p = 0.007) improvement in 1- and 2-year survival, respectively, when patients receiving the lowest dose (39 and 20%) were compared with those receiving either of the two highest dosages (68 and 52%). Comparison of the immunologic data between progressive versus stable or better disease revealed increased IFN-γ reactivity of the patients’ T cells after vaccination in patients with positive clinical responses. Although the use of the vaccine as the stimulating agent makes it impossible to determine if the resultant immune response is to the tumor-associated antigens or the allotypic differences between the vaccine’s lung cancer cell lines and the patient, the data are encouraging and are possibly reflective of the vaccine’s mechanism of action. Safety data reveals this to be a well-tolerated vaccine with only two grade III adverse events consisting of edema at the injection site and one patient who developed chronic myelogenous leukemia, which was thoroughly vetted as not related to the study vaccine. The benign safety profile of this treatment and improved survival rates of the patients receiving the higher dosages of the vaccine initiated the Phase III trial using the middle dose of the vaccine.
For the Phase III trial, inclusion criteria allow only stage IIIA (T3N2 only), IIIB and IV patients to participate in a placebo-controlled, randomized trial, in which patients receive 18 monthly immunizations followed by two booster injections in 3-month intervals. Prior to enrollment, patients must have responded to or have stable disease following chemotherapy with a platinum-based regimen. The primary end point is overall survival with secondary end points of progression-free survival, quality of life, time to progression, best overall tumor response, response duration, incidence of CNS metastasis and safety. The estimated enrollment is 700 patients and the estimated study completion date is October 2011.
The STOP trial is unique in that it incorporates a strategy to counteract the immuno-regulatory effects of tumor-derived TGF-β2 on the induction of antitumor immunity. Using the four tumor cells as a vaccine allows the inclusion of a multitude of relevant tumor antigens without the need to identify them a priori and, thus, may be applicable to all patients suffering with NSCLC. Evaluation of microarray data of lung cancer reveals that the majority of the genes are co-expressed amongst different tumor classifications with only a limited set of genes capable of delineating differing subclasses of NSCLC [26,27]. As a class, genetically altered whole-tumor cell vaccines have enjoyed early success with promising data from trials involving the B7-transfected NSCLC tumor cell vaccine, GVAX®, and an ongoing trial at our institution incorporating a NSCLC cell line transfected with a fusion protein of gp96 and a modified immunoglobulin protein to induce secretion of tumor antigens bound to gp96 for presentation on dendritic cells [25,28,29]. An important distinction separating the STOP trial from the GVAX trials is the use of allogeneic tumor cell lines as a vaccine to augment reactive immunity through mismatched MHC molecules akin to the aforementioned B7 and ongoing gp96 vaccine trials. Therefore, the use of the allogenic NSCLC tumor cell lines in the STOP trial may provide a strong vaccine with the greatest applicability to the breadth of patients with NSCLC.
These three ongoing Phase III clinical trials should begin to determine the impact of immunotherapy on survival in NSCLC patients. The studies collectively encompass both early-stage disease for the treatment of micrometastases and late-stage disease where only palliative care options exist. Of particular note, the inclusion of vaccines consisting of tumor antigens of lipopolypeptides (START), full-length proteins (MAGRIT) or tumor cell lines (STOP) may also allow comparison of the efficacy that different antigen formulations have on vaccine potency. Results from these trials will improve our knowledge concerning the use of vaccines for the treatment of NSCLC and, hopefully, have a positive impact on the overall survival of these patients.
Cuban humoral EGF vaccine
EGF plays a central role in many NSCLC patients’ tumors [30] and its receptor is the target of approved biological therapy, including erlotinib [31] and gefitinib [32]. Use of a vaccine incorporating the EGF protein with the intent of creating a humoral response to antagonize the EGF–EGF receptor axis is the basis of several studies completed in Cuba p. Results of these trials led to the vaccine’s approval by the Cuban government for the treatment of advanced-stage lung cancer.
In stage IIIB/IV NSCLC, three trials [10,33,34] evaluated the effect a vaccine consisting of human EGF had on patients’ overall survival. The vaccine consisted of the full-length human EGF protein complexed to the Neisseria meningitides P64K protein for added immunogenicity. The three trials differ only by the adjuvant used to emulsify the EGF protein, with one using alum, one using Montanide ISA 51, and one using Montanide ISA 51 and the use of a single dose of cyclophosphamide prior to vaccination to positively condition the patient’s immune system [23]. Patients were immunized with four weekly injections and an additional inoculation on day 51 followed by booster vaccinations if titers decreased.
