Abstract
Background and Purpose
Mineralocorticoid receptor (MR) antagonists have protective effects in rodent models of ischemic stroke, but the cell-type-specific actions of these drugs are unknown. In the present study, we examined the contribution of myeloid cell MR during focal cerebral ischemia using myeloid-specific MR knockout (MyMRKO) mice.
Methods
MyMRKO mice were subjected to transient (90 minutes) middle cerebral artery occlusion (MCAo) followed by 24 hours reperfusion (n = 5–7/group). Ischemic cerebral infarcts were identified by hematoxylin and eosin staining and quantified with image analysis software. Immunohistochemical localization of microglia and macrophages was performed using Iba1 staining, and the expression of inflammatory markers was measured after 24 hours of reperfusion by qRT-PCR.
Results
MyMRKO resulted in a 65 % reduction in infarct volume (P = 0.005) following MCAo. This was accompanied by a significant reduction in activated microglia and macrophages in the ischemic core. Furthermore, MyMRKO suppressed classically activated M1 macrophage markers TNFα, IL-1β, MCP1, MIP1α and IL-6 while partially preserving the induction of alternatively activated, M2, markers Arg1 and Ym1.
Conclusions
These data demonstrate that myeloid MR activation exacerbates stroke and identify myeloid MR as a critical target for MR antagonists. Further, these data indicate that MR activation has an important role in controlling immune cell function during the inflammatory response to stroke.
Keywords: mineralocorticoid receptor, ischemia, macrophage, stroke, inflammation
Introduction
Mineralocorticoids can cause vascular inflammation and hypertension which lead to vascular damage and remodeling1. During ischemic stroke, mineralocorticoid receptor (MR) activation results in increased vascular damage and ischemia2. Not surprisingly, several studies have shown that MR antagonists, at doses that do not alter blood pressure, are protective in rodent models of ischemic stroke. Treatment with the MR antagonist spironolactone was shown to reduce vascular damage and decrease mortality3. Similarly, another MR antagonist, eplerenone, decreases superoxide production and reduces infarct volume in animal models of ischemic stroke4. This indicates that MR blockade might have clinical potential as a therapeutic agent for stroke. However, the mechanisms of pharmacologic control and, importantly, the cell-type-specific actions of MR antagonists have not been identified and characterized.
In addition to its classical role in the kidney, MR has also been identified in other tissues, including the heart, brain, and inflammatory cells such as macrophages and microglia5, 6. In many of these cells, particularly brain and hematopoietic cells, the ligand for MR is thought to be glucocorticoids. MR has two high affinity physiologic ligands, mineralocorticoids such as aldosterone, and glucocorticoids such as corticosterone in rodents7. Since glucocorticoids circulate at levels 100–1000-fold higher than mineralocorticoids, MR binding sites are thought to be occupied by glucocorticoids in the absence of 11β-hydroxy-steroid dehydrogenase 2 (11βHSD2) which inactivates corticosterone to 11β-dehydrocorticosterone. Neurons and hematopoietic cells lack 11βHSD2 and so the majority of MR molecules are predicted to be occupied by glucocorticoids8.
Inflammation has an important role in the pathogenesis of ischemic stroke. A reduction in immune cells, inflammatory cytokines, and adhesion molecules reduces stroke injury9, 10 where as increases in anti-inflammatory cytokines such as IL-10 and IL-1RA are protective during models of cerebral ischemia11, 12. Several strategies to reduce the damaging inflammatory response following ischemic stroke have targeted immune cells and immune cell recruitment. Decreasing neutrophil infiltration reduces infarct volumes and neuronal cell death in mice following focal cerebral ischemia10. However, there was no neuroprotection found in clinical trials which tested agents that reduced neutrophil activity13. Similarly, adhesion molecules are important for leukocyte trafficking and infiltration into ischemic regions, and the use of monoclonal antibodies against intercellular adhesion molecule-1 (ICAM-1) has been successful in animal models of ischemic stroke 14, 15. Again, this treatment failed to translate to the clinical condition, but this was possibly due to the use of murine immunoglobulin. Targeting nuclear receptors that alter inflammation may be a viable alternative.
