Abstract
Sleep disordered breathing with recurrent apneas is one of the most frequently encountered breathing disorder in adult humans and preterm infants. Recurrent apnea patients exhibit several co-morbidities including hypertension and persistent sympathetic activation. Intermittent hypoxia (IH) resulting from apneas appears to be the primary stimulus for evoking autonomic changes. The purpose of this article is to briefly review the effects of IH on chemo-and baro-reflexes and circulating vasoactive hormones and their contribution to sympathetic activation and blood pressures. Sleep apnea patients and IH-treated rodents exhibit exaggerated arterial chemo-reflex. Studies on rodent models demonstrated that IH leads to hyperactive carotid body response to hypoxia. On the other hand, baro-reflex function is attenuated in patients with sleep apnea and in IH-treated rodents. Circulating vasoactive hormone levels are elevated in sleep apnea patients and in rodent models of IH. Thus, persistent sympathetic activation and hypertension associated with sleep apneas seems to be due to a combination of altered chemo-and baro-reflexes resulting in sympathetic activation and action of elevated circulating levels of vasoactive hormones on vasculature.
Keywords: hypoxia, apneas, carotid body, baro-receptors, sleep-apneas, vasoactive mediators
1. Introduction
Sleep disordered breathing with recurrent apneas is a commonly encountered respiratory problem in adult humans and infants born preterm. Recurrent apneas are characterized by transient, repetitive cessations of breathing, each episode lasting no more than tens of seconds and arise either as a consequence of obstruction of the upper-airway leading to cessation of airflow (Obstructive Sleep Apnea or OSA) or due to defective respiratory rhythm generation by the central nervous system (central sleep apneas). Adult patients with either form of recurrent apnea exhibit several co-morbidities including increased risk for developing hypertension and display persistent activation of the sympathetic nervous system (see review Narkiewicz and Somers., 1997; Peppard et al., 2000). Considerable evidence suggests that intermittent hypoxia (IH) resulting from apneas is the primary stimulus for evoking sympathetic excitation (see review Prabhakar et al., 2007). Hypercapnia that occurs during apneas and apnea by itself (i.e., absence of pulmonary afferent feed back) might also contribute to sympathetic excitation. Reflexes arising from peripheral arterial chemo and baro-receptors regulate blood pressure by altering the sympathetic tone. Studies on recurrent apnea patients and rodent models provide important insights into the effects of IH on arterial chemo- and baro-receptors and their role in evoking persistent sympathetic activation and elevation of blood pressures. In addition to the sympathetic tone, circulating vasoactive hormones/mediators profoundly impact blood pressure by altering peripheral vascular resistance. Recent studies indicate that IH also affects the production and release of aminergic and peptidergic vasoactive hormones, which contribute to elevated blood pressures by directly acting on blood vessels. The purpose of this article is to briefly review the studies related to the effects of IH on chemo- and baro-reflexes and vasoactive hormones and their contribution to sympathetic activation and blood pressures.
2. Blood pressures and Sympathetic nerve activity
Sleep apnea patients
Much of the information on the consequences of recurrent apneas on blood pressures and sympathetic nerve activity has come from studies on OSA patients. Sleep apnea patients exhibit elevated blood pressures and elevated sympathetic tone as measured by muscle sympathetic nerve activity (MSNA), which is reflective of reflex control of systemic vascular resistance (Carlson et al., 1993; Hedner et al. 1988; Leuenberger et al., 1995; Somers et al. 1995). Elevated MSNA is seen even in day time wherein breathing pattern and arterial blood gas values are normal without any evidence for apneas. Although occurrence of sleep apneas is more prevalent in obese individuals, the elevated sympathetic tone reported in OSA patients seems independent of obesity (Narkiewicz and Somers, 1997).