The results demonstrated that 83% of patients doubled their anti-EGF titers and showed that use of the adjuvant Montanide ISA 51 induced a higher percentage seroconversion and anti-EGF titers than patients vaccinated using alum as the adjuvant. Use of cyclophosphamide pretreatment further increased the maximal titers achieved in these patients when compared with patients who received Montanide ISA 51 or alum adjuvants. Evaluating the combined survival data of all three trials, patients with seroconversion had an 11.1- versus 5.7-month survival (p = 0.005) and, importantly, those patients who achieved titers higher than the median had a greater survival advantage than those who achieved titers below the median (12.2 vs 8.1 months, respectively; p = 0.036). Unfortunately, these titers were transient, with levels beginning to decrease 10 weeks after the final vaccination, but were generally recoverable with booster vaccinations. These vaccines were well tolerated by all patients with only grade I or II adverse events confined to injection-site reactions, constitutional symptoms, tremors, nausea and hot flashes that abated with appropriate intervention. The significant improvement in survival led to the vaccine’s approval for treatment of NSCLC patients in Cuba.
A follow-up study of 20 advanced-stage NSCLC patients reported an important finding concerning the role that chemotherapy has on the efficacy of the vaccine [35]; an intervention that many immunotherapists fear may decrease a vaccine’s potency through the indiscriminant destruction of all proliferating cells, including reactive immune cells. In this study, two doses of the EGF vaccine in Montanide ISA 51 with cyclophosphamide pretreatment were delivered prior to standard chemotherapy and three additional doses were given starting 1 month after vaccination. This vaccine was, again, well tolerated, with vaccine-related adverse events confined to grade II or less. Vaccination induced high titers of anti-EGF antibodies and, importantly, did not decrease with the application of chemotherapy. This study demonstrated a decrease in circulating EGF concentrations with increased anti-EGF titers and almost half of the induced antibodies specifically blocked the interaction of EGF with its receptor. Of the 20 patients, two achieved a complete response and five achieved a partial response based on Response Evaluation Criteria In Solid Tumors (RECIST) criteria with a median survival consistent with the other three trials of 12.8 months. Analysis of mean survival found that subjects who achieved antibody levels greater than the mean titer for all patients enjoyed a 25.6 month survival compared to 10.5 months for those whose titers fell below the mean. Thus, the strength of the induced immunity correlates with the outcome.
Phase I/II trials
Numerous Phase I and II trials have been completed to date (Table 1), evaluating various forms of antigen-specific immunotherapy for NSCLC. They include vaccines of multiple forms that encompass the breadth of vaccine technologies available to researchers, including peptide-, protein-, dendritic cell-, tumor cell-, viral vector- and DNA-based vaccines. Most report clinical responses in vaccinated patients; however, the studies are predominantly designed to evaluate safety parameters and lack control groups, which makes attributing these effects to the vaccine subjective. Importantly, many vaccines use antigens considered to be self proteins and, in doing so, raise questions of potential autoimmunity. The strength of this data, regardless of clinical effect, is the benign safety profile associated with these vaccines. Although it could be argued that the lack of autoimmune toxicity is reflective of the lack of antitumor clinical effect, the lack of serious adverse events in patients treated with the recently approved prostate cancer vaccine, sipuleucel-T [1] and reports of tumor antigen-reactive immune responses in cancer patients’ peripheral blood [36] indicates that efficacy may not need to suffer at the expense of autoimmunity. In effect, these trials have demonstrated vaccine therapy to be safe and well tolerated with a continued need to develop more potent methods of augmenting antitumor immunity to improve the vaccine’s efficacy and affect outcomes in NSCLC.
Table 1.
