Summary
Whether the recently identified innate lymphocyte population co-expressing natural killer cell receptors (NKRs) and the nuclear receptor RORγt is part of the NK or lymphoid tissue inducer (LTi) cell lineage remains unclear. Using adoptive transfer of genetically tagged LTi-like cells, we demonstrate that NKR−RORγt+ innate lymphocytes but not NK cells were direct progenitors to NKR+RORγt+ cells in vivo. Genetic lineage tracing revealed that the differentiation of LTi-like cells was characterized by the stable upregulation of NKRs and a progressive loss of RORγt expression. Whereas interleukin-7 (IL-7) and intestinal microbiota stabilized RORγt expression within such NKR-LTi cells, IL-12 and IL-15 accelerated RORγt loss. RORγt+ NKR-LTi cells produced IL-22, whereas RORγt− NKR-LTi cells released IFN-γ and were potent inducers of colitis. Thus, the RORγt gradient in NKR-LTi cells serves as a tunable rheostat for their functional program. Our data also define a previously unappreciated role of RORγt− NKR-LTi cells for the onset or maintenance of inflammatory bowel diseases.
Introduction
The evolutionarily ancient innate immune system is the first barrier against infections and tumors. It is equipped with two principal hematopoietic cell lineages - myeloid and lymphoid. In addition to natural killer (NK) cells, two additional innate lymphocyte subsets have been described -lymphoid tissue inducer (LTi) cells and natural helper cells (also known as type 2 innate lymphocytes or nuocytes) (Moro et al., 2010; Neill et al., 2010; Price et al., 2010). Development of LTi cells, NK cells and natural helper cells depends on the transcription factor inhibitor of DNA binding 2 (Id2), suggesting that the innate lymphocyte lineages share a common transcriptional and developmental program (Moro et al., 2010; Yokota et al., 1999). Specific transcription factors and cytokines unique to LTi and NK cell fate decisions have also been determined. Commitment to the LTi lineage requires the orphan nuclear receptor RORγt and interleukin-7 (IL-7), whereas the NK cell fate is determined by the recently identified transcription factor E4bp4 (Nfil3) and IL-15 (Gascoyne et al., 2009; Kennedy et al., 2000; Luther et al., 2003; Meier et al., 2007; Sun et al., 2000).
NK cells are cytotoxic lymphocytes that express activating immunoreceptors (i.e., NKG2D; NCR1, also known as NKp46; NKR-P1C, also known as NK1.1) allowing them to eliminate infected, transformed or stressed cells (Lanier, 2005). In addition, NK cells are potent producers of interferon-γ (IFN-γ). During embryonal development, LTi cells are indispensable for lymphorganogenesis (Mebius et al., 1997; Sun et al., 2000). Lymphocytes phenotypically resembling LTi cells can also be identified after birth but their role is not well defined. LTi-like cells within the intestinal lamina propria of adult mice serve as inducer cells of tertiary lymphoid organs such as cryptopatches and intestinal lymphoid follicles which are required for T cell-independent IgA-synthesis (Bouskra et al., 2008; Tsuji et al., 2008). LTi cells are also an important innate source of IL-22 and IL-17 (Cupedo et al., 2009; Takatori et al., 2009) and may be involved in repairing tissue damage after viral infections (Scandella et al., 2008).
A population of IL-22-producing lymphocytes co-expressing RORγt and activating NKRs (i.e., NKp46+RORγt+ cells) that localized within the intestinal lamina propria was identified (Cella et al., 2009; Cupedo et al., 2009; Hughes et al., 2009; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008). In mice, these cells are involved in safeguarding epithelial homeostasis (Sanos et al., 2009) and in protecting against various forms of experimental colitis (Satoh-Takayama et al., 2008; Zenewicz et al., 2008). Similar to LTi cells, NKp46+RORγt+ cells depend on RORγt for their differentiation and/or development and are potent producers of IL-22. In contrast, conventional (c) NK cells (i.e., NKp46+RORγt−) are independent of RORγt and do not produce IL-22 (Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008). It is unclear whether NKp46+RORγt+ cells are part of the NK cell or the LTi cell lineage or, alternatively, constitute a third independent lineage of innate lymphocytes (Cooper et al., 2009).
Chronic inflammatory disorders such as inflammatory bowel diseases (Crohn’s disease, ulcerative colitis) are caused by lymphocytes that are inappropriately activated and destroy self-tissues. Recent data have assigned a disease-promoting role to IL-23, a cytokine of the IL-12 family (Duerr et al., 2006; Izcue et al., 2009). Such forms of autoimmunity have often been perceived to be exclusively caused by lymphocytes of the adaptive immune system, but recent evidence suggests that IL-23-responsive innate lymphocytes may be sufficient to cause colitis in mice (Buonocore et al., 2010; Hue et al., 2006; Uhlig et al., 2006). In mice lacking all lymphocytes of the adaptive immune system (Rag2−/−), experimental colitis can be triggered by CD40-mediated activation of myeloid cells or by infection with Helicobacter hepaticus (Buonocore et al., 2010; Hue et al., 2006; Uhlig et al., 2006). CD40-triggered colitis is dependent on IL-23, IFN-γ and tumor necrosis factor (TNF) (Uhlig et al., 2006) and cannot be triggered in animals genetically lacking RORγt (Buonocore et al., 2010). However, the colitogenic, IL-23-responsive innate lymphocyte subset remains undefined (Diefenbach and Vonarbourg, 2010).
Using a combination of genetic lineage tracing and in vivo transfer of genetically tagged cells, we report that RORγt+ innate lymphoid cells (i.e., LTi-like cells) and not cNK cells are direct progenitors to NKp46+RORγt+ lymphocytes in vivo. We now refer to these as NKR-LTi cells. NKR-LTi cells progressively loose RORγt expression, and the resulting gradient of RORγt expression determines a distinct phenotype and functional program. RORγt+ NKR-LTi cells produced IL-22, whereas RORγt− NKR-LTi cells released large amounts of IFN-γ. Interestingly, intestinal IFN-γ-producing RORγt− NKR-LTi cells, but not cNK cells, were potent inducers of experimental colitis. Thus, graded expression of RORγt serves as a molecular rheostat to generate functional plasticity within the NKR-LTi population.
Results
NKp46−RORγt+ LTi-like cells are progenitors of NKp46+RORγt+ cells
Consistent with the available data, three models can be proposed for the differentiation of intestinal NKp46+RORγt+ cells (Figure S1A). One model (LTi lineage model) is that NKp46+RORγt+ cells are derived from NKR−RORγt+ LTi-like cells that upregulate NKRs. Another model (NK lineage model) proposes that NK cells, similar to IL-17-producing CD4 T cells (i.e., Th17 cells), upregulate RORγt expression as part of a distinct differentiation program endowing these cells with a particular functional profile (i.e., IL-22 production). NKp46+RORγt+ cells could also represent a distinct third innate lymphocyte lineage independent of NK and LTi-like cells. It is currently unknown which of these models is correct.
