Abstract
The prevalence of obesity has reached epidemic proportions, making finding effective solutions to reduce obesity a public health priority. One part of the solution could be for individuals to increase consumption of nonoilseed pulses (dry beans, peas, chickpeas, and lentils), because they have nutritional attributes thought to benefit weight control, including slowly digestible carbohydrates, high fiber and protein contents, and moderate energy density. Observational studies consistently show an inverse relationship between pulse consumption and BMI or risk for obesity, but many do not control for potentially confounding dietary and other lifestyle factors. Short-term (≤1 d) experimental studies using meals controlled for energy, but not those controlled for available carbohydrate, show that pulse consumption increases satiety over 2–4 h, suggesting that at least part of the effect of pulses on satiety is mediated by available carbohydrate amount or composition. Randomized controlled trials generally support a beneficial effect of pulses on weight loss when pulse consumption is coupled with energy restriction, but not without energy restriction. However, few randomized trials have been conducted and most were short term (3–8 wk for whole pulses and 4–12 wk for pulse extracts). Overall, there is some indication of a beneficial effect of pulses on short-term satiety and weight loss during intentional energy restriction, but more studies are needed in this area, particularly those that are longer term (≥1 y), investigate the optimal amount of pulses to consume for weight control, and include behavioral elements to help overcome barriers to pulse consumption.
Introduction
The worldwide prevalence of obesity has reached epidemic proportions (1). Because excess body fat is associated with the development of life-threatening chronic conditions such as heart disease, type 2 diabetes, and certain types of cancer (2,3), viable and sustainable solutions for sustainable weight loss and prevention of weight gain are urgently needed. Generally, selection of a diet high in fiber, low in energy density and glycemic load, and moderate in protein is thought to be particularly important for weight control (4). Such a diet may be achieved by regularly consuming food from certain food groups, including fruits, vegetables, and whole grains; some lean meats, nuts, and legumes; and limited consumption of food from other groups, including high-fat meats, sugar-sweetened beverages, bakery items, and highly processed foods. In this review, we will focus of the role of legumes, particularly the nonoilseed pulses (see terminology below), in energy regulation and successful weight control, highlighting the work that has been published in the past 10 y.
Terminology: legumes, pulses, and beans
The pods or fruits of plants in the botanical family Fabaceae, or Leguminosae, are commonly known as legumes. Legumes include alfalfa, clover, lupin, green beans and peas, peanuts, soybeans, dry beans, broad beans, dry peas, chickpeas, and lentils. According to the FAO (5), a pulse is a type of legume that is exclusively harvested for the dry grain and therefore excludes peanuts and soybeans, which are harvested for their oil. Pulses are also sometimes referred to as grain legumes or pulse grains. The published literature often refers to the Phaseolus vulgaris species; these include kidney beans, haricot beans, pinto beans, and navy beans. Figure 1 shows a simplified scheme of the classification of different legumes.
Figure 1.
Legume classifications (5).
The health effects of soybeans and peanuts have been well studied. Although soybeans share some of the nutritional properties of pulses [e.g. high in fiber and protein, low glycemic index (GI)], they are thought to have unique health effects due to their high content of certain phytoestrogens such as isoflavones and other bioactive compounds (6). The effects of soybeans and soy protein (7–9) and peanuts (10–14) on body weight have been reviewed elsewhere and will not be covered here.
Macronutrient and phytochemical profiles of pulses and energy regulation
Pulses have a unique nutritional profile consistent with several dietary composition factors thought to assist with weight control. They also contain several antinutrients that have been suggested to play a role in energy regulation (Tables 1 and 2). In particular, they are high in fiber, providing ∼7 g/0.5 c (120 mL) serving. Pulses are also relatively low in energy density (1.3 kcal/g or 5.3 kJ/g) and a good source of digestible protein (average of 7.7 g of protein/0.5 c). Pulse carbohydrates are slowly digested (see below), which allows some of the lowest GI among carbohydrate-containing foods. Pulse GI typically range from ∼29 to 48 (using glucose as the standard) compared with GI of 32–36 for dairy, 39–64 for fruit, 42–72 for grains, 49–80 for breakfast cereals, and 49–97 for root vegetables (15–17). The relatively slow digestibility and hence low GI of pulses has been attributed to several constituents, including carbohydrate composition, protein content and protein-starch matrix, and antinutrient factors such as enzyme inhibitors (e.g. amylase inhibitor, trypsin inhibitor), phytates, lectins, saponins, and tannins (18,19). The following section provides a brief description of several of these constituents. The roles of fiber, energy density, carbohydrate type, GI and glycemic load, and protein in energy regulation and weight control in general have been reviewed in detail elsewhere (20–29).
Table 1.
Selected nutrients per 1/2 c (120 mL) serving of some pulses1
| Black bean | Cowpea (Blackeye) | Great Northern bean | Kidney bean, all types | Lima bean, large | Navy bean | Pinto bean | Chickpea | Lentil | Peas, split | Average | |
| Weight, g | 86 | 86 | 89 | 89 | 89 | 91 | 86 | 82 | 99 | 98 | 90 |
| Energy,2kcal | 114 | 100 | 104 | 112 | 108 | 127 | 122 | 134 | 115 | 116 | 115 |
| Protein, g | 7.6 | 6.6 | 7.4 | 8.4 | 7.3 | 7.5 | 7.7 | 7.3 | 8.9 | 8.2 | 7.7 |
| Fat, g | 0.5 | 0.5 | 0.4 | 0.4 | 0.4 | 0.6 | 0.6 | 2.1 | 0.4 | 0.4 | 0.6 |
| Carbohydrate, g | 20.4 | 17.9 | 18.7 | 20.1 | 19.6 | 23.7 | 22.4 | 22.5 | 19.9 | 20.7 | 20.6 |
| Dietary fiber, g | 7.5 | 5.6 | 6.2 | 5.7 | 6.6 | 9.6 | 7.7 | 6.2 | 7.8 | 8.1 | 7.1 |
| Protein,3% energy | 27 | 26 | 28 | 30 | 27 | 24 | 25 | 22 | 31 | 28 | 27 |
| Fat,3% energy | 4 | 5 | 3 | 3 | 3 | 4 | 4 | 14 | 3 | 3 | 5 |
| Carbohydrate,3% energy | 72 | 72 | 72 | 72 | 73 | 75 | 73 | 67 | 69 | 71 | 72 |
| Energy density, kcal/g | 1.3 | 1.2 | 1.2 | 1.3 | 1.1 | 1.4 | 1.4 | 1.6 | 1.2 | 1.2 | 1.3 |
Values are from USDA Standard Reference 22 and are based on edible portions of mature seeds, cooked, boiled, without salt.