Phase I and II clinical trials of immunotherapy for non-small-cell lung cancer.
| Author | Vaccine | n | Stage | Clinical response | Immunologic response | Safety data | Ref. |
|---|---|---|---|---|---|---|---|
| Barve et al. | Nine class I peptides (from CEA, p53, HER-2/neu, MAGE-2 and -3) with PADRE | 63 | IIIB–IV or recurrent | OS: 17.3 months; 1-year survival: 60% | CTL responses | One grade III fever/chills | [51] |
| Ramlau et al. | Modified Ankara virus containing MUC-1 and IL-2 | 65 | IIIB–IV | Median OS: 12.7 months | CTL responses | Grade III/IV possible/probably related: anemia/rash | [52] |
| Bolonaki et al. | Optimized class I hTERT peptide p572Y and native peptide p572 | 22 | III–IV | SD achieved in 36.4% | CTL responses | One grade II nausea | [53] |
| Hirschowitz et al. | Immature DCs pulsed with apoptotic bodies from NSCLC cell line | 14 | I–IIIB | NED in 64% at 12 months | CTL responses | Mild injection site reactions | [54] |
| Meyer et al. | Seven ras peptides encompassing predicted mutations of codon 12 | 4 | I or IV | No relapse after 1 year in three patients with early-stage disease | DTH responses | One subject with PE | [55] |
| Brunsvig et al. | hTERT class I peptide p611 and class II peptide p540 with GM-CSF | 26 | IIB–IV | SD achieved in 28% | T-cell proliferation | Mild flu-like symptoms, fever and chills | [56] |
| Nemunaitis et al. | L523S gene immunized in a plasmid followed by a viral vector | 13 | IB–IIB | Median OS: 290 days | Humoral responses | Two grade II: neck pain and arm pruritis | [57] |
| Nemunaitis et al. | Autologous tumor cells with K562 cells transfected with GM-CSF | 86 | III–IV | Median OS: 7.0 (5.4)† months; 1-year survival: 31% (22%)† | DTH/humoral responses | Grade III or IV, possibly related: dyspnea, PE, tachycardia, back pain, chest pain | [58] |
| Morse et al. | Dexosomes pulsed with MAGE-A3, -A4, -A10 and -3DPO4 peptides | 12 | IIIB–IV | Survival range: 52–502 days at 2-year follow-up | DTH responses | Grade II or less: injection site reactions, flu-like symptoms | [59] |
| Tsuboi et al. | Class I WT1 peptide p235 | 2 | IV | Decreased tumor markers, one PR | CTL/DTH responses | Mild injection site reactions | [60] |
| Mine et al. | SART-1, -2 and -3, CypB, Lck, and ART-1 and -4 peptides | 10‡ | IV or recurrent | 1-year survival: 60% | CTL/DTH responses | Three grade II injection site reactions, one grade II colitis, one grade II dyspnea | [61] |
| Raez et al.§ | B7.1 and HLA A1- or A2-transfected allogenic NSCLC cell line | 15 | IIIB–IV | Median OS: 18 months; SD in 27% | CTL responses | Mild injection site reactions | [25] |
Intention-to-treat numbers are in parentheses.
Includes one patient with small-cell lung cancer.
Phase II trial currently enrolling.
CTL: Cytotoxic T lymphocyte; DC: Dendritic cell; DTH: Delayed-type hypersensitivity; GM-CSF: Granulocyte macrophage colony stimulating factor; NED: No evidence of disease; NSCLC: Non-small-cell lung cancer; OS: Overall survival; PE: Pulmonary embolism; PR: Partial response; SD: Stable disease.
Immune system modulators
The theory that the immune system eradicates a burgeoning tumor cell until the cancer evades it implicates the eventual quiescence of the immune system’s reaction to the tumor cells. Multiple lines of evidence point to the active and continual participation of the tumor in suppressing antitumor immunity to allow its survival despite the presence of immune effector cells that are capable of reacting to it [36]. Thus, the lack of effective tumor immunity may lie in the functionality of the cells and not the specificity. Use of agents to nonspecifically stimulate the immune system or remove the tumor-induced immunosuppression may rekindle effective antitumor immunity and produce a change in clinical outcomes. Two compounds have been employed in advanced clinical trials to attempt to revitalize antitumor immunity.
CpG oligonucleotides
Unmethylated, juxtaposed cytosine and guanine residues are found in much lower frequencies in eukaryotic than prokaryotic DNA [37], and form the pathogen-associated molecular pattern that binds TLR9 and acts as a ‘danger signal’ to the innate immune system [38]. Interaction of these CpG oligonucleotides (CpG ODN) with TLR9 induces the elaboration of type I interferons, maturation of dendritic cells and, when used in mouse models of cancer, beneficial effects at preventing the outgrowth of tumor cells [39].
