Abstract
Objective
To evaluate the association between prenatal alcohol exposure and the rate of Conduct Disorder in exposed compared to unexposed adolescents.
Method
Data for these analyses are from a longitudinal study of prenatal substance exposures. Women were interviewed at their 4th and 7th prenatal months, and with their children, at birth, 8 and 18 months, 3, 6, 10, 14, and 16 years postpartum. Offspring were interviewed with the Diagnostic Interview Schedule-IV; maternal and adolescent diagnoses were made using DSM-IV criteria at age 16. The sample was 592 adolescents and their mothers/caretakers.
Results
Prenatal alcohol exposure is significantly associated with an increased rate of Conduct Disorder in the adolescents. This effect was detected above an average exposure of 1 or more drinks/day in the first trimester. The effect remained significant after controlling for other significant variables including measures of the environment, maternal psychopathology, and other prenatal exposures.
Conclusion
Prenatal alcohol use in the first trimester is a risk factor for Conduct Disorder in the exposed offspring.
Introduction
Alcohol use during pregnancy is common and remains a significant threat to the development and health of exposed offspring. A recent survey by the National Birth Defects Prevention Study 1 found that nearly one-third of women drank alcohol at some time during their pregnancy; 22.5% drank during the first month of pregnancy and 25% drank during the first trimester. Longitudinal analyses of the 1991–2005 Behavioral Risk Factor Surveillance System surveys indicate that the rate of past-month alcohol use among pregnant women was unchanged across this 14-year period 2. Thus, drinking during pregnancy remains prevalent despite an advisory from the Surgeon General 3 to limit alcohol use if pregnant or contemplating pregnancy.
Alcohol exposure during gestation is associated with varied effects on development, depending on the amount and timing of exposure. Heavy alcohol use during pregnancy is the cause of Fetal Alcohol Syndrome (FAS), a well-defined syndrome that includes growth deficits, facial dysmorphology, and CNS abnormalities 4. However, most women who consume alcohol during pregnancy are light-to-moderate drinkers in early pregnancy and quit or decrease their alcohol use by mid-pregnancy. 5–7 Moderate or light alcohol consumption during pregnancy is associated with fewer and less severe effects.
Children with FAS and those with high levels of prenatal alcohol exposure (PAE) have higher scores on the aggression subscales of the Child Behavior Checklist (CBCL) 8–11. Sood et al., 12 found a dose-response association between PAE and behavior problems. At low levels of PAE, offspring had more aggressive and externalizing behaviors. Higher levels of exposure predicted more delinquent behaviors and a higher total problem score on the CBCL. By contrast, a recent longitudinal study, 13 found no effects of low levels of PAE on problem behaviors; moderate first trimester PAE predicted scores in the clinical range of aggression on the CBCL, and both moderate and heavy exposure in late pregnancy predicted total externalizing behaviors. Using a large representative U.S. sample, D'Onofrio et al. 14 examined the association of PAE to maternal ratings of behavior problems among siblings with differing levels of exposure, a study design that made it possible to control for unmeasured environmental and genetic confounders. They reported a dose-response relation between PAE and externalizing behaviors and concluded the association was causal.
Conduct Disorder (CD) in DSM-IV is a diagnosis 15 that describes a pattern of severe behavior problems persisting for more than 12 months with at least one behavior present in the past six months. The diagnostic criteria include aggression toward people and animals, destruction of property, deceitfulness or theft, and serious rule violations. The cumulative prevalence of DSM-IV CD by age 16 in a population sample was 9% 16, with a higher rate among boys than girls (14.1% versus 3.8%). In the National Comorbidity Survey-Replication, the lifetime prevalence of CD among the subsample of adults aged 18–44 years was 9.5%, with a higher rate among males (12%) than females (7.1%). 17
CD was significantly more prevalent among a small sample of heavily alcohol-exposed children when compared to a matched control group. 18 Using a retrospective measure of prenatal exposure to alcohol and tobacco, Disney et al. 19 assessed the relation of PAE to symptoms of DSM-IV CD in a population sample of adolescents from the Minnesota Twin Family Study. PAE was associated with a greater number of CD symptoms when prenatal tobacco exposure (PTE) and parental psychopathology (Substance Use Disorders and Antisocial Personality Disorder) were controlled.