During sleep, normal subjects (i.e., subjects without apneas) display low levels of sympathetic activity, reductions in heart rate and blood pressures (Hornyak et al., 1991; Okada et al., 1991; Somers et al., 1993). On the other hand, recurrent apnea patients, do not exhibit reductions in sympathetic activity and blood pressures during sleep (Narkiewicz and Somers, 1997). Sympathetic nerve activity and blood pressures progressively increase during each episode of apnea and the magnitude of increase is most pronounced during REM sleep, wherein the duration of apneas were longer than in non-REM sleep (Narkiewicz and Somers, 1997). Preventing recurrent apneas through the use of continuous positive airway pressure (CPAP) lowers sympathetic activity and blood pressures in OSA patients (Carlson et al., 1993; Imadojemu et al.2007; Somers et al.,1995).
Rodent models of IH
Greenberg et al (1999) examined the effects of chronic IH on sympathetic nerve activity in a rodent model. These investigators recorded cervical sympathetic nerve activity in 2 month old male Sprague-Dawley rats exposed to 30d of IH (30s of hypoxia and 30s of normoxia) for 8h/d during day time (which is sleep time in rodents). Baseline blood pressures and cervical sympathetic nerve activity were elevated in IH treated rats compared to controls. Although the relevance of cervical sympathetic nerve activity to changes in blood pressure in IH treated rats can be argued, subsequent studies reported similar increases in renal (Huang et al., 2009), splanchnic (Dick et al., 2007), thoracic (Zoccal et al., 2008) and lumbar (Marcus et al., 2010) sympathetic nerve activities, which are more relevant to blood pressure regulation. Zoccal et al (2008) further noted altered coupling of central sympathetic-respiratory outputs in chronic IH treated rats. These investigators reported that increased sympathetic nerve activity was more pronounced in late expiratory phase of respiration, a finding consistent with an earlier study by Dick et al (2007). c-fos is an immediate early gene, and the expression of the encoded protein c-Fos is a marker of increased neural activity in the central nervous system (CNS). Chronic IH activates various areas of the CNS associated with regulation of sympathetic nervous system and blood pressure in rats as evidenced by increased c-Fos expression (Sica et al., 2000).
3. Intermittent hypoxia augments arterial chemoreceptor reflex
Arterial chemoreceptors, especially the carotid bodies are the primary sensors for hypoxia and the ensuing reflex activates sympathetic nerve activity, elevates blood pressure and stimulates breathing. Because apneas are associated with IH, several studies examined the potential role of chemo-reflex in sympathetic activation in sleep apnea patients and in IH treated rodents. The following section summarizes some of these studies.
Studies on sleep-apnea patients
Acute hyperoxia, which decreases carotid body sensory activity, reduces blood pressure in normoxic OSA patients but not in normoxic control subjects (Narkiewicz et al, 1998), suggesting heightened chemo-reflex function. Furthermore, hypoxia-evoked ventilatory and pressor responses were more pronounced in OSA patients compared to control subjects (Hedner et al., 1992). MSNA activation during hypoxic episodes (apneas) at night is markedly augmented in OSA patients (Leuenberger et al. 1995). Also, acute hypoxia-induced sympathetic excitation was more pronounced in OSA patients under wakefulness compared to control subjects (Smith et al., 1996). Lusina et al. (2006) reported augmented MSNA responses to acute hypoxia in normal subjects treated with 1h of hypoxia per day for 10 days. Acute IH, akin to sleep apneas with and without CO2 leads to persistent sympathetic activation in normal subjects (Cutler et al. 2004; Morgan et al., 1995; Xie et al., 2000). Repetitive hypoxia-induced persistent sympathetic activation might explain day time sympathetic activity reported in sleep apnea patients.