1 kcal = 4.184 kJ.
Values do not add to 100%, because carbohydrate is determined by difference and the assumed Atwater factors of 4, 9, and 4 kcal/g for protein, fat, and carbohydrate, respectively, may not always apply to individual foods.
Table 2.
Summary of pulse nutritional and antinutritional components that may help with weight management
| Component | Brief description of mechanism |
| Carbohydrate | Slowly digestible, low glycemic |
| Dietary fiber | Soluble, viscous fiber gels in small intestine, slowing gastric emptying and hence absorption (ileal brake). Fermentable fiber is digested in the colon by bacteria, which liberates SCFA that may be used for energy, thereby sparing protein and glucose. SCFA may also suppress hepatic glucose production. The SCFA propionate may also stimulate satiety. |
| Starch make-up | High amylose:amylopectin ratio compared with other vegetables and root crops. Amylose also ferments in the colon and therefore may have effects similar to fiber. |
| Resistant starch | Amylose starch and the external branches amylopectin starch gelatinize as a result of heating, but they reassociate or retrograde upon cooling, thereby becoming resistant to digestion. α-Glucosidase enzymes are less apt to reach the areas needed to digest the starch. The starch-protein matrix may also reduce starch digestion rate by limiting enzyme accessibility to the starch. |
| Oligosaccharides | Considered prebiotic, therefore may facilitate the growth of bacteria in the large intestine, which may help to spare protein and glucose use for energy and stimulate satiety the liberation of SCFA as described above. |
| Protein | About 25% energy from protein; bound to starch in matrix, limiting the accessibility of both to digestive enzymes; limiting in amino acids lysine and methionine but when coupled with complementary protein sources such as corn or grain (higher in these 2 amino acids) becomes a complete protein source. Dietary protein may stimulate gut hormones to increase satiety, increase the energy cost of digesting and absorbing a meal high in protein, and spare fat-free mass during energy restriction. |
| Digestive enzyme inhibitors | Protease inhibitors may inhibit enzymes needed for protein digestion (e.g. trypsin and chymotrypsin); however, they are mostly destroyed by cooking and are not thought to play an important role in weight regulation. Amylase inhibitors may inhibit pancreatic amylase, reducing carbohydrate digestion, but these are also thought to be mostly destroyed during cooking and processing of whole pulses. However, extracts from Phaseolus vulgaris may be heat treated and prepared in such a way as to preserve the amylase inhibitor activity. |
| Phytochemicals | Phenolic compounds may reduce enterocyte glucose absorption by interfering with glucose transporters that may help delay postprandial glucose absorption, which may contribute to satiety and delay the return of hunger. Phytic acid (myo-inositol hexaphosphate), the major storage form of phosphate in plant cells, may help delay postprandial glucose absorption, which could contribute to satiety and delay the return of hunger. |
| Energy density | Moderate energy density may contribute to satiety, helping to limit energy intake. |
Pulse carbohydrates
Starches account for 22–45% of pulse grain weight depending on the source, whereas starch content is low in the oilseeds (30). As is typical of other grains, pulse starches are composed of amylose, a linear α-1,4-linked glucan with few branches in the molecular weight range of 105–106, and amylopectin, a highly branched and much larger molecule (molecular weight 107–109) composed of α-1,4-linked glycosyl units of varying lengths connected by α-1,6 branch points. Architecturally, amylopectin is divided into clusters containing the exterior branches and short linear chains, and an internal region containing longer linear chains linking clusters. Both the amylose long linear chain and the amylopectin external linear chains reassociate or “retrograde” on cooling following gelatinization, although this retrogradation occurs much faster in amylose. Pulse starches generally have a higher content of amylose compared with cereal and tuber starches; this factor plus their associated high capacity for retrogradation may reduce the starch digestion rate, rendering them either slowly digestible and/or resistant to digestion.
Slowly digestible starch and resistant starch.
A number of reports infer a slowly digestible or resistant character of the starch in pulses (31–34). Slowly digestible starch is a term given to that fraction of starch that is not rapidly digested but digests and absorbs slowly throughout the course of the small intestines. The term resistant starch applies to the fraction that is not digested by the human α-glucosidases, reaching the colon undigested with a general fate to be fermented by saccharolytic bacteria. These 2 nutritional classes of starch are perhaps best measured in vitro as described by Englyst et al. (35) as starch digested by their system between 20 and 120 min and that undigested at 120 min, respectively.
The basis for the moderated digestion rate of pulse starches remains rather unclear. Reports generally implicate cellular and cotyledon tissue structures in impeding enzyme access to the starch or their comparably high amylose content, as discussed above (34,36,37). Certainly, processing the grains to disrupt native macrostructures increases the rate and extent of starch digestion, thus supporting the idea that slow digestion is at least partly related to digestive enzymes’ poor access to starch (38,39).
Dietary fiber.
A recent thorough review of dietary fiber in pulses can be found in Tosh and Yada (40). In their raw state, pulses are high in fiber, with ∼15–32% total dietary fiber; of this, approximately one-third to three-quarters is insoluble fiber and the remaining is soluble fiber. Insoluble fiber is associated with fecal bulking through its water-holding capacity, whereas soluble fiber ferments, positively affecting colon health through production of SCFA, lowered pH, and potential microbiota changes. Viscous soluble fiber may also increase gastric distention and help to slow gastric emptying rate (20).
Oligosaccharides.