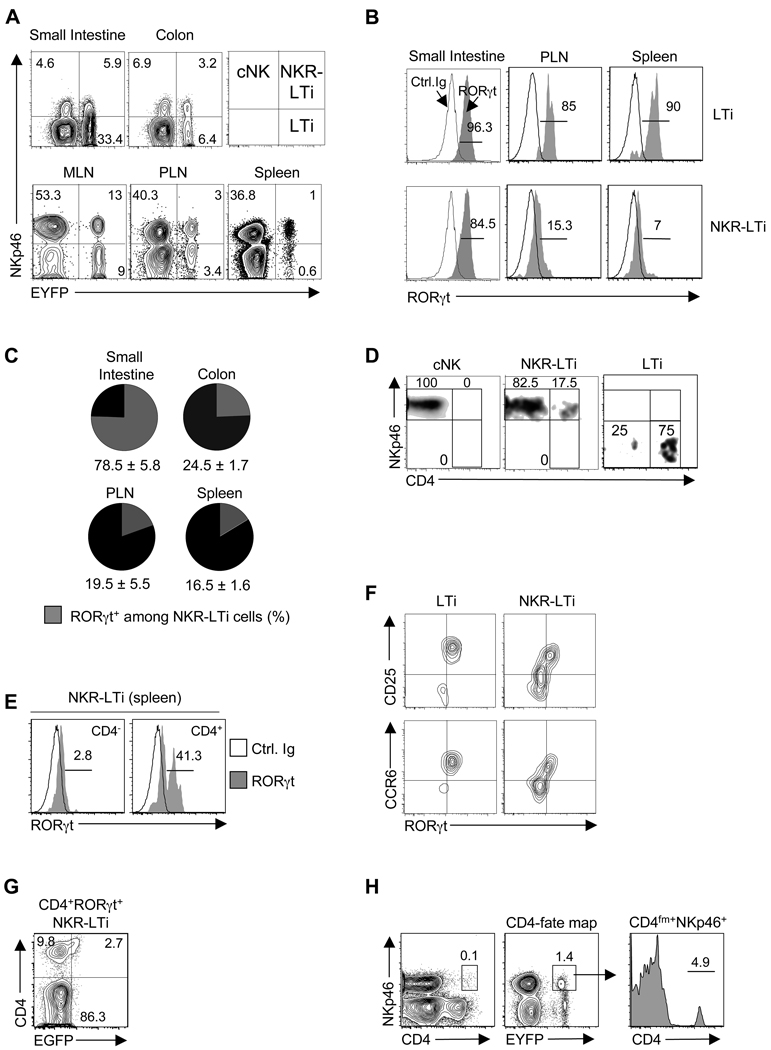
These models can be experimentally probed in cell transfer experiments employing donor cells from mice with a reporter gene (EGFP) knocked into the Rorc locus (encoding RORγt) (Eberl et al., 2004). As previously reported, RORγt expression serves as a faithful marker of intestinal LTi-like cells in adult mice (Eberl and Littman, 2004). Other innate lymphocyte lineages (i.e., natural helper cells) do not express RORγt (Moro et al., 2010). LTi cells and NKp46+RORγt+ cells were represented in the lamina propria of the small intestine, colon and in mesenteric LNs (Figure 1A) (Eberl and Littman, 2004; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008). We highly purified (purity >98%) NK cells (NKp46+RORγt−) and genetically tagged LTi-like cells (NKp46−RORγt+) from the lamina propria of the small intestine of Rorcgfp/+ mice (H-2b) (Figure S1B,C) and transferred them into Rag2−/−Il2rg−/− mice (H-2d) that lack all lymphocytes including NK cells and LTi cells. Transferred (H-2b) NK cells did not appreciably upregulate RORγt expression and remained NKp46+RORγt− cells even six months after transfer (Figure 1B and data not shown). In contrast, transferred NKp46−RORγt+ cells differentiated into NKp46+ cells but a subpopulation remained negative for NKp46 even six months after transfer (Figure 1B and data not shown). Upregulation of NKp46 was already observed two weeks after transfer and the fraction of NKp46+ LTi-derived cells further increased after four weeks (Figure 1B). Although virtually all of the donor-derived cells found in the small intestine remained RORγt+, a considerable fraction of LTi-derived NKp46+ cells that homed to the colonic lamina propria or the spleen turned off RORγt expression demonstrating that the extent of RORγt downregulation was dependent on the tissue environment (Figure 1B). Very similar results were obtained when transferring colonic NK cells or LTi cells (Figure S2A).
Figure 1. NKp46+RORγt+ cells are derived from NKp46−RORγt+ precursors.
(A) Flow cytometry analysis of NKp46 and RORγt (EGFP) expression by CD3−CD19− lymphocytes from the indicated organs of Rorcgfp/+ mice. Numbers represent percent cells in quadrant. n.d.: not done.
(B) 2×104 of the indicated cell populations from the small intestine of Rorcgfp/+ mice (H-2b) were transferred into Rag2−/−Il2rg−/− mice (H-2d). At the indicated timepoints, donor-derived lymphocytes were analyzed for NKp46 and RORγt (EGFP) expression. Numbers represent percent cells in quadrant.
(C) Sorted cell populations from the intestinal lamina propria of Rorcgfp/+ mice were cultured with and without feeder cells for seven days in vitro. Contour plots show NKp46 and RORγt (EGFP) expression and were electronically gated on CD45+ cells. Numbers represent percent cells in quadrant. Data are representative of three (C) or four (A,B) independent experiments.
Our in vivo data was confirmed by an in vitro culture system. Highly purified LTi-like cells (NKp46−RORγt+) were cultured with or without a stromal feeder cell layer for seven days. A substantial fraction of LTi cells readily upregulated NKp46 whereas NK cells did not gain RORγt expression in vitro (Figure 1C). Although an appreciable proportion of LTi-derived cells became RORγt-negative when the cells were cultured on an OP-9 stromal cell layer, LTi-derived cells cultured in the absence of stromal cells maintained RORγt expression (Figure 1C). This further corroborates the view that environmental cues influence maintenance or loss of RORγt expression by LTi-derived NKp46+ cells.
Lymph node LTi cells differentiate into NKR+ cells
NKp46+RORγt+ cells were originally identified in the intestinal immune system but it is unknown whether a similar cell type exists in other organs. Although an appreciable population of CD3−CD19−NKp46−RORγt+ cells was detectable in spleen and peripheral LNs (PLN) of RORγt reporter mice, only a very small proportion of RORγt-expressing NKp46+ lymphocytes was present (Figure 1A). The NKp46−RORγt+ subset represented adult-type LTi-like cells as they co-expressed various LTi markers and displayed surface lymphotoxin α1β2 (sLTα1β2), but did not express perforin or granzyme B (Figure S2B,C). After transfer into Rag2−/−Il2rg−/− mice, virtually all PLN-derived LTi cells differentiated into NKp46+ cells, whereas NK cells did not upregulate RORγt expression (Figure S2D). The acquisition of NKp46 by PLN LTi-like cells was confirmed by in vitro culture (Figure S2E,F). The differentiation of PLN-derived LTi-like cells into NKp46+ cells that lost RORγt ocurred more rapidly and more completely compared to intestinal LTi cells (Figures 1B,C,S2A vs. S2D–F). Conventional NK cells from PLN or spleen did not upregulate RORγt (Figure S2F), even under culture conditions that induce RORγt expression in CD4+ T cells (Figure S2G). Thus, NKp46+RORγt+ cells differentiate from NKp46−RORγt+ precursor cells (i.e., LTi-like cells) whereas cNK cells do not acquire RORγt expression. Consequently, we will refer to these cells as RORγt+ NKR-expressing LTi-like (NKR-LTi) cells.
Genetic lineage tracing reveals two distinct NKR-expressing lymphocyte lineages
Based on our in vivo transfer data (Figure 1B,S2A,D), we considered that RORγt expression of NKp46+ cells may be transient and become undetectable in RORγt reporter mice. We used genetic lineage tracing (“fate mapping”) to visualize in lymphoreplete mice all NKp46+ cells derived from RORγt+ progenitors, including those which had lost RORγt. Mice expressing Cre recombinase under the control of the Rorc locus control elements (Rorc-CreTg) (Eberl and Littman, 2004) were crossed with Rosa26-reporter mice (R26R-EYFP) (Srinivas et al., 2001). The R26R-EYFP strain expresses a fluorescent reporter under the control of the ubiquitously active Rosa26 promoter once the LoxP-flanked STOP cassette is excised (Figure S3A). In Rorc-CreTg;R26R-EYFP mice, all cells derived from RORγt+ precursors are permanently and heritably marked by the EYFP reporter (i.e., RORγtfm+), even if they subsequently loose RORγt expression. RORγt expression by RORγtfm+ cells can be analyzed through co-staining with an RORγt-specific antibody.
Analysis of innate lymphocytes from RORγt-fate map mice revealed that a fraction of NKp46+ cells in all organs tested was RORγtfm−, demonstrating that these cells did not express RORγt at any time during their lineage development (Figure 2A). NKp46+RORγtfm− cells displayed cell surface markers consistent with NK cells (Figure S3B,C) and expressed perforin and granzyme B, which are required for cell-mediated cytotoxicity (Figure S3D). Taking into consideration that under no conditions did NKp46+RORγt− cells upregulate RORγt (Figure 1B,C, S2A,D–G), we conclude that NKp46+RORγtfm− cells constitute a lymphocyte lineage developmentally distinct from RORγt-expressing lymphoid cells. We refer to NKp46+RORγtfm− cells as cNK cells (Figure 2A).
Figure 2. Genetic lineage tracing reveals progressive loss of RORγt in NKR-LTi cells.
(A) Flow cytometry analysis of NKp46 and RORγt-fate map (EYFP) in CD3−CD19− lymphocytes from the indicated organs. Numbers represent percent cells in each quadrant.
(B) Lymphocytes from the indicated organs of RORγt-fate map mice were stained with antibodies specific for CD3, CD19, NKp46 and RORγt or isotype control antibody. Histograms are electronically gated on CD3−CD19− cells and represent staining of the indicated cell populations with RORγt (grey) or isotype control Ab (open). Numbers indicate percentages of RORγt+ cells.
(C) Percentage (± SEM, n=7) of RORγt+ cells among all NKR-LTi cells in the indicated organs.