Studies on experimental models of IH
Arterial chemo-reflex is augmented in IH treated cats (Rey et al, 2004) and mice (Peng et al. 2006) as evidenced by exaggerated ventilatory responses to hypoxia. Huang et al (2009) examined renal sympathetic nerve and arterial blood pressure responses to acute hypoxia and hypercapnia in rats treated with 3 weeks of IH. These investigators found exaggerated renal nerve responses to both hypoxia and hypercapnia in IH treated rats. Juvenile rats (3 week old rats) treated with 10d of IH showed enhanced thoracic sympathetic and phrenic nerve responses to cyanide, a chemo-receptor stimulant in working heart-brainstem preparation (Braga et al. (2006) and a subsequent study concluded that the augmented chemo-reflex contributes to enhanced sympathetic nerve activity in juvenile rats (Zoccal et al. 2008). Furthermore, ablation of sinus nerves, which innervate the carotid body, prevents IH-induced hypertension (Fletcher 2001) and long-lasting increases in splanchnic nerve activity (Prabhakar et al. 2005) in rats. The findings from rodent models and sleep apnea patients suggest that IH associated with sleep apneas contribute to exaggerated arterial chemo-reflex.
4. Intermittent hypoxia results in hyperactive carotid body
Recent studies provide compelling evidence for altered carotid body function in experimental animals treated with chronic IH. Direct recording of sensory activity of the carotid body in chronic IH exposed rats revealed an exaggerated response to hypoxia but not to hypercapnia (Peng et al., 2003). Similar sensitization of the carotid body response to hypoxia was also reported in chronic IH treated cats (Rey et al., 2004) and mice (Peng et al., 2006).
In control rats, challenging the carotid bodies with acute intermittent hypoxia (AIH; 15s of hypoxia followed by 5min of re-oxygenation, 10 episodes) augmented the sensory activity during each episode of hypoxia and after terminating AIH, sensory activity promptly returned to baseline. On the other hand, in chronic IH treated rats, sensory activity progressively increased with each episode of hypoxia, and more importantly, baseline activity remained elevated for about 60min during the post-AIH period. This long-lasting increase in baseline sensory activity has been termed as sensory long-term facilitation i.e., sensory LTF (Peng et al., 2003).
The effects of chronic IH on carotid body function including the sensitization of the hypoxic response and the induction of sensory LTF developed over time and reversed completely after re-exposure to normoxia (Peng et al., 2003). The reversible nature of the carotid body responses to chronic IH might explain why CPAP therapy reverses the adverse cardio-sympathetic effects in OSA patients (Carlson et al., 1993; Somers et al., 1995).
5. Mechanisms associated with IH-induced carotid body hyperactivity
Chronic IH-induced sensitization of the hypoxic response as well as sensory LTF could be evoked in rat and mice carotid bodies ex vivo (Peng et al., 2003, 2006) suggesting that these effects are not secondary to cardiovascular changes such as elevated blood pressures seen in animals exposed to IH (Fletcher., 2001, Kumar et al., 2006, Peng et al., 2006). Furthermore, IH-evoked changes in the carotid body function were not associated with morphological changes in the chemoreceptor tissue (Peng et al. 2003). It has been proposed that reactive oxygen species (ROS) generated during the re-oxygenation phase mediate cellular responses to IH (Prabhakar, 2001). ROS levels were elevated in IH treated carotid bodies as evidenced by decreased aconitase enzyme activity (Peng et al., 2003), a robust biological marker of ROS. Further studies identified NADPH-oxidase, especially Nox2 (Peng et al. 2009) and inhibition of mitochondrial electron transport chain at the complex I (Peng et al. 2003) as potential sources of ROS generation in carotid bodies from chronic IH treated rats. Concurrent systemic administration of a stable superoxide dismutase mimetic [manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride (MnTMPyP), 5 mg/kg, i.p.], a potent scavenger of ROS every day prior to subjecting animals to 8h of IH prevented sensitization as well as the sensory LTF of the carotid body (Peng et al., 2003 and Prabhakar and Peng, 2004). These studies suggest that ROS mediate chronic IH-induced sensitization of the hypoxic response as well as the induction of sensory LTF of the carotid body.