Maternal smoking during pregnancy also has been associated with externalizing behaviors and with CD. Wakschlag et al. 20 reported that smoking more than a half pack/day during pregnancy increased the risk of CD 4.4 times in boys compared to boys with no prenatal tobacco exposure (PTE). Using a dimensional rating of DSM-IV psychiatric symptoms, Fergusson et al. 21 reported a significant association between PTE and CD symptoms after adjusting for confounders such as socioeconomic factors, harsh discipline, child abuse, and parental criminality. Other research indicates that when confounders such as parental psychopathology and PAE are controlled, there is no significant effect of PTE on externalizing behaviors. D’Onofrio et al. 22 compared the rate of conduct problems among tobacco-exposed offspring and their non-exposed siblings from the National Longitudinal Survey of Youth and concluded that the effect of PTE on conduct problems was not significant when genetic and environmental influences on externalizing problems were controlled. In a study of a population-based birth cohort, the Generation R Study, Roza et al. 23 similarly found that after adjustment for confounds such as socioeconomic factors and parental psychopathology, the association between prenatal exposure to maternal and paternal smoking and offspring externalizing behaviors was not significant. Another study examined risk for externalizing disorders among youth at high or low risk for developing alcoholism; prenatal alcohol and tobacco exposure were not significantly related to externalizing behaviors when family history of alcohol dependence was considered. 24
Twin studies provide support for genetic transmission of CD. 25,26 Parental history of alcohol or drug dependence has also been associated with externalizing behavior, possibly through a shared genetic liability for deviant behaviors. 24, 27 Using a twin-family study design, Hicks et al. 28 explored the familial resemblance of four externalizing disorders (CD, adult antisocial behavior, alcohol and drug dependence) among parents and twin-offspring and concluded that a general vulnerability to the four externalizing disorders exists that is highly heritable. Other common risk factors identified include male gender, IQ, race, poverty, home environment, parenting, life events, single parenthood, and parental psychopathology. 29–31
This report will focus on the relation of PAE to a diagnosis of CD in a birth cohort. We hypothesized that PAE would significantly predict CD after adjusting for the effects of other significant risk factors including other prenatal exposures. In contrast to other reports, trimester-specific exposures were ascertained and the outcome was CD diagnosis, rather than a count of CD symptoms, offering a more rigorous test of the hypothesis. Covariates included prenatal exposure to tobacco, marijuana, cocaine, and other illicit drugs, income, race, gender, parenting style, life events, home environment, family history of alcohol problems, and maternal lifetime psychopathology.
Method
Data are from two longitudinal studies of the effects of prenatal substance exposure begun in 1982. Women 18 years and older were interviewed when they attended their fourth prenatal month visit at an urban hospital prenatal clinic. Women not interviewed at this time were contacted again at their fifth month visit. Any eligible woman who did not complete an interview by the fifth month visit was not contacted again.
A total of 1360 women was screened. Fifteen percent declined to participate. From this sample of pregnant women, two cohorts were selected. The alcohol cohort included all women who consumed an average of three or more drinks/week in the first trimester and the next woman interviewed who drank alcohol less often or abstained from drinking. A second cohort was selected to study the effects of marijuana use during pregnancy. All women who used marijuana at the rate of two or more joints per month during the first trimester and the next woman who used less than this amount or did not use marijuana were selected. The two study protocols were identical. Sampling was done with replacement, so women could be in either or both of the cohorts; the overlap between the two cohorts was 47%. For the present analysis, the two cohorts were combined (n= 829). Subsequent assessments of the women occurred at the 7th prenatal month, and with their children, at delivery, 8 and 18 months, 3, 6, 10, 14, 16, and 22 years postpartum. The study protocol was approved by the Institutional Review Boards of the Magee-Womens Hospital and the University of Pittsburgh. Informed consent was obtained from participants at each phase.
There were 763 live singleton births, 97% of the original sample. The 63 losses included 21 women who moved out of the Pittsburgh area, 16 who were lost to follow-up, 8 who refused the delivery interview and newborn exam, 2 multiple births, 15 deaths, and 1 adoption.
Retention rates have been excellent: At 16 years postpartum, 592 adolescents and their mothers/caregivers were interviewed (78% of the birth cohort). For those adolescents not in the care of their biological parent (16% of the sample), the current caregiver completed the parent interview. Subject loss between birth and this assessment phase included those who declined to participate in the current phase (n = 52), moved out of the Pittsburgh region (n = 35), or were lost to follow-up (n = 69). Additionally, six children died, eight were adopted, and one child was institutionalized. Reports of the care-givers are included in the analyses. For convenience, we refer to the group of care-givers and mothers as mothers. Compared to the birth sample, significantly more African-Americans (54.5% versus 42.4%, respectively, p = 0.004) and females (52.6% versus 41.4%, respectively, p = 0.007) attended the 16-year follow-up.
Twenty adolescents who were interviewed did not complete a valid diagnostic assessment; three were missed, the diagnostic interview (Diagnostic Interview Schedule for DSM-IV; DIS-IV) 32 was not administered to seven who had mental handicaps or who left early, five had an incomplete DIS-IV in which the Conduct Disorder module was not administered, and five DIS-IV interviews were excluded because of questionable validity (e.g., the adolescent was unable to answer the questions or was not paying attention). Those who were not seen or did not complete the diagnostic interview at the 16-year follow-up (n = 191) did not differ from those who completed the assessment (n = 572) with respect to first trimester alcohol, marijuana, and tobacco exposure, maternal age at delivery, household income, marital status, and education.
