Abstract
Asthma and chronic sinusitis are inexplicably common airway diseases that are linked to atopy and allergic inflammation. T helper type 2 (Th2) cells and their associated cytokines are believed to play critical pathogenic roles in asthma, but the environmental factors that instigate allergic airway disease remain poorly understood. Environmental proteinases are highly allergenic and are candidate inducers of airway Th2 responses. Determining the proteinases and their sources that are relevant to airway disease, however, remains challenging. In this review, we summarize the evidence that implicates fungi as both a relevant source of allergenic proteinases and a potential cause of asthma, atopy and chronic sinusitis through airway infection. Clarification of the extrinsic causes of these processes will markedly improve diagnosis, prognosis and therapy.
Allergic inflammation in asthma: aberrant or appropriate?
Allergic asthma and related respiratory tract allergic disorders such as chronic sinusitis are among the most common of all human medical conditions and afflicts up to 10% of adults in the United States. These disorders cause facial pain, difficulty breathing, cough, and, in the severest forms of asthma, death due to profound airway obstruction. Asthma and chronic sinusitis are associated with diverse inflammatory markers including eosinophilia, immunoglobulin E (IgE), and lung T helper type 2 (Th2) cells that secrete the signature cytokines IL-4, IL-5, and IL-131–4.
Studies conducted in several laboratories have demonstrated that airway obstruction is intimately tied to Th2 cells, the cytokines interleukin (IL) −4 and IL-13, and numerous related effector molecules5–11. Two fundamentally distinct but related processes can be conceived for how asthma arises: an environmental exposure and the immune response to that exposure that perpetuates the pathological inflammation. A widely held perception of asthma pathogenesis is that airway allergic responses arise aberrantly following inhalation of putatively benign environmental agents, including fungal antigens. This view powerfully influences clinical practice in asthma in which the principle objective is to reduce allergic inflammation through immunosuppressive medications. In this Opinion, we summarize the growing literature that challenges the prevailing view of asthma as an aberrant inflammatory condition and propose an alternative model in which inflammation is appropriately directed against an insidious fungal infection of either the upper or lower airway. Fungi are known to cause airway allergic diseases, but entities such as allergic bronchopulmonary aspergillosis (ABPA) are currently viewed as clinically distinct from asthma. Our recent analyses of mice experimentally infected with household fungi, and of human subjects undergoing surgery for fungal-related sinus disease, emphasize why this distinction might be misplaced and how further studies have the potential to radically alter how asthma and sinusitis are diagnosed and treated.
Exogenous causes of asthma: what triggers Th2 responses?
Numerous hypotheses have been put forward to explain the proximate causes of asthma and related disorders, most of which have not survived close scrutiny12. Nonetheless, a fruitful approach to understanding the environmental causes of asthma has been to consider cases that have been definitively assigned a specific etiology. Examples of such natural human experiments are rare, but have the potential to be etiologically informative. Perhaps the most interesting of such investigations involved detergent factory workers that were exposed to proteinases of bacterial origin, an additive that greatly improves the performance of many cleaning products. Asthma with clear allergic features was documented exclusively in the workers that handled the proteinases but not other chemicals such as the detergent itself13–16. Similar cases of occupational asthma have been reported with workers handling proteinases of plant origin17.
These reports establish that proteinase exposure can cause human asthma, but such cases are confined to industrial settings and are less informative of the exposures that are typical of most asthma subjects. The strongest risk factor for asthma is atopy, which is defined as the exaggerated tendency to mount symptomatic immune reactions, i.e., immediate hypersensitivity, against common environmental antigens. Atopy is usually quantified in terms of total and antigen-specific serum IgE levels and immediate cutaneous reactions to panels of antigens (“allergen skin testing”)18. A surprising feature of the many human allergens that have been structurally and biochemically defined is that proteinase activity is a common feature (Fig. 1). For example, the allergen most commonly associated with atopy in asthma, Der p 1, a common dust mite allergen19, is a cysteine proteinase that is capable of cleaving numerous host proteins with immune relevance20–23. Other proteinases that have been linked to human atopy and allergic disease include the major allergens from cat dander (Fel d 1) and honey bee venom (Api m 7) and numerous other allergens from fungi and dust mites (Table 1) (http://fermi.utmb.edu/cgi-bin/SDAP/sdap_07?dB_Type=0&Code=10).