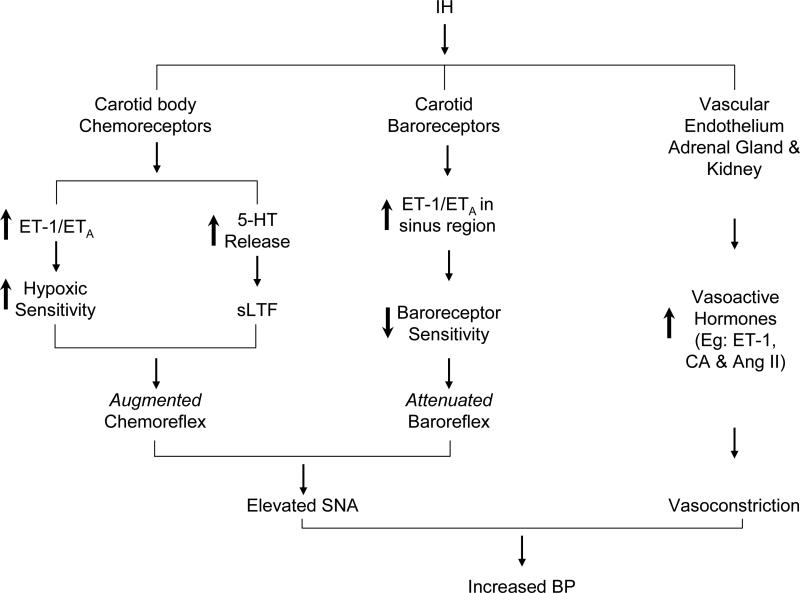
ROS signaling mechanisms mediating IH-evoked sensitization of the hypoxic response, however, seem to differ from those evoking the sensory LTF of the carotid body. IH-evoked up-regulation of hypoxic sensitivity of the carotid body involves ROS-dependent up-regulation of endothelin-1 (ET-1; Pawar et al. 2009), and ETA but not ETB receptors (Rey et al. 2006; Pawar et al., 2009; Fig.1). However, ET-1 seems not to contribute to the induction of sensory LTF (Peng & Prabhakar, unpublished observations, 2010). On the other hand, 5-HT mediates sensory LTF by activating NADPH oxidase, especially Nox2 and the ensuing ROS generation (Peng et al. 2009; Fig.1).
Figure 1.
Schematic illustration of the effects and mechanisms associated with alterations in chemo-and baro-reflexes by intermittent hypoxia (IH) and the effects of IH on circulating vasoactive hormones. ET-1-Endothelin-1; ETA = Endothelin receptor A; 5-HT= 5-hydroxytryptamine; sLTF= sensory long-term facilitation of the carotid body activity. CA= catecholamines; Ang II= angiotensin II; SNA= sympathetic nerve activity; BP= Arterial blood pressure.
6. Significance of hyperactive carotid body function
The above studies demonstrate that chronic IH has two major effects on carotid body function that include: a) sensitization of the carotid body response to acute hypoxia, and b) long-lasting activation of baseline sensory activity i.e., sensory LTF. It is likely that the sensory LTF of the carotid body via reflex activation contributes to sustained day time elevations of sympathetic activity reported in sleep-apnea patients. Supporting such a possibility are the findings that inhibition of sympathetic nerve evoked by hyperoxic stimulus (which inhibits carotid body activity) was less pronounced in sleep-apnea patients compared to control subjects (Leuenberger et al. 1995; Narkiewicz et al. 1998). Furthermore, pharmacological blockade of carotid body prevents augmented chemo-reflex in IH-treated rats (Marcus et al. 2010). These observations support the idea that IH associated with apneas indeed augment chemoreceptor response to hypoxia and mediate sympathetic excitation.
7. Evidence for down-regulation of baro-reflex by intermittent hypoxia
Besides chemo-reflex, arterial baro-reflex is another major regulator of the sympathetic tone and arterial blood pressure. Unlike chemoreceptors, activation of baroreceptors inhibits sympathetic tone. The following studies suggest that baro-reflex function is down regulated in sleep-apnea patients and in rodents treated with chronic IH.
