Abstract
In this study, we developed a microfluidic cell co-culture platform that permits individual manipulation of the microenvironment of different cell types. Separation of the cell culture chambers is controlled by alternating the position of a microfabricated valve, which serves as a barrier between each chamber. This unique feature of our platform allowed us to maintain healthy co-cultures of hippocampal neurons and glia for several weeks under optimal conditions. Controlled fluidic exchange between the cell culture chambers provided neurons with a continuous supply of in situ conditioned glia media that was critical for their survival. Using the barrier valve, we transfected neurons in the adjacent chambers with green fluorescent protein (GFP) and mCherry cDNA, respectively, with a transfection efficiency of approximately 40%. Co-culture with glia further enhanced the transfection efficiency of neurons to almost 60%. Thus, the microfluidic devices offer a novel platform for the long-term culture, transfection, and individual treatment of central nervous system cells.
Keywords: hippocampal neurons, co-culture, glia, microfluidic device, transfection
1. Introduction
Microfluidic devices are emerging as powerful tools in neurobiology because of their ability to precisely control and manipulate the microenviroment of cells (Taylor and Jeon, 2010). The Campenot chamber is one of the first microfluidic platforms that was developed for neuronal studies (Campenot, 1977). Subsequent devices have been described for culturing neurons and examining neuronal processes such as neurite formation, outgrowth, and regeneration (Chang and Sretavan, 2008; Ivins et al., 1998; Ravula et al., 2007; Rhee et al., 2005; Taylor et al., 2005; Taylor et al., 2003; Vahidi et al., 2008). While these platforms are very useful, it is difficult to differentially manipulate cells in their culture chambers, which would open new avenues for interesting neurobiological applications. A recent design partially overcame this limitation by incorporating long microchannels (900 µm) between the neuronal chambers that provided for their separate treatment as long as a constant hydrostatic pressure was maintained (Taylor et al., 2010). In these devices, bulky syringe pumps are required to sustain the hydrostatic pressure. Moreover, diffusion between the chambers may limit their ability to achieve complete separation because there is no physical barrier between them, pointing to a need for the development of new, compact microfluidic platforms to address this.
Additionally, several microfluidic devices have been developed for the co-culture of neurons with other nervous system cells, such as glia and oligodendrocytes (Hosmane et al., 2010; Park et al., 2009; Taylor et al., 2005; Yang et al., 2009). In these designs, the cultured cells are intermixed and not physically separated; therefore, all of the nervous system cells are maintained under similar conditions. In all, these devices lack the ability to house multiple cell populations in a manner that adequately facilitates manipulation of individual cell types.
Here, we describe a novel, cost-effective microfluidic platform for co-culturing neurons and glia. With this device, we can reversibly isolate distinct cell populations without the need for expensive and complex syringe pump systems. In addition, the platform is amendable to high resolution microscopy and is conducive to high efficiency transfection of neurons, making it ideal for many neurobiological applications.
2. Materials and Methods
2.1 Fabrication and assembly of the microfluidic platforms
The microfluidic platforms were fabricated by soft-lithography techniques using replica molding (McDonald and Whitesides, 2002; Xia and Whitesides, 1998) and were comprised of two layers of polydimethylsiloxane (PDMS) (Sylgard 184, Dow Corning, MI) assembled onto a glass coverslip (No. 1, VWR Vista Vision, Suwanee, GA). The first layer of PDMS created the cell culture chambers and supporting channels, while the second formed the pressure chamber.
The mold for the first PDMS layer was created using a photo-sensitive material (SU-8) patterned by two transparent masks and positioned over a silcon wafer (McDonald and Whitesides, 2002). The masks were printed at a resolution of 20,000 dpi using a high-resolution printer (Cad/Art Services, Bandon, OR). The first transparent mask generated microchannels with dimensions of 100 µm by 5 µm (width and height) (Fig. 1Ai). The second transparent mask generated two cell culture chambers, wells for media reservoirs and for waste, and defined the length of the connecting channels (100 µm in width, 10 mm in length) that allowed for media exchange between the chambers (Fig. 1Aii). The dimensions of the cell chambers were 6 mm by 800 µm by 100 µm (length, width, and height). A barrier was created between the cell chambers by inserting a strip of PDMS 100 µm wide, which had multiple connecting channels at the bottom that could be open or closed.
Fig 1.