Early clinical trials in malignant melanoma [40], metastatic renal cell carcinoma [41] and cutaneous T-cell lymphoma [42] revealed modest antitumor effects of a specific CpG ODN, CpG 7909, when used as a single-agent modality to treat each disease. Animal data suggested that CpG ODNs may perform better when used in combination with conventional therapeutic modalities including chemotherapy [43]. Theoretically, the cellular destruction that accompanies the use of chemotherapy may provide tumor antigens to dendritic cells within the draining lymph nodes, which are conditioned by TLR9 agonists to produce effective anticancer immunity.
To date, the most advanced clinical trial involving CpG ODNs resulted from a Phase II clinical trial in NSCLC, in which 112 patients with stage IIIB and IV disease underwent randomization to four to six cycles of taxane/platinum chemotherapy with subcutaneous injections of the CpG ODN versus chemotherapy or CpG 7909 alone [44]. The CpG ODNs were given subcutaneously 7 and 14 days after the initial chemotherapy infusion of each cycle. In the experimental arms, patients reported mild injection-site reactions with serious adverse side effects including thrombocytopenia, neutropenia and anemia. Over 57% of patients in the experimental arm suffered from grade IV neutropenia but infections and febrile neutropenia were reportedly minor and were not specifically associated with CpG 7909 administration.
Using a modified intention-to-treat analysis with objective response rate as the primary end point, the investigator-assessed objective response rate was 30% in the experimental arm and 19% in the chemotherapy-alone arm (p = 0.043). A blinded, independent review of the radiographic evidence available for 90 patients decreased these numbers to 19 and 11%, respectively (p = 0.32). Survival data revealed an improvement in median survival from 6.8 to 12.3 months with the inclusion of the CpG 7909; however, the overall survival hazard ratio of 0.75 had a non-significant p-value of 0.19. The improved median survival prompted the initiation of the Phase III trial despite the toxicity profile.
The trial utilized a similar cohort of patients with stage IIIB and IV NSCLC with good performance statuses and who were chemotherapy naive. A total of 839 patients were randomized to receive up to six cycles of CpG 7909, gemcitabine and cisplatin versus chemotherapy alone in a Phase III trial [45]. CpG 7909 was administered subcutaneously after infusion of both chemotherapy agents as part of the standard chemotherapy cycle. The primary end point of this study was overall survival with objective response rate, safety profile and progression-free survival listed as secondary end points.
Interim analysis by an external data safety monitoring board led to the early termination of the study caused by significant serious adverse side effects in the experimental arm and no change in overall survival, with a hazard ratio of 0.98. Compared with the control arm, the experimental arm suffered increased rates of thrombocytopenia (61 vs 33%), neutropenia (69 vs 47%) and need for transfusion (19 vs 10%). Final analysis is still pending the completion of data collection for all patients involved in the study; however, this study strongly suggests that CpG 7909 as an adjuvant to chemotherapy was not effective in treating advanced-stage NSCLC.
ONTAK®
ONTAK (denileukin diftitox) is a fusion protein of diptheria toxin fragments A and B with IL-2, designed to deliver the cytocidal bacterial proteins to cells expressing the IL-2 receptor including regulatory T cells that express the high-affinity IL-2 receptor, CD25 [46]. In mouse studies, ONTAK has demonstrated enhanced T-cell-specific antitumor immunity via the reduction in regulatory T cells [47], and has promising efficacy in patients with cutaneous T-cell lymphoma and Sézary syndrome [48].
Given these favorable results, a Phase II trial enrolled stage IIIB/IV patients with NSCLC whose disease progressed despite chemotherapy [49]. Although 32% of the enrolled patients were unable to complete at least two cycles, of the remaining patients 44% achieved stable disease and 24% exhibited progressive disease. The median survival of 5.8 months (0.3–11.3 months) is difficult to interpret in lieu of a control group; however, this trial had increased toxicity ascribed to the drug. One patient’s death was attributed to myocarditis, possibly associated with ONTAK and 19 others had grade III and IV adverse events with gastrointestinal toxicity, constitutional syndromes and vascular-leak syndrome. The minor clinical response and toxicity associated with ONTAK revealed its limited usefulness in the treatment of patients with NSCLC.