Patients with OSA exhibit reduced baroreflex sensitivity as evidenced by attenuated heart rate and vascular resistance responses to activation of baroreceptors (Bonsignore et al., 2002; Carlson et al. 1993; Cooper et al., 2007; Monahan et al., 2006) and CPAP treatment restores baroreflex sensitivity (Bonsignore et al., 2002). Lai et al (2006) examined the effects of several days of IH treatment on baro-reflex function in rats. Spectral analysis of heart rate responses indicate attenuated baro-reflex function in IH treated rats with a concomitant increase in chemo-reflex as evidenced by augmented ventilatory response to hypoxia. In a recent preliminary study, we examined the effects of IH (15s hypoxia followed by 5 min normoxia) on splanchnic nerve responses to phenylephrine (PE) in adult male Sprague-Dawley rats (Peng et al., 2010). PE-induced sympathetic inhibition was markedly attenuated in IH treated rats, indicating down regulation of baro-reflex function. However, Greenberg et al (1999) reported no change in cervical sympathetic nerve response to PE in adult rat exposed to 30d of IH. These authors used a IH paradigm consisting of alternating cycles of hypoxia and normoxia 30s each, wherein inspired O2 levels with each episode were ~7% O2 with a dwell time of ~6s. Measurements of arterial blood O2 saturation levels showed that IH paradigm by Greenberg et al (1999) produced 90-95% O2 saturations; whereas our IH paradigm resulted in 75-80% O2. The relatively lower O2 saturations in response to IH paradigm used by Greenberg et al is conceivably due to a combination of shorter hypoxic dwell time (i.e., ~6s) and lesser intensity of hypoxia (i.e., ~7% O2) as compared to longer hypoxic dwell time (15s) and more intense hypoxia (5% O2) used in our IH paradigm. Zoccal et al (2008) found no alteration in baro-reflex control of sympathetic nerve activity in a working heart-brainstem preparation in IH treated juvenile rats. Thus, the studies outlined above suggest that depending on the severity of the stimulus and developmental stage of the animals, chronic IH up-regulates chemo- and down-regulates baro-reflexes.
8. Effects of IH on baro-receptor Activity
The attenuated baroreflex sensitivity described above could in part be due to the effects of IH on carotid and/ or aortic baroreceptors. Gu et al (2007) reported no change in aortic baroreceptor activity in IH treated Fischer-344 rats. A recent preliminary study examined the effects of chronic IH on carotid baro-receptor activity (Peng et al., 2010). These investigators employed an ex vivo rat carotid sinus preparation in order to set the initial conditioning pressure to the same level in the control and IH treated rats. In control rats, baroreceptor activity progressively increased in a sigmoidal fashion in response to step increases in sinus pressure. In IH-treated preparation, the magnitude of increase in baroreceptor activity at higher pressures was markedly attenuated primarily due to reduced saturation and threshold pressures. ET-1 was up-regulated in endothelial cells in the carotid sinus region and ETA receptor antagonist prevented IH-induced attenuated carotid baro-receptor sensitivity. These findings suggest that IH attenuates baro-reflex function, which is in part due to its effects on carotid baro-receptors (Fig.1). Despite the preliminary nature of these findings, it is remarkable that IH-evoked up-regulation of ET-1 exerts diametrically opposing effects on carotid chemo and baroreceptors, in that it exaggerates chemoreceptor responses to hypoxia whereas it depresses baroreceptor responses to increased sinus pressures (Fig.1).
9. Significance of altered chemo-baro-receptor function by IH
The studies outlined above suggest that depending on the severity of the stimulus and age chronic IH up-regulates chemo- and down-regulates baro-reflexes. It is likely that the imbalance between these two opposing reflex pathways play a major role in evoking persistent sympathetic activation in response to chronic IH (Fig.1).
10. Effects of IH on vasoactive hormones/ mediators
In addition to chemo-and baro reflex regulation of blood pressure via sympathetic nervous system, several vasoactive hormones/mediators by directly acting on resistance vessels also contribute to changes in blood pressure. In the periphery, the vascular endothelium, adrenal gland, and kidney are the major sites of production of vasoactive hormones/mediators. Catecholamines derived from the adrenal medulla, renal renninangiotensin system and endothelins from the vascular endothelial cells are major vasoconstrictors. Several studies on patients with OSA and IH-treated rodents explored the roles of vasoactive mediators in elevated blood pressures.