(D) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46 and CD4. Density plots are electronically gated on CD3−CD19− cells and depict the proportion of CD4 and NKp46 expressing cells within the indicated cell populations.
(E) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46, CD4 and RORγt (grey) or with isotype control antibody (open). Histograms are gated on CD3− CD19− cells and depict expression of RORγt within CD4+ and CD4− NKR-LTi cells. Numbers indicate percentages of RORγt+ cells.
(F) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46, CD25, CCR6 and RORγt. Density plotes are electronically gated on CD3−CD19− cells and show co-expression of RORγt and CD25 (top) or CCR6 (bottom).
(G) 2×104 splenic CD4+RORγt+ NKR-LTi cells of Rorcgfp/+ mice (H-2b) were transferred into Rag2−/−Il2rg−/− mice (H-2d). Two weeks later, CD4 and RORγt (EGFP) expression by donor-derived cells (H-2b) was determined. Numbers represent percent cells in quadrants.
(H) Splenocytes from CD4-fate map mice were stained for CD3, CD19, NKp46 and CD4. Contour plots are gated on CD3−CD19− cells and depict expression of NKp46 and CD4 or CD4-fate map (EYFP). Histogram shows the percentage of NKp46+CD4fm+ cells expressing CD4. Data are representative of ten (A), seven (C), four experiments (B, D–G) or two experiments (H).
RORγtfm+ cells can be further subdivided into an NKp46+ and an NKp46− subset (Figure 2A). NKp46−RORγtfm+ cells uniformly expressed RORγt (Figure 2B) and sLTα1β2 (Figure S3E) but were negative for perforin and granzyme B (Figure S3D) and thus are identical to the NKp46−RORγt+ LTi-like cell population. This is also reflected by the absolute cell numbers of the NKp46−RORγt+ and the NKp46−RORγtfm+ subsets that were virtually identical (Figure S3F). Our transfer data demonstrated that LTi-derived cells upregulate NKp46 and consecutively lost RORγt (Figure 1B,S2A,D). Thus, the entire NKp46+ LTi cell-derived population should be contained within the NKp46+RORγtfm+ subset. Co-staining of RORγt in NKp46+RORγtfm+ cells from various organs revealed the extent of RORγt downregulation (Figure 2B,C) and exactly correlated with our transfer experiments. Approximately 80% of NKp46+RORγtfm+ cells of the small intestine were RORγt+, but <25% of cells from colon, spleen or PLNs expressed RORγt (Figure 2B,C). This was corroborated by analysis of absolute cell numbers. In Rorcgfp/+ mice only the RORγt+ NKR-LTi cells can be visualized which were lower in numbers compared to the NKp46+RORγtfm+ subset from RORγt-fate map mice (Figure S3G). This difference was most visible in organs permissive for RORγt loss (e.g., colon). Analysis of the “NK cell” population revealed the corresponding result. In Rorcgfp/+ mice, the number of NKp46+RORγt− cells was always larger compared to the NKp46+RORγtfm− population because the NKp46+RORγt− population contains cNK cells and RORγt− NKR-LTi cells (Figure 2A–C,S3G).
We aimed to identify markers of NKR-LTi cells that were influenced by RORγt expression. In spleen and PLNs, CD4 was expressed by the majority of LTi cells (Kim et al., 2008; Mebius et al., 1997) and by a subpopulation of NKR-LTi cells whereas cNK cells were negative (Figure 2D). CD4− NKR-LTi cells did not express RORγt, but the CD4+ subset contained all RORγt+ NKR-LTi cells (Figure 2E). However, CD4 did not clearly discriminate between the RORγt+ and the RORγt− NKR-LTi subsets in PLNs and only a small subpopulation of LTi cells and NKR-LTi cells in the small intestine of adult mice expressed CD4 (Figure S3H). Interestingly, CD25 (IL-2Rα chain) and CCR6 (C-C chemokine receptor 6) expression distinguished between RORγt+ and RORγt− NKR-LTi cells (Figure 2F). RORγt+ NKR-LTi cells also expressed high sLTα1β2 and CD127, which were diminished (but not absent) in RORγt− NKR-LTi cells (Figure S3E,I). RORγt− NKR-LTi cells expressed perforin and granzyme B, indicating that they may mediate cell-mediated cytotoxicity (Figure S3D). Thus, RORγt expression of NKR-LTi cells profoundly influences gene expression of cell surface markers and effector molecules.
RORγt+ NKR-LTi cells are direct progenitors to RORγt− NKR-LTi cells
As noted above, transfer of intestinal LTi cells into alymphoid mice led to the generation of both RORγt+ and RORγt− NKR-LTi cells. To determine whether RORγt+ NKR-LTi cells are the direct progenitors to RORγt− NKR-LTi cells, we transferred highly purified RORγt+ NKR-LTi cells (Figure S1B,C) from the small intestine of RORγt-reporter mice (H-2b) into alymphoid mice (H-2d). Two weeks after transfer, most of the donor-derived NKR-LTi cells in the small intestine retained RORγt expression whereas, after four weeks, a substantial fraction had downregulated RORγt (Figure 1B). In the environment of the colon or spleen the same donor cells almost entirely lost RORγt expression (Figure 1B). Similarly, CD4+RORγt+ NKR-LTi cells from PLNs differentiated into CD4+RORγt− and further into CD4−RORγt− NKR-LTi cells (Figure 2G). These data indicate that the CD4+ subset of NKR-LTi cells contains RORγt+ NKR-LTi cells and those that have recently lost RORγt. This is further supported by data from a CD4-fate map in which most of the CD4fm+ NKR-LTi cells did no longer express CD4 demonstrating that CD4− NKR-LTi cells have a CD4+ progenitor (Figure 2H). Thus, LTi cells differentiate in a two step differentiation program characterized by the stable upregulation of NKRs and the progressive loss of RORγt expression: RORγt+ LTi cells → RORγt+ NKR-LTi cells → RORγt− NKR-LTi cells. Importantly, LTi cell differentiation was restricted to NKR+ cells as the LTi cells did not differentiate into any other lymphocyte or myeloid cell population under homeostatic conditions in vivo (Figure S3J–L).
Commensal microflora and IL-7 stabilize RORγt expression within NKR-LTi cells
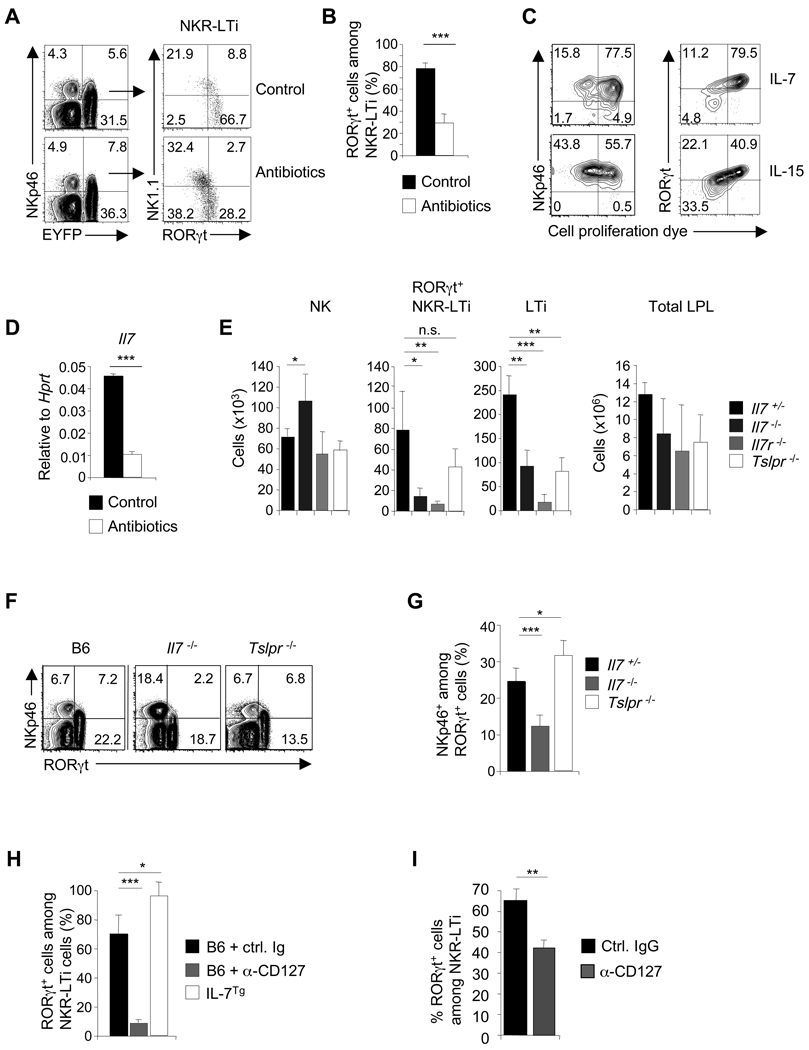
We and others had previously shown that the population of RORγt+ NKR-LTi cells was diminished in germ-free mice (Sanos et al., 2009; Satoh-Takayama et al., 2008). This could reflect reduced differentiation of LTi-like cells into RORγt+ NKR-LTi cells or microbiota-dependent stabilization of RORγt expression, slowing the progression to RORγt− NKR-LTi cells. Eradication of the intestinal microflora in RORγt-fate map mice did not lead to substantial differences in cNK, LTi or NKR-LTi cell populations both in relative and absolute numbers (Figure 3A and data not shown). However, analysis of RORγt expression within the NKR-LTi subset revealed that RORγt+ NKR-LTi cells were significantly diminished in the absence of microbiota (Figure 3A,B). Thus, the commensal microflora contributes to the stabilization of RORγt within the NKR-LTi cell subset and not to their differentiation from LTi-like progenitors.