Schematic of the microfluidic platform. (A) Fabrication of the first PDMS layer. (i) SU-8 was patterned on a silicon wafer to define the connecting channels. (ii) A second layer of SU-8, which created the cell culture chambers, channels and wells, was then aligned to the patterned SU-8. (iii) A pre-polymer of PDMS was poured over the silicon wafer containing the SU-8 patterns. (iv) The solidified PDMS layer was peeled from the wafer, and holes were punched into the PDMS layer. (B) Fabrication of the second PDMS layer. (i) A slab of glass was attached to the silicon wafer. (ii) A pre-polymer of PDMS was poured over the silicon wafer. (iii) The polymerized PDMS layer was peeled from the wafer, and a small hole was punched into the PDMS to create an inlet for a microbore tube. (C) A schematic of the assembled microfluidic platform.
The mold for the second PDMS layer was made by attaching a slab of glass with dimensions of 4 mm by 12 mm by 1 mm (width, length, and height) to a silicon wafer (Fig. 1Bi). A pre-polymer of PDMS was then mixed with a curing agent at a 12:1 ratio and poured over both molds (Fig. 1Aiii and 1Bii). Spacers were used to ensure that the thickness of the first and the second PDMS layers were 1 mm and 3 mm, respectively. After degassing, the PDMS layers were allowed to solidify over their molds at 70°C for 2 h.
The solidified layers of PDMS were then peeled from their molds, and sharp metal punchers were used to generate holes for the media reservoir, waste wells, and a microbore tube (Cole-Parmer, Vermon Hills, IL) (Fig. 1Aiv and Biii). The first layer of PDMS was bonded to an acid-washed glass coverslip after the surfaces were treated with oxygen plasma. Similarly, the second layer of PDMS was manually aligned and bonded onto the first layer, creating the pressure chamber. A microbore tube was glued on top of the pressure chamber using liquid PDMS, and two Pyrex cloning cylinders (Fisher Scientific, Pittsburgh, PA) were attached to the loading wells to make the reservoirs (Fig.1C). The entire device was placed in an oven for 1 h at 75°C to cure. During the curing process, 200 µl of sterile, deionized water was added to each reservoir to retain the hydrophilic nature of the microchannels. The assembled microfluidic platforms were then sterilized under ultraviolet light in a laminar flow hood for 1–2 h.
The pressure chamber can be filled with 0.2–0.3 ml of air or water to create either a pneumatic or a hydraulic chamber, which controlled the connecting channels underneath the barrier valve. When the pressure chamber was filled, the channels underneath the barrier were closed and the valve was activated, causing separation of the two cell chambers from each other. Removal of water or release of air from the pressure chamber opened the connecting channels (valve deactivated) and reconnected the cell culture chambers.
2.2 Loading primary culture of hippocampal neurons into the microfluidic platforms
Dissociated hippocampal neurons were isolated from E19 rats embryos as previously described (Goslin et al., 1998). Prior to loading neurons into the microfluidic platforms, the glass coverslips were coated with 1 mg/ml poly-L-lysine (PLL) (Sigma-Aldrich, St.Louis, MO) for 12 h at 37°C. Uncoated PLL was removed by washing with sterile, deionized water. After equilibrating the chambers with B27 Neurobasal™ media (GIBCO™ Invitrogen, Carlsbad, CA), 1 × 105 hippocampal neurons, suspended in 20 µl of media, were seeded into the cell culture chambers. Neurons were allowed to attach to the PLL-coated coverslips for 2–3 h at 37°C, and then 300 µl of B27 Neurobasal™ media (neuronal media) was added to one reservoir and 150 µl to the other reservoir. By keeping the media level in one reservoir higher than in the other, a continuous flow through the microchannels connecting the two chambers was maintained, which ensured sufficient fresh nutrient media near the barrier and in the connecting channels.
2.3 Transfection of neurons in the microfluidic devices
The cDNAs for GFP and mCherry were cloned into a neuronal expression vector, provided by Freda Miller, which had a neuronal-specific α1-tubulin promoter (Gloster et al., 1999). Neurons in the microfluidic platform were transfected at day 3–4 in culture using a calcium phosphate method as previously described with the following modifications (Zhang et al., 2003). GFP or mCherry cDNA (2.5–4.0 µg) was added to a tube containing 45 µl of 120 mM CaCl2, and a calcium phosphate-DNA precipitate was allowed to form by slowly bubbling 45 µl of HEPES-buffered solution containing 42 mM HEPES, 274 mM NaCl, 9.5 mM KCl, 15 mM glucose (HBS), pH 7.05 into the tube. After adding an additional 45 µl of glia-conditioned B27 media, the mixture was allowed to stand for 5 min at room temperature to facilitate precipitate formation. Before adding the transfection mixture to the microfluidic platform, the pneumatic chamber was filled, and the valve barrier was activated to completely separate the two chambers. After removing the media from the reservoirs and waste wells, 45 µl of the transfection mixture was loaded into the respective reservoirs. A drop of media was added to the waste well 10–15 min later to reduce the fluid flow-rate, which promoted the deposition of the precipitates onto the cells. The chambers were then incubated with the transfection mixture for 1–1.5 h at 37°C. Following transfection, the microfluidic platforms were washed with pre-warmed HBS, pH 7.15 for 1 h. The cell chambers were kept isolated for the entire duration of the transfection procedure. After washing the neurons, the air in the pressure chamber was released, thereby deactivating the valve. The reservoir cylinders were subsequently filled with 300 µl of fresh glia-conditioned B27 media. For neuron-glia co-cultures, the reservoirs were filled with unconditioned B27 Neurobasal media as described below.