Catecholamines
Several studies reported elevated circulating catecholamines in OSA patients (Carlson et al., 1993; Fletcher et al.1987; Garcia-Rio et al. 2000; Marrone et al. 1993; Somers et al., 1995). Both norepinephrine and epinephrine levels were elevated in plasma and urine in these patients (Carlson et al., 1993; Fletcher et al. 1987; Marrone et al., 1993). Marrone et al (1993) reported urinary epinephrine levels decreased in the absence of apneas in these subjects. Chronic CPAP treatment reduced circulating catecholamines in OSA patients (Suzuki et al., 1993). A similar increase in circulating norepinephrine and epinephrine was also reported in rats and mice exposed to chronic IH (Bao et al., 1997; Kumar et al., 2006; Peng et al., 2006).
Adrenal medulla is a major source of catecholamines. Recent studies examined the effects of IH on acute-hypoxia evoked catecholamine secretion from adrenal medulla in rats (Kumar et al., 2006; Souvannakitti et al., 2009) and mice (Kuri et al., 2007). Hypoxia-evoked catecholamine secretion from adrenal medulla was markedly potentiated in IH treated rats. Studies on mouse adrenal medullary chromaffin cells (AMC) showed that chronic IH increases the readily releasable pool of secretory vesicles via ROS-mediated activation of protein kinase C (Kuri et al., 2007). Studies on rat AMC showed that the effects of IH involve ROS-dependent facilitation of Ca2+ influx as well as mobilization of intracellular Ca2+ stores (Souvannakitti et al. 2009). In a recent study, Souvannakitti et al (2010) examined the source of ROS and the cellular mechanisms associated with Ca2+ homeostasis in IH treated AMC. They identified NADPH oxidases, especially Nox2 and Nox 4 isoforms as potential sources of ROS generation. They further showed that IH-evoked Ca2+ flux is mediated in part via up-regulation of low-threshold T-type Ca2+ channels and mobilization of intracellular Ca2+ stores involving transcriptional up-regulation as well as post-translational modification of ryanodine receptors (RyRs). These studies suggest that IH facilitates catecholamine secretion from AMC involves ROS-dependent signaling mechanisms, which might account in part to the elevated circulating catecholamines reported in sleep-apnea patients and IH treated rodents.
Endothelins
Vascular endothelial cells express endothelin-1 (ET-1), a potent vasoconstrictor peptide with mitogenic properties (Howard et al., 1992). Circulating ET-1 levels are elevated in patients with OSA compared to healthy humans (Gjorup et al., 2007; Phillips et al., 1999; Saarelainen et al., 1997; Zamarron-Sanz, et al, 2006), and CPAP treatment restores ET-1 levels. However, some studies reported no change in plasma ET-1 levels in OSA patients (Grimpen et al., 2000; Moller et al., 2003). Interestingly, Jordan et al., (2005) reported elevations of plasma big ET-1 (a precursor of ET-1) but not ET-1 level in OSA patients, and CPAP therapy decreased big-ET-1 levels to controls. Kanagy et al., (2001) reported elevated plasma ET-1 levels and blood pressure in rats treated with chronic IH combined with hypercapnia. Endothelin type A receptor antagonist normalized blood pressure in IH treated rats (Allahdadi et al., 2008).
Renin-Angiotensin System
Activation of the renin-angiotensin system causes vasoconstriction, via the angiotensin I (AT-1) receptor. Patients with OSA exhibit elevated plasma angiotensin II levels (Moller et al., 2003). Fletcher et al. (2002) reported that AT-1 receptor antagonist prevents elevated blood pressures in rats treated with chronic IH.
11. Summary and Perspective
In this review, we highlighted recent studies addressing the mechanisms by which recurrent apnea causes persistent sympathetic activation and elevated blood pressure. Studies on experimental models of IH provided significant mechanistic insights underlying the autonomic changes associated with recurrent apneas. Importantly, these studies uncovered that augmented chemoreflex and attenuated baroreflex as one of the major reflex alteration for evoking persistent sympathetic excitation by IH. In addition, IH also increases the synthesis and release of various vasoactive hormones leading to vasoconstriction. Thus, interplay between the sympathetic activation and vasoactive hormones mediate the elevated blood pressure caused by IH as illustrated in Figure 1.