We have recently identified MR as a regulator of macrophage polarization, and deletion of MR from macrophages induces an alternatively activated macrophage phenotype, sometimes called M2, while suppressing the classically activated, M1, phenotype16. Decreasing the M1/M2 ratio was associated with abrogation of L-NAME/Angiotensin-II-induced cardiac and vascular hypertrophy, fibrosis and inflammation. Myeloid MR is also important in DOCA/salt-induced cardiac fibrosis17. Importantly, our previous work showed these effects to be independent of blood pressure lowering and, rather, are proposed to be a result of MR control of macrophage activation. We therefore hypothesized that the neuroprotective effects of MR antagonists during cerebral ischemia are at least partially due to a modulation in myeloid cell response, particularly the M1/M2 polarization of macrophages and microglia. To test this, we examined the effects of myeloid MR knockout (MyMRKO) in a model of focal cerebral ischemia.
Methods
Mice
MyMRKO mice on a C57BL/6 background were bred by crossing homozygous floxed MR mice with homozygous floxed MR mice containing LysM-Cre (MRfl/fl;LysM-Cre×MRfl/fl). MRfl/fl;LysM-Cre (knockouts) and littermate MRfl/fl (floxed controls (FC)) were used for all experiments. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 80–23) and were approved by the University Committee on Use and Care of Animals of the University of Michigan.
Middle Cerebral Artery Occlusion
Male MyMRKO mice weighing between 25–32 g were used. MCA occlusion was performed using the intraluminal filament method as previously described18. The mice were anesthetized with 1–3 % isoflurane and a 6-0 silicon rubber-coated nylon monofilament (Docoll Corporation, CA) was inserted into the right internal carotid artery. The right MCA was occluded for 90 minutes at which point the monofilament was removed and mice were allowed to recover.
Measurement of Infarct Volume
See supplemental methods.
Immunohistochemistry
See supplemental methods.
Quantitative real-time RT-PCR
See supplemental methods.
Statistics
Data are presented as mean ± SEM. Comparison of mean values between groups was performed using an unpaired, Student’s t test or by a two-way ANOVA with a Bonferroni post-test as indicated. P < 0.05 was considered statistically significant.
Results
MyMRKO
There are no obvious phenotypic differences in MyMRKO mice compared to floxed controls. Since MR is known classically to regulate blood pressure and this can affect stroke, we determined if MyMRKO affected blood pressure. We observed no significant change in baseline systolic and diastolic blood pressure between freely moving, unanesthetized MyMRKO and floxed control groups as measured by arterial pressure transducers monitored by radiotelemetry (Figure 1A and B). There is also no change in heart rate between the groups (Figure 1C). This would indicate that differences in neurologic outcome between the floxed controls and MyMRKO are unlikely to be related to blood pressure.
Figure 1.
Effect of MyMRKO on blood pressure. Data represents the mean systolic pressure (A), diastolic pressure (B), and heart rate (C) of FC and MyMRKO mice during day and night cycles determined by implanted arterial pressure transducers. n = 4 per group
MyMRKO reduces infarct volume
We examined the effect of MyMRKO on ischemic lesion size during focal cerebral ischemia. MyMRKO resulted in a significant reduction in infarct size 24 hours after a 90 minute transient occlusion of the right MCA. The infarct volume was determined in H&E stained serial coronal sections using Image J software and a significant decrease in ischemic infarct size was detected in MyMRKO sections (Figure 2A) relative to floxed controls (Figure 2B). Quantification of infarct volumes in serial coronal sections shows a significant reduction in MyMRKO (Figure 2C). The total infarct size of the ischemic hemisphere in the MyMRKO group was 11%, which was significantly less (P=0.005) than floxed controls, which had a total infarct volume of 32% (Figure 2D). This represented a highly significant 65% reduction in ischemic infarct volume in the MyMRKO group. No differences in pH, PO2, or PCO2 were detected before or during ischemia (Supplemental Table I). Cerebral blood flow in the MCA territory was reduced to less than 50% baseline during ischemia, but no differences were seen in perfusion between floxed control and MyMRKO mice.
Figure 2.
Quantification of infarct volume following transient cerebral ischemia. Representative photographs of MyMRKO (A) and FC (B) showing a reduced infarct size in the MyMRKO group. Quantification of infarct volume in serial coronal sections of FC and MyMRKO mice (C) and quantification of total ischemic infarct size in whole brain hemispheres (D) also showed a significant reduction in infarct size in the MyMRKO group. n = 5–7 per group. **P < 0.01, *** P < 0.001, Bonferroni post-test.