The oligosaccharides of pulses are often considered a negative attribute due to their high fermentability, with their associated rapid gas production and discomfort. Technically known as α-galactosides, they are derived from sucrose and have 1–3 α-1,6-linked galactosyl units attached. They are commonly known as raffinose (1 galactosyl unit), stachyose (2 galactosyl units), and verbascose (3 galactosyl units). Although generally considered a problem, and methods have been developed to partially remove them, the oligosaccharides may also be considered prebiotics (34), which are thought to be beneficial for health. α-Galactosidase, found in the product Beano, can be used to digest the galactosyl units from these oligosaccharides, leaving sucrose for further digestion.
Pulse proteins
The 2010 review by Boye et al. (41) provides comprehensive information on protein ranges in pulses, types of pulse proteins, their functional properties, and the effects of processing. Briefly, the amount of protein in pulses is ∼17–35% on a dry weight basis. In terms of solubility in specific solvents, pulse proteins fall primarily into the albumin (water-soluble) and globulin (salt-soluble) classes. The storage proteins legumin and vicillin are globulins, and the albumins comprise the heterogeneous group of enzymes, amylase inhibitors, and lectins. In general, macronutrient studies have shown that protein is more satiating than carbohydrates or lipids (42,43). Moreover, the protein in pulses (44) and soybeans (7) has been implicated in providing satiety; however, little is known regarding whether it is a specific property or more than 1 property of these proteins that elicit this effect or simply the inherent high amounts. Sufian et al. (45) found that pepsin-derived peptides from “country” beans (Dolichos lablab) stimulated secretion of cholecystokinin, a gut hormone related to satiety. Thus, pulse proteins as consumed may contain bioactive components that contribute to satiety.
Protease and amylase inhibitors.
Protein-based protease inhibitors found in pulses act on either or both of the serine proteases trypsin and chymotrypsin (46). They are found in comparably high amounts in the pulses compared with other plant foods and negatively affect digestibility of food proteins if not processed (i.e. mainly cooked) properly (41). They do not appear to have an important role in weight management. On the other hand, α-amylase inhibitors found in pulses, specifically dry beans (up to 2–4 g/kg), reduce starch digestibility and thus energy availability (46). Isolated α-amylase inhibitor lowers postprandial glycemic responses (47), although it may be inactivated through cooking or other processing methods (47). As discussed later in this review, several pulse extracts prepared with processing methods thought to retain amylase inhibitor activity have been tested in randomized, placebo-controlled trials for their potential to affect weight and fat loss.
Pulse phytochemicals
Phenolic compounds.
Pulses contain a range of phenolic compounds, with the darker grains such as black beans and red kidney beans generally having higher amounts. The phenolic compounds in pulses are generally polyphenols and include tannins, phenolic acids, and flavonoids (46). Antioxidant activity is related to total phenolic content (48). Their potential role in weight management is unclear, although studies indicate that certain phenolics interfere with enterocyte glucose absorption through interference with the glucose transporters (49,50).
Phytates.
Phytic acid, also known as myo-inositol hexaphosphate, is the major storage form of phosphate in plant cells (51). Pulses are one of the primary sources of phytate in the diet, the others being cereals, oilseeds, and nuts. Phytate has been shown to reduce the in vitro rate of starch digestion and delay postprandial glucose absorption in humans (52), which could contribute to satiety and delay the return of hunger, as discussed below.
Pulse consumption and energy regulation
It is unknown which of the pulse components described above has the strongest influence on appetite regulation and potentially energy balance or whether multiple factors work in consort. Nonetheless, several lines of evidence exist that suggest that pulse consumption could potentially increase satiety and help with weight control when consumed regularly. These studies are reviewed below.
Pulse consumption, glycemic response, and satiety
Most studies (15,31,53–60), although not all (61,62), consistently show lower glucose and insulin responses to consumption of controlled amounts of pulses compared with other foods. The glycemic response (peak and area under the curve) to pulses is at least 45% lower than that of other carbohydrate-containing foods such as cereals, grains, pasta, biscuits, and tuberous vegetables (31). Whether a low glycemic response per se is mechanistically related to a reduction in satiety is a topic of much debate (63–66), although there are some data to support a slower return of hunger in response to low-GI meals in general (67–69).
Additionally, a second meal effect has been observed whereby a reduced glycemic response to a second meal occurs after consumption of a first meal low in GI (70,71). In those studies, a first meal containing barley and lentils improved glucose tolerance to a second meal compared with a first meal containing whole-meal bread. The second meal effect is thought to be due primarily to an increase in colonic fermentation and only minimally to a reduction in gastric emptying rate (72,73). Fermentation in the colon produces SCFA, which can be oxidized and used for energy (74) in preference to glucose. SCFA may also suppress hepatic glucose production (75). Both of these mechanisms could lead to more stable glucose patterns over time, which some have hypothesized may result in reduced appetite and energy intake (76,77). Furthermore, an increase in satiety has been linked with the consumption of bread prepared with the SCFA propionate (78). Colonic fermentation and its effects may be long-lasting, as it has been observed to occur up to 13 h after the previous meal (73).
Few studies have specifically measured the satiety and appetite responses to pulse consumption (Table 3). In 2 different experiments, Leathwood and Pollet (56) fed participants hachis parmentier (the French version of shepherd’s pie) made with either potato puree or bean puree. Glycemic responses to the bean meals were significantly lower than with the potato meals. In addition, participants reported significantly less hunger at 180 min, greater satiety at 240 min, and a lower desire to consume “something tasty” after consuming the bean meals. In this study, the meals were not matched for carbohydrate content, with the bean meals containing 7.5 g less carbohydrate than the potato meals. In another study, Holt et al. (79) measured satiety responses over 2 h to 38 foods. All foods were served in 240-kcal portions (1 MJ). Of the protein-rich foods, ling fish led to greater satiety compared with lentils and baked beans. Also, baked beans and eggs led to greater satiety than did white bread.
Table 3.