Figure 3. Commensal microflora and IL-7 stabilize RORγt expression in NKR-LTi cells.
(A,B) Lymphocytes from the small intestine of conventional and antibiotic-treated RORγt-fate map mice were stained with antibodies specific for CD3, CD19, NKp46, NK1.1 and RORγt. Contour plots (left) are electronically gated on CD3−CD19− cells and depict expression of RORγtfm (EYFP) and NKp46. Dot plots (right) are electronically gated on NKR-LTi cells and represent staining for RORγt and NK1.1. Numbers indicate percentages. (B) Percentage (± SEM, n=3) of RORγt+ cells among NKR-LTi cells in conventional and antibiotic-treated RORγt-fate map mice.
(C) Highly purified RORγt+ NKR-LTi cells from the small intestine were labelled with a cell proliferation dye and cultured in IL-7 or IL-15 for six days. Cells were stained for NKp46 and RORγt. Numbers indicate percentages in each quadrant.
(D) Quantitative RT-PCR analysis of Il7 expression in small intestine of control mice and germ-free mice.
(E) Absolute cell numbers (± SD, n=5) of the indicated cell populations in the lamina propria of the small intestine.
(F,G) Lymphocytes of the small intestine were stained with antibodies specific for CD3, CD19, NKp46 and RORγt. Contour plots are electronically gated on CD3−CD19− cells and numbers indicate percentages within quadrants (F). (G) Percentage (± SD, n=5) of NKp46+ cells among all RORγt+ cells.
(H) 2×104 highly purified RORγt+ NKR-LTi cells (CD45.1) from the small intestine were transferred into groups of B6 (CD45.2) or IL-7Tg mice (CD45.2). B6 mice were treated with IL-7Rα antibodies or control Ig. Percentages (± SEM, n=3) of RORγt+ cells among donor-derived cells (CD45.1+) were determined two weeks after transfer.
(I) RORγt-fate map mice were treated with either IL-7Rα antibodies or control Ig. Percentages (± SEM, n=3) of RORγt+ cells among NKR-LTi cells were determined after six days. Data are representative of at least three independent experiments.
An important question is whether the differentiation of RORγt+ into RORγt− NKR-LTi cells requires proliferation. We labelled highly purified intestinal RORγt+ NKR-LTi cells with a cell proliferation dye and analyzed proliferation in the context of RORγt expression after in vitro culture. Cells that downregulated RORγt expression had proliferated, whereas RORγthi NKR-LTi cells did not (Figure 3C). RORγt+ NKR-LTi cells cultured in IL-7 retained RORγt expression and proliferated less, while cells cultured in IL-15 proliferated more and downregulated RORγt expression (Figure 3C). Thus, loss of RORγt expression by NKR-LTi cells required proliferation and IL-7 may be an important factor for stabilizing RORγt.
IL-7 expression was reduced in the intestine of germ-free mice, suggesting a mechanism for the de-stabilization of RORγt expression of NKR-LTi cells (Figure 3D). We analyzed mice deficient in IL-7, IL-7Rα and TSLPR (thymic stromal lymphopoietin receptor) for the presence of intestinal LTi-like cells, RORγt+ NKR-LTi cells and NKp46+RORγt− cells. In extension of previously published data, intestinal LTi-like and RORγt+ NKR-LTi cells were virtually absent in Il7r−/− mice that lack IL-7 and TSLP signalling, whereas NK cell development was not impacted (Figure 3E) (Satoh-Takayama et al., 2010). In contrast, LTi-like cells developed in Il7−/− or Tslpr−/− mice, albeit at reduced numbers (Figure 3E,F). Thus, IL-7 and TSLP play redundant roles for development and/or survival of intestinal LTi cells. Despite equal representation of LTi-like cells in Il7−/− and Tslpr−/− mice, RORγt+ NKR-LTi cells were significantly reduced in Il7−/− (but not in Tslpr−/−) mice, whereas the numbers of NKp46+RORγt− cells (composed of cNK cells and RORγt− NKR-LTi cells) were substantially increased (Figure 3E–G). We considered that IL-7 stabilizes RORγt expression in NKR-LTi cells. We transferred RORγt+ NKR-LTi cells from Rorcgfp/+ mice into mice treated with a blocking antibody to the IL-7R or into mice overexpressing IL-7 (IL-7Tg mice). Although the majority of donor-derived NKR-LTi cells retained RORγt expression in mice treated with control Ig, RORγt expression was virtually lost in the absence of IL-7 signalling. In contrast, the generation of RORγt− NKR-LTi cells was diminished in mice overexpressing IL-7 (Figure 3H). These data were confirmed by blocking IL-7R signalling in RORγt-fate map mice (Figure 3I,S4A). Collectively, our data demonstrates that IL-7 but not TSLP stabilizes RORγt expression in NKR-LTi cells.
No significant differences in absolute and relative numbers of LTi cells, RORγt+ NKR-LTi cells and NKp46+RORγt− cells were found in Il6−/−, Il1r−/− and Il23p19−/− mice (Figure S4B–I). Interestingly, mice deficient for Il12a (encoding IL-12p35) or Il12rb2, which is required for IL-12 but not IL-23 signalling, showed a significant increase in the fraction of intestinal RORγt+ NKR-LTi cells (Figure S4F–I). This may imply that IL-12 destabilizes RORγt expression in NKR-LTi cells.
Human “NK-22” but not NK cells developmentally depend on IL7R signalling
Human IL-22-producing CD56+NKp44+ cells isolated from tonsils expressed IL22 and RORC, whereas conventional CD56+NKp44− NK cells did not (Figure 4A) (Cella et al., 2010). A four stage differentiation program for human “NK cells” from progenitor cells within secondary lymphoid organs was proposed and it was found that “stage 3 NK cells” contained IL-22-producing cells (Freud et al., 2006; Hughes et al., 2009). Although stage 1, 2 and 4 NK cells from peripheral blood and tonsils did not express IL22 or RORC, “stage 3 NK cells” expressed high RORC and IL22 (Figure 4B and data not shown). As cNK cells and their progenitors did not express RORγt (Figure 1,2A), we reasoned that human IL-22-producing RORC+CD56+ cells may represent human NKR-LTi cells. Mutations of the IL7R gene in humans causes severe combined immunodeficiency disease (SCID) and analysis of immune cells from these patients revealed a substantial reduction of T cells, whereas NK cells and B cells developed normally (Figure 4C) (Lai et al., 1997; Puel et al., 1998). We investigated the representation of RORC+ IL-22-producing “stage 3 NK cells” in humans with IL7R-deficiency. “Stage 3 NK cells” were virtually absent in patients with IL7R deficiency whereas stage 1, 2 and 4 were represented normally (Figure 4D). Considering our mouse data showing that cNK cells at no time express RORγt and that they do not require IL-7 for their development, these data indicate that human IL-22-producing lymphocytes are not part of the cNK cell lineage but may be a subset of human NKR-LTi cells.
Figure 4. Human IL-22-producing cells depend on IL-7 for their development.
(A,B) Quantitative RT-PCR analysis of IL22 and RORC expression by the indicated, populations from human tonsils (A) or peripheral blood (B). Stage 1: CD34+CD117− CD94−, Stage 2: CD34+CD117+CD94−, Stage 3: CD34−CD117+CD94−, Stage 4: CD34−CD117+CD94+.