Measures
Trimester-specific substance use was assessed using measures developed for this study. Alcohol consumption was assessed from conception to pregnancy recognition, from recognition to confirmation of pregnancy, and from confirmation to the end of the first trimester 33. From this, a measure of first trimester PAE was created that is weighted by the amount of alcohol consumed and the amount of time spent in those three time periods. At each assessment, the usual, minimum and maximum quantity and frequency were measured for beer, wine, liquor, wine coolers and beer coolers. Alcohol use was expressed as the average number of drinks per day or average daily volume (ADV). The assessment of marijuana was parallel to alcohol; marijuana, sinsemilla and hashish were combined to represent marijuana use. Marijuana exposure was expressed as average daily joints or ADJ. Tobacco use was measured as the number of cigarettes smoked per day. Use of other illicit drugs such as cocaine, amphetamines, barbiturates, hallucinogens, etc. was also assessed; Cocaine use was dichotomized for this analysis (any/none) and use of any of the other illicit drugs was combined into one variable representing other illicit drug use (any/none).
Current and lifetime psychiatric disorders were assessed in the mothers and offspring with the Diagnostic Interview Schedule-IV (DIS-IV) 32 at the 16-year follow-up. The DIS-IV is a structured interview using DSM-IV criteria 15 that is appropriate for use with lay interviewers. The interviewers were trained to administer the computerized DIS-IV as an interview by a trained and experienced clinician. DIS-IV interviews were audiotaped; reliability of the interview administration was maintained through weekly review of a random selection of audiotaped, de-identified interviews to ensure that the protocol standard was maintained. In response to any deviation in the interview administration, staff was immediately retrained to the protocol standard. The adolescents and mothers were interviewed separately regarding their own history of symptoms.
We created a measure of the home environment to parallel the Home Observation for Measurement of the Environment-Short Form (HOME) 34 designed for younger children and used in earlier phases. The correlation (r) between the created measure and the HOME at age 14 was 0.3. The adolescents were asked whether their biological father or other male adult was involved in their life, whether they regularly ate meals with their family, participated in family activities and did household chores. In addition, they reported on their participation in sports, school activities, music, and hobbies. Each of these activities was given one point on a scale. Scores ranged from 1–10, with higher scores reflecting a more optimal home environment.
Positive and negative events were assessed using a scale adapted from the PERI Life Events Scale 35. From a list of 34 common life events, the mothers indicated which had occurred in the past year. The number of endorsed events was summed.
Race was based on maternal report at delivery. At all phases, the mothers reported on their marital status, education, work status, and monthly family income. Child IQ was measured using the Stanford Binet Intelligence Scale (SBIS). 36 The adolescents rated their parents using the My Parents questionnaire 37, which assesses parental acceptance and involvement, strictness and supervision, and psychological autonomy granting.
Data Analysis
To maximize the sample size, we restricted our focus to first and third trimester alcohol exposure. Fewer women participated in the second trimester assessment and alcohol use at this phase was highly correlated with third trimester use. We evaluated exposure to first trimester cocaine and to other illicit drugs but did not analyze the data on third trimester exposures to these drugs since there were too few users.
Significant differences were identified for adolescents with and without a CD diagnosis in 1) demographic variables at enrollment and 16 years, 2) prenatal exposure to alcohol, tobacco, marijuana, cocaine and other illicit drugs, and 3) measures of parenting practices (youth report), home environment, and past-year life events (maternal report). Conservatively, we included variables that were related to CD at p < 0.10 in a multivariable logistic model to identify independent predictors of a lifetime CD diagnosis in the adolescents. To test for consistency in covariate selection, the logistic regression was repeated using backward elimination with the same results.
For the subset of mother-child pairs (n=487) on which we had diagnostic assessments for both biological mothers and offspring, associations between maternal lifetime psychiatric diagnoses and adolescent CD diagnosis were tested for statistical significance using chi-squares. This allowed us to consider the contribution of genetic liability for deviant behaviors. Maternal lifetime diagnoses with p-values < 0.10 were included in a multivariate logistic regression model along with variables identified as significantly related to CD diagnosis.
Results
The study began in 1982. The sample is representative of the population attending the prenatal clinic. At study enrollment, 73% of the women had completed high school, 26% were working or going to school. Their average monthly income was $346; average age was 23 years (range 18–42); 55% were African-American, and 68% were single. The mean alcohol use was 0.6 drinks/day (range 0–20); mean marijuana use was 0.4 joints/day (range 0–9); mean number of cigarettes was 8/day (range 0–50); 8% of the women reported illicit drug use other than marijuana, and 3% reported cocaine use.
At the 16-year follow-up, the average age of the mothers was 41 years (range 19–79), 50% were married or living with a male partner and 72.5% worked or attended school. They had, on average, 12.2 years of education; the average monthly household income was $2134, and the women reported an average of three life events in the past year. Fifty-five percent of the mothers met criteria for a lifetime DSM-IV diagnosis including Conduct Disorder (9.5 %), Antisocial Personality Disorder (ASPD; 5.8 %), and any Substance Use Disorder (SUD; 27%).
The average age of the adolescents was 16.8 years (range 15.9–19.2), mean IQ was 91.8, and grade in school was 10.8. Sixteen percent did not live with their biological mother. The lifetime prevalence of CD was 11.7% and 5% met criteria for a current diagnosis. Age of CD onset ranged from 5 to 16 years.
