Abstract
Inositol-requiring 1 (IRE1)/X-box-binding protein 1 (XBP1)-mediated signalling represents the most conserved branch of the unfolded protein response. A series of recent studies reveal novel and potentially ancient roles for this pathway in the coordination of metabolic and immune responses.
Keywords: unfolded protein response (UPR), Toll-like receptors (TLR), Insulin, Phosphoinositide 3-kinase (PI3K), Inflammation
In eukaryotic cells, the endoplasmic reticulum (ER) is the site for the synthesis, folding and modification of proteins that traffic to the secretory apparatus. The ER has a tremendous ability to adjust its protein-folding capacity to meet the demands of the cell. Increases in protein synthesis or altered cellular homeostatic conditions that disrupt protein folding in the ER cause the accumulation of unfolded/misfolded proteins, leading to activation of the unfolded protein response (UPR) pathway. In mammals, three ER transmembrane protein sensors mediate UPR signals: inositol-requiring 1 (IRE1), PKR-like ER kinase (PERK) and activating transcription factor 6 (ATF6). These sensors remain inactive while bound to the ER protein chaperone immunoglobulin heavy chain-binding protein (BiP). Accumulation of misfolded proteins that bind to and sequester BiP leads to IRE1 dimerization and activation of its kinase and endoribonuclease activities. The activated endoribonuclease domain cleaves a 26 base intron from the mRNA encoding the transcription factor XBP1 (X-box-binding protein 1), which results in a translational frame-shift and the production of a potent transcriptional activator (Zhang & Kaufman, 2008). IRE1/XBP1 is the most conserved branch of the UPR and has been suggested to play crucial roles in a wide range of biological processes including development, metabolism, immunity, inflammation and neurodegeneration. A string of recent studies suggests that IRE1/XBP1 signalling from the ER represents a crucial node that coordinates metabolic and immune responses (Martinon et al, 2010; Park et al, 2010; Richardson et al, 2010; Winnay et al, 2010, Fig 1).
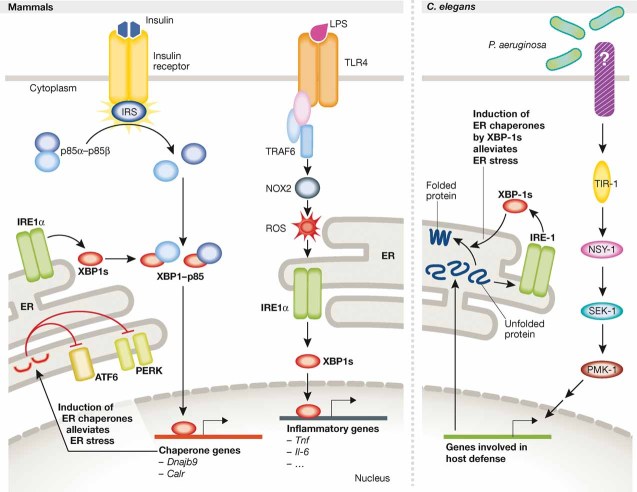
Figure 1. IRE1/XBP1 links ER homeostasis to metabolism and innate immunity.
Upon insulin stimulation, heterodimers of p85α–p85β, the regulatory subunits of PI3K, dissociate so that p85 can interact with XBP1, leading to its nuclear translocation (left panel, left part). XBP1 in the nucleus induces expression of ER chaperones thereby alleviating ER stress, preventing chronic UPR activation and improving insulin sensitivity. In macrophages, TLR2/4 specifically activates IRE1α-dependent production of XBP1s to induce transcription of inflammatory response genes, including Il6 and Tnf, by binding to their promoter regions (left panel, right part). Upon TLR activation, the adaptor protein TRAF6 mediates the NOX-2-dependent production of reactive oxygen species (ROS), which are required for the activation of IRE1α. When Caenorhabditis elegans larvae are infected by Pseudomonas aeruginosa, activation of the PMK-1-mediated immune response induces expression of a large set of genes that increases the protein-folding load on the ER (right panel). The accumulation of unfolded proteins activates IRE-1, thus leading to the production of XBP1s, which enhances productive protein folding by chaperone gene induction and protects the larvae from immune response-induced ER stress.