Short-term (<1 d) studies on pulses, satiety, and appetite and subsequent energy intake responses1
| Reference | n | BMI, kg/m2 | Meals | Meals matched for | Measurement duration, h | Satiety effect? | Subsequent ad libitum energy intake effect? |
| Leathwood and Pollet (56), study 1 | 6 | 14.5–30.4 | Hachis parmentier (shepherd’s Pie) topped with either bean puree or potato puree | Energy | 4 | Bean < potato (P < 0.10 for hunger at min 180)2 | Not measured |
| Leathwood and Pollet (56), study 2 | 6 | 19.2–27.2 | Hachis parmentier (shepherd’s Pie) topped with either bean puree or potato puree | Energy | 4 | Bean > potato (stomach fullness at min 240); Bean < potato (hunger and desire to eat “something tasty” at mins 180 and 240)2 | Not measured |
| Holt et al. (79) | 11 | 24.3 ± 3.1 (SD) | Canned lentils, canned baked beans, other foods | Energy | 2 | Fish > lentils and baked beans; baked beans = eggs > white bread | Not measured |
| Sparti et al. (57) | 14 | 19–25 | High- vs. low-unavailable carbohydrate breakfast, lunch, and dinner; included pulses in lunch and dinner | Energy, macronutrients | 24 | High unavailable > low unavailable at lunch and dinner | Not measured |
| Johnson et al. (62) | 11 | 24.7 ± 0.8 (SEM) | Bread made with conventionally processed chickpea flour vs. extruded chickpea flour (more subject to retrogradation) | Available carbohydrate, fat | 2 | NS | NS |
| Pai et al. (44) | 32 | 16–31 | Traditional Indian (Asian) breakfasts: Rice-pulse vs. rice based vs. wheat based | Energy | 2 | Rice-pulse > all others | Not measured |
| Wong et al. (60), study 1 | 14 | 20–25 | Canned beans, homemade beans vs. glucose | Available carbohydrate | 2 | Homemade beans > glucose | (Pizza meal) Canned < homemade and glucose |
| Wong et al. (60), study 2 | 14 | 20–25 | Canned: maple, pork, and molasses, or tomato sauce vs. homemade: pork and molasses vs. white bread | Available carbohydrate | 2 | Homemade > white bread (P = 0.09) | (Pizza meal) N = no |
| Wong et al. (60), study 3 | 15 | 20–25 | Pulse variety: chickpeas, lentils, navy beans, yellow peas vs. white bread vs. water | Energy | 2 | All pulse varieties = white bread > water | (Pizza meal) All varieties = white bread; chickpeas = water |
All studies were performed in healthy adults (no chronic disease) ≤ 45 y of age.
Comparisons were planned a priori only for min 180 and 240.
Sparti et al. (57) fed 14 healthy, normal-weight participants 3 meals that were either high or low in unavailable carbohydrate and measured metabolic and appetitive responses to the meals over 24 h in a metabolic chamber. The lunch and dinner meals from the high-unavailable carbohydrate regimen contained pulses (chickpea salad at lunch and red bean salad at dinner). The diets were controlled for energy and macronutrient distribution but differed in fiber (60 vs. 3 g), slowly and rapidly digestible starch (63 vs. 24 g of slowly digestible starch), and resistant starch (18 vs. 4 g). Hunger was significantly greater and fullness significantly less after the low-unavailable carbohydrate lunch and dinner, but not breakfast, compared with the respective high-unavailable carbohydrate meals. The diet high in unavailable carbohydrates also resulted in a significantly lower rapid rise in postprandial carbohydrate oxidation, and the difference in carbohydrate oxidation between diets was inversely correlated with hunger ratings. The investigators concluded that delayed carbohydrate oxidation associated with the diet high in unavailable carbohydrate resulted in less hunger. Because it is impossible to determine whether the different responses to the 2 diets were attributable to the difference in pulse content per se, it is recommended that more studies designed to better isolate the effects of pulses and incorporating the scope of measurements included in this study be conducted.
Wong et al. (60) conducted a series of 3 preload experiments to study the effects of processing, recipe, and pulse variety on glycemic and appetitve responses and energy intake of pizza meals 2 h later. In the first 2 experiments, preload meals were matched for carbohydrate amount (50 g) and in the 3rd, they were matched for energy. In the first experiment, 2 types of canned navy beans (plain) were compared with navy beans that were soaked as one would prepare at home and made into a baked bean recipe. A glucose drink served as the control. The glycemic responses to the bean meals were not significantly different, and, not unexpectedly, all were significantly lower than the glucose drink. Appetite response was significantly lower only for the homemade beans compared with glucose, and the amount of pizza consumed in a test meal 2 h later was lower after the 2 canned beans but not the homemade beans. In the second experiment, 3 different recipes made with canned navy beans (tomato sauce, maple style, pork, and molasses) and one made with homemade navy beans (pork and molasses) were compared with white bread as the control. Only 1 of the canned recipes (tomato sauce) and the homemade recipe led to a glycemic response that was significantly lower than that of white bread. The appetite responses tended to be lower for these same recipes, but these differences did not reach significance (P = 0.09) and pizza intake did not differ among treatments. Finally, in the 3rd experiment, which compared responses to different pulse varieties (chickpeas, lentils, navy beans, yellow peas), water and white bread, glycemic responses varied somewhat and tended to be lowest for lentils and chickpeas, but differences in appetite or subsequent pizza intake were not observed. This study provided only weak evidence and mixed findings for a satiety-promoting effect of pulses depending on how they are processed and prepared. Additional studies are needed comparing different recipes and processing methods of pulses to meals with alternative protein sources such as meat.
Glycemic and insulinemic responses and energy intake subsequent to consumption of breads made with normally processed chickpea flour, extruded chickpea flour, or white wheat flour were measured (62). The extrusion process involves higher temperatures and shear compared with those used during normal processing. Gelatinized starch during extrusion processing may fragment and align differently than when normally processed and, by retrogradation, potentially increase the resistant starch content of the chickpea flour. The results showed a significantly higher insulin response and a nonsignificant tendency toward a lower glycemic response after ingestion of the chickpea bread (normal process). However, neither satiety nor energy intake differed among bread type at a buffet meal 2 h later, perhaps due to the variation in food form (chickpea flour) compared with other studies that have used whole pulses. In addition, the starch and fiber contents of the breads were not matched, which could have affected the results. Further studies on foods made with pulse flours may be of interest, given its potential as a functional ingredient (59) and the expanding variety of products made with pulse flours now available, such as tortillas, pasta, and bakery products.