2.4 Co-culture of glia and neurons in the microfluidic platforms
Glia were isolated from the brains of post-natal day 2 pups as previously described (Goslin et al., 1998). The cell culture chambers of the microfluidic platform were separated by activating the pressure chamber with sterile, deionized water (hydraulic valve). The glia chamber was then coated with 10 µg/ml type I collagen (BD Biosciences, San Jose, CA) for 12 h at 37°C. Excess collagen was removed by flowing sterile phosphate-buffered saline (PBS) solution through the devices for at least an hour at 37°C. The neuronal chamber was coated with PLL as described above. Approximately 30,000 glial cells in 75 µl of Minimum Essential Medium (MEM) (Invitrogen, Carlsbad, CA), containing 10% horse serum and penicillin/streptomycin, were loaded into the glia chamber. After 2–3 days, glial cells typically reached 80–90% confluence, which was sufficient to support the growth of neurons. After the glia reached near confluence, neurons were loaded into the other chamber of the microfluidic platform, as described above. After the neurons attached, the valve between the two chambers was released (deactivated). Fresh neuronal media was added to the reservoirs for both the glial (300 µl) and neuronal (100 µl) chambers. The flow of media from the glia to the neurons effectively provided glia-conditioned media to the neurons.
2.5 Microscopy
Glia and neurons were fixed as previously described with minor modifications (Wegner et al., 2008). Briefly, after media was removed from the reservoirs and waste wells, 4% paraformaldehyde/sucrose in PBS, pH 7.4 was flowed through the chambers for 30 min at room temperature. The platforms were then washed with PBS, and cells were imaged using a Retiga EXi CCD camera (QImaging, Surrey, BC) connected to an inverted Olympus IX71 microscope (Melville, NY) fitted with 10× (NA 0.3) and 20× (NA 0.5) objectives. Image acquisition and analysis were performed using MetaMorph software (Molecular Devices, Sunnyvale, CA). GFP was visualized with a GFP Bandpass filter cube (excitation HQ470/40, emission HQ525/50, Q495LP dichroic mirror) (Chroma, Brattleboro, VT), and mCherry was seen with a TRITC/Cy3 cube (excitation HQ545/30, emission HQ610/75, Q570LP dichroic mirror).
3. Results and Discussion
3.1 Loading, culturing, and transfecting neurons in the microfluidic platform
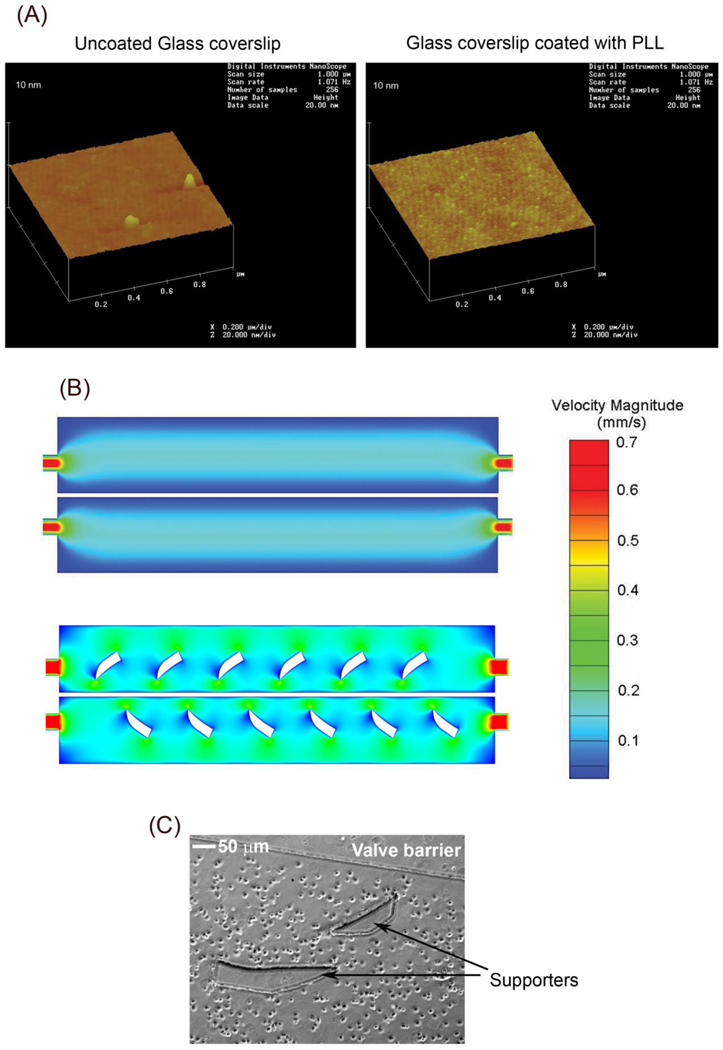