In the bivariate analyses, CD diagnosis was significantly related to gender (Table 1). Nearly 60% of those with the diagnosis were male, compared to 46% of those without CD (chi-square = 4.6, p < .05). Mothers of offspring with CD were marginally less likely to have been married when their child was born (chi square = 3.2, p < .10). Youth who rated their parents as more strict (t = 3.1, p< .005) and more involved (t = 2.9, p < .005) were less likely to have CD. Home environment, used as a continuous measure (range, 1–10), was less optimal among adolescents with CD (mean 5.34) compared with youth without CD (mean 6.07) (t=3.25, p=.005). Adolescents whose mothers reported a greater number of life events in the past year were more likely to have a CD diagnosis than those whose mothers reported fewer events (3.7 vs. 2.8, t = 3.4, p = .005) (Table 1).
Table 1.
Sample Characteristics by Lifetime Conduct Disorder Diagnosis at Age 16
| Adolescent Conduct Disorder |
||||
|---|---|---|---|---|
| No N=505 |
Yes N=67 |
p | ||
| Child Measures | ||||
| Race (% Caucasian) | 45.7 | 43.3 | NS | |
| Gender (% male) | 45.7 | 59.7 | χ2=4.6, p<.05 | |
| Child’s age at 16 year phase (mean) | 16.9 | 16.8 | NS | |
| Stanford Binet Composite Score | 91.9 | 91.4 | NS | |
| Maternal Measures | ||||
| Maternal marital status | ||||
| At delivery (% married) | 36.4 | 25.4 | χ2=3.2, p<.10 | |
| At 16- year phase (% married) | 39.5 | 35.9 | NS | |
| Maternal education level (mean) | ||||
| First Trimester | 11.8 | 11.6 | NS | |
| At 16-year phase | 12.2 | 12.0 | NS | |
| Mother’s work/school status | ||||
| In pregnancy (% working) | 26.7 | 19.4 | NS | |
| At 16-yr phase (% working) | 72.4 | 73.4 | NS | |
| Household Income/month (mean) | ||||
| At delivery | 329 | 289 | NS | |
| At 16-year phase | 2148 | 2029 | NS | |
| Family history of alcohol problems (%) | 76.6 | 79.1 | NS | |
| Parental Strictness | 19.0 | 17.4 | t=3.1, p<.005 | |
| Parental Involvement | 30.3 | 28.6 | t=2.9, p<.005 | |
| Parental Autonomy | 24.2 | 23.5 | NS | |
| Mother's age (mean) | ||||
| At delivery | 23.0 | 22.9 | NS | |
| At 16-year phase | 41.3 | 40.1 | NS | |
| Home environment scale (mean) | 6.07 | 5.34 | t=3.5, p<.005 | |
| Life events in past year (mean) | 2.8 | 3.7 | t=3.4, p<.005 | |
Note: NS = not significant.
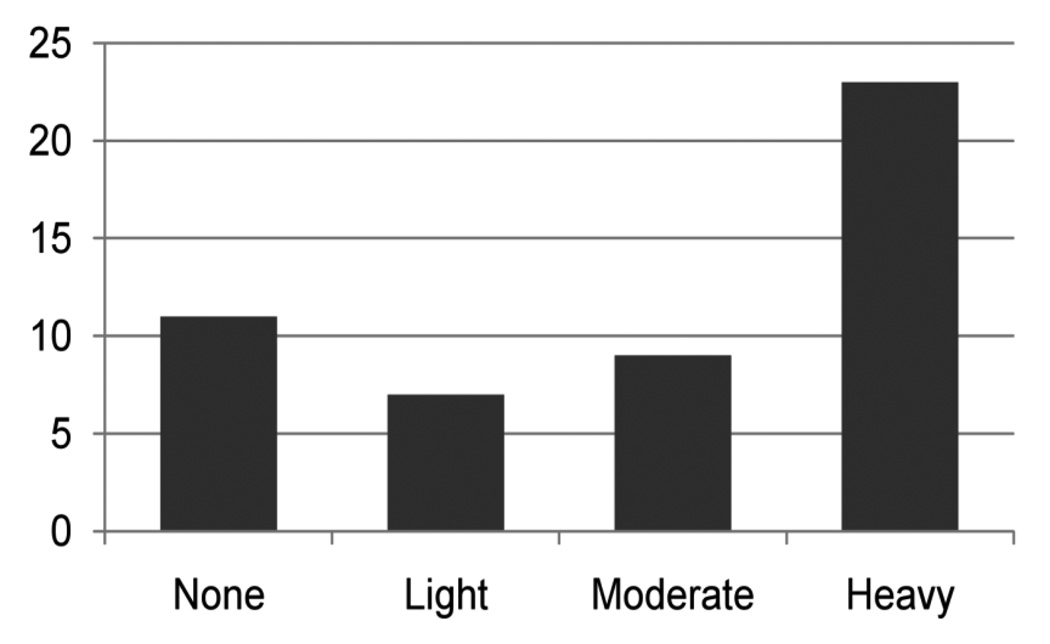
The relation of PAE to CD was not linear, so we categorized PAE as none (ADV=0), light (ADV≤0.4), moderate (0.4<ADV≤0.89) (Figure 1), and heavy (ADV>0.89), and assessed the bivariate associations between PAE and CD at each level. The category (ADV>0.89) is equivalent to drinking one or more alcoholic drinks per day. This was calculated using the formula: 7 drinks/week X 4 weeks/month)/ 31 days/month. Thirty-six percent of children with CD were exposed to at least one drink/day during the first trimester compared to 16% among the adolescents who did not have CD (Fisher’s Exact = 14.7, p = 0.002). There were no significant differences at lower levels of exposure. Therefore, in the multivariate analysis, PAE was dichotomized at 1 or more drinks/day. Third trimester alcohol use did not predict offspring CD. Mothers who smoked cigarettes at the rate of a half a pack or more/day during the first trimester were marginally more likely to have an adolescent with CD (chi-square = 2.86, p = 0.09). Exposure to marijuana, cocaine or other illicit drugs during gestation did not increase the risk of CD (Table 2). The relation of first and third trimester binge drinking to CD was not significant (data not shown).