Activation of myeloid derived microglia/macrophages following MCAo
Following MCAo, there were no differences in the number of microglia in the non-ischemic, contralateral hemisphere between floxed control and MyMRKO groups (Figure 3A). There was a robust increase in Iba1+ cells in the ischemic, ipsilateral core when compared to the non-ischemic, contralateral hemisphere in floxed controls, indicating an increase in microglia activation and/or macrophage recruitment. However, this response was reduced in MyMRKO mice. Quantification of Iba+ cells/field showed a statistically significant reduction (P=0.018) in microglia/macrophages in MyMRKO in the ischemic core (Figure 3B). A regional comparison of Iba1+ cells show that significant differences in microglia/macrophages are largely confined to the subcortical basal ganglia (Table 1), which is within the ischemic core.
Figure 3.
Immunohistochemical analysis of activated microglia and macrophages following MCAo. Representative photomicrographs of non-ischemic contralateral (Contra) and ischemic ipsilateral (Ipsi) regions from coronal sections of floxed control and MyMRKO (A). Quantification of immunoreactive Iba1+ cells in the ischemic core showed a significant decrease in macrophages/microglia in of MyMRKO mice (B). n = 5–7 per group.
Table 1.
Anatomical localization of Iba1+ cells following MCAo.
| Anatomical Region | Iba1+ cells / field |
||
|---|---|---|---|
| FC |
MyMRKO |
||
| Mean ± S.E. | Mean ± S.E. | P-value | |
| Basal ganglia | |||
| Medial | 123 ± 5 | 44 ± 8 | < 0.001 |
| Lateral | 101 ± 11 | 54 ± 11 | 0.011 |
| Cortex | |||
| Primary Motor | 19 ± 2 | 21 ± 1 | 0.329 |
| Secondary Motor | 20 ± 1 | 21 ± 1 | 0.100 |
| Primary somatosensory | 29 ± 2 | 22 ± 3 | 0.088 |
| Secondary somatosensory | 31 ± 8 | 27 ± 2 | 0.280 |
| Olfactory area | 32 ± 11 | 28 ± 2 | 0.332 |
| Hypothalamic area | 31 ± 15 | 25 ± 4 | 0.337 |
Values represent mean ± S.E. of Iba1+ cells observed in different anatomical regions within the cerebrum. Cells were counted in a 40X field. N = 5–7 per group.
MyMRKO alters the inflammatory response to stroke
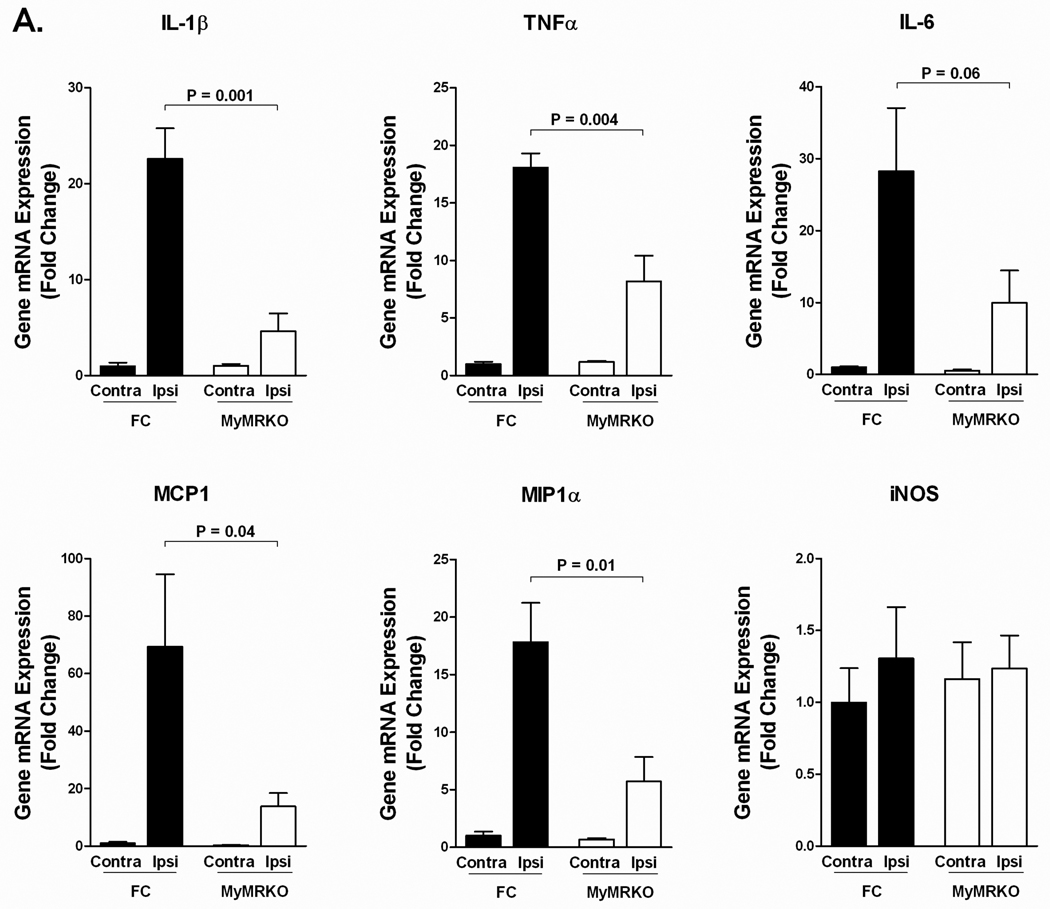
We have demonstrated previously that myeloid MR controls macrophage polarization and alters the inflammatory response during cardiac inflammation and fibrosis 16. To determine whether MyMRKO alters the inflammatory response to ischemic stroke, we measured the expression of classical and alternative macrophage markers 24 hours after MCAo using qRT-PCR. There is a strong induction in pro-inflammatory cytokines in the ischemic hemisphere of the floxed control group. However, MyMRKO demonstrated profound suppression of classically activated M1 markers TNFα, IL-1β, MCP1 and MIP1α (Figure 4A). These pro-inflammatory mediators are generally associated with exacerbation of tissue damage.
Figure 4.
MyMRKO shows an altered inflammatory response during MCAo. mRNA expression of M1 classically activated macrophage markers (A) M2 alternatively activated macrophage markers (B) and IL-17 (C) following MCAo. All genes were normalized to β-actin. n = 4 per group.