»…IRE1/XBP1 mediates an inflammatory response in macrophages…«
IRE1/XBP1 is essential for plasma cell differentiation as well as for dendritic cell and intestinal Paneth cell development and survival (Kaser et al, 2008; Todd et al, 2008), all of which are involved in innate immunity and host defence. As such, this signalling pathway is anticipated to play a key role in regulation of immune responses. Recent studies reported by Martinon et al further support this notion by showing that IRE1/XBP1 mediates an inflammatory response in macrophages (Martinon et al, 2010).
These authors show that Toll-like receptors (TLR) 2 and 4 specifically activate IRE1α-dependent production of spliced XBP1 (XBP1s) to induce transcription of the inflammatory response genes Il6 and Tnf. The functional significance of XBP1 signalling in innate immunity is highlighted by the observation that XBP1 deficiency in murine haematopoietic cells increases the bacterial burden after Francisella tularensis infection. The authors further show that signalling from TLR to Xbp1 splicing requires the adaptor proteins TIRAP and MyD88 (TLR2), MyD88 or TRIF (TLR4) and TRAF6 (TLR2/4), but not the downstream targets NF-κB, p38 mitogen-activated protein kinase (MAPK) or JNK. Intriguingly, TLR2/4-mediated activation of IRE1/XBP1 depends on the activity of NADPH oxidase NOX2, although the mechanism remains unknown. In future experiments, it would be interesting to test whether activation of NOX2 activity is sufficient to initiate Xbp1 splicing independently of TLR activation and whether perturbation of BiP levels in the ER lumen, or pharmacological manipulation with antioxidants could interfere with the proinflammatory response triggered by TLR ligation in macrophages.
»…The important and potentially ancient role of IRE1/XBP1 in innate immunity…«
The important and potentially ancient role of IRE1/XBP1 in innate immunity is further underscored by recent studies in worms (Richardson et al, 2010). Specifically, when Caenorhabditis elegans larvae are infected by Pseudomonas aeruginosa, activation of the p38 MAPK family member 1 (PMK-1)-mediated immune response induces IRE1/XBP1 signalling. However, in contrast to mammals and flies, the immune response in C. elegans depends mainly on the p38 MAPK pathway, whereas TOL-1, the worm homologue of TLR, does not appear to be essential (Irazoqui et al, 2010). Richardson et al show that loss-of-function mutation in xbp-1 severely impairs development and viability on P. aeruginosa. However, P. aeruginosa accumulation in the intestine was not altered in the absence of XBP-1. Surprisingly, the lethality of xbp-1 mutants appears due to hyperactivation of PMK-1, even in the absence of pathogen infection. The results suggest that XBP-1 suppresses the detrimental effect of PMK-1 activation during the immune response, but does not facilitate the elimination of the pathogen.
These findings generate many questions. How does the PMK-1-mediated immune response activate the IRE1/XBP1 pathway, and how does C. elegans XBP-1 prevent the detrimental effects of PMK-1 hyperactivation? In response to P. aeruginosa infection, C. elegans activates more than 300 genes, of which nearly half encode proteins that require folding, assembly and modification in the ER. Since XBP-1 is required for survival in response to protein misfolding (Shen et al, 2001), one possibility is that XBP-1 may protect the larvae from P. aeruginosa infection by enhancing productive protein folding upon activation of the immune response, a situation reminiscent of the essential requirement for XBP1 in intestinal Paneth cells to promote high level secretion of anti-microbial peptides (Kaser et al, 2008). An alternative possibility is that, in the absence of XBP-1, accumulation of unfolded proteins may lead to production of reactive oxygen species (ROS) (Malhotra et al, 2008), which are known to activate PMK-1 (Inoue et al, 2005). Accordingly, IRE1/XBP1-mediated improvement in ER function would limit ROS production and, thus, prevent PMK-1 hyperactivation.
Future studies should dissect how IRE1/XBP1 responds to different pathogens/toxins in different life stages (i.e. larva and adulthood). It also remains to be determined whether IRE1/XBP1 signalling interacts with the p38 MAPK pathway in a cell type- and/or context-specific manner in different pathophysiological processes known to be associated with p38 and IRE1/XBP1 in mammals.