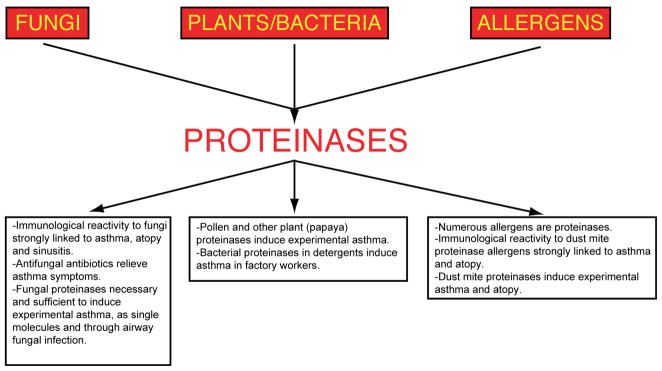
Figure 1.
Evidence linking environmental sources of proteinases to allergic airway disease. Virtually all organisms produce proteinases, but three major sources are most likely to be inhaled, releasing proteinase activity in the respiratory tract that then elicits allergic disease. These proteinase sources include filamentous fungi inhaled as spores, seasonally prevalent plant pollens, and perennially present allergens derived from common household organisms such as dust mites. Some of the experimental and human data linking these proteinase sources and allergic airway disease are summarized in the boxes.
Table 1.
Exemplary Proteolytically Active Allergens
| Allergen | Source | Allergen | Source |
|---|---|---|---|
| Api m 7 | Apis mellifera (honey bee) venom | Der m 1 | D. microceras (dust mite) |
| Asp f 10 | Aspergillus fumigatus (fungus) | Fel d 1 | Felis domesticus (domestic cat) |
| Asp f 13 | A. fumigatus (fungus) | Pen b 13 | Penicillium citrinum (fungus) |
| Asp f 18 | A. fumigatus (fungus) | Pen c 13 | P. brevicompactum (fungus) |
| Asp fl 13 | A. flavus (fungus) | Pen c 2 | P. citrinum (fungus) |
| Asp n 18 | A. niger (fungus) | Pen ch 13 | P. chrysogenum (fungus) |
| Asp o 13 | A. oryzae (fungus) | Pen ch 18 | P. chrysogenum (fungus) |
| Der f 2 | D. farinae (dust mite) | Pen o 18 | P. oxalicum (fungus) |
| Der p 1 | D. pteronyssinus (dust mite) | Tri t 4 | T. tonsurans (fungus) |
All organisms produce proteinases, so it is perhaps not surprising that many allergens are proteolytically active, an association that therefore may arise merely by chance. However, the organisms that have been most strongly linked to asthma-like diseases and atopy (fungi, pollens) are unique in that they must secrete highly durable proteinases into their environments and become airborne as a matter of survival. Thus, abundant circumstantial evidence associates exogenous proteinases with human allergic disease and suggests that at least some organismal proteinases are intrinsically allergenic (Fig. 1). However, in order to prove the allergenic nature of proteinases, we must turn again to experimental models.