Even fewer studies have measured satiety responses to pulse consumption in studies lasting longer than 1 d. McCrory et al. (80) recently completed a randomized intervention comparing 3 doses of pulse consumption on weight loss and adherence to 30% reduction in baseline energy intake over 6 wk. The doses were 1 Tbsp/d (15 mL), 0.5 c/d (120 mL), or 1.8–2.5 c/d (432--600 mL) for 6 d/wk. There was a significant time-pulse dose group interaction effect on average daily satiety ratings, where satiety ratings were highest in the group receiving 0.5 c/d, particularly over the first 3 wk. In another recent study using a nonrandomized design in which participants served as their own controls (81), individuals were followed for a period of 4 wk on their usual diet, followed by 12 wk of chickpea supplementation (mean 104 g/d, which is just over 0.5 c/d), then another 4 wk on their usual diet. Participants reported a significant increase in feelings of satiation in the chickpea phase relative to the first habitual phase and a significant decrease in satiation in the second habitual phase relative to the chickpea phase. However, it is unclear when these ratings were collected during the study (i.e. daily, weekly, or at the end of each phase).
In summary, short-term studies (mostly single-meal studies) indicate reduced hunger and increased satiety 2–4 h after pulse consumption when meals were controlled for energy but not when controlled for available carbohydrate. This suggests that at least part of the effect of pulses on satiety may be mediated by available carbohydrate amount or composition. Across all of these studies, the control or comparison foods varied widely, from potato to cereal grains to glucose and white bread. This raises the question of the optimal control food to use in determining whether pulse consumption helps to increase satiety in real-world situations. One possibility is to include a food that might otherwise be consumed in normal daily life instead of pulses, such as another protein source like meat, poultry, or fish, or an alternative carbohydrate source, such as whole grains. The effects of pulse consumption on subsequent energy intake are still largely uncertain, because very few of these studies measured subsequent energy intake. In addition, more studies are needed to identify whether regular daily pulse consumption can help to increase satiety on a regular basis relative to other foods and potentially help with weight control in the longer term.
Pulse consumption and body weight
Observational studies.
One way to determine whether longer term pulse consumption may affect body weight is to determine whether an association exists between pulse consumption and body weight cross-sectionally. Very few studies have examined this relationship. Papanikolaou and Fulgoni (82) reported on the association of consumption of beans (a subgroup of pulses, as described above) with dietary quality and obesity risk in >8000 adult participants in the NHANES 1999–2002 using data from a single, multiple pass, 24-h dietary recall. They found that individuals who had consumed variety beans or baked beans had significantly lower body weights compared with those who had not consumed beans. In addition, the odds of being obese (BMI ≥ 30 kg/m2) was significantly lower in variety bean consumers and baked bean consumers compared with nonconsumers (odds ratio = 0.78 and 0.77, respectively). Interestingly, when variety bean consumers were analyzed separately from baked bean consumers, the reduced risk of overweight or obesity was no longer observed in the baked bean consumers compared with nonconsumers. There were several dietary differences associated with bean intake that could have mediated these relationships. Compared with bean nonconsumers, both baked bean consumers and variety bean consumers had significantly higher intakes of total legumes, fiber, and minerals and lower intakes of discretionary fat (trend toward significance in variety bean consumers). The variety bean consumers had lower intakes of meat and added sugars, whereas the baked bean consumers had lower intakes of total grains, whole grains, and vegetables and higher intakes of added sugars. These dietary confounders were not controlled for in analyses, so it is difficult to determine the degree to which the relative leanness in variety bean and baked bean consumers may be attributed specifically to bean consumption. Minimally, this study showed that bean consumption is associated with an overall dietary pattern and lifestyle that tends to be associated with relative leanness. This suggestion is also supported by other studies described below.
A few studies examined relationships between dietary patterns incorporating pulses but did not specifically isolate pulse consumption associations in their analyses. Most (83–88), although not all (89,90), showed an inverse association of the dietary pattern incorporating pulses with BMI or BMI increase over time. Other types of studies examined overall dietary patterns of high fruit and vegetable intake or a vegetarian lifestyle typically incorporating large amounts of pulses. However, pulse intake was not quantified in those studies. As reviewed (91), most studies show that vegetarians weigh less than nonvegetarians. In addition, vegans and those on macrobiotic diets generally weigh less than lacto-ovo vegetarians. Others reviewing studies on fruit and vegetable consumption (92,93) note that despite methodological inconsistencies among different studies leading to somewhat mixed findings, a pattern of higher fruit and vegetable intake has generally been associated with lower body weight.
In summary, studies examining the potential associations between pulse consumption and weight status consistently show that individuals with lower BMI consume a greater amount of pulses as part of their usual diet. However, very few studies have been conducted and only 1 considers beans separately from other pulses or legumes or from other food groups. Finally, these studies should be interpreted with caution, because confounding may exist, as is usually the case with cross-sectional associations. Cause and effect cannot be assumed, because pulse consumption may be part of an overall lifestyle that confers maintenance of healthy weight. Experimental study designs are necessary to determine whether pulses can be implicated as having independent effects on body weight.
Experimental studies in humans
Pulse consumption and weight loss during intentional energy restriction.
Very few interventions testing effectiveness of whole pulses for weight loss during intentional caloric restriction have been published (Table 4). The earliest study was conducted in 1987 (94) in 15 type 2 diabetics with a mean BMI of 24.8 kg/m2; about one-half of the participants were normal weight (BMI > 25 kg/m2) and one-half were overweight or obese (BMI ≥ 25 kg/m2). A 3-wk crossover design was used. Seven participants began with the control diet and 8 began with a legume-based diet. It is not stated whether the order of the diets was randomized; however, each participant switched to the other diet after 3 wk and there was no wash-out period. Diets were designed to provide ∼1600 kcal/d (6.7 MJ/d). Because of the wide BMI range in this study, a 1600-kcal/d intake would likely be anywhere from a 15–40% energy deficit, depending on initial energy requirement (95). Both diets were 20/34/56% of energy from protein/carbohydrate/fat, and the legume-based diet contained ∼21% of energy from legumes. Based on 115 kcal (481 kJ) per 0.5 cup of pulses (Table 1), the legume dose was probably about 1.5 c/d (21% of 1600 kcal = 336 kcal/d or 1.4 MJ/d of legumes). The legume-based diet contained mostly pulses (green peas, brown beans, white beans, chickpeas, lentils, and yellow peas), but there was also a small amount of soybeans and green beans included. After 3 wk of consuming each diet, there was a small but significant weight loss after consuming the control diet but not the legume-based diet (no values given). Compliance monitoring was not mentioned in the publication.