(C,D) Mononuclear cells were stained with antibodies specific for the indicated markers and for CD3 and CD19. Dot plots (C) were electronically gated on CD3−CD19− cells. Contour plots (D) exclude all CD3+ and CD19+ cells and are electronically gated on CD34+ (stage 1 and stage 2) or CD34− cells (stage 3 and stage 4). Numbers indicate percentage of cells within the adjacent gates. Data are representative of two independent experiments.
RORγt levels assign distinct functional programs to NKR-LTi cells
We found a previously unappreciated plasticity within RORγt+ innate lymphoid cells, allowing us to identify discrete LTi cell-derived populations that develop along a decreasing gradient of RORγt. IL-12 and IL-23 are related heterodimeric cytokines acting on NK and LTi-derived cells that signal through related receptors (Oppmann et al., 2000). We investigated the expression of the IL-12 and IL-23 receptors (R) by cNK cells and LTi-derived cells. The IL-12R and IL-23R complexes share the IL-12 and IL-23R β1 chain that forms a heterodimer with the IL-12-specific IL-12Rβ2 chain or the IL-23-specific IL-23R. The β1 chain was detected on the surface of all cNK cells, LTi cells and NKR-LTi cells from PLNs, spleen and intestine (Figure 5A and data not shown). Expression of Il23r correlated with RORγt expression and was highest in LTi cells and CD4+ NKR-LTi cells whereas CD4− (i.e., RORγt−) NKR-LTi cells expressed very low and cNK cells had almost undetectable amounts of Il23r (Figure 5B). In contrast, expression of Il12rb2 inversely correlated with RORγt expression (Figure 5C).
Figure 5. RORγt expression within NKR-LTi cells determines distinct functional fates.
(A) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46, IL-12/23Rβ1 (grey) or isotype control antibody (open). Histograms depict electronic gating on the indicated cell populations after exclusion of CD3+ and CD19+ cells.
(B,C,G,H) Quantitative RT-PCR analysis of the expression of Il23r (B,G) and Il12rb2 (C,H) in sorted cell populations from spleen (B,C) or colon (G,H) of RORγt-fate map mice.
(D,E) Cytokine production of highly purified cell populations from spleens of RORγt-fate map mice (D) or Rorcgfp/+ mice (E) stimulated for 24 hours with the indicated cytokines. Contour plots depict staining with anti-IL-22 and anti-IFN-γ. The numbers indicate the percentage of cells in each quadrant.
(F) Groups of RORγt-fate map mice were injected with LPS. 18 hours later, splenocyte suspensions were stained with antibodies specific for CD3, CD19, NKp46, CD4, IL-22 and IFN-γ. Contour plots are electronically gated on the indicated cell populations after exclusion of all CD3+ and CD19+ cells. Numbers indicate percentage of cells in each quadrant.
(I) Highly purified lamina propria lymphocytes from the colon of RORγt-fate map mice were cultured for 24h in IL-23. Contour plots depict expression of IFN-γ and RORγt. Numbers indicate the percentage of cells in each quadrant.
We stimulated highly purified lymphocytes from PLNs of RORγt-fate map mice with IL-12 or IL-23. Stimulation of LTi cells and CD4+ NKR-LTi cells (containing all RORγt+ NKR-LTi cells) with IL-23 led to IL-22 but not IFN-γ production (Figure 5D). CD4− (i.e., RORγt−) NKR-LTi cells and cNK cells did not produce IL-22 or IFN-γ in response to IL-23, and LTi cells did not produce either cytokine in response to IL-12, which is in agreement with their very low Il23r or Il12rb2 expression, respectively (Figure 5B–D). After IL-12 stimulation, highly purified cNK cells produced IFN-γ but not IL-22 (Figure 5D). Consistent with their substantial expression of Il12rb2, CD4− (i.e., RORγt−) NKR-LTi cells also produced IFN-γ in response to IL-12 stimulation but had lost the potential to produce IL-22 (Figure 5D). CD4+ NKR-LTi cells that contain both RORγt+ and RORγt− NKR-LTi subsets (Figure 2E) showed a more complex cytokine production pattern in response to IL-12. Although a substantial population of CD4+ NKR-LTi cells produced IL-22, we noted a similar fraction of IFN-γ producers and even a highly reproducible subset producing both cytokines (Figure 5D). CD4+ NKR-LTi cells are composed of RORγt+ NKR-LTi cells and RORγt− NKR-LTi cells that have very recently lost RORγt expression (Figure 2E,G,H). We reasoned that the cytokine production profiles may be goverened by the different amounts of RORγt. Indeed, CD4+RORγt+ NKR-LTi cells produced IL-22, whereas CD4+RORγt− NKR-LTi cells produced either IFN-γ alone or both IFN-γ and IL-22 (Figure 5E). Very similar data was obtained after in vivo stimulation of NKR-LTi cells. Splenic LTi cells and CD4+ NKR-LTi cells robustly produced IL-22 (Figure 5F). While LTi cells did not produce IFN-γ, a small fraction of IFN-γ-producing cells was detected within the population of CD4+ NKR-LTi cells that contains RORγt− NKR-LTi cells. As in the in vitro experiments, cNK cells and CD4− (i.e., RORγt−) NKR-LTi cells did not produce IL-22 but were instead IFN-γ producers (Figure 5F). Our data demonstrates that the gradual loss of RORγt expression within NKR-LTi cells functions as a molecular rheostat for their responsiveness to IL-12 family cytokines and for their cytokine expression program.
RORγt− NKR-LTi cells are potent initiators of experimental innate colitis
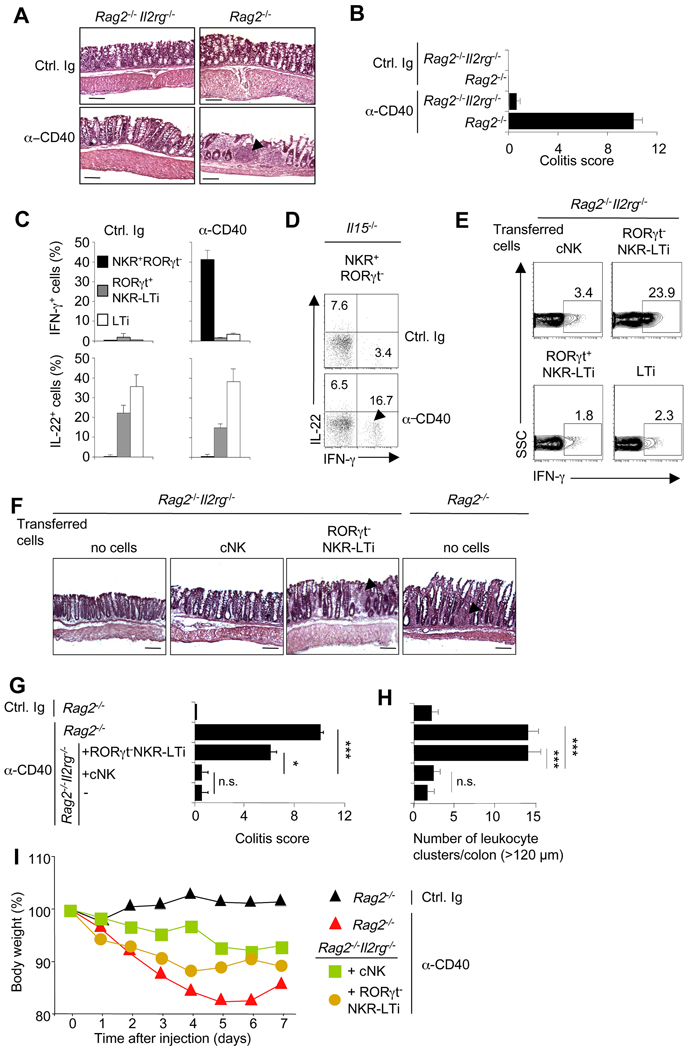
RORγt+ NKR-LTi cells and LTi cells are constitutive IL-22 producers and promote epithelial homeostasis by regulating expression of tissue protective genes (e.g., Reg3 genes) (Sanos et al., 2009). We attempted to assign function to the previously unrecognized population of RORγt− NKR-LTi cells which is the dominating subset of NKR-LTi cells in the colon (Figure 2C). In contrast to RORγt− NKR-LTi cells from PLN, colonic RORγt− NKR-LTi cells retained Il23r expression (Figure 5B,G,H). Interestingly, colon RORγt− NKR-LTi cells but not RORγt+ NKR-LTi, LTi or cNK cells produced IFN-γ in response to IL-23 (Figure 5I and data not shown). IL-23-dependent IFN-γ production is required for the development of experimental colitis in Rag2−/− mice after systemic administration of CD40 antibodies but the colitogenic cell type is poorly defined (Buonocore et al., 2010; Diefenbach and Vonarbourg, 2010; Uhlig et al., 2006). Rag2−/− mice injected with anti-CD40 developed extensive colitis but Rag2−/−Il2rg−/− mice that lack all lymphocytes did not (Figure 6A,B). Colitis onset in Rag2−/− mice correlated with the presence of IFN-γ producing cells absent in Rag2−/−Il2rg−/− mice (Figure S5A). Thus, an innate IFN-γ-producing lymphocyte subset is required for pathogenesis.