Figure 1.
First Trimester Average Daily Volume of Alcohol
Table 2.
Prenatal Exposures by Adolescent Conduct Disorder Diagnosis at Age 16
| Adolescent Conduct Disorder Diagnosis |
||||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Alcohol | ||||||
| First trimester ADV | N | % | N | % | ||
| None | 185 | 36.6 | 22 | 32.8 | p=0.002a | |
| > 0 - ≤ 0.40 | 162 | 32.1 | 13 | 19.4 | ||
| >0.4 - ≤ 0.89 | 78 | 15.4 | 8 | 11.9 | ||
| > 0.89 | 80 | 15.8 | 24 | 35.8 | ||
| Third Trimester ADV | ||||||
| None | 351 | 69.5 | 42 | 62.7 | p=0.55 | |
| > 0 - ≤ 0.40 | 120 | 23.8 | 20 | 29.9 | ||
| >0.4 - ≤ 0.89 | 15 | 3.0 | 3 | 4.5 | ||
| > 0.89 | 19 | 3.8 | 2 | 3.0 | ||
| Cigarette Smoking | ||||||
| First Trimester | ||||||
| None to < ½ pack per day | 346 | 68.5 | 39 | 58.2 | p=0.09 | |
| ≥ ½ per day | 159 | 31.5 | 28 | 41.8 | ||
| Third Trimester | ||||||
| None to < ½ pack per day | 328 | 65.0 | 40 | 59.7 | p=0.40 | |
| ≥ ½ per day | 177 | 31.5 | 27 | 40.3 | ||
| Marijuana | ||||||
| First Trimester | ||||||
| None | 307 | 59.4 | 38 | 65.7 | p=0.80 | |
| Light >0 – 0.4 joints/day | 92 | 19.6 | 13 | 19.4 | ||
| Heavy > .4 joints/day | 106 | 21.0 | 16 | 23.9 | ||
| Third Trimester | ||||||
| None | 408 | 80.2 | 54 | 80.6 | p=0.24 | |
| Light >0 – 0.4 joints/day | 52 | 10.3 | 10 | 19.4 | ||
| Heavy > .4 joints/day | 48 | 9.5 | 3 | 23.9 | ||
| Cocaine | ||||||
| First Trimester | ||||||
| No use | 488 | 96.6 | 66 | 98.5 | p=0.24 | |
| Use | 17 | 3.4 | 1 | 3.01 | ||
| Other Illicit Drugs. | ||||||
| First Trimester | p=0.82 | |||||
| No use | 463 | 91.7 | 61 | 91.0 | ||
| Use | 42 | 8.3 | 6 | 8.4 | ||
Note: ADV = Alcohol Daily Volume
Determined using Chi-square and Fisher’s exact test.
In the multivariate model, PAE (ADV>0.89) during the first trimester significantly increased the likelihood of CD in the 16-year-old offspring (Odds Ratio (OR) = 2.74; 95% Confidence Interval (CI) = 1.50–5.01). Other significant predictors included strict parenting (OR = 0.90; CI = 0.83–0.96) and past year life events (OR = 1.20; CI = 1.07–1.34) (Table 3).
Table 3.
Multivariate Models: Significant Predictors of Adolescent Lifetime Conduct Disorder Diagnosis
| Odds Ratio |
95% CI of Odds Ratio |
||
|---|---|---|---|
| Biological Mothers and Current Caregivers (n=572) | |||
| Strict parenting | 0.90 | 0.83 | 0.96 |
| Life events | 1.20 | 1.07 | 1.34 |
| First trimester alcohol exposure (ADV> 0.89) | 2.74 | 1.50 | 5.01 |
| Biological Mothers Only (n=487) | |||
| Strict parenting | 0.88 | 0.82 | 0.95 |
| First trimester alcohol exposure (ADV>0.89) | 2.47 | 1.30 | 4.69 |
| Lifetime history of Sedative/Hypnotic/Opiate Dependence | 4.93 | 1.60 | 15.2 |
Note: ADV = Alcohol Daily Volume; CI = confidence interval.