Table 4.
Intervention studies on pulses and weight loss with intentional energy restriction1
| Reference | Design | BMI, kg/m2 | Pulse dosage and/or types | Other intervention components | Δ Weight, kg | Significant difference between groups? |
| Karlstrom et al. (94) | 3-wk crossover design, unclear if order randomized (n = 15 type 2 diabetics with inadequate control) | 19–33 | 1.4 c/d legumes (mostly green peas, brown beans, white beans, chickpeas, lentils, yellow peas, but also soybeans and green beans) | Provided 1600 kcal/d2; both diets were 20/56/34% energy from P/C/F; fiber 23.7 vs 36.7 g/d for control and legume diet, respectively | Legume: −0.4 kg control: −0.7 kg (SD not provided) | Yes (legume < control) |
| Sicheiri et al. (96) | 8 wk RCT (n = 40 women) | ≥27 | Rice and beans vs. lean meat twice a day (dose unclear) | Provided 1800 kcal/d2; P/C/F was 14/71/15% energy (rice and bean), 18/57/25 (lean meat) | 4 wk, rice/bean: −2.4 kg lean meat: −0.9 kg (SD not provided) 8 wk, rice/bean: −3.8 ± 1.8 kg lean meat: −1.5 ± 0.9 kg | Yes at 4 wk, no at 8 wk (35% drop-out at 8 wk) |
| McCrory et al. (80) | 6 wk RCT (n = 42 men and women) | 25–35 | 3 Treatments: all 6 d/wk high pulse 1.8 c/d women, 2.5 c/d men; medium pulse 0.5 c/d; low pulse (control): 1 T/d | Prescribed 30% energy deficit; pulses provided | High pulse: −2.3 ± 2.3 kg medium pulse: −3.9 ± 2.2 kg low pulse: −1.8 ± 1.9 kg | Yes, medium vs. low |
| Abete et al. (100) | 8 wk RCT (n = 35 men) | 31.8 ± 3 | 4 Treatments: legume 4 d/wk and avoid fish vs. fatty fish 3 d/wk and avoid legumes vs. high protein (meat, eggs, and lean dairy) vs. control (avoid legumes and fatty fish) | Prescribed 30% energy deficit; P/C/F was 17/40/30% energy for legume, fatty fish and control, and 30/40/30% energy for high protein; fiber was 26.5 g/d in legume diet vs 18.1–20.3 g/d in the other diets | Legume: −8.3 ± 2.9% fatty fish: −6.4 ± 2.6% high protein: −8.4 ± 1.2% control: −5.5 ± 2.5% of initial weight | Yes (legume = high protein > control) |
| Hermsdorff et al. (101) | 8 wk RCT (n = 30 men and women) | 32.5 ± 4.5 | Pulse: 4 servings/wk = 3.6–5.2 c/wkvs. control: limit pulse intake | Prescribed 30% energy deficit; 17/53/30% energy from P/C/F for both diets; fiber was 26.0 vs. 18.0 g/d for pulse vs. control diet | Pulse: −7.8 ± 2.9% control: −5.3 ± 2.7% of initial weight | Yes |
RCT, randomized controlled trial; P, protein; C, carbohydrate; F, fat.
1 kcal = 4.184 kJ.
The study by Sichieri et al. (96) was originally published in Portuguese but was summarized in English in a subsequent paper (85). They conducted a randomized controlled trial in which 40 overweight or obese women (BMI ≥ 27 kg/m2) were provided with an 1800-kcal/d (7.5 MJ/d) diet incorporating either rice and beans twice a day (with no meat) or lean meat twice a day. Diets had protein/carbohydrate/fat distributions of 14/15/71% of energy (beans and rice) or 18/25/57% of energy (lean meat). The rice and beans diet resulted in greater weight loss after 1 mo (2.4 vs. 0.9 kg; P = 0.04); however, the difference was not significant after 2 mo (3.8 vs. 1.5 kg; P = 0.10). The lack of a difference at 2 mo was likely due to loss of follow-up, which was 35% in the rice and beans group and 45% in the lean meat group. Potential reasons for the high drop-out rate were not discussed. However, the results of that study strongly suggest that pulses could aid weight loss during intentional caloric restriction if drop-out could be prevented or substantially reduced.
In the 6-wk intervention trial by McCrory et al. (80) described above, 49 overweight and obese participants (BMI 25–35 kg/m2) were randomized to consume either 1 Tbsp/d (low), 0.5 c/d (medium), or 1.8–2.5 c/d (high) of pulses for 6 d/wk while reducing their energy intake by 30% of baseline energy requirements daily. Foods with the requisite amount of pulses were provided as 4 servings/d [250 kcal (1.0 MJ) and 300 kcal (1.3 MJ) for women and men, respectively]. The foods were familiar foods such as casseroles and pasta dishes, and a variety of pulses were used including (but not limited to) chickpeas, lentils, black beans, pinto beans, navy beans, and split peas. Thus, 1000 (4.2 MJ/d) kcal/d were provided to women and 1200 kcal/d (5.0 MJ/d) were provided to men. Participants were counseled about how to choose the remainder of their energy intake (to meet the target intake) by following an exchange list. In addition to weight loss, adherence to the energy intake target was an outcome of interest. Seven participants dropped out of the study (2 from each group and 1 prior to randomization). Interestingly, the results showed there was no dose-response effect of pulse consumption on weight loss, with the weight loss being 1.8 ± 1.9 kg, 3.9 ± 2.2 kg, and 2.3 ± 2.3 kg in the low-, medium-, and high-pulse groups, respectively, and differing significantly only between the low- and medium-pulse groups. Energy intake from the multiple pass 24-h recall dietary intake assessments also reflected a higher energy intake in the high-pulse group. The reasons for the lack of a greater weight loss in the high-pulse group are several and could be due to a “halo” effect (97,98) of the high pulse provision on energy intake, an adaptation to the high pulse intake that disrupted appetite regulation mechanisms (99) or lack of compliance with the high pulse intake. However, “flatulence and intestinal gas” ratings assessed by a 9-point rating scale were highest in the high-pulse group, suggesting that the group as a whole compliant with their intake of provided pulses.