Figure 6. RORγt− NKR-LTi cells induce CD40-triggered colitis.
(A–D) Groups of Rag2−/−, Il15−/− and Rag2−/−Il2rg−/− mice were injected with control Ig or anti-CD40 and were analyzed seven days later. (A) Histological analysis of colon sections (H&E stain). Arrowhead points to a cluster of infiltrating leukocytes that can only be found in mice with colitis. Bar = 100µm. (B) Clinical colitis score (± SEM; n=5). (C,D) Lamina propria lymphocytes from colon were stained for CD45, NKp46, RORγt, IL-22 and IFN-γ. (C) Bar diagrams show percentages (± SEM; n=5) of IFN-γ or IL-22-producing cells within the indicated lymphocyte populations. (D) Dot plots depict expression of IFN-γ and IL-22 by NKR+RORγt− cells. Numbers indicate the percentage of cells in each quadrant.
(E–I) 5×104 highly purified cells from the intestine of RORγt-fate map mice (H-2b) were transferred into Rag2−/−Il2rg−/− mice (H-2d). (E) Five days after transfer, mice were injected with anti-CD40. Seven days after CD40 injection, donor cells were analyzed for IFN-γ production. Numbers indicate percentage of IFN-γ+ cells. (F–I) Two weeks after transfer mice were injected with control Ig or anti-CD40 and anlyzed seven days later. (F) Histological analysis of colon sections (H&E stain). Arrowheads point to clusters of infiltrating leukocytes that can only be found in mice with colitis. Bar = 100µm. (G) Clinical colitis score (± SEM; n=3). (H) Absolute numbers (± SEM; n=3) of leukocyte clusters (> 120 µm) per colon. (I) Weight as a percentage of the initial weight at day 0. Data represents the mean weight and is pooled from three independent experiments (n=5 mice per group). Error bars were omitted for clarity. Data represent the results of three independent experiments.
CD40 injection into Rag2−/− mice led to the induction of IFN-γ production by NKp46+RORγt− cells containing cNK cells and RORγt− NKR-LTi cells (Figure 6C). RORγt+ NKR-LTi cells and LTi cells did not produce IFN-γ but instead released IL-22 (Figure 6C). It should be noted that the fraction of IL-22 or IL-17-producing cells was not augmented after CD40 stimulation (Figure 6C,S5B), which is in line with previous data indicating that IL-17 is not involved in the pathogenesis of CD40 colitis (Buonocore et al., 2010). To determine whether RORγt− NKR-LTi cells or cNK cells are a cellular source of IFN-γ in this model, we injected anti-CD40 into Il15−/− mice that lack all cNK cells (Kennedy et al., 2000) but normally develop LTi-like and NKR-LTi cells (Sanos et al., 2009). Anti-CD40 injection into Il15−/− mice led to IFN-γ production by NKp46+ cells, indicating that RORγt− NKR-LTi cells may be the IFN-γ producers (Figure 6D). However, it remained possible that both cNK cells and RORγt− NKR-LTi cells contributed to the pool of IFN-γ-producing, colitis-promoting cells in normal mice. To definitively identify the colitogenic IFN-γ-producing cell type, we transferred highly purified cells from RORγt-fate map mice into alymphoid mice and injected CD40 antibodies five days later. During this time, LTi cells and RORγt+ NKR-LTi cells did not appreciably differentiate into RORγt− cells (data not shown). Only RORγt− NKR-LTi cells produced IFN-γ after CD40 injection whereas cNK cells, RORγt+ NKR-LTi cells or LTi cells did not (Figure 6E). Thus, RORγt− NKR-LTi cells are the main source of IFN-γ during experimental colitis. The colitis-promoting role of RORγt− NKR-LTi cells was further confirmed in cell transfer experiments. Only mice transferred with RORγt− NKR-LTi cells developed colitis comparable to Rag2−/− mice (Figure 6F–I). Our data extend previous work reporting that the colitogenic cells reside within a Thy1+Sca1+ lymphocyte subset (Buonocore et al., 2010). The Thy1+Sca1+ population is heterogenous and contains RORγtfm− and RORγtfm+ cells including IFN-γ-producing RORγt− NKR-LTi cells (Figure S5C). Thus, RORγt− NKR-LTi cells but not cNK cells trigger innate colitis.
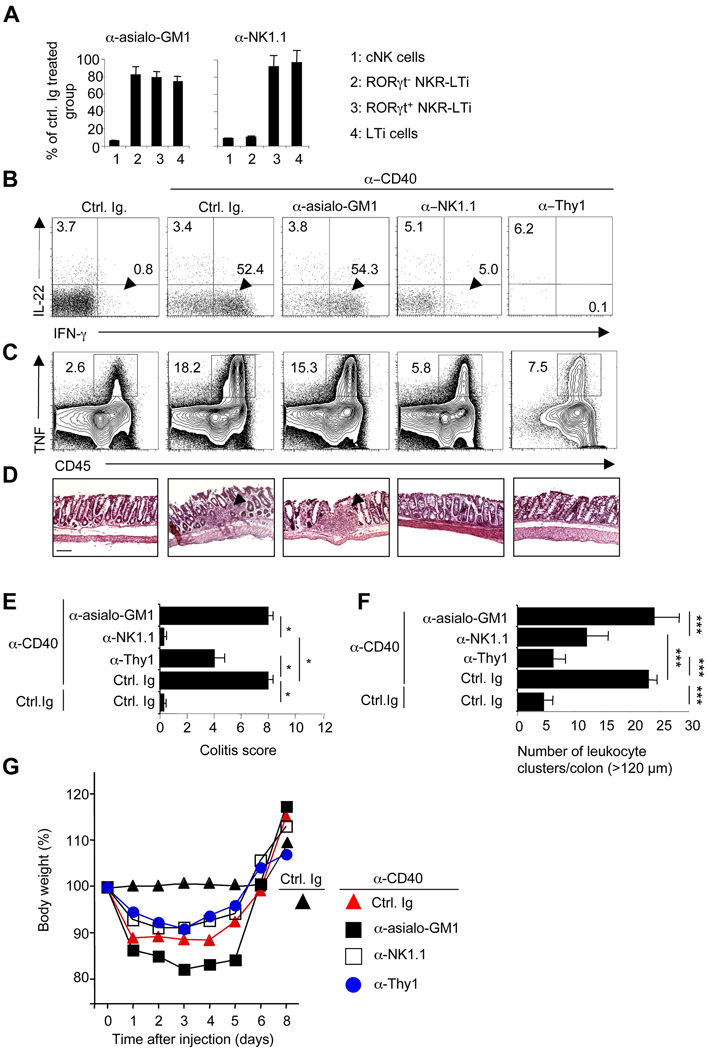
Finally, we investigated colitis development in Rag2−/− mice depleted of cNK cells or NKR-LTi cells. Application of anti-asialo-GM1 resulted in the depletion of most cNK cells, whereas NKR-LTi cells and LTi cells were largely unaffected (Figure 7A). Although almost all cNK cells were depleted, only a third of cNK cells could be stained with the asialo-GM1 antiserum (Figure 7A,S6A). It is appreciated that the isolation procedure from the intestinal lamina propria involving enzymatic digests may interfer with the consecutive detection of antigens (Sanos and Diefenbach, 2010). Intestinal RORγt− NKR-LTi cells and cNK cells expressed high levels of NK1.1 and were depleted after injection of anti-NK1.1, whereas RORγt+ NKR-LTi cells were NK1.1dim and similar to NK1.1− LTi cells were not depleted (Figure 7A,S6B). NK cells, NKR-LTi cells and LTi-like cells expressed high Thy1 (Figure S5C,S6C) and anti-Thy1 injections led to the depletion of virtually all innate lymphocyte populations (Figure 7B). Depletion of cNK cells by anti-asialo-GM1 injection did not reduce the fraction of IFN-γ or TNF-producing cells in the colon and did not ameliorate colitis development (Figure 7B–G). TNF was produced by myeloid cells phenotypically resembling macrophages or monocyte-derived cells (Figure S6D) (Bogunovic et al., 2009; Varol et al., 2009). In contrast, depletion of NK1.1+ cells, including RORγt− NKR-LTi cells, led to significant amelioration of the disease (Figure 7B–G). Not surprisingly, the depletion of all innate lymphocytes by injection of Thy1 antibodies also prevented colitis development (Figure 7B–G). Collectively, these data show that IFN-γ-producing RORγt− NKR-LTi cells are potent inducers of experimental colitis.