Distinct mechanisms for Th2 cell generation in vivo
The apparent requirement for proteolytic activity in some environmental allergens must be reconciled with the well-known ability of chicken egg ovalbumin, which possesses no biochemical activity, to induce allergic disease in rodents. Many non-proteolytic human allergens are also known. Local lung immune mechanisms consisting of immunosuppressive dendritic cells that preferentially induce Tregs, powerfully suppress effector immune responses against such innocuous antigens in the absence of immuminization24,25. Experimental allergic lung disease due to ovalbumin can only be induced if mice are initially immunized against this protein through a route that is remote from the lung, i.e., peritoneum or skin, before lung delivery is able to elicit allergic inflammation26. It is possible that sensitization to non-enzymatically active antigens implicated in atopy and asthma occurs through routes remote from the lung, i.e., through the skin and gut. However, a more likely possibility is that some allegens contain an adjuvant substance that is perceived by the airway immune system as a danger signal and which overcomes normally tolerogenic mechanisms to induce inflammatory responses27.
The molecular basis for immune adjuvants is now well understood, at least for Th1 and Th17 responses, and involves recognition of structural features of microbial cell wall components, nucleic acids and some proteins, termed pathogen associated molecular patterns (PAMPs), by Toll like receptors (TLRs) and related immune receptors28–33. While there is evidence that TLRs participate in experimental Th2 and allergic responses as well as asthma under some conditions34–37, other studies refute the requirement of TLRs for allergic disease38–41. In contrast, our laboratory demonstrated that allergic lung disease and lung Th2 responses can be induced by proteinases of fungal and plant origin acting as Th2 adjuvant factors42. This observation, subsequently confirmed and extended to proteinases from other plants, animals, and microbes 43–48, is important because it experimentally validates the circumstantial link between proteinases of diverse sources and asthma (Fig. 1). Moreover, in contrast to ovalbumin, proteinases need only be delivered through the respiratory tract to induce allergic disease and thus are presumably more physiologically relevant42. The adjuvant activity of proteinases lies in their enzymatic activity, not the proteinase itself, thus allergenic proteinases are manifestly not PAMPs. Nonetheless, a link between allergenic proteinases and TLRs remains possible and deserves additional study.
Common fungi induce allergic lung disease through active infection and secretion of proteinases
A major challenge of mucosal immunology research is to understand how agents as diverse as ovalbumin, proteinases and TLR ligands relate to Th2 cell-dependent human diseases such as asthma and also to other important disease features such as atopy. We began this undertaking by first determining that proteinase activity can be found in the dust of virtually all Houston-area homes and that fungi were a major source of the proteinases43. Although we have identified more than 25 distinct fungal species from these samples, by far the most commonly isolated organisms were Aspergillus spp. Surprisingly, although Der p 1 protein could be detected in the majority of households by immunoassay, its enzymatic activity could not be detected by zymography, leaving fungi as the major, if not sole, source of active proteinases in most house dust samples43.
To understand the relationship between fungi and their secreted proteinases to allergic disease, we challenged mice with relatively low-doses (2000–400,000 conidia/day) of the conidia of A. niger to determine if this organism could grow in the airway and induce allergic lung disease. In a dose-dependent manner, the viable conidia of A. niger readily induced many salient aspects of asthma-like disease, including airway hyperreactivity, goblet cell metaplasia, highly polarized lung Th2 responses and serum fungus-specific antibodies. Active fungal infection was the underlying cause of the allergic lung phenotype because these responses were all either attenuated or abrogated in mice that received irradiated conidia that were incapable of producing hyphae49–51. We further showed that the conidia convert to filamentous hyphae in the airway, proving that the fungi were actively growing in the airways. Thus, A. niger readily infects the mouse airway and induces a disease phenotype closely resembling allergic asthma.
Fungi secrete or contain numerous potentially allergenic substances in addition to proteinases, including chitin52, b-glucans53,54 and many proteins linked to atopy. To specifically address the importance of proteinases, we challenged mice with induced mutant strains of A. niger in which either the aspergillopepsin I gene was specifically deleted or all detectable proteinase activity had been deleted55. Not only were these proteinase mutants capable of growing well in vitro and in mouse airways in vivo, but also the aspegillopepsin I mutant was more difficult to clear from the lungs of mice relative to animals infected with wild type strains. More importantly, neither mutant A. niger strain was capable of inducing robust allergic lung disease in mice. These studies are remarkable because they demonstrate that proteinases are necessary for allergic lung disease in response to airway infection with a fungus that possesses many distinct allergy-inducing factors. Combined with our prior finding that proteinases are sufficient as single molecules for the induction of experimental asthma, the observations indicate that fungal proteinases secreted during active airway infection are critical factors in allergic disease pathogenesis42,43.