Prior to cell loading, PLL was flowed through microfluidic platforms to provide a surface for neuronal attachment to glass coverslips. The coverslips were then subjected to atomic force microscopy (AFM) to determine whether PLL coated these surfaces. PLL attached to the coverslips as demonstrated by an increased roughness on the glass surface (Fig. 2A). After PLL coating, 1 × 105 hippocampal neurons, which were suspended in 20 µl of neuronal media, were added to the microfluidic platforms. The pressure difference between the reservoirs and the downstream (waste) wells generated a continuous fluid flow that distributed neurons to the cell culture chambers and provided nutrient media for cells. The small volume was necessary to ensure a slow flow rate, which maximized the retention of neurons within the cell culture chambers. Semilunar/crescent shaped PDMS supporters were included within the cell culture chambers to facilitate an even distribution of neurons (neuronal suspension) and media. Computational fluid dynamics (CFD) modeling of the flow field showed that the semilunar supporters increased the velocity of the flow at the edges of the chamber (Fig. 2B), which promoted a more uniform distribution of cells (Fig. 2C). Moreover, the supporters prevented the roofs of the cell culture chambers from collapsing when air or water pressure was generated within the pressure chambers.
Fig 2.
Preparation of microfluidic platforms for neuronal culturing. (A) Prior to cell loading, borate buffer or 1 mg/ml PLL in borate buffer was flowed through microfluidic platforms attached to glass coverslips. The coverslips were then scanned using AFM to determine if PLL coated the glass surfaces. A scan of a glass coverslip treated with buffer alone (uncoated) (left panel) or PLL (right panel) is shown. Note that the surface of the PLL coated glass coverslip shows remarkable roughness compared to the relatively smooth uncoated glass surface, indicating attachment of PLL to the coverslip. (B) To promote an even distribution of neurons in the microfluidic platforms, crescent shaped PDMS supporters were integrated into the design of the cell culture chambers. CFD simulations of the flow field velocity are shown for microfluidic platforms without (top panel) and with (bottom panel) the supporters. The supporters redirect the flow more evenly around the edges of the culture chambers, which aids in the uniform distribution of coating molecules, culture media, and cells (C).
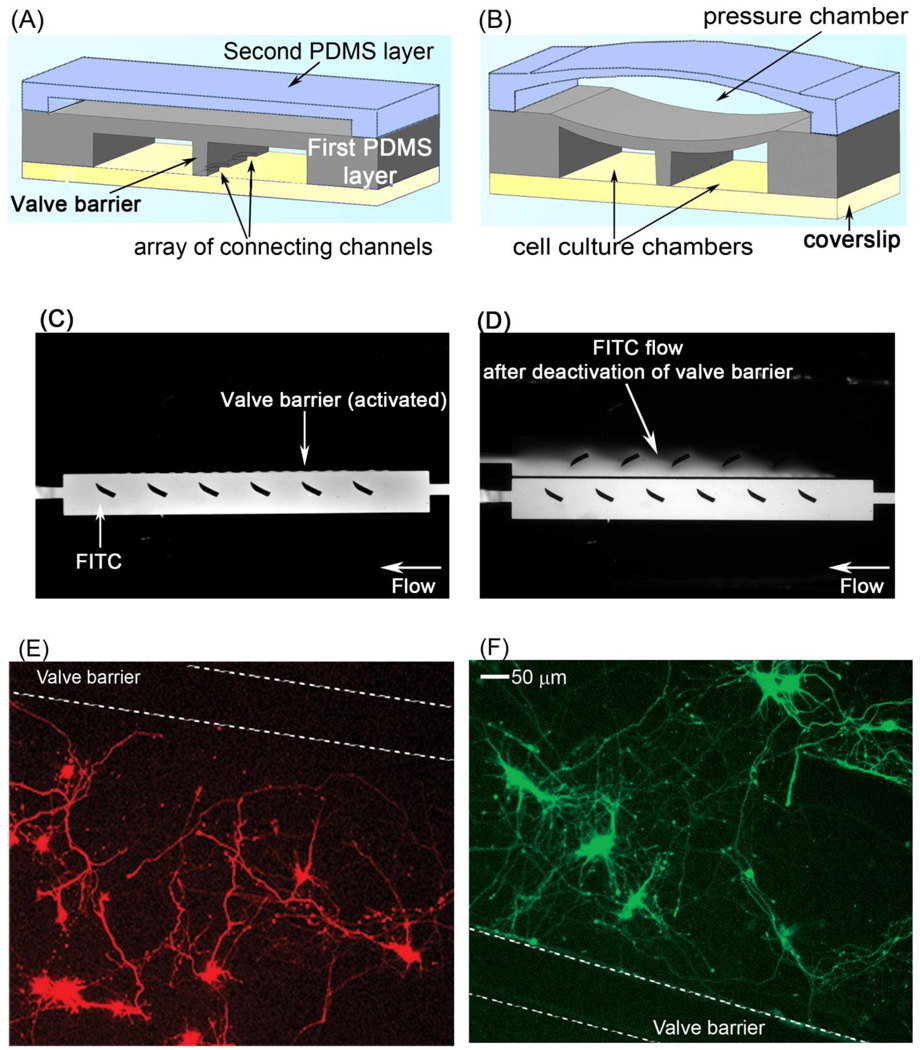
When culturing neurons, the valve barrier between the chambers remained deactivated, and the cell culture chambers were connected through the microchannels between them for adequate media exchange (Fig. 3A). When separation of the cell culture chambers was desired, such as during transfection, the pressure chamber was filled to activate the barrier valve (Fig. 3B). To verify the valve completely separated the two cell culture chambers, it was activated, and fluorescein isothiocyanate (FITC) was loaded into one chamber. FITC did not diffuse or flow to the other cell culture chamber, indicating that the barrier valve effectively separated the two chambers (Fig. 3C). However, after the barrier valve was deactivated, FITC