In the subset of 487 biological mother-child pairs, six maternal disorders including lifetime alcohol dependence and sedative/hypnotic/opiate dependence were significantly associated with adolescent CD in the bivariate analyses (Table 4). The multivariate model above was rerun with these six variables included. In the multivariate model, maternal lifetime history of sedative/hypnotic/opiate dependence was significantly related to the adolescent's risk of having a CD diagnosis (OR = 4.93, 95% CI = 1.60–15.2) while maternal lifetime alcohol dependence was not. First trimester PAE (ADV>0.89) remained the strongest predictor of CD in the adolescents (OR = 2.47; 95% CI = 1.3–4.7). Parental strictness and supervision also significantly predicted having a CD diagnosis (OR = 0.88; 95% CI = 0.82–0.95) in this multivariate model, and past-year life events (OR = 1.19, 95% CI =1.06–1.33) were marginally related to CD.
Table 4.
Maternal Lifetime Diagnoses by Adolescent Conduct Disorder Diagnosis
| Adolescent Conduct Disorder (%Positive) |
|||
|---|---|---|---|
| Mother’s Lifetime DSM-IV Diagnosis (% Yes) | No N=428 |
Yes N=59 |
P |
| Conduct Disorder | 9.1 | 8.5 | NS |
| Antisocial Personality | 5.2 | 5.1 | NS |
| Major Depression | 35.0 | 39.0 | NS |
| Posttraumatic Stress Disorder | 15.0 | 22.0 | NS |
| Generalized Anxiety | 10.0 | 13.6 | NS |
| Alcohol Dependence |
12.2 | 22.0 | p<.05 |
| Abuse | 6.6 | 3.4 | NS |
| Withdrawal | 2.6 | 8.5 | p<.05 |
| Cannabis Dependence |
5.2 | 3.4 | NS |
| Abuse | 3.5 | 1.7 | NS |
| Cocaine Dependence |
7.3 | 15.3 | p<.05 |
| Withdrawal | 5.2 | 11.9 | p<.05 |
| Abuse | 1.2 | 0 | NS |
| Amphetamine/PCP/Hallucinogen/Other Illicit Drug Dependence |
2.1 | 3.4 | NS |
| Withdrawal | 1.9 | 3.4 | NS |
| Abuse | 1.6 | 1.7 | NS |
| Sedative/Hypnotic/Opiate Dependence |
2.1 | 10.2 | p<.005 |
| Opiate Withdrawal | 2.3 | 8.5 | p<.05 |
| Abuse | 0.7 | 1.7 | NS |
Note: NS = Not significant.
Discussion
Adolescents exposed to an average of one or more drinks of alcohol per day in the first trimester of pregnancy were three times more likely to meet criteria for a lifetime diagnosis of CD than were adolescents whose mothers drank less than that amount or abstained. This finding was consistent whether we used the full cohort of mothers, caregivers, and adolescents, or the subgroup of adolescents who were living with their biological mother. The effect of PAE on CD was significant after controlling for maternal, family and environmental factors. This result agrees with the few previous reports of the association between CD and PAE.18,19
There are a number of confounders that must be considered before this result can be considered reliable. Among these are 1) the association between maternal substance use, and familial transmission of CD and SUDs, 2) the effects of postnatal environmental factors, and 3) the effects of prenatal tobacco and other substances.
Genetic studies have demonstrated the familial transmission of CD 25, 26 and of SUDs. Moreover, parents with SUDs can transmit disruptive behavior to the offspring. 38 Because of this, researchers have argued that there is an underlying vulnerability across externalizing disorders. 24, 25, 27, 39 In our cohort, the mothers of offspring with CD had a significantly higher rate of SUDs in the bivariate analyses, but PAE remained a significant predictor of offspring CD after controlling for SUDs in the multivariate analyses. Similar findings have been reported by other researchers. 10, 14 The rates of maternal CD did not differ between the offspring with CD and those without CD. Unfortunately, data are not available on the fathers of these adolescents, but maternal CD and ASPD did not affect the association between PAE and CD in this cohort. Thus, the role of heredity, at least from the mothers, was not strong.
Environmental factors also play a significant role in the development of CD. We controlled for the environment by including other prenatal exposures and measures of the postnatal environment in the model. After controlling for these factors, the effects of PAE on CD remained significant. This result has also been reported by other researchers. Although we controlled for SES, it is difficult to evaluate the effects of SES in this cohort as almost all of the mothers were lower SES.
There was a significant effect of gender at the bivariate level, but not in the multivariate analyses, which is at odds with the epidemiology of CD. 16 PAE, however, has a biological effect, which may not differ by gender. We also found a significant association between a maternal lifetime history of sedative/hypnotic/opiate dependence among the biological mothers and CD in the offspring. This result is based on small numbers and may not be reliable, and it did not affect the association between PAE and CD.