»…Immunity, inflammation and metabolism are intertwined biological processes…«
Immunity, inflammation and metabolism are intertwined biological processes. The significance of IRE1α/XBP1 in providing a fundamental role in regulating metabolism is suggested by observations that this pathway orchestrates insulin sensitivity (Ozcan et al, 2004), hepatic lipogenesis (Lee et al, 2008) and in vitro adipogenesis (Sha et al, 2009). Recent studies by Park et al and Winnay et al now suggest that p85α and p85β, the regulatory subunits of phosphoinositide 3-kinase (PI3K), potentiate the UPR by interacting with XBP1 and increase its nuclear translocation (Park et al, 2010; Winnay et al, 2010). Upon growth factor stimulation, the PI3K p110 catalytic subunit is recruited to the membrane through interaction of the PI3K p85 regulatory subunit (α, β or γ) with phosphotyrosine residues on activated receptor tyrosine kinases (RTKs) or receptor-associated adaptor proteins, including the insulin receptor substrate (IRS). Park et al find that insulin stimulation disrupts p85α–p85β heterodimers, so that p85 can interact with XBP1 to facilitate its nuclear translocation, independently of PI3K catalytic activity. Accordingly, fibroblasts deficient in p85 exhibit diminished nuclear accumulation of XBP1 and blunted UPR induction following ER stress. A similar phenotype was observed in p85α-deleted hepatocytes in vivo (Winnay et al, 2010). Park et al propose that p85 promotes XBP1 nuclear translocation to induce expression of ER chaperones thereby alleviating ER stress and preventing chronic UPR activation, which is associated with proinflammatory and pro-apoptotic responses (Park et al, 2010; Zhang & Kaufman, 2008).
Park et al extend these observations by studying how the p85–XBP1 interaction may regulate insulin responses. They show that in insulin resistant ob/ob mice, the interaction between p85 and XBP1 is disrupted and the nuclear translocation of XBP1 is severely impaired. Interestingly, over-expression of p85 in liver significantly improved glucose tolerance in ob/ob mice. Although these findings are not consistent with previous reports on the effects of p85 deletion, knock-down, and over-expression on insulin sensitivity (Taniguchi et al, 2006, 2007), the studies extend our appreciation of how insulin signalling may intersect with the UPR to modulate ER homeostasis, and how insulin resistance and chronic ER stress may establish a vicious cycle in the development of multi-organ inflammation and metabolic syndrome.
Although intriguing, the studies of Winnay et al and Park et al leave many questions unexplored. The majority of these studies rely on over-expression of p85, so further studies are required to characterize the XBP1 interaction and nuclear localization under more physiological conditions. If insulin facilitates the p85–XBP1 interaction, do other growth factors/RTKs promote XBP1-mediated transcription induction in a similar manner? In addition to the UPR, XBP1-mediated transcriptional induction is required in embryonic liver development (Reimold et al, 2000), hepatic lipogenesis (Lee et al, 2008), adipocyte differentiation (Sha et al, 2009) and the macrophage proinflammatory response (Martinon et al, 2010). Therefore, it might be predicted that p85-mediated nuclear translocation will regulate XBP1 function in multiple cell types and various biological processes. The recent flurry of discoveries have provided potentially important insights into the significance of IRE1/XBP1 and ER homeostasis as a signalling platform involved in health and disease and should stimulate further intense research in this field.
Acknowledgments
RJK is an Investigator of the Howard Hughes Medical Institute and supported by NIH grants DK42394, HL52173 and PO1 HL057346.
The authors declare that they have no conflict of interest.
References
- Inoue H, et al. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui JE, et al. Nat Rev Immunol. 2010;10:47–58. doi: 10.1038/nri2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, et al. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, et al. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, et al. Proc Natl Acad Sci USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, et al. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, et al. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Park SW, et al. Nat Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold AM, et al. Genes Dev. 2000;14:152–157. [PMC free article] [PubMed] [Google Scholar]
- Richardson CE, et al. Nature. 2010;463:1092–1095. doi: 10.1038/nature08762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha H, et al. Cell Metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, et al. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, et al. Proc Natl Acad Sci USA. 2006;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, et al. Mol Cell Biol. 2007;27:2830–2840. doi: 10.1128/MCB.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ, et al. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- Winnay JN, et al. Nat Med. 2010;16:438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]