In contrast, the induction of M2 markers was at least partially preserved in MyMRKO. There was a significant increase in M2 markers, Arg1 and Ym1, in the ischemic hemisphere of both floxed control and MyMRKO groups (Figure 4B); interestingly, no significant differences in these M2 markers in the ischemic hemisphere were observed between the MyMRKO and floxed controls. In MyMRKO, there was a minimal suppression of Arg1 and Ym1 (less than 2-fold) where as all of the M1 markers were suppressed by greater than 2-fold and IL-1β and MCP had a 5-fold suppression relative to controls. Other M2 markers such as the mannose receptor (Mrc1) and Mgl1 showed no significant changes whereas the IL-1 receptor antagonist (IL-1RA) was significantly lower in the MyMRKO group (Figure 4B). Aldosterone has been shown to induce IL-17 mediated neuroinflammation19, but we did not see any significant change in the expression of IL-17 (Figure 4C).
Since oxidative stress is a critical mediator of reperfusion injury, we determined if MyMRKO altered the expression of genes associated with oxidative stress. We found no significant stroke-induced or strain-dependent differences in several genes (NADPH oxidase 2 (NOX2), manganese superoxide dismutase (MnSOD), catalase (Cat), and peroxiredoxin-2 (Prdx2)) (Figure 5) that are known to contribute to oxidative damage and that are associated with inflammation. In cardiac inflammation and fibrosis, myeloid MR was shown to exacerbate tissue remodeling and increase fibrosis. However, no changes in collagen 1A (Col1A1), collagen 3 (Col3), and fibronectin (Fn) expression were detected, nor did we see any difference in the expression of matrix metalloproteinase 9 (MMP9) (Figure 5).
Figure 5.
Markers of oxidative stress and fibrosis. MyMRKO did not show any differences in the mRNA expression of markers of oxidative stress (NOX2, MnSOD, Cat, Prdx2), nor did it show a difference in the expression of genes associated with tissue remodeling and fibrosis (Col1A
Discussion
In the present study, we demonstrated an important role for myeloid MR during ischemic stroke using cell-type-specific knockout and a model of focal cerebral ischemia. We found that deletion of MR from cells of myeloid lineage significantly reduced stroke infarct volume following MCAo. Further, a reduction in activated microglia/macrophages was observed along with a concomitant decrease in pro-inflammatory markers associated with the classically activated M1 macrophage phenotype. In addition, there was a partial preservation of the alternatively activated M2 macrophage phenotype. These data indicate that MyMRKO confers neuroprotection by modulating the immune response to ischemic stroke. Furthermore, we identify myeloid cells, which includes macrophages and microglia, as critical targets for MR antagonists and MR-regulation of myeloid cells as a potential mechanism for neuroprotection exhibited in previous studies.