Additional studies are required to determine what are the essential host genes that recognize the presence of foreign proteinase and trigger allergic responses. Two general models by which fungal proteinases may interact with host proteins are depicted in Figure 2. Cell-associated proteins that may be targeted directly or indirectly by proteinases potentially include tight junctions of airway epithelial cells20 and various immune receptors, including CD23 and CD2556, proteinase activated receptor 2 (PAR2)57, and the C3a anaphylatoxin receptor58. One soluble factors that has been implicated as a proteinase substrate in allergic disease is complement protein 3 (C3)59. Moreover, our recent discovery that matrix metalloproteinase 7 (MMP7) cleaves and activates IL-25 to facilitate induction of allergic lung disease suggests that exogenous proteinases might activate IL-25 or conversely deactivate Th1-inducing cytokines (e.g., IL-1260) to induce allergic disease61.
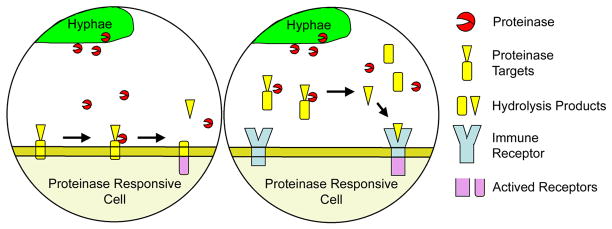
Figure 2.
Models for fungal proteinase-induced activation of allergic airway inflammation. Left, fungal proteinase cleaves a cell-associated target, thereby activating or modifying intracellular signaling pathways to favor Th2 responses. Right, proteinase cleaves a soluble target to release a ligand capable of activating an immune receptor controlling Th2 responses. Although depicted as membrane bound, this putative receptor could equally be intracellular or soluble. Candidate cell-associated proteins pertinent to this model include tight junctions of airway epithelial cells, CD23, CD25, proteinase activated receptor 2 (PAR2) and the C3a anaphylatoxin receptor. Soluble factors potentially include complement protein 3 (C3) and IL-25 (see text).
Experimental atopy, human chronic sinusitis and antimicrobial clinical trials further connect asthma to fungal infection
Additional studies from humans and mice further add to the growing link between fungi and asthma. Atopy is currently believed to represent a primary, genetically-controlled hypersensitivity to antigens that predisposes to secondary immune-related disorders such as asthma18,62–64. However, we have recently demonstrated that atopy can, like allergic lung disease, be secondary to a primary fungal airway infection43. Whereas ovalbumin behaves as a typical innocuous antigen and fails to elicit inflammation when delivered alone to the respiratory tract, when combined with A. niger conidia, atopy to ovalbumin is induced. Strangely, however, although Th2 responses to low-dose A. niger exposures were seen in these studies, atopy to the fungus was not detectable, indicating that antibody responses to fungi are an inefficient means of revealing active infection. The combined immune response to both low-dose A. niger infection and ovalbumin was, however, sufficient to induce the full spectrum of allergic lung disease, whereas either antigen administered alone was insufficient. Thus, the combination of a bystander antigen such as ovalbumin and viable conidia is synergistic and establishes the possibility that both atopy and asthma could represent secondary manifestations of the same primary fungal airway infection (Fig. 3).
Figure 3.