Figure 7. Depletion of RORγt− NKR-LTi cells ameliorates colitis.
(A) Groups of RORγt-fate map were treated twice with the indicated antibodies. Two days after the last injection, intestinal lamina propria cells were stained with NKp46, RORγt, CD3 and CD19. After exclusion of all CD3+ and CD19+ cells, the indicated populations were quantified. The data represents the percentage (± SD; n=5) of remaining cells compared to control Ig-treated groups.
(B–G) Groups of Rag2−/− mice were treated with the indicated antibodies and injected with control Ig or anti-CD40. Seven days later, lamina propria lymphocytes from colon were stained for CD45, NKp46, RORγt, IL-22, IFN-γ or TNF. The dot plots (B) represent the analysis of CD45+NKp46+RORγt− cells that contain both cNK cells and RORγt− NKR-LTi cells. Numbers represent percentage of cells in the respective quadrants. The contour plots (C) represent the analysis of all CD45+ cells. Numbers next to the areas indicate percentage of TNF+ cells within gates. (D) H&E staining of sections from the distal colon. Arrowheads point to inflammatory foci. Bar = 100 µm. (E) Clinical colitis score (± SEM, n=3). (F) Mean numbers (±SEM, n=3) of leukocyte clusters (> 120 µm) per colon. (G) Weight as a percentage of the initial weight at day 0. Data represents the mean weight and is pooled from three independent experiments (n=5 mice per group). Error bars were omitted for clarity.
Discussion
It is an important and unresolved question where to position the recently identified population of IL-22-producing NKp46+RORγt+ cells on hematopoietic lineage maps (Cooper et al., 2009). As Id2-deficient mice lack both LTi-like cells and NK cells and as LTi-like cells could be differentiated into NK-like cells in vitro, it was conceivable that LTi cells might be progenitors to all NKR-expressing cells (Mebius et al., 1997; Satoh-Takayama et al., 2010; Yokota et al., 1999). Lineage tracing data via an RORγt-fate map excludes the development of cNK cells and recently identified “thymic NK cells” (Vosshenrich et al., 2006) from RORγt+ precursors. Thus, cNK cells and LTi-like cells are developmentally distinct and constitute separate innate lymphocyte lineages.
Do NKp46+RORγt+ cells then differentiate from NK cell or LTi cell precursors or do they constitute a distinct third lineage of innate lymphocytes? An intriguing hypothesis was that NK cells upregulated RORγt under the influence of the intestinal micromilieu and were instructed to a particular functional fate (i.e., IL-22 production). However, we failed to detect upregulation of RORγt by cNK cells after in vivo transfer or under any in vitro conditions tested, including those known to induce RORγt expression by CD4 T cells. Thus, cNK cells are not the progenitors to NKp46+RORγt+ cells.
We next considered that NKp46+RORγt+ cells may represent LTi-like cells that upregulate NKRs. This view was supported by our previous data showing that LTi-like cells and NKp46+RORγt+ cells share a similar developmental program (Sanos et al., 2009). In addition, LTi cells can upregulate NKRs when cultured under specific in vitro conditions (Cupedo et al., 2009; Mebius et al., 1997). However, these conclusions relied on in vitro experiments and on the definition of LTi cells by a combination of cell surface markers, some of which (e.g., CD127, c-kit, CD25) have now been shown to be also expressed by natural helper cells (Moro et al., 2010). RORγt is the lineage-defining transcription factor of LTi-like cells and is not expressed by any other of the recognized innate lymphocyte lineages (Moro et al., 2010). Our lineage tracing data also show that LTi-like cells permanently express RORγt. Therefore, RORγt can be used to genetically mark LTi-like cells (Eberl et al., 2004; Moro et al., 2010). Using a combination of genetic lineage tracing for RORγt and cell transfer experiments of genetically tagged LTi cells, we demonstrated that NKR−RORγt+ cells cells are direct progenitors to NKR+RORγt+ cells but do not have the potential to develop into myeloid cells, B cells or T cells in vivo. These data firmly place LTi-like cells within the lymphocyte lineage and demonstrate that the differentiation potential of LTi cells in vivo is more limited than previously appreciated. Although the marker profile of NKR−RORγt+ (LTi-like) cells was quite homogenous and the development of the entire population depended on RORγt, subsets could be discriminated on the basis of CD4 expression and different levels of CD127 and c-kit. However, their shared developmental program and data from CD4-fate mapping and in vivo transfer studies support the view that these subsets represent differentiation states of LTi-like cells. Future work will need to address the developmental relationships of these subsets within the NKR−RORγt+ population of innate lymphoid cells.
Do IL-22+ NKR-LTi cells constitute a stable cell fate? We identified three stages of LTi cell differentiation that proceeded along a decreasing gradient of RORγt expression. In the first transition, LTi-like cells (stage 1) acquired NKRs while maintaining RORγt expression (stage 2). It is remarkable that a fraction of LTi-like cells in the small intestine did not acquire NKRs even six months after transfer. Perhaps, a subpopulation of NKR−RORγt+ LTi-like cells has self-renewing potential. While NKR expression remained stable, NKR-LTi cells progressively lost RORγt and this transition to the third stage may require proliferation. The fraction of RORγt− NKR-LTi cells varied from organ to organ, suggesting that molecular cues from the microenvironment may either stabilize RORγt expression or promote its loss. Although the exact nature of these cues is poorly defined, we now demonstrate that signals from the commensal microbiota stabilize RORγt expression in NKR-LTi cells. This provides a mechanistic explanation for our previous observation that the numbers of RORγt+ NKR-LTi cells were decreased whereas those of NKR+RORγt− cells were increased in germ-free mice (Sanos et al., 2009; Satoh-Takayama et al., 2008). The finding that the fraction of RORγt− NKR-LTi cells was less represented in the colon than in the small intestine indicates that stabilization of RORγt is not simply proportional to the number of bacteria. Perhaps, specific microbiota present in the small intestine but not in the colon promote RORγt expression. Furthermore, we have uncovered an important role for IL-7, but not for the related cytokine TSLP, in stabilizing RORγt expression within the intestinal NKR-LTi cell population. IL-7 amounts were significantly decreased in germ-free mice providing a framework for the action of IL-7 in stabilizing RORγt expression in NKR-LTi cells.
The level of RORγt expression determined the phenotype and function of NKR-LTi cells. While RORγt+ LTi-like cells and RORγt+ NKR-LTi cells were potent producers of IL-22 and expressed sLTα1β2, RORγt− NKR-LTi cells instead expressed IFN-γ and upregulated expression of perforin and granzyme B. Graded expression of RORγt served as a rheostat for the responsiveness of NKR-LTi cells to the related cytokines IL-12 and IL-23. These results are reminiscent of a previous report regarding the plasticity of Th17 cells that can downregulate RORγt expression and become Th1-like IFN-γ-producing cells that promote autoimmune colitis (Lee et al., 2009).
Development and/or survival of intestinal and peripheral LTi-like cells strictly required IL-7R signalling (Luther et al., 2003; Meier et al., 2007; Satoh-Takayama et al., 2010; Schmutz et al., 2009). Although LTi-like cells in PLN and spleen were absent in Il7−/− mice (Schmutz et al., 2009), intestinal LTi-like cells were present. Our data demonstrate that TSLP and IL-7 play redundant roles for the differentiation of intestinal LTi-like cells. This extends our previous data showing that overexpression of TSLP in Il7−/− mice can rescue LTi cell differentiation (Chappaz and Finke, 2010). As LTi cells and NKR-LTi cells but not cNK cells strictly required IL-7R signalling for their development, we investigated the development of cNK cells and IL-22-producing NKR+ cells in patients lacking IL-7R expression. Patients with IL7R deficiency had a normal cNK cell compartment but selectively lacked IL-22-producing NKR+ cells. These data demonstrate that human IL-22-producing NKR+ cells share a common developmental program with LTi-like cells.
Collectively, our data also enable us to more accurately name these cells. Previous attempts (NK-22, NCR22) only took into account their production of IL-22 and the expression of NKRs (Cella et al., 2009; Satoh-Takayama et al., 2010). However, IL-22-producing NKR-LTi cells are directly derived from LTi-like progenitors and are just one discrete stage in a wider ranging differentiation program. Thus, the designation NKR-LTi cells more accurately reflects their lineage relationships and the designation NK-22 or NCR22 may only be appropriate for the subpopulation of IL-22-producing RORγt+ NKR-LTi cells.