Acknowledgement
We thank Drs. T.E. Dick, A.P. Fox, Y-J. Peng, Xin Zhang, A. Pawar and D. Souvannakitti, for their contributions to the experiments from authors’ laboratory. Research from authors’ laboratory is supported by grants from National Institutes of Health, Heart, Lung and Blood Institute HL-90554, HL-76537, HL-86493, HL-089616.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, Kanagy NL. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H434–H440. doi: 10.1152/ajpheart.91477.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J. Appl. Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- Bonsignore MR, Parati G, Insalaco G, Marrone O, Castiglioni P, Romano S, Di Rienzo M, Mancia G, Bonsignore G. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2002;166:279–286. doi: 10.1164/rccm.2107117. [DOI] [PubMed] [Google Scholar]
- Braga VA, Soriano RN, Machado BH. Sympathoexcitatory response to peripheral chemoreflex activation is enhanced in juvenile rats exposed to chronic intermittent hypoxia. Exp. Physiol. 2006;91:1025–1031. doi: 10.1113/expphysiol.2006.034868. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner JA, Elam M, Ejnell H, Sellgren J, Wallin G. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Elliott MW, Pearson SB, Taylor CM, Mohammed MM, Hainsworth R. Daytime variability of baroreflex function in patients with obstructive sleep apnoea: implications for hypertension. Exp. Physiol. 2007;92:391–398. doi: 10.1113/expphysiol.2006.035584. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J. Appl. Physiol. 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar NR. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp. Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J. Appl. Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep. 1987;10:35–44. doi: 10.1093/sleep/10.1.35. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J. Appl. Physiol. 2002;92:627–633. doi: 10.1152/japplphysiol.00152.2001. [DOI] [PubMed] [Google Scholar]
- Garcia-Rio F, Racionero MA, Pino JM, Martinez I, Ortuno F, Villasante C, Villamor J. Sleep apnea and hypertension. Chest. 2000;117:1417–1425. doi: 10.1378/chest.117.5.1417. [DOI] [PubMed] [Google Scholar]
- Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am. J. Hypertens. 2007;20:44–52. doi: 10.1016/j.amjhyper.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J. Appl. Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- Grimpen F, Kanne P, Schulz E, Hagenah G, Hasenfuss G, Andreas S. Endothelin-1 plasma levels are not elevated in patients with obstructive sleep apnoea. Eur. Respir. J. 2000;15:320–325. doi: 10.1034/j.1399-3003.2000.15b17.x. [DOI] [PubMed] [Google Scholar]
- Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster RD, Chapleau MW, Ma X, Cheng ZJ. Selective Impairment of Central Mediation of Baroreflex in Anesthetized Young-Adult Fischer 344 Rats Following Chronic Intermittent Hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H2809–2818. doi: 10.1152/ajpheart.00358.2007. [DOI] [PubMed] [Google Scholar]
- Hedner JA, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J. Hypertens. 1988;6(Suppl):5529–5531. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE. A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am. Rev. Respir. Dis. 1992;146:1240–1245. doi: 10.1164/ajrccm/146.5_Pt_1.1240. [DOI] [PubMed] [Google Scholar]
- Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG. Sympathetic muscle nerve activity during sleep in man. Brain. 1991;114(Pt 3):1281–1295. doi: 10.1093/brain/114.3.1281. [DOI] [PubMed] [Google Scholar]
- Howard PG, Plumpton C, Davenport AP. Anatomical localization and pharmacological activity of mature endothelins and their precursors in human vascular tissue. J. Hypertens. 1992;10:1379–1386. doi: 10.1097/00004872-199211000-00010. [DOI] [PubMed] [Google Scholar]
- Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y, Weiss JW. Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir. Physiol. Neurobiol. 2009;166:102–106. doi: 10.1016/j.resp.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA, Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131:1406–1413. doi: 10.1378/chest.06-2580. [DOI] [PubMed] [Google Scholar]