To address the effect that myeloid MR has on the inflammatory response to stroke, we measured the expression of classical M1 and alternatively activated, M2, markers following MCAo. We observed infarct-induced-MyMRKO suppression of TNFα, IL-1β, MCP1, IL-6 and MIP1α, which are expressed principally by macrophages and are markers of classical M1 macrophage activation. Several of the M1 markers such as MCP1, IL-1β, and MIP1α were highly suppressed by 5-, 5- and 3-fold changes, respectively. However, the expression of M2 markers, Arg1 and Ym1, was partially preserved in MyMRKO mice with both being suppressed by less than 2-fold. The suppression of M2 markers was less than all of the M1 markers tested. This indicates there may be a higher ratio of M2 polarized myeloid cells within the brain and suggests that macrophage polarization may have an important role in neurologic outcome. Other M2 markers that were previously found to be regulated by MR during cardiac inflammation such as Mrc1, Mgl1 and Fizz1 were not upregulated during ischemia. This is likely due to the fact that different phenotypes of M2 polarization can exist based on the external stimuli that activate macrophages or expression of these genes in other cell types.
Immunohistochemical staining for Iba1 indicates a significant change in the macrophage/microglia response. However, Iba1 does not differentiate between macrophages and microglia, and it is difficult to differentiate the two cell types based on morphology. Changes in Iba1 staining were mainly confined to subcortical regions, although changes in infarct size are largely defined by differences in the cortex. This could indicate that MR control of the M1/M2 phenotype, rather than increases in the total number of myeloid cells, are more important in determining infarct size in the cortex.
There is evidence that microglia also adopt different functional phenotypes similar to the classical and alternative macrophage polarization20, 21. However, microglia do not express LysM until they become activated, and even upon activation there is only partial gene recombination and deletion22. Microglia containing LysM-Cre are able to undergo partial recombination during isolation and culturing, but we were unable to detect any suppression of MR expression in cultured microglia (Supplemental Figure I). This would suggest that resident macrophages or infiltrating myeloid cells might have a more dominant role in reducing inflammation and lesion size. It also remains to be determined whether MR activation can affect the population of circulating monocytes, which are recruited following ischemic stroke. Therefore, future studies aimed at identifying the individual contribution of monocytes, macrophages and microglia are warranted.
Iwanami et al.4 have shown that the MR antagonist eplerenone reduces macrophage associated oxidative stress following MCAo4. Further, high levels of aldosterone, a physiologic MR ligand, increase oxidative stress in circulating monocytes, as well as isolated macrophages23, 24. Our data show that myeloid MR does not affect the expression of genes associated with oxidative stress and the production of reactive oxygen species (ROS) 24 hours after ischemic stroke. However, many enzymes that generate ROS can be directly activated within minutes of ischemia and reperfusion. Furthermore, aldosterone is capable of activating NADPH oxidase by non-genomic mechanisms25. We have not directly measured the levels of ROS and therefore it could be possible that MR affects ROS production in this manner.
Although mineralocorticoid excess clearly affects stroke, in a normal physiological setting where glucocorticoids are significantly higher than aldosterone, as mentioned above, myeloid MR is thought to be mainly occupied by glucocorticoids. Glucocorticoids have been implicated as important regulators of MR function in other tissues that lack 11β-HSD2, including the brain and heart. In a model of myocardial infarction, the glucocorticoid cortisol was shown to increase the size of infarction and myocyte cell death26. This response was blocked by the MR antagonist spironolactone indicating potential actions of glucocorticoids on MR. Therefore, in our model of ischemic stroke, it is possible that glucocorticoids have a significant role in mediating the pro-inflammatory effects of myeloid MR. However, it is unclear how either aldosterone or glucocorticoids alter inflammation in macrophages and other myeloid cells. Future studies need to be aimed at understanding the mechanisms of myeloid phenotypic control by MR and whether MR is a direct transcriptional activator of pro-inflammatory genes.
