Abstract
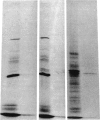
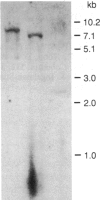
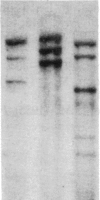
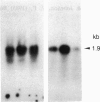
Administration of ethanol to rabbits is known to induce a unique liver microsomal cytochrome P-450, termed isozyme 3a or P-450ALC, which is responsible for the increased oxidation of ethanol and other alcohols and the activation of toxic or carcinogenic compounds such as acetaminophen and N-nitrosodimethylamine. To further characterize this cytochrome P-450 we have identified cDNA clones to isozyme 3a by immunoscreening, DNA hybridization, and hybridization-selection. The cDNA sequence determined from two overlapping clones contains an open reading frame of 1416 nucleotides, and the first 25 amino acids of this reading frame correspond to residues 21-45 of cytochrome P-450 3a. The complete polypeptide, including residues 1 to 20, contains 492 amino acids and has a molecular weight of 56,820. Cytochrome P-450 3a is approximately 55% identical in sequence to P-450 isozymes 1 and 3b and 48% identical to isozyme 2. Hybridization of clone p3a-2 to electrophoretically fractionated rabbit liver poly(A)+ RNA revealed multiple bands, but, with a probe derived from the 3' nontranslated portion of this cDNA, only a 1.9-kilobase band was observed. Treatment of rabbits with imidazole, which increases the content of isozyme 3a, resulted in a transient increase in form 3a mRNA, but this was judged to be insufficient to account for the known 4.5-fold increase in form 3a protein. Genomic DNA analysis indicated that the cytochrome P-450 3a gene does not belong to a large subfamily.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza J. P., Felver M. E., Veech R. L. The metabolism of acetone in rat. J Biol Chem. 1984 Jan 10;259(1):231–236. [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Dieter H. H., Johnson E. F. Functional and structural polymorphism of rabbit microsomal cytochrome P-450 form 3b. J Biol Chem. 1982 Aug 25;257(16):9315–9323. [PubMed] [Google Scholar]
- Ding X. X., Koop D. R., Crump B. L., Coon M. J. Immunochemical identification of cytochrome P-450 isozyme 3a (P-450ALC) in rabbit nasal and kidney microsomes and evidence for differential induction by alcohol. Mol Pharmacol. 1986 Oct;30(4):370–378. [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W., Hardwick J. P., Kasper C. B. Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J Biol Chem. 1985 Jun 25;260(12):7435–7441. [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I., Eliasson E., Norsten C., Ingelman-Sundberg M. Hydroxylation of acetone by ethanol- and acetone-inducible cytochrome P-450 in liver microsomes and reconstituted membranes. FEBS Lett. 1986 Feb 3;196(1):59–64. doi: 10.1016/0014-5793(86)80214-9. [DOI] [PubMed] [Google Scholar]
- Johansson I., Ingelman-Sundberg M. Carbon tetrachloride-induced lipid peroxidation dependent on an ethanol-inducible form of rabbit liver microsomal cytochrome P-450. FEBS Lett. 1985 Apr 22;183(2):265–269. doi: 10.1016/0014-5793(85)80790-0. [DOI] [PubMed] [Google Scholar]
- Koop D. R., Casazza J. P. Identification of ethanol-inducible P-450 isozyme 3a as the acetone and acetol monooxygenase of rabbit microsomes. J Biol Chem. 1985 Nov 5;260(25):13607–13612. [PubMed] [Google Scholar]
- Koop D. R., Coon M. J. Purification of liver microsomal cytochrome P-450 isozymes 3a and 6 from imidazole-treated rabbits. Evidence for the identity of isozyme 3a with the form obtained by ethanol treatment. Mol Pharmacol. 1984 May;25(3):494–501. [PubMed] [Google Scholar]
- Koop D. R., Crump B. L., Nordblom G. D., Coon M. J. Immunochemical evidence for induction of the alcohol-oxidizing cytochrome P-450 of rabbit liver microsomes by diverse agents: ethanol, imidazole, trichloroethylene, acetone, pyrazole, and isoniazid. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4065–4069. doi: 10.1073/pnas.82.12.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop D. R. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P-450 isozyme 3a. Mol Pharmacol. 1986 Apr;29(4):399–404. [PubMed] [Google Scholar]