Previously, RORγt− NKR-LTi cells have gone unnoticed because they were contained within the population of “NK cells” (i.e., NKp46+CD3−) that in fact is a composite of two developmentally and functionally distinct lymphocyte lineages -cNK cells and LTi-derived NKR+ cells. We attempted to assign function to RORγt− NKR-LTi cells. In mice lacking all lymphocytes of the adaptive immune system (Rag2−/−), autoimmune colitis can be triggered by the application of CD40 antibodies, suggesting that components of the innate immune system are sufficient for the induction of colitis (Uhlig et al., 2006). Colitis development required production of IFN-γ or TNF but the colitogenic cell type was unknown (Uhlig et al., 2006). A recent report demonstrated that CD40 colitis could not be triggered in mice genetically lacking RORγt (Buonocore et al., 2010). These mice lack all LTi-derived cells but also do not have lymph nodes or intestinal lymphoid clusters (Sun et al., 2000). It was not explored whether colitis was diminished because of the absence of LTi-derived cells or because of the lack of anatomical sites for leukocyte interactions (Diefenbach and Vonarbourg, 2010). Using gain- and loss-of-function experiments, we demonstrate that RORγt− NKR-LTi cells are the colitogenic, IFN-γ-producing innate lymphocyte subset. Thus, RORγt− NKR-LTi cells are required and sufficient for the development of innate colitis. Why are RORγt− NKR-LTi cells such potent inducers of colitis? An important pathological feature of colitis, as well as of other chronic inflammtory processes, are de novo generated pro-inflammatory leukocyte clusters (Aloisi and Pujol-Borrell, 2006; Izcue et al., 2009). RORγt− NKR-LTi cells may still have lymphoid tissue-inducing potential as they express sLTα1β2. Perhaps, the composite qualities of pro-inflammatory cytokine expression and induction of ectopic lymphoid tissues make these cells uniquely positioned to induce and maintain inflammation.
Experimental Procedures
Mice
C57BL/6 mice and gene-targeted mice were purchased from Janvier, Charles River Laboratories, Taconic Farms or were provided by other laboratories. A complete list of mouse strains used is available in the Supplemental Experimental Procedures. All mice were used at 8–16 weeks of age. Antibiotic treatment was performed as described (Rakoff-Nahoum et al., 2004). Experiments were approved by and were in accordance with local animal care and use committees.
Cell isolation and flow cytometry
The isolation of lymphocytes from lamina propria and other organs as well as staining of cell surface markers with fluorophore-conjugated antibodies were performed as previously described (Sanos et al., 2009; Sanos and Diefenbach, 2010). The strategy for the analysis of innate lymphoid cells is depicted in Figure S1E,F. Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated after Pancoll density gradient centrifugation (Biotech). Human tonsillar tissue was obtained from patients undergoing tonsillectomy and blood samples were from healthy volunteers. Informed written consent was obtained prior to sample acquisition from all donors and all investigations have been conducted according to the principles expressed in the Declaration of Helsinki. A list of antibodies used throughout the study is available in the Supplemental Experimental Procedures.
In vivo differentiation
For the in vivo transfer experiments, lymphocytes were isolated from the indicated organs and mice (H-2b), for LNs and spleen CD3+ and CD19+ cells were removed using magnetic beads (Miltenyi) and cells were sorted twice on a MoFlo sorter (Beckman Coulter). Cells used were >98% purity in the post-sort analysis (Figure S1B–D) and were injected intravenously into Rag2−/−Il2rg−/− mice (H-2d). Donor-derived lymphocyte populations were analyzed at the indicated time points. In some experiments, mice were injected every three days with a blocking IL-7Rα (0.5 mg) antibody (clone A7R34).
In vitro differentiation
For in vitro differentiation, 1,000–5,000 double sorted cells (purity >98%) were seeded into microtiter plates that were coated with irradiated OP-9 cells (30 Gy) or left uncoated in medium supplemented with IL-15 (50 ng/ml), IL-7, Flt3L and/or SCF (20 ng/ml, each; all Peprotech). Cells were analyzed after the indicated time points. In some experiments cells were labelled prior to the culture with a cell proliferation dye according to the manufacturer’s instructions (eBioscience).
In vitro stimulation and intracellular cytokine staining
The indicated lymphocyte populations were stimulated overnight with 50 ng/ml IL-12 or 10 ng/ml IL-23 (Peprotech). Brefeldin A (Sigma) was added for the last 4–6 h of the stimulation period. Cytokine expression was analyzed by intracellular staining.
Experimental colitis
CD40 colitis was induced as described (Uhlig et al., 2006). For transfer colitis, 5×104 cells of the indicated cell populations were transferred into Rag2−/−Il2rg−/− mice and two weeks later, colitis was induced. Mice were sacrificed seven days after CD40 injection eand colitis was evaluated according to clinical and histological parameters (see Supplemental Experimental Procedures). In some experiments NKR+ cells were depleted by injections of 250 µg anti-Thy1 (clones 30H12 and M5/49.4.1), 250 µg anti-NK1.1 (clone PK136) or 100 µg anti-asialo GM1 at days −1, +1 and +4.
Real Time PCR
Real-time PCR was performed as previously described (Sanos et al., 2009). The amount of mRNA was normalized to that of the ‘housekeeping’ gene Hprt1 (encoding hypoxanthine guanine phosphoribosyl transferase). Primer sequences are reported in the Supplemental Experimental Procedures.
Statistical analyses
ANOVA test was used to determine significance of the clinical colitis scores and of leukocyte clusters. Student’s t test was used for all other data sets. * p < 0.05, ** p < 0.01, *** p < 0.001.
Highlights
LTi-like cells are progenitors to NK cell receptor-expressing (NKR-LTi) lymphocytes
Progressive loss of RORγt assigns distinct functional fates to NKR-LTi cells
RORγt− NKR-LTi cells are potent inducers of colitis
Human “NK-22” cells share a developmental program with LTi cells
Supplementary Material
Acknowledgments
We thank Georg Häcker for support and for critically reading the manuscript, the members of the Diefenbach laboratory for valuable discussions, Alain Fischer, Hanspeter Pircher and Katie Connor for comments on the manuscript, Jürgen Brandel for help with the figures, and Nathalie Göppert and Karin Oberle for technical assistance. We are grateful to Dan Littman, Fabio Santori, Gabi Niedermann and Caroline Johner for experimental support, and to Marie Follo, Klaus Geiger and Jan Wersing for cell sorting. The work was supported by the Deutsche Forschungsgemeinschaft (GRK1104, SGBM, CRC620 to A.D., A.M., T.H., E.A.K., M.F.), the BMBF (CCI, A.D.), the Max-Planck-Society (IMPRS-MCB), the Swiss National Science Foundation grant PP00A-116894/1 (D.F.), the National Institutes of Health (grants R37AI033068, CA069381 and AI088445 to C.F.W.) and and the Cluster of Excellence Inflammation at Interfaces (Borstel-Kiel-Lübeck-Plön; EXC306 to C.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Data
Supplemental Data include 6 figures, extended Experimental Procedures and additional references.
References
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappaz S, Finke D. The IL-7 Signaling Pathway Regulates Lymph Node Development Independent of Peripheral Lymphocytes. J Immunol. 2010;184:3562–3569. doi: 10.4049/jimmunol.0901647. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009;10:1103–1110. doi: 10.1038/embor.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Vonarbourg C. Innate lymphocytes induce inflammatory bowel disease. Immunol Cell Biol. 2010;88:694–696. doi: 10.1038/icb.2010.82. [DOI] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORγt+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–4010. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Rossi S, Withers D, McConnell F, Toellner KM, Gaspal F, Jenkinson E, Anderson G, Lane PJ. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–174. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SY, Molden J, Goldsmith MA. Shared gamma(c) subunit within the human interleukin-7 receptor complex. A molecular basis for the pathogenesis of X-linked severe combined immunodeficiency. J Clin Invest. 1997;99:169–177. doi: 10.1172/JCI119144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Diefenbach A. Isolation of NK cells and NK-like cells from the intestinal lamina propria. Methods Mol Biol. 2010;612:505–517. doi: 10.1007/978-1-60761-362-6_32. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- Schmutz S, Bosco N, Chappaz S, Boyman O, Acha-Orbea H, Ceredig R, Rolink AG, Finke D. Cutting edge: IL-7 regulates the peripheral pool of adult ROR gamma+ lymphoid tissue inducer cells. J Immunol. 2009;183:2217–2221. doi: 10.4049/jimmunol.0802911. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.