Paradoxical immune responses during fungal airway infection. As assessed in mouse studies, the nature of airway immune responses to inhaled antigens are strongly dependent on concomitant airway fungal infection. Left panel: high airway fungal burdens induce robust fungal-specific Th2 and atopic (IgG or IgE) responses. Middle panel: low-grade fungal burdens induce only Th2 responses; atopic responses are not detectable. Right panel: Inert bystander antigens if inhaled alone induce no inflammation or detectable immune response (not shown). In contrast, low-grade fungal airway infection induces specific Th2 responses against the bystander antigen, but not the fungus itself. This example, which is most representative of antigen encounters likely to be seen by humans, potentially explains the lack of fungal atopy in many asthma and CRS patients.
In addition to atopy, asthma is linked to upper respiratory tract inflammatory disorders such as rhinitis and chronic rhinosinusitis (CRS)65–69. CRS exists in several clinical variants, including CRS with and without nasal polyposis and, when associated with biopsy-proven fungi and fungal atopy, is termed allergic fungal rhinosinusitis (AFRS). In addition to the mucosal inflammation, mucopurulent sinus drainage, nasal obstruction and facial pain that are typical of this syndrome, most (> 60%) CRS patients are highly atopic and otherwise exhibit immunologic features that are characteristic of AFRS, including nasal eosinophilia and Th2 cell polarization70–72. Moreover, using specialized methods, virtually all CRS patients can be shown to have fungal culture-positive nasal secretions or possess hyphae in sinus specimens even in the absence of fungal atopy70, a finding with remarkable parallels to our inability to demonstrate fungal atopy to low-grade fungal lung infection in mice (Fig. 3). Thus, analyses of mice and humans with respiratory tract allergic disease suggest that the degree of fungal-related disease, including atopy, is strongly related to the fungal burden. In addition to simply correlating fungal isolation with allergic disease, future clinical studies are likely to benefit from quantitating the degree of fungal infection, although the techniques required for this remain at the experimental stage73.
Despite the strong association between the growth of fungi in the upper respiratory tract, allergic inflammation, and CRS, many investigators view the link to fungi in terms of mucosal colonization and not true infection74. However, the fungi are present in close association with profoundly abnormal, highly inflamed sinus tissue that causes airway obstruction. Moreover, in contrast to healthy control subjects that are largely non-reactive, the majority of AFRS patients manifest fungal antigen-specific Th2 responses when peripheral blood cells are stimulated with fungal antigens75. Together, we interpret these findings as indicative of true fungal infection, and not mucosal colonization. These observations further suggest that peripheral blood T cell-based immunodiagnosis may prove useful for the future diagnosis of fungal-related allergic airway disease.
A final clue suggesting fungal infection in asthma and CRS is the salutary effect of anti-fungal therapy on asthma severity. Two randomized clinical trials performed on fungus-sensitized asthmatic subjects have demonstrated that standard antibiotics (fluconazole, itraconazole) ameliorate disease overall and, for itraconazole, radically improve the quality of life of more than 50% of patients with severe disease76,77. These and related studies78,79 support the concept that active fungal infection in part drives the expression of asthma and related respiratory tract allergic diseases.
Concluding remarks
The heterogeneity in clinical expression for which asthma is notorious suggests to many investigators that asthma is not a specific disease, but rather a syndrome consisting of etiologically distinct entities with shared clinical features80. Viewed from an immune perspective, however, even clinically diverse asthma cases demonstrate a far more homogeneous nature in which Th2 cell-driven allergic inflammation is a consistent finding18,81. Experimental studies further validate that diverse antigens administered to the respiratory tract of widely divergent species and animal strains provoke a stereotypical immune and physiological reaction that strongly resembles allergic asthma. Thus, a substantially similar pathophysiology nonetheless appears to underlie the clinical heterogeneity of asthma and CRS, giving hope that relatively few, highly specific therapies may be developed that far exceed the utility of current treatment approaches.