The association between PAE and CD was not linear and was significant only above the level of one or more drinks/day during the first trimester. This could suggest a threshold effect. An alternative explanation is that at higher levels of PAE, the exposure is sufficient to lead to multiple symptoms and a diagnosis of CD, while fewer symptoms of CD are expressed at lower levels. This parallels the findings for FAS where lower levels of exposure predict symptoms, but the number and severity of symptoms are not sufficient to reach criteria for FAS. The evidence in the literature supports the latter explanation. Sood et al. 12 found that at lower levels of PAE, the symptoms were less severe than at moderate and higher levels; other researchers found a dose-response relation between PAE and conduct problems. 14
Women who use alcohol while pregnant are more likely to use other substances 40. Investigators have reported a significant association between prenatal tobacco exposure and externalizing behaviors. 20, 21 After controlling for PTE and other illicit drugs, the significant association between PAE and CD remained. Other investigators have also found that the effects of PTE were not significant when they controlled for factors such as PAE and/or maternal psychopathology. 22, 23 In this cohort, PAE was a significant predictor of CD after controlling for PTE and other prenatal substance exposures.
This study has limitations. The sample was from a prenatal clinic and the mothers were generally lower SES. The lifetime prevalence of CD was 11.7%, which is within the range of 9% 16 and 9.5% 17 reported by population samples. Few women had SUDs and although we had the entire spectrum of use, the level of alcohol, tobacco, and other substance use was mostly light to moderate. We did not have information about the psychiatric status of the biological fathers and were unable to control for this variable.
The data from this study are longitudinal, thus we avoid the bias of retrospective reporting of substance use during pregnancy over lengthy periods. The clinic population was half African-American and half white, and the sample reflects that distribution. Subject retention has been excellent: 78% of the birth cohort was assessed at the 16-year follow-up, and attrition bias has been kept to a minimum. Moreover, these analyses used a diagnostic measure rather than a symptom scale and considered the effects of other prenatal substance exposures as well as maternal psychopathology, parenting practices, and quality of the home environment.
In summary, we have shown that prenatal alcohol exposure above the level of 1 drink/day predicts a three-fold increase in the rate of Conduct Disorder in exposed offspring at 16 years of age. This finding was stable after controlling for other covariates including other prenatal exposures and maternal psychopathology. This finding has been noted before, but usually only with symptom measures and/or in specially chosen populations.
From a clinical perspective, PAE should be considered as another risk factor for Conduct Disorder. The next steps in research should be to define the interactions between prenatal exposures, environmental factors, and heritability. This would allow a more complete picture of the relations between PAE and CD. From a public health perspective, while the effects of PAE are small, they are significant on a population basis.
Acknowledgments
Funding was provided by National Institute of Alcohol Abuse and Alcoholism grants AA00312 (C.L.) and AA06666 (N.D.), and National Institute on Drug Abuse grant DA03874 (N.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Larkby, Goldschmidt, Hanusa, and Day report no biomedical financial interests or potential conflicts of interest.
Bibliography
- 1.Ethen M, Ramadhani T, Scheuerle A, et al. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Alcohol Use among Pregnant and Nonpregnant Women of Childbearing Age -- United States, 1991–2005. MMWR. 2009;58:529–532. [PubMed] [Google Scholar]
- 3.Office of the Surgeon General. Advisory on Alcohol Use in Pregnancy. Department of Health and Human Services Press Release; 2005. Feb 21, [Google Scholar]
- 4.Bertrand J, Floyd RL, Weber MK. Guidelines for identifying and referring persons with Fetal Alcohol Syndrome. MMWR. 2005;54(RR11):1–10. [PubMed] [Google Scholar]
- 5.Day NL, Jasperse D, Richardson G, et al. Prenatal exposure to alcohol: Effect on infant growth and morphologic characteristics. Pediatrics. 1989;84:536–541. [PubMed] [Google Scholar]
- 6.Ebrahim SH, Luman ET, Floyd RL, Murphy CC, Bennett EM, Boyle CA. Alcohol consumption by pregnant women in the United States during 1988–1995. Obstet Gynecol. 1998;92(2):187–192. doi: 10.1016/s0029-7844(98)00205-1. [DOI] [PubMed] [Google Scholar]
- 7.Serdula M, Williamson DF, Kendrick JS, Anda RF, Byers T. Trends in alcohol consumption by pregnant women. 1985 through 1988. J Am Med Assoc. 1991;20(265(7)):876–879. [PubMed] [Google Scholar]
- 8.Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 9.Aronson M, Hagberg B. Neuropsychological disorders in children exposed to alcohol during pregnancy: A follow-up study of 24 children born to alcoholic mothers in Goteborg, Sweden. Alcohol Clin Exp Res. 1998;22:321–324. doi: 10.1111/j.1530-0277.1998.tb03655.x. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael-Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- 11.Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcohol Clin Exp Res. 2000;24:226–231. [PubMed] [Google Scholar]