The results of these two trials of nonspecific immunotherapy were not promising as none of the drugs demonstrated any clinical improvement and both drugs showed significant toxicity. Although the theory for use is valid, the application of this class of immunotherapy has yet to demonstrate its potential in NSCLC. These results should not be interpreted as the failure of the class but as a failure of the individual agents as singular modalities or adjuvants to chemotherapy. Nonexistent or weak nascent immune responses in NSCLC patients [50] make it unlikely that nonspecific stimulation of the immune system or removal of immunosuppression will create changes in clinical outcomes. We believe work in this area will, ultimately, prove quite useful when promising antigen-specific vaccines are combined with agents that positively modulate the immune system of cancer patients.
Conclusion
With the recent approval of the prostate cancer vaccine, Sipuleucel-T, immunotherapy for NSCLC becomes more conceivable, and it is encouraging that three large Phase III trials are currently enrolling to determine the role it may play in the treatment of this otherwise predominantly fatal disease. The approval of the EGF vaccine in Cuba provides additional hope for patients that improvement in the longstanding and unvarying 5-year mortality rate for advanced-stage NSCLC will be forthcoming. In the next 5 years, the results from the Phase III clinical trials, in addition to a multitude of earlier phase trials, will continue to advance this field as the hope for a cure continues.
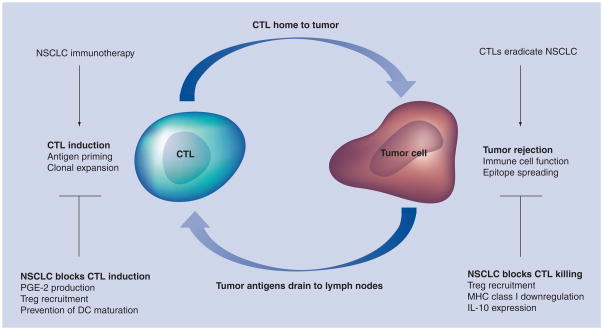
It is interesting to note that one hypothesis for the tumorigenesis of NSCLC involves the appropriation of an immunosuppressive micro-environment at the site of the individual’s cancer. To date, most studies in NSCLC immunotherapy have focused on the induction of potent antitumor immunity in patients without addressing the immunosuppressive environment at the targeted tumor. Thus, the finding of tumor-reactive T cells in the peripheral blood of vaccinated patients without an objective clinical response may reflect this limitation in immunization strategy. The use of ONTAK focused on the reversal of the immunosuppressive nature of the tumor state without dealing with the need for specific augmentation of antitumor immunity and may also have failed because it did not address both issues. It is our contention that successful immunotherapy will only occur when both the lack of an effective antitumor immunity and the immunosuppressive nature of cancer are addressed in a rational vaccine strategy (Figure 1). With advances in both improved vaccine efficacy and delineation of the mechanisms used by tumors to antagonize the immune system, we can design the next generation of vaccines to encompass both strategies.
Figure 1. Schematic demonstrating the methods used by non-small-cell lung cancer to counteract tumor immunity through both the blockade of cytotoxic T-lymphocyte killing of tumors and inhibition of cytotoxic T-lymphocyte priming.
CTL: Cytotoxic T-lymphocyte; DC: Dentritic cell; NSCLC: Non-small-cell lung cancer; PGE: Prostaglandin E; Treg: Regulatory T cell.
Future perspective
Compared with chemotherapy, NSCLC immunotherapy is a newer concept that suffers from a lack of long-term clinical experience. As many of the vaccines target self proteins, concerns of autoimmunity have defined and limited evaluation, despite the fact that curative therapy does not exist for the majority of NSCLC patients. As the literature in this field matures, fears of autoimmunity have been unfounded and recent Phase II trials in NSCLC have begun to show clinical promise. In addition to earlier phase trials evaluating multiple aspects of NSCLC immunotherapy, results of the three ongoing Phase III trials should allow proper evaluation of vaccines for NSCLC and advance the science.
As NSCLC is a complicated disease that restricts both the induction of immune responses and the ability of reactive immune cells to affect tumor cells, successful immunotherapy will not be a simple endeavor. The complexity of the pathways that led to the development of a clinically relevant tumor will demand strategies to target the multiple facets of NSCLC to be completely efficacious. As these barriers continue to be identified and new innovations aid in the development of newer therapeutic modalities, we can design the next generation of vaccines to address the need to augment antitumor immunity, overcome tumor-mediated immunosuppression and surmount tumor-mediated barriers to effector cell function.