In an 8-wk trial (100), 35 overweight or obese men were randomized to consume a control diet, a legume diet, a fatty fish diet, or a high-protein diet. The control, legume, and fatty fish diets were designed to provide 17/30/53% of energy as protein/carbohydrate/fat, whereas the high-protein diet was designed to provide 30/30/40% of energy from protein/carbohydrate/fat. Protein sources in the high-protein diet were primarily meat, eggs, and lean dairy products. For the legume diet, neither legume dose nor type was mentioned, but legumes were required 4 d/wk, fish was not allowed, and other animal protein intake was decreased. All diets were designed to produce a 30% energy deficit. Compliance was monitored weekly by interview with a dietitian and 3-d weighed food intake records at the week prior to the intervention and at wk 7. Based on reported intakes, compliance with the prescribed diets appeared to be quite good. Results showed significant weight loss in all 4 groups, but the legume group (−8.3% of initial weight) and high-protein group (−8.4% of initial weight) lost significantly more than the control group (−5.5% of initial weight). Absolute changes in body weight were not reported.
In the most recently published trial (101), 30 overweight and obese participants consumed a reduced energy intake diet for 8-wk prescribed at 30% energy restriction based on initial energy requirements. Participants were randomized to a treatment group that consumed 4 servings/wk of pulses or to a control group that restricted pulses during this time period. One serving was defined as 160–235 g of cooked pulses, depending on the prescribed energy intake. Because pulses average 90 g/0.5 c (120 mL) (Table 1), 1 serving in this study ranged from ∼0.9 to 1.3 c (216-312 mL). Therefore, the total dose ranged from 3.6 to 5.2 c (756-1092 mL) of pulses/wk. The prescribed macronutrient distribution was the same for both groups, which was a protein/carbohydrate/fat distribution of 17/53/30 percent of energy. Compliance with the assigned pulse consumption and macronutrient distribution was good, as indicated by weekly monitoring by a dietitian and by 3-d weighed food records during the week prior to the intervention and during wk 7. Results showed significantly greater decreases in BMI and body weight expressed as percent of initial value in the pulse-consuming group compared with the control group (−2.0 vs. −0.9 kg/m2 and −7.8 vs. −5.3%, respectively). Percentage body fat and waist circumference decreases, however, did not differ significantly between groups. Group values for reported energy intake or diet composition during the intervention were not reported; therefore, it is uncertain whether the differences in weight loss between groups could be ascribed to differences in energy intake or other metabolic effects of pulses on energy expenditure.
Pulse consumption and body weight without energy restriction
The effects of pulse consumption on body weight can also be explored by examining the results of pulse intervention studies in which chronic disease risk factors were the primary outcomes yet body weight was measured. Most of these studies were designed to provide energy in an amount necessary to maintain body weight. Hence, no significant effects of pulse consumption on body weight were observed over 3- to 7-wk periods (102–111). Pulse consumption under less rigorous feeding conditions (e.g. ad libitum dietary intake, except for the provided pulses and control foods, with advice given to maintain usual dietary and exercise habits) over 2–16 wk also did not affect body weight (112–116). Similarly, body weight was not affected when pulses were added to a prescribed low-fat diet (28–32% of energy) (117). The lack of an effect of pulses on body weight under all of these study conditions is consistent with the suggestion that an increase in fruit and vegetable intake is likely to have only very small or modest effects on body weight loss unless advice on reducing energy intake is provided simultaneously (118). This may not be the case, however, if the fruit and vegetable load is very high or if multiple dietary changes are made at once. In support of this idea, and as Berkow and Barnard reviewed (91), controlled interventions that place participants on a vegetarian or vegan diet for several weeks have resulted in weight changes of 2.5–7.2 kg, with maintenance of these changes shown in a few uncontrolled studies.
Studies using pulse extracts
A few human clinical trials have examined the effects of pulse “extracts” taken as a dietary supplement on body weight and related parameters. During normal starch digestion, amylases break α-1,4 bonds to allow it to be broken into more easily digested and absorbable units. The main ingredient in these extracts is thought to be α-amylase inhibitor; thus, the extracts are often referred to as starch blockers. Depending on the method of preparation, other components may also be present in the extracts. The effectiveness of pulse extracts were recently reviewed by Preuss (119). Briefly, extract preparations in the early 1980s were crude and not very effective at blocking starch digestion due at least in part to low amylase inhibitor activity (120). However, extract preparations in the later 1980s and beyond showed greater α-amylase inhibitor activity and, hence, effectiveness at blocking starch digestion in short-term human studies [e.g. (121)]. Also, initially there was some concern that the extract preparations contained lectins, which may be harmful to health; however, in animal studies, the extracts have been shown to be safe and the current preparation method is thought to result in the destruction of lectins (122,123). As with all dietary supplements, however, the commercially available preparations are not monitored regularly by the FDA for safety or content.
Several human trials testing the effectiveness of these extracts have been conducted in the last several years (124–130). All but 1 (126) used a randomized, double-blind, placebo-controlled design (Table 5) and all included only initially overweight or obese participants. Intervention length ranged from 4 to 12 wk. Extract doses varied from pills to powders mixed in water taken with 1 or 2 carbohydrate-rich meals a day. In all studies, there was greater weight loss in the treatment group compared with the placebo group, but this difference was significant in only 3 studies. Overall, the mean weight loss among the 6 randomized controlled trials was 0.4 ± 0.2 kg/wk (0.5 ± 0.1% of initial weight/wk) in the treatment groups compared with 0.2 ± 0.2 kg/wk (0.2 ± 0.2% of initial weight/wk) in the placebo groups. Variable results among these studies could be due to factors such as differences in dietary composition, degree of energy deficit, compliance with the prescribed dietary regimens, and extract preparation/composition. Therefore, results look promising, but more research is needed to determine the effectiveness of pulse extract preparations. In addition, the relevance of these trials to consumption of whole pulses is uncertain. As mentioned earlier in this review, the activity of α-amylase inhibitor is thought to be destroyed during cooking and/or processing (46), although low levels of activity may remain (47). Few studies have been conducted on the effects of repeated consumption of processed/cooked pulses and other legumes, which may contain some low level of α-amylase inhibitor activity; therefore, this is an area that could be further researched.