flowed into the adjacent cell culture chamber, indicating that liquid media is exchanged between the chambers under these conditions (Fig. 3D). When the position of the barrier valve was controlled by a pneumatic chamber, separation of the two cell culture chambers could be maintained for 4–6 h while a hydraulic chamber could isolate the chambers for over a week. This is most likely because PDMS is a gas permeable material, but is impermeable to water (Merker et al., 2000).
Fig 3.
The valve barrier allows for the reversible separation of the two cell culture chambers in the microfluidic platform. (A) Deflation of the pressure chamber deactivates the valve barrier, which permits connection of the cell culture chambers through microchannels. (B) Filling the pressure chamber with air or water pushes the barrier valve down (activates), resulting in the separation of the cell culture chambers. (C) To test its effectiveness, the barrier valve was activated, and FITC was loaded into one chamber. The activated valve blocked the flow of FITC to the adjacent chamber, indicating that it can completely separate the two cell culture chambers. (D) When the barrier valve was deactivated, FITC flowed to the opposite cell culture chamber. (E,F) Hippocampal neurons were loaded into the cell culture chambers of the microfluidic platform. On day 3 in culture, the barrier valve was activated, and neurons in the adjacent chambers were transfected with mCherry (E) and GFP (F) cDNA, respectively. Expression of mCherry and GFP was confined to their respective chamber.
The inclusion of the barrier valve in the design of the microfluidic platform provided us with the unique opportunity to transfect neurons in the two cell culture chambers with different cDNAs. On day 3 in culture, the barrier valve was activated and neurons in the adjacent chambers were transfected with GFP and mCherry cDNA, respectively, using a calcium phosphate method. Following transfection, the barrier valve was deactivated, reconnecting the two chambers. Neuronal media was added to the reservoirs, and neurons were maintained in culture in the platforms for an additional 3 days. Expression of mCherry was confined to neurons in the culture chamber that received the mCherry cDNA transfection mixture (Fig. 3E). Similarly, GFP expression was seen in neurons in one chamber, but not observed in the other chamber (Fig. 3F). These results indicate that the barrier valve prevented cross flow between the two chambers and successfully separated them. The microfluidic platforms are conducive to the long-term culture of neurons after transfection. Indeed, healthy cultures of neurons can be maintained in the microfluidic platforms for at least three weeks after transfection, making these devices very amenable for studying processes, such as synapse formation.