- Koop D. R., Morgan E. T., Tarr G. E., Coon M. J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982 Jul 25;257(14):8472–8480. [PubMed] [Google Scholar]
- Koop D. R., Nordblom G. D., Coon M. J. Immunochemical evidence for a role of cytochrome P-450 in liver microsomal ethanol oxidation. Arch Biochem Biophys. 1984 Nov 15;235(1):228–238. doi: 10.1016/0003-9861(84)90272-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton J. K., DeBrunner-Vossbrinck B. A., Kemper B. Isolation and sequence analysis of three cloned cDNAs for rabbit liver proteins that are related to rabbit cytochrome P-450 (form 2), the major phenobarbital-inducible form. Biochemistry. 1984 Jan 17;23(2):204–210. doi: 10.1021/bi00297a005. [DOI] [PubMed] [Google Scholar]
- Mizukami Y., Sogawa K., Suwa Y., Muramatsu M., Fujii-Kuriyama Y. Gene structure of a phenobarbital-inducible cytochrome P-450 in rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3958–3962. doi: 10.1073/pnas.80.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. T., Koop D. R., Coon M. J. Catalytic activity of cytochrome P-450 isozyme 3a isolated from liver microsomes of ethanol-treated rabbits. Oxidation of alcohols. J Biol Chem. 1982 Dec 10;257(23):13951–13957. [PubMed] [Google Scholar]
- Morgan E. T., Koop D. R., Coon M. J. Comparison of six rabbit liver cytochrome P-450 isozymes in formation of a reactive metabolite of acetaminophen. Biochem Biophys Res Commun. 1983 Apr 15;112(1):8–13. doi: 10.1016/0006-291x(83)91789-8. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Fujii-Kuriyama Y., Okada Y., Sogawa K., Hirose T., Inayama S., Omura T. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4647–4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okino S. T., Quattrochi L. C., Barnes H. J., Osanto S., Griffin K. J., Johnson E. F., Tukey R. H. Cloning and characterization of cDNAs encoding 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible rabbit mRNAs for cytochrome P-450 isozymes 4 and 6. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5310–5314. doi: 10.1073/pnas.82.16.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J., Heinemann F. S., Johnson E. F. The complete amino acid sequence of a constitutive form of liver microsomal cytochrome P-450. J Biol Chem. 1985 May 10;260(9):5427–5434. [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. E., Koop D. R., Thomas P. E., Coon M. J., Levin W. Evidence that isoniazid and ethanol induce the same microsomal cytochrome P-450 in rat liver, an isozyme homologous to rabbit liver cytochrome P-450 isozyme 3a. Arch Biochem Biophys. 1986 May 1;246(2):633–644. doi: 10.1016/0003-9861(86)90319-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab G. E., Johnson E. F. Two catalytically distinct subforms of cytochrome P-450 3b as obtained from inbred rabbits. Biochemistry. 1985 Dec 3;24(25):7222–7226. doi: 10.1021/bi00346a030. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Black S. D., Fujita V. S., Coon M. J. Complete amino acid sequence and predicted membrane topology of phenobarbital-induced cytochrome P-450 (isozyme 2) from rabbit liver microsomes. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6552–6556. doi: 10.1073/pnas.80.21.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey R. H., Okino S., Barnes H., Griffin K. J., Johnson E. F. Multiple gene-like sequences related to the rabbit hepatic progesterone 21-hydroxylase cytochrome P-450 1. J Biol Chem. 1985 Oct 25;260(24):13347–13354. [PubMed] [Google Scholar]
- Yang C. S., Tu Y. Y., Koop D. R., Coon M. J. Metabolism of nitrosamines by purified rabbit liver cytochrome P-450 isozymes. Cancer Res. 1985 Mar;45(3):1140–1145. [PubMed] [Google Scholar]
- Zaphiropoulos P. G., Folk W. R., Coon M. J. Isolation and characterization of a novel cytochrome P-450-like pseudogene. Biochem Biophys Res Commun. 1986 Jan 29;134(2):499–505. doi: 10.1016/s0006-291x(86)80448-x. [DOI] [PubMed] [Google Scholar]