In summary, this study has identified a previously unknown role for myeloid MR activation during ischemic stroke. Using MyMRKO mice, we demonstrated that MR activation in myeloid cells exacerbates inflammation and alters the M1/M2 inflammatory response to stroke. Moreover, these experiments indicate that MR control of immune cell function significantly affects stroke lesion size.
Supplementary Material
Acknowledgments
Sources of Funding: This study was supported by grants R01HL083201 from NIH-NHLBI, GM007767 from NIH-NIGMS, NS054724 and NS062816 from NIH-NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: R.A.F. wrote the manuscript, performed the gene expression analysis, immunohistochemical analysis, and analyzed the data. H.M. performed the MCAo surgeries. S.Z.D. analyzed the data. S.B. and G.S. provided the floxed MR mice and edited the manuscript. G.X. and Y.H. measured the blood gases and analyzed the data. M.M.W. and R.M.M. designed the study, analyzed the data, and edited the manuscript.
Conflicts of Interest/Disclosures: Grant.
References
- 1.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 2.Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension. 2006;47:590–595. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- 3.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT., Jr Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension. 1998;31:451–458. doi: 10.1161/01.hyp.31.1.451. [DOI] [PubMed] [Google Scholar]
- 4.Iwanami J, Mogi M, Okamoto S, Gao XY, Li JM, Min LJ, Ide A, Tsukuda K, Iwai M, Horiuchi M. Pretreatment with eplerenone reduces stroke volume in mouse middle cerebral artery occlusion model. Eur J Pharmacol. 2007;566:153–159. doi: 10.1016/j.ejphar.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Armanini D, Strasser T, Weber PC. Characterization of aldosterone binding sites in circulating human mononuclear leukocytes. Am J Physiol. 1985;248:E388–E390. doi: 10.1152/ajpendo.1985.248.3.E388. [DOI] [PubMed] [Google Scholar]
- 6.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 7.Krozowski ZS, Funder JW. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci U S A. 1983;80:6056–6060. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol. 1994;105:R11–R17. doi: 10.1016/0303-7207(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 9.Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ. Cerebral protection in homozygous null icam-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, Mayadas TN. Mice deficient in mac-1 (cd11b/cd18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 11.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. Il-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 13.Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA. Acute stroke therapy by inhibition of neutrophils (astin): An adaptive dose-response study of uk-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- 14.Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- 15.Zhang RL, Chopp M, Li Y, Zaloga C, Jiang N, Jones ML, Miyasaka M, Ward PA. Anti-icam-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 16.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, Lumeng CN, Mortensen RM. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Herrada AA, Contreras FJ, Marini NP, Amador CA, Gonzalez PA, Cortes CM, Riedel CA, Carvajal CA, Figueroa F, Michea LF, Fardella CE, Kalergis AM. Aldosterone promotes autoimmune damage by enhancing th17-mediated immunity. J Immunol. 184:191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 20.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in ad and in mouse models of ad. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. Il-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J Neurochem. 2007;101:771–781. doi: 10.1111/j.1471-4159.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- 22.Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ. Role of microglial ikkbeta in kainic acid-induced hippocampal neuronal cell death. Brain. 2008;131:3019–3033. doi: 10.1093/brain/awn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahokas RA, Warrington KJ, Gerling IC, Sun Y, Wodi LA, Herring PA, Lu L, Bhattacharya SK, Postlethwaite AE, Weber KT. Aldosteronism and peripheral blood mononuclear cell activation: A neuroendocrine-immune interface. Circ Res. 2003;93:e124–e135. doi: 10.1161/01.RES.0000102404.81461.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage nadph oxidase and increases atherosclerosis development: A possible role for angiotensin-converting enzyme and the receptors for angiotensin ii and aldosterone. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi H, Kobara M, Abe M, Tanaka N, Gouda E, Toba H, Yamada H, Tatsumi T, Nakata T, Matsubara H. Aldosterone nongenomically produces nadph oxidase-dependent reactive oxygen species and induces myocyte apoptosis. Hypertens Res. 2008;31:363–375. doi: 10.1291/hypres.31.363. [DOI] [PubMed] [Google Scholar]
- 26.Mihailidou AS, Loan Le TY, Mardini M, Funder JW. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension. 2009;54:1306–1312. doi: 10.1161/HYPERTENSIONAHA.109.136242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.