3.2 Co-culture of glia and neurons in the microfluidic platform
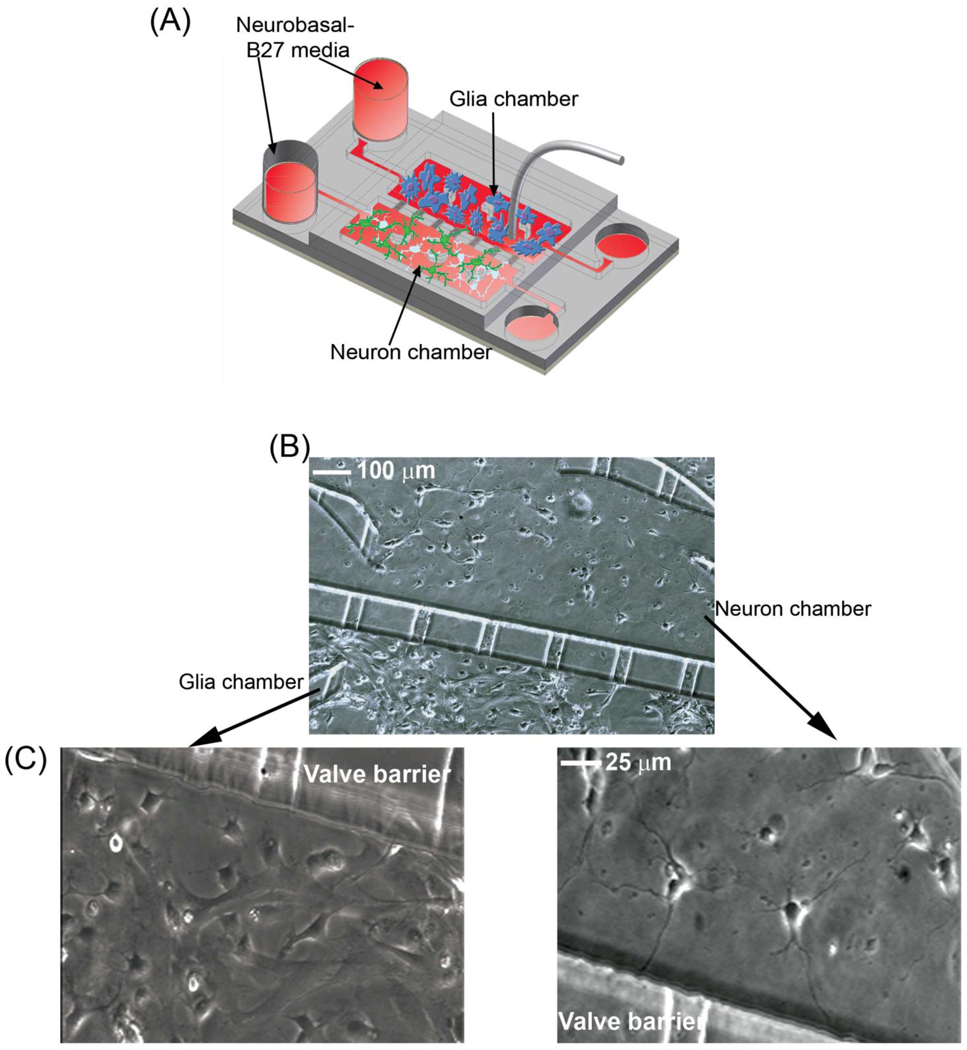
The microfluidic platforms are designed to support the culture of distinct cell populations in close proximity to each other, making them ideal for co-culturing neurons and glia. To set up these co-cultures, the chambers were separated by activating the valve using the hydraulic pressure chamber. The two cell culture chambers were coated with PLL and type I collagen, respectively, to promote the attachment of neurons and glia to the glass coverslips. After washing away uncoated substrate, neurons and glia were loaded and cultured in separate chambers for 2–3 days. This allowed the glia to reach near confluence and be an effective nutrient source for neurons (Fig. 4A). During this time, each cell type was provided with their respective culture media. After glial cells reached near confluence, the chambers were connected to allow for exchange of nutrients between the chambers, and both reservoirs were filled with neuronal media. The media level in the glia reservoir was kept higher than that in the neuronal reservoir to promote the flow of media from the glia to the neuronal chamber, and thus facilitate an in-situ conditioning of the neuronal media. In the absence of glia or glia-conditioned media, neurons were not healthy and could not survive for more than a week. In the co-culture platform, cells in both chambers were healthy and viable for the duration of our experiments (more than 3 weeks), indicating that glia provided the necessary nutrients for neuronal survival (Fig. 4B–C).