- Jordan W, Reinbacher A, Cohrs S, Grunewald RW, Mayer G, Rüther E, Rodenbeck A. Obstructive sleep apnea: Plasma endothelin-1 precursor but not endothelin-1 levels are elevated and decline with nasal continuous positive airway pressure. Peptides. 2005;26:1654–1660. doi: 10.1016/j.peptides.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–515. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR, Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J. Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri BA, Khan SA, Chan SA, Prabhakar NR, Smith CB. Increased secretory capacity of mouse adrenal chromaffin cells by chronic intermittent hypoxia: involvement of protein kinase C. J. Physiol. 2007;584:313–319. doi: 10.1113/jphysiol.2007.140624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol. 2006;100:1974–1982. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J. Appl. Physiol. 1995;79:581–588. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- Lusina SJ, Kennedy PM, Inglis JT, McKenzie DC, Ayas NT, Sheel AW. Long-term intermittent hypoxia increases sympathetic activity and chemosensitivity during acute hypoxia in humans. J. Physiol. 2006;575(Pt 3):961–970. doi: 10.1113/jphysiol.2006.114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir. Physiol. Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103:722–727. doi: 10.1378/chest.103.3.722. [DOI] [PubMed] [Google Scholar]
- Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am. J. Hypertens. 2003;16:274–280. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J. Physiol. 2006;574(Pt 2):605–613. doi: 10.1113/jphysiol.2006.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J. Appl. Physiol. 1995;79:205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J. Hypertens. 1997;15:1613–1619. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- Okada H, Iwase S, Mano T, Sugiyama Y, Watanabe T. Changes in muscle sympathetic nerve activity during sleep in humans. Neurology. 1991;41:1961–1966. doi: 10.1212/wnl.41.12.1961. [DOI] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R735–R742. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J. Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J. Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Raghuraman G, Wang N, Nanduri J, Kumar GK, Prabhakar NR. Up-regulation of endothelin-1 mediates chronic intermittent hypoxia-evoked down-regulation of carotid baro-receptor activity. Am.J.Respir. crit.Care Med. 2010;181:A4198. [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J. Hypertens. 1999;17:61–66. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J. Appl. Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J. Appl. Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp. Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin. Exp. Pharmacol. Physiol. 2005;32:447–449. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J. Physiol. 2004;560(Pt 2):577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res. 2006;1086:152–159. doi: 10.1016/j.brainres.2006.02.082. [DOI] [PubMed] [Google Scholar]
- Saarelainen S, Seppala E, Laasonen K, Hasan J. Circulating endothelin-1 in obstructive sleep apnea. Endothelium. 1997;5:115–118. doi: 10.3109/10623329709079869. [DOI] [PubMed] [Google Scholar]
- Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir. Physiol. 2000;121:173–84. doi: 10.1016/s0034-5687(00)00126-2. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J. Neurophysiol. 2009;101:2837–2846. doi: 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and Ryanodine Receptors mediate the Augmented Exocytosis of Catecholamines from Intermittent Hypoxia treated Neonatal Rat Chromaffin Cells. J. Neurosci. 2010 doi: 10.1523/JNEUROSCI.2307-10.2010. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J. Auton. Nerv. Syst. 1996;56:184–190. doi: 10.1016/0165-1838(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Otsuka K, Guilleminault C. Long-term nasal continuous positive airway pressure administration can normalize hypertension in obstructive sleep apnea patients. Sleep. 1993;16:545–549. doi: 10.1093/sleep/16.6.545. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J. Appl. Physiol. 2000;89:1333–1339. doi: 10.1152/jappl.2000.89.4.1333. [DOI] [PubMed] [Google Scholar]
- Zamarron-Sanz C, Ricoy-Galbaldon J, Gude-Sampedro F, Riveiro-Riveiro A. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Arch. Med. Res. 2006;37:552–555. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J. Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]