- 12.Sood B, Delaney-Black V, Covington C, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose-response effect. Pediatrics. 2001;108(2):E34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary CM, Nassar N, Zubrick SR, Kurinczuk JJ, Stanley F, Bower C. Evidence of a complex association between dose, pattern and timing of prenatal alcohol exposure and child behaviour problems. Addiction. 2009;105:74–86. doi: 10.1111/j.1360-0443.2009.02756.x. [DOI] [PubMed] [Google Scholar]
- 14.D'Onofrio BM, Van Hulle CA, Waldman ID, et al. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Arch Gen Psychiat. 2007;64:1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder. 4th ed. Washington DC: American Psychiatric Publishing Inc.; 1994. [Google Scholar]
- 16.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiat. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 17.Nock MK, Kazdin AE, Hiripi E, Kessler RC. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychol Med. 2006;36:699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- 19.Disney ER, Iacono W, McGue M, Legrand L. Strengthening the case: Prenatal alcohol exposure is associated with increased risk for conduct disorder. Pediatrics. 2008;122(6):e1225–e1230. doi: 10.1542/peds.2008-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of Conduct Disorder in boys. Arch Gen Psychiat. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- 21.Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiat. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- 22.D'Onofrio BM, Van Hulle CA, Waldman ID, et al. Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roza SJ, Verhulst FC, Jaddoe VW, et al. Maternal smoking during pregnancy and child behaviour problems: the Generation R Study. Int J Epidemiol. 2009;38:680–689. doi: 10.1093/ije/dyn163. [DOI] [PubMed] [Google Scholar]
- 24.Hill SY, Lowers L, Locke-Wellman J, Shen SA. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J Stud Alcohol. 2000;61:661–668. doi: 10.15288/jsa.2000.61.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bornovalova MA, Hicks BM, Iacono WG, McGue M. Familial transmission and heritability of childhood disruptive disorders. Am J Psychiatry. 2010 July 15; doi: 10.1176/appi.ajp.2010.09091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scourfield J, Van den Bree M, Martin N, McGuffin P. Conduct problems in children and adolescents: A twin study. Arch Gen Psychiat. 2004;61:489–496. doi: 10.1001/archpsyc.61.5.489. [DOI] [PubMed] [Google Scholar]
- 27.Marmorstein NR, Iacono WG, McGue M. Alcohol and illicit drug dependence among parents: associations with offspring externalizing disorders. Psychol Med. 2008;39:149–155. doi: 10.1017/S0033291708003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Arch Gen Psychiat. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- 29.Frick PJ, Lahey BB, Loeber R, Stouthamer-Loeber M, Christ MA, Hanson K. Familial risk factors to oppositional defiant disorder and conduct disorder: Parental psychopathology and maternal parenting. J Consult Clin Psych. 1992;60:49–55. doi: 10.1037//0022-006x.60.1.49. [DOI] [PubMed] [Google Scholar]
- 30.Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional Defiant and Conduct Disorder: A review of the past 10 years, Part I. J Am Acad Child Psych. 2000;39:1468–1484. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Simons-Morton B, Chen R, Hand LS, Haynie DA. Parenting behavior and adolescent conduct problems -- reciprocal and mediational effects. J Sch Violence. 2008;7:3–25. doi: 10.1300/J202v07n01_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for DSM-IV. St. Louis, MO: Washington University School of Medicine, Department of Psychiatry; 2000. [Google Scholar]
- 33.Day N, Wagener D, Taylor P. Measurement of substance use during pregnancy: methodologic issues. NIDA Research Monograph. 1985;59:36–47. [PubMed] [Google Scholar]
- 34.Baker PC, Mott FL. National Longitudinal Study of Youth Child Handbook. Ohio State University: Center for Human Resource Research; 1989. [Google Scholar]
- 35.Dohrenwend BS, Askenasy AR, Krasnoff L, Dohrenwend BD. Exemplification of a method for scaling life events: The PERI Life Events Scale. J Health Soc Behav. 1978;19(2):205–229. [PubMed] [Google Scholar]
- 36.Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. 4th ed. Chicago, IL: Riverside Pub Co.; 1986. [Google Scholar]
- 37.Steinberg L, Lamborn S, Dornbusch S, Darling N. Impact of parenting practices on adolescent achievement: Authoritative parenting, school-involvement, and encouragement to success. Child Dev. 1992;63:1266–1281. doi: 10.1111/j.1467-8624.1992.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 38.Lahey BB, Piacentini JC, McBurnett K, Stone P, Hartdagen S, Hynd G. Psychopathology in the parents of children with conduct disorder and hyperactivity. J Am Acad Child Psychol. 1988;27:163–170. doi: 10.1097/00004583-198803000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Kruger RE RF, Caspi A, Moffitt TE, Silva PA. the structure and stability of common mental disorders: a longitudinal-epidemiological study. J Abnorm Psychol. 1998;107:216–227. doi: 10.1037//0021-843x.107.2.216. [DOI] [PubMed] [Google Scholar]
- 40.Stratton K, Howe C, Battaglia FC, editors. Committee to Study Fetal Alcohol Syndrome. Diagnosis, Epidemiology, Prevention, and Treatment. Washington DC: National Academy Press; 1996. Institute of Medicine. [Google Scholar]