For such a fortuitous outcome to yet be realized, a much greater understanding of the exogenous factors that initiate allergic airway inflammation is required. We have reviewed the growing literature that indicates a prominent role for common environmental fungi in the pathophysiology of both upper and lower respiratory tract allergic diseases. Our research has further uncovered the essential molecular basis by which fungi elicit allergic inflammation through secreted proteinases. Despite these insights, asthma and chronic sinusitis remain strongly tied to atopy and the knowledge that many non-fungal environmental antigens likely play prominent roles in disease expression. Here again, however, the discovery that primary fungal airway infection can lead to atopic responses against bystander antigens provides a remarkably parsimonious explanation for these phenomena.
We have emphasized here the role of fungal proteinases during airway infection, but viruses such as human rhinovirus are also linked to both asthma and sinusitis and proteinases of this organism can also elicit allergic lung disease44. Thus, in addition to delineating the molecular basis by which diverse proteinases elicit allergic inflammation, future studies should focus on confirming whether active airway infection by fungi and viruses in fact underlies concomitant respiratory tract allergic disease.
Acknowledgments
Supported by NIH grants HL75243 and AI057696 (to D.B.C.), AI070973 (F.K. and D.B.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Horn BR, et al. Total eosinophil counts in the management of bronchial asthma. N Engl J Med. 1975;292 (22):1152–1155. doi: 10.1056/NEJM197505292922204. [DOI] [PubMed] [Google Scholar]
- 2.Johansson SG. Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet. 1967;2 (7523):951–953. doi: 10.1016/s0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- 3.Bradley BL, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88(4):661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 5.Corry DB, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4(5):344–355. [PMC free article] [PubMed] [Google Scholar]
- 6.Grunig G, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282 (5397):2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavett SH, et al. Interleukin-4 receptor blockade prevents airway responses induced by antigen challenge in mice. Am J Physiol. 1997;272(2 Pt 1):L253–261. doi: 10.1152/ajplung.1997.272.2.L253. [DOI] [PubMed] [Google Scholar]
- 8.Keane-Myers A, et al. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J Immunol. 1997;158(5):2042–2049. [PubMed] [Google Scholar]
- 9.Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282 (5397):2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 10.Corry DB, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183(1):109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corry DB. Emerging immune targets for the therapy of allergic asthma. Nature Rev Drug Discov. 2002:1, 55–64. doi: 10.1038/nrd702. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey CD, Celedon JC. The hygiene hypothesis and asthma. Current Opinion in Pulmonary Medicine. 2005;11 (1):14–20. doi: 10.1097/01.mcp.0000145791.13714.ae. [DOI] [PubMed] [Google Scholar]
- 13.Pepys J, et al. Allergic reactions of the lungs to enzymes of Bacillus subtilis. Lancet. 1969;1 (7607):1181–1184. doi: 10.1016/s0140-6736(69)92166-7. [DOI] [PubMed] [Google Scholar]
- 14.Pepys J. Immunological and clinical findings in workers and consumers exposed to enzymes of Bacillus subtilis. Proc Royal Soc Med. 1973;66(9):930–932. doi: 10.1177/003591577306600950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepys J, et al. Clinical and immunological responses to enzymes of Bacillus subtilis in factory workers and consumers. Clin Allergy. 1973;3 (2):143–160. doi: 10.1111/j.1365-2222.1973.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 16.Cullinan P, et al. An outbreak of asthma in a modern detergent factory. Lancet. 2000;356 (9245):1899–1900. doi: 10.1016/s0140-6736(00)03264-5. [DOI] [PubMed] [Google Scholar]
- 17.Baur X, Fruhmann G. Allergic reactions, including asthma, to the pineapple protease bromelain following occupational exposure. Clinical Allergy. 1979;9 (5):443–450. doi: 10.1111/j.1365-2222.1979.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 18.Burrows B, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320(5):271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 19.Sporik R, et al. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323(8):502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 20.Wan H, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104(1):123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough L, et al. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999;190(12):1897–1902. doi: 10.1084/jem.190.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz O, et al. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med. 1998;187(2):271–275. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King C, et al. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161(7):3645–3651. [PubMed] [Google Scholar]