Fig 4.
Co-culture of neurons and glia in the microfluidic platform. (A) A schematic showing the co-culture of neurons and glia. Note that the culture media level of the glia side is higher than that of the neuronal side to introduce flow from the glia to the neuronal chamber. (B) Image of neurons and glia from the co-culture platform is shown. (C) Higher magnification images of glia (left panel) and neurons (right panel) are shown.
3.3 Co-culture with glia enhances the transfection efficiency of neurons
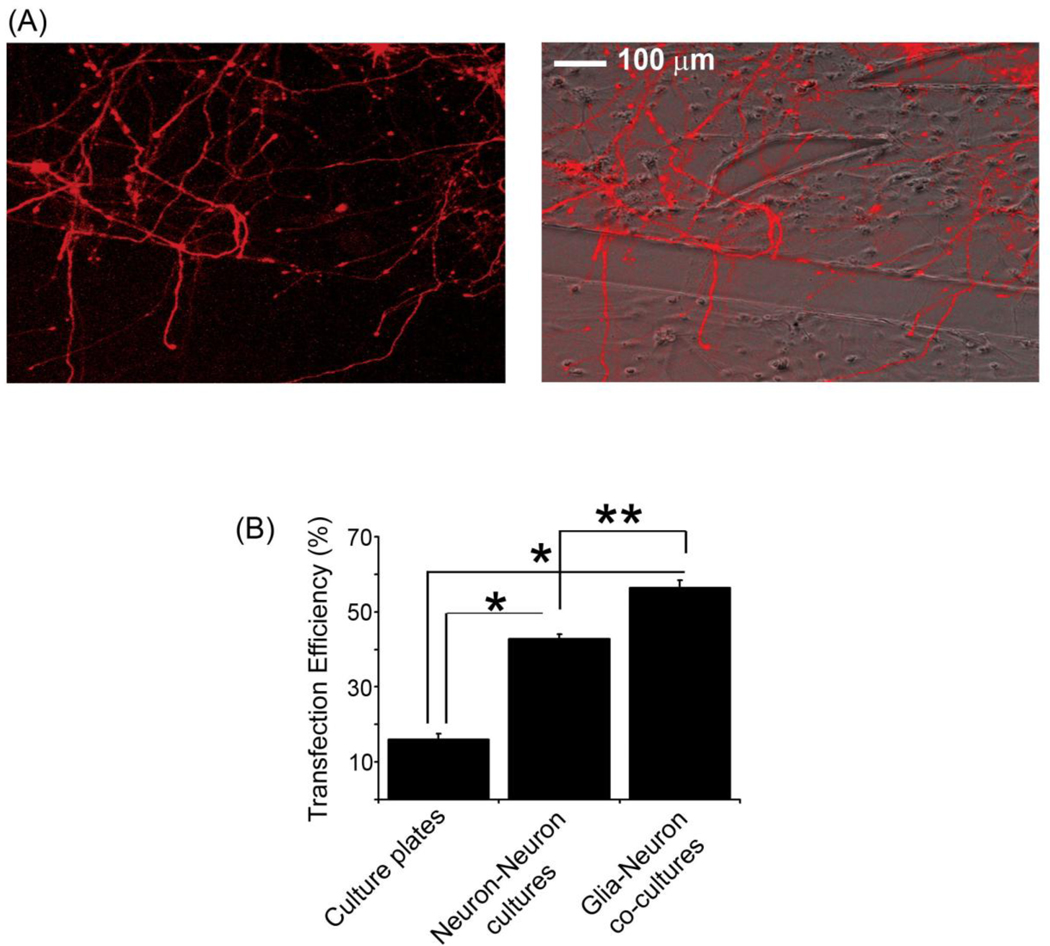
Next, neurons in the co-culture platform were transfected with mCherry cDNA as described above to determine if the presence of glia affected this process. Interestingly, the overall transfection efficiency of neurons in the microfluidic platform was increased 3–4-fold compared to that obtained on traditional culture plates under identical experimental conditions (Fig. 5B), suggesting the devices are highly conducive to neuronal transfection. When neurons were co-cultured with glia in the platform, the transfection efficiency was 60%, which was significantly higher than that observed with neurons alone (Fig. 5B). This result most likely reflects the improved general health of neurons when cultured with glia. After transfection, neurons were healthy and extended axons and dendrites from the cell body, which in some cases crossed the barrier and grew into the adjacent chamber of glial cells (Fig. 5A). Collectively, these results show that the microfluidic platforms are effective tools for growing healthy neuronal cultures, which can be consistently transfected with high efficiency. The design of the microfluidic device allows for the co-culture of neurons and glia, and thus provides a novel platform for studying neuron-glia interactions in the nervous system under physiologically relevant conditions.