Early trials necessarily needed to test individual agents in Phase I safety trials and, in addition to subjective clinical responses, were proven to have benign safety profiles. Recent trials are beginning to combine modalities to address both the need to augment antitumor immunity and counteract tumor-mediated immunosuppression. As additional agents are developed and tested, future immunotherapy trials will need to combine more powerful interventions to target all aspects of NSCLC immunology and hopefully, one day, be part of the cure.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
ER Podack has financial interest in cell-based lung cancer vaccines. LE Raez receives research support from Merk–Serono. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, Phase 3 trials of active cellular immunotherapy with Sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. First US FDA-approved immunotherapy for the treatment of prostate cancer. [DOI] [PubMed] [Google Scholar]
- 2.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 3.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber TH, Raez L, Rosenblatt JD, Podack ER. Tumor immunogenicity and responsiveness to cancer vaccine therapy: the state of the art. Semin Immunol. 2010;22(3):105–112. doi: 10.1016/j.smim.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Vansteenkiste J, Zielinski M, Linder A, et al. Final results of a multi-center, double-blind, randomized, placebo-controlled Phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(18S):7554. Phase II trial of the MAGE-A3 vaccine in non-small-cell lung cancer (NSCLC) showing trends towards improved disease-free interval that led to the MAGE-A3 as Adjuvant NSCLC Immunotherapy (MAGRIT) trial. [Google Scholar]
- 8▪▪.Butts C, Murray N, Maksymiuk A, et al. Randomized Phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23(27):6674–6681. doi: 10.1200/JCO.2005.13.011. Phase II trial in NSCLC using the BLP25 vaccine, which demonstrates a trend towards improved median survival in NSCLC patients and led to the Stimulating Targeted Antigenic Responses To NSCLC (START) trial. [DOI] [PubMed] [Google Scholar]
- 9▪▪.Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor β-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24(29):4721–4730. doi: 10.1200/JCO.2005.05.5335. Phase II trial using allogenic NSCLC cell lines transfected with antisense TGF-β2; prolonged survival rates were observed in cohorts receiving the higher doses of vaccine, which led to the Survival; Tumor-free, Overall; and Progression-free (STOP) trial. [DOI] [PubMed] [Google Scholar]
- 10▪▪.Gonzalez G, Crombet T, Neninger E, Viada C, Lage A. Therapeutic vaccination with epidermal growth factor (EGF) in advanced lung cancer: analysis of pooled data from three clinical trials. Hum Vaccin. 2007;3(1):8–13. doi: 10.4161/hv.3.1.3537. Compilation of the three Cuban trials using the humoral EGF vaccine, which led to the approval of this vaccine for advanced NSCLC patients in Cuba. [DOI] [PubMed] [Google Scholar]
- 11.Gure AO, Chua R, Williamson B, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11(22):8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 12.Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83(2):409–417. doi: 10.1016/j.athoracsur.2006.08.046. discussion 17–18. [DOI] [PubMed] [Google Scholar]
- 13.Varlotto JM, Recht A, Flickinger JC, Medford-Davis LN, Dyer AM, Decamp MM. Factors associated with local and distant recurrence and survival in patients with resected nonsmall cell lung cancer. Cancer. 2009;115(5):1059–1069. doi: 10.1002/cncr.24133. [DOI] [PubMed] [Google Scholar]
- 14.Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5(2):220–228. doi: 10.1097/JTO.0b013e3181c814e7. [DOI] [PubMed] [Google Scholar]
- 15.Kruit WH, Suciu S, Dreno B, et al. Immunization with recombinant MAGE-A3 protein combined with adjuvant systems AS15 or AS02B in patients with unresectable and progressive metastatic cutaneous melanoma: a randomized open-label Phase II study of the EORTC Melanoma Group (16032–18031) J Clin Oncol. 2008;26(Suppl 15):9065. [Google Scholar]
- 16.Atanackovic D, Altorki NK, Stockert E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172(5):3289–3296. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 17.Murad YM, Clay TM, Lyerly HK, Morse MA. CPG-7909 (PF-3512676, ProMune): Toll-like receptor-9 agonist in cancer therapy. Expert Opin Biol Ther. 2007;7(8):1257–1266. doi: 10.1517/14712598.7.8.1257. [DOI] [PubMed] [Google Scholar]