- 24.Tsitoura DC, et al. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163(5):2592–2600. [PubMed] [Google Scholar]
- 25.Akbari O, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8(9):1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 26.Corry DB, Irvin CG. Promise and pitfalls in animal-based asthma research: Building a better mousetrap. Immunol Res. 2006;35(3):279–294. doi: 10.1385/IR:35:3:279. [DOI] [PubMed] [Google Scholar]
- 27.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296 (5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov R, et al. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388 (6640):394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 29.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11(1):13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 30.Schnare M, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2(10):947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 31.Kim YG, et al. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28 (2):246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Ramet M, et al. Drosophila scavenger receptor CI is a pattern recognition receptor for bacteria. Immunity. 2001;15 (6):1027–1038. doi: 10.1016/s1074-7613(01)00249-7. [DOI] [PubMed] [Google Scholar]
- 33.Thomas CA, et al. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med. 2000;191(1):147–156. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabbagh K, et al. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002;168(9):4524–4530. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 36.Wan GH, et al. Airborne endotoxin exposure and the development of airway antigen-specific allergic responses. Clin Exp Allergy. 2000;30 (3):426–432. doi: 10.1046/j.1365-2222.2000.00730.x. [DOI] [PubMed] [Google Scholar]
- 37.Redecke V, et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172(5):2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 38.Bortolatto J, et al. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clinical & Experimental Allergy. 2008;38 (10):1668–1679. doi: 10.1111/j.1365-2222.2008.03036.x. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez D, et al. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol. 2003;171(2):1001–1008. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- 40.Velasco G, et al. Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am J Respir Cell Mol Biol. 2005;32(3):218–224. doi: 10.1165/rcmb.2003-0435OC. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe J, et al. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003;278(43):42361–42368. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- 42.Kheradmand F, et al. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169(10):5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 43.Porter P, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2(6):504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh M, et al. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. Journal of Allergy & Clinical Immunology. 2010;125 (6):1369–1378. doi: 10.1016/j.jaci.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cates EC, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173(10):6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 46.Sokol CL, et al. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9(3):310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kukreja N, et al. Effect of proteolytic activity of Epicoccum purpurascens major allergen, Epi p 1 in allergic inflammation. Clin Exp Allergy. 2008;154 (2):162–171. doi: 10.1111/j.1365-2249.2008.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudha VT, et al. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy. 2008;63 (6):768–776. doi: 10.1111/j.1398-9995.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 49.Kolattukudy PE, et al. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect Immun. 1993;61(6):2357–2368. doi: 10.1128/iai.61.6.2357-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith JM, et al. Virulence of Aspergillus fumigatus double mutants lacking restriction and an alkaline protease in a low-dose model of invasive pulmonary aspergillosis. Infect Immun. 1994;62(12):5247–5254. doi: 10.1128/iai.62.12.5247-5254.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattila PE, et al. Dectin-1 Fc targeting of aspergillus fumigatus beta-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008;52(3):1171–1172. doi: 10.1128/AAC.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447 (7140):92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan GH, et al. An airbone mold-derived product, beta-1,3-D-glucan, potentiates airway allergic responses. Eur J Immunol. 1999;29(8):2491–2497. doi: 10.1002/(SICI)1521-4141(199908)29:08<2491::AID-IMMU2491>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 54.Yoon J, et al. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. Journal of Immunology. 2008;181 (4):2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattern IE, et al. Isolation and characterization of mutants of Aspergillus niger deficient in extracellular proteases. Mol Gen Genet. 1992;234(2):332–336. doi: 10.1007/BF00283855. [DOI] [PubMed] [Google Scholar]