Fig 5.
Glia increase the transfection efficiency of neurons. (A) Neurons in the co-culture platform were transfected with mCherry cDNA. A fluorescent image (left panel) and a fluorescent/phase overlay (right panel) of neurons expressing mCherry are shown. Following transfection, cells were healthy and extended neuronal processes from the cell body. (B) Transfection efficiencies for neurons cultured in traditional culture dishes and in microfluidic platforms containing neuron-neuron and neuron-glia cultures are shown. Error bars represent S.E.M. for 30–40 neurons from three separate experiments (*, p <0.0001; **, p <0.001).
4. Conclusions
Our microfluidic devices are versatile, compact cell co-culture platforms that can be used for the long-term culture of primary central nervous cells such as neurons and glia. The inclusion of a barrier valve in our platform design permits the reversible separation of the cell culture chambers and thus allows for the optimal co-culture and transfection of different cell types. Co-culture with glia provides nutrient media for maintaining healthy neuronal cultures, eliminating the need to supply neurons with pre-conditioned glia media. Glia also significantly enhance the transfection efficiency of neurons in the platform. In addition, the microfluidic platforms are amendable to high resolution microscopy because they are constructed on glass coverslips. These attractive features of our microfluidic platforms make them ideal for studying central nervous system interactions on both a cellular and a molecular level.
Research Highlights
Novel microfluidic device for the long-term co-culture of neurons and glia
Unique device design allows for reversible separation of cell culture chambers
High transfection efficiency of neurons in the microfluidic platform
Microfluidic platform allows for the differential manipulation of neurons and glia
Microfluidic device is amenable to high resolution live-cell imaging
Acknowledgements
We are grateful to Lan Hu for providing technical assistance in preparing neuronal and glial cultures. This work was supported by grants MH071674 from NIH and S10RR025524 from the National Center for Research Resources at NIH to D.J.W. and grant CBET0643583 from NSF to D.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Sretavan DW. Novel high-resolution micropatterning for neuron culture using polylysine adsorption on a cell repellant, plasma-polymerized background. Langmuir. 2008;24:13048–13057. doi: 10.1021/la8021479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloster A, El-Bizri H, Bamji SX, Rogers D, Miller FD. Early induction of Talpha1 alpha-tubulin transcription in neurons of the developing nervous system. J Comp Neurol. 1999;405:45–60. doi: 10.1002/(sici)1096-9861(19990301)405:1<45::aid-cne4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low-density culture. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Hosmane S, Yang IH, Ruffin A, Thakor N, Venkatesan A. Circular compartmentalized microfluidic platform: Study of axon-glia interactions. Lab Chip. 2010;10:741–747. doi: 10.1039/b918640a. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Bui ET, Cotman CW. Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis. 1998;5:365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Accounts Chem Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- Merker TC, Bondar VI, Nagai K, Freeman BD, Pinnau I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane) J. Polymer Sci. 2000;38:415–434. [Google Scholar]
- Park J, Koito H, Li J, Han A. A multi-compartment CNS neuron-glia Co-culture microfluidic platform. J Vis Exp. 2009 doi: 10.3791/1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravula SK, Wang MS, Asress SA, Glass JD, Bruno Frazier A. A compartmented neuronal culture system in microdevice format. J Neurosci Methods. 2007;159:78–85. doi: 10.1016/j.jneumeth.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Rhee SW, Taylor AM, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Patterned cell culture inside microfluidic devices. Lab Chip. 2005;5:102–107. doi: 10.1039/b403091e. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron. 2010;66:57–68. doi: 10.1016/j.neuron.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Jeon NL. Micro-scale and microfluidic devices for neurobiology. Curr Opin Neurobiol. 2010;20:640–647. doi: 10.1016/j.conb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Rhee SW, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Microfluidic Multicompartment Device for Neuroscience Research. Langmuir. 2003;19:1551–1556. doi: 10.1021/la026417v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahidi B, Park JW, Kim HJ, Jeon NL. Microfluidic-based strip assay for testing the effects of various surface-bound inhibitors in spinal cord injury. J Neurosci Methods. 2008;170:188–196. doi: 10.1016/j.jneumeth.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem. 2008;283:15912–15920. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YN, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Horwitz AF. Synapse formation is regulated by the signaling adaptor GIT1. J Cell Biol. 2003;161:131–142. doi: 10.1083/jcb.200211002. [DOI] [PMC free article] [PubMed] [Google Scholar]