- 18.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind Phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 19.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100(11):2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer M, Parker J, Modi S, et al. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer. 2001;3(1):49–57. doi: 10.3816/clc.2001.n.018. discussion 8. [DOI] [PubMed] [Google Scholar]
- 21.Beatson RE, Taylor-Papadimitriou J, Burchell JM. MUC1 immunotherapy. Immunotherapy. 2010;2(3):305–327. doi: 10.2217/imt.10.17. [DOI] [PubMed] [Google Scholar]
- 22.Cluff CW. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Adv Exp Med Biol. 2009;667:111–123. doi: 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]
- 23.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 24.Ju S, Qiu H, Zhou X, et al. CD13+CD4+CD25hi regulatory T cells exhibit higher suppressive function and increase with tumor stage in non-small cell lung cancer patients. Cell Cycle. 2009;8(16):2578–2585. doi: 10.4161/cc.8.16.9302. [DOI] [PubMed] [Google Scholar]
- 25.Raez LE, Cassileth PA, Schlesselman JJ, et al. Allogeneic vaccination with a B7.1 HLA-A gene-modified adenocarcinoma cell line in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22(14):2800–2807. doi: 10.1200/JCO.2004.10.197. [DOI] [PubMed] [Google Scholar]
- 26.Hayes DN, Monti S, Parmigiani G, et al. Gene expression profiling reveals reproducible human lung adenocarcinoma subtypes in multiple independent patient cohorts. J Clin Oncol. 2006;24(31):5079–5090. doi: 10.1200/JCO.2005.05.1748. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharjee A, Richards WG, Staunton J, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA. 2001;98(24):13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemunaitis J, Sterman D, Jablons D, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004;96(4):326–331. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber TH, Deyev VV, Rosenblatt JD, Podack ER. Tumor-induced suppression of CTL expansion and subjugation by gp96–Ig vaccination. Cancer Res. 2009;69(5):2026–2033. doi: 10.1158/0008-5472.CAN-08-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi K, Ito F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010;277(2):316–326. doi: 10.1111/j.1742-4658.2009.07450.x. [DOI] [PubMed] [Google Scholar]
- 31.Iyer R, Bharthuar A. A review of erlotinib – an oral, selective epidermal growth factor receptor tyrosine kinase inhibitor. Expert Opin Pharmacother. 2010;11(2):311–320. doi: 10.1517/14656560903551283. [DOI] [PubMed] [Google Scholar]
- 32.Reck M. Gefitinib in the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009;9(4):401–412. doi: 10.1586/era.09.1. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez G, Crombet T, Torres F, et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14(3):461–466. doi: 10.1093/annonc/mdg102. [DOI] [PubMed] [Google Scholar]
- 34.Crombet T, Torres O, Neninger E, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor. Cancer Biother Radiopharm. 2001;16(1):93–102. doi: 10.1089/108497801750096122. [DOI] [PubMed] [Google Scholar]
- 35.Neninger E, Verdecia BG, Crombet T, et al. Combining an EGF-based cancer vaccine with chemotherapy in advanced nonsmall cell lung cancer. J Immunother. 2009;32(1):92–99. doi: 10.1097/CJI.0b013e31818fe167. [DOI] [PubMed] [Google Scholar]
- 36.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 38.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60(7):795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Murad YM, Clay TM. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs. 2009;23(6):361–375. doi: 10.2165/11316930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Pashenkov M, Goess G, Wagner C, et al. Phase II trial of a Toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24(36):5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 41.Thompson JA, Kuzel T, Bukowski R, Masciari F, Schmalbach T. Phase Ib trial of a targeted TLR9 CpG immunomodulator (CpG 7909) in advanced renal cell carcinoma (RCC) J Clin Oncol. 2004;22(Suppl 14):4644. [Google Scholar]
- 42.Kim Y, Giardi M, Duvic M, et al. TLR9 agonist immunomodulator treatment of cutaneous T-cell lymphoma (CTCL) with CPG7909. Presented at: 46th Annual Meeting of the American Society of Hematology; CA, USA. 4–7 December 2004; (Abstract 743) [Google Scholar]