- 56.Shakib F, et al. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19 (7):313–316. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 57.Schmidlin F, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169(9):5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 58.Drouin SM, et al. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169(10):5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 59.Drouin SM, et al. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2001;167(8):4141–4145. doi: 10.4049/jimmunol.167.8.4141. [DOI] [PubMed] [Google Scholar]
- 60.Trinchieri G, et al. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Progress in Growth Factor Research. 1992;4 (4):355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 61.Goswami S, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nature Immunology. 2009;10 (5):496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Postma DS, et al. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995;333(14):894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 63.Mansur AH, et al. Association study of asthma and atopy traits and chromosome 5q cytokine cluster markers. Clin Exp Allergy. 1998;28 (2):141–150. doi: 10.1046/j.1365-2222.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 64.Kabesch M, et al. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol. 2006;117(2):269–274. doi: 10.1016/j.jaci.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 65.Guerra S, et al. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109(3):419–425. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, et al. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope. 2003;113 (7):1199–1205. doi: 10.1097/00005537-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 67.Tang P, et al. Allergic fungal sinusitis associated with Trichoderma longibrachiatum. J Clin Microbiol. 2003;41(11):5333–5336. doi: 10.1128/JCM.41.11.5333-5336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tosca MA, et al. Improvement of clinical and immunopathologic parameters in asthmatic children treated for concomitant chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2003;91(1):71–78. doi: 10.1016/s1081-1206(10)62062-5. [DOI] [PubMed] [Google Scholar]
- 69.Tsao CH, et al. Concomitant chronic sinusitis treatment in children with mild asthma: the effect on bronchial hyperresponsiveness. Chest. 2003;123 (3):757–764. doi: 10.1378/chest.123.3.757. [DOI] [PubMed] [Google Scholar]
- 70.Ponikau JU, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74(9):877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 71.Shin SH, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. Journal of Allergy & Clinical Immunology. 2004;114 (6):1369–1375. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Carney AS, et al. Th2 immunological inflammation in allergic fungal sinusitis, nonallergic eosinophilic fungal sinusitis, and chronic rhinosinusitis. American Journal of Rhinology. 2006;20 (2):145–149. [PubMed] [Google Scholar]
- 73.Vallor AC, et al. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob Agents Chemother. 2008;52(7):2593–2598. doi: 10.1128/AAC.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borish L, et al. Fungi in chronic hyperplastic eosinophilic sinusitis: reasonable doubt. Clin Rev Allergy Immunol. 2006;30(3):195–204. doi: 10.1385/CRIAI:30:3:195. [DOI] [PubMed] [Google Scholar]
- 75.Luong A, et al. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009;23 (3):281–287. doi: 10.2500/ajra.2009.23.3311. [DOI] [PubMed] [Google Scholar]
- 76.Ward GW, Jr, et al. Treatment of late-onset asthma with fluconazole. J Allergy Clin Immunol. 1999;104(3):541–546. doi: 10.1016/s0091-6749(99)70321-0. [DOI] [PubMed] [Google Scholar]
- 77.Denning DW, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179(1):11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 78.Pasqualotto AC, et al. The effects of antifungal therapy on severe asthma with fungal sensitization and allergic bronchopulmonary aspergillosis. Respirology. 2009;14 (8):1121–1127. doi: 10.1111/j.1440-1843.2009.01640.x. [DOI] [PubMed] [Google Scholar]
- 79.Vicencio AG, et al. Severe asthma with fungal sensitization in a child: response to itraconazole therapy. Pediatrics. 2010;125(5) doi: 10.1542/peds.2009-2443. [DOI] [PubMed] [Google Scholar]
- 80.Lemanske RF, Jr, Busse WW. Asthma. Jama. 1997;278 (22):1855–1873. [PubMed] [Google Scholar]
- 81.Humbert M, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med. 1996;154(5):1497–1504. doi: 10.1164/ajrccm.154.5.8912771. [DOI] [PubMed] [Google Scholar]