- 43.Vicari AP, Luu R, Zhang N, et al. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58(4):615–628. doi: 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manegold C, Gravenor D, Woytowitz D, et al. Randomized Phase II trial of a Toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(24):3979–3986. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Readett D, Denis L, Krieg A, Benner R, Hanson D. PF-3512676 (CPG 7909) a Toll-like receptor 9 agonist – status of development for non-small cell lung cancer (NSCLC). Presented at: 12th World Congress on Lung Cancer; Seoul, Korea. 2–6 September 2007; Results of the Phase III CpG 7909 trial in NSCLC with equivocal clinical results but significant adverse effects. [Google Scholar]
- 46.Manoukian G, Hagemeister F. Denileukin diftitox: a novel immunotoxin. Expert Opin Biol Ther. 2009;9(11):1445–1451. doi: 10.1517/14712590903348135. [DOI] [PubMed] [Google Scholar]
- 47.Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110(9):3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaminetzky D, Hymes KB. Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Biologics. 2008;2(4):717–724. doi: 10.2147/btt.s3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪▪.Gerena-Lewis M, Crawford J, Bonomi P, et al. A Phase II trial of Denileukin Diftitox in patients with previously treated advanced non-small cell lung cancer. Am J Clin Oncol. 2009;32(3):269–273. doi: 10.1097/COC.0b013e318187dd40. Phase II trial of ONTAK® in NSCLC patients demonstrated subjective clinical responses and significant adverse events. [DOI] [PubMed] [Google Scholar]
- 50.Holt GE, Disis ML. Immune modulation as a therapeutic strategy for non-small-cell lung cancer. Clin Lung Cancer. 2008;9(Suppl 1):S13–S19. doi: 10.3816/clc.2008.s.003. [DOI] [PubMed] [Google Scholar]
- 51.Barve M, Bender J, Senzer N, et al. Induction of immune responses and clinical efficacy in a Phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;26(27):4418–4425. doi: 10.1200/JCO.2008.16.6462. [DOI] [PubMed] [Google Scholar]
- 52.Ramlau R, Quoix E, Rolski J, et al. A Phase II study of Tg4010 (Mva–Muc1–IL2) in association with chemotherapy in patients with stage III/IV non-small cell lung cancer. J Thorac Oncol. 2008;3(7):735–744. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 53.Bolonaki I, Kotsakis A, Papadimitraki E, et al. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol. 2007;25(19):2727–2734. doi: 10.1200/JCO.2006.10.3465. [DOI] [PubMed] [Google Scholar]
- 54.Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer. 2007;57(3):365–372. doi: 10.1016/j.lungcan.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer RG, Korn S, Micke P, et al. An open-label, prospective Phase I/II study evaluating the immunogenicity and safety of a ras peptide vaccine plus GM-CSF in patients with non-small cell lung cancer. Lung Cancer. 2007;58(1):88–94. doi: 10.1016/j.lungcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Brunsvig PF, Aamdal S, Gjertsen MK, et al. Telomerase peptide vaccination: a Phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55(12):1553–1564. doi: 10.1007/s00262-006-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nemunaitis J, Meyers T, Senzer N, et al. Phase I trial of sequential administration of recombinant DNA and adenovirus expressing L523S protein in early stage non-small-cell lung cancer. Mol Ther. 2006;13(6):1185–1191. doi: 10.1016/j.ymthe.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13(6):555–562. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 59.Morse MA, Garst J, Osada T, et al. A Phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuboi A, Oka Y, Osaki T, et al. WT1 peptide-based immunotherapy for patients with lung cancer: report of two cases. Microbiol Immunol. 2004;48(3):175–184. doi: 10.1111/j.1348-0421.2004.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 61.Mine T, Gouhara R, Hida N, et al. Immunological evaluation of CTL precursor-oriented vaccines for advanced lung cancer patients. Cancer Sci. 2003;94(6):548–556. doi: 10.1111/j.1349-7006.2003.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.American Cancer Society. Cancer Facts & Figures. 2010 www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf.
- 102.GSK1572932A Antigen-Specific Cancer Immunotherapeutic as Adjuvant Therapy in Patients With Non-Small Cell Lung Cancer. http://clinicaltrials.gov/ct2/show/NCT00480025.
- 103.Cancer Vaccine Study for Unresectable Stage III Non-small Cell Lung Cancer. http://clinicaltrials.gov/ct2/show/NCT00409188.
- 104.Phase III Lucanix™ Vaccine Therapy in Advanced Non-small Cell Lung Cancer (NSCLC) Following Front-line Chemotherapy (STOP) http://clinicaltrials.gov/ct2/show/NCT00676507.