Table 5.
Studies on white bean extract and weight loss1
| Reference | Product | Design | BMI, kg/m2 | Dosage | Other intervention components | Δ Weight, kg | Significant difference between groups? |
| Thom (124) | Phaseolamin (white bean extract, 200 mg; inulin, 200 mg; Garcinia cambogia, 50 mg) | 12-wk RCT, double-blind (n = 20 active; n = 20 placebo) | Overweight + obese | 2 tablets immediately after each meal (breakfast, lunch, and dinner) | Prescribed 1200 kcal/d2 low-fat diet | Active: −3.5 ± 2.0 placebo: −1.3 ± 1.4 | Yes (34 completed; intent to treat analysis) |
| Udani et al. (125) | Phase 2 (previously sold as Phaseolamin) | 8-wk RCT, double-blind (n = 20 active; n = 19 placebo) | Obese | 1500 mg + 8 fl oz water with lunch and dinner | Prescribed high-fiber, low-fat diet; instructed to eat majority of CHO at lunch and dinner | Active: −1.7, placebo: −0.8 (SD not given) | No (intent to treat analysis) |
| Koike et al. (126) | Phaseolamin 1600 (white bean extract, 750 mg; clove, 200 mg; lysine, arginine, alanine, 20 mg each) | 8-wk open label, no control group (n = 10 active) | Normal weight + overweight | 2 capsules taken 30 min before lunch and dinner with water | Normal meals and exercise routine | Active: −1.8 (SD not given) | n/a |
| Opala et al. (127) | Nutrifin appeal PLUS (Finzelberh, GmbH & Co. KG) tablet 1: extracts of asparagus, green and black teas, guarana, mate, and kidney bean; tablet 2: extracts of kidney bean pods, Garcinia cambogia, chromium yeast | 12-wk RCT, double blind (n = 47 active; n = 51 placebo) | Overweight + obese | 2 tablets with each main meal (1 before and 1 after each meal) | Prescribed 1500 kcal/d;2 daily walking | Active: −2.0 ± 2.6 placebo: −1.5 ± 3.5; corrected for hours of exercise; active:−54 ± 73 g/h; placebo:−30.1 ± 68 g/h | No for kg; yes for g/h exercise; yes for percentage body fat (not shown) |
| Celleno et al. (128) | Phase 2 Starch neutralizer (Phaseolamin 2250) with 445 mg Phaseolus vulgaris extract, 0.5 mg chromium picolinate, calcium phosphate, vitamin B-3, and others | 30-d RCT, double-blind (n = 30 active; n = 20 placebo) | 5–15 kg overweight, mean BMI ∼26.0 ± 2 kg/m2 | 1 tablet/d before the main meal | Prescribed 2000–2250 kcal/d,2 carbohydrate rich | Active: −2.9 ± 1.6 placebo: −0.4 ± 0.4 | Yes |
| Udani and Singh (129) | Phase 2 Starch neutralizer (Phaseolamin 2250) supplied by Pharmachem Labs | 4-wk RCT, double-blind (n = 13 active; n = 12 placebo) | Normal weight, overweight, and obese | 2 x 500 mg capsules each at beginning of breakfast and lunch | 1800 kcal/d; provided breakfast and lunch, prescribed dinner; prescribed exercise 30 min x 4d/wk; behavior groups 1x/wk | Active: −2.7, placebo: −2.1; for highest CHO tertiles, active: −3.9, placebo: 0.7 (no SD given) | No overall, yes for highest CHO tertiles (no for 2 lower tertiles) |
| Wu et al. (130) | Phase 2 Starch neutralizer | 60-d RCT, double-blind (n = 51 active; n = 50 placebo) | Overweight + obese | 2 x 500 mg tablets 15 min before each meal (breakfast, lunch, and dinner) | No details provided | Active: −1.9 ± 0.2 placebo: −0.4 ± 0.1 | Yes |
RCT, randomized controlled trial.
1 kcal = 4.184 kJ.
Pulses in the U.S. diet
Based on recent analyses of dietary intake data from the NHANES, few U.S. adults consume pulses in their usual diet. The proportion of U.S. adults estimated to consume pulses or legumes over 1–2 d varies from 8–30%, depending on the data set used [NHANES 1999–2002 (82,131) vs. NHANES 2003–2004 (132)], the number of days of intake available (1 in NHANES 1999–2002 and 2 in NHANES 2003–4), and the type of legume analyzed, i.e. dried beans (82), nonsoy legumes (131), or legumes including soy beans and green beans (132). None of the estimates appear to have included food products that may contain pulse fractions such as pea fiber in baked goods and pasta.
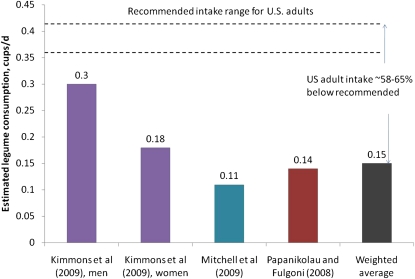
The USDA-recommended amount of legume consumption for most adults aged 19 y and older is 3 c/wk, with the exception of women aged 51 y and older for which the recommendation is 2.5 c/wk (133). Perhaps not surprisingly, pulse consumption in the US is markedly below the recommendation (Fig. 2). The weighted average intake of legumes based on the available studies (82,131,132) is ∼0.15 c/d (36 mL), which is ∼58–65% lower than the recommended amount. Kimmons et al. (132) estimated that of the 30% of adults who do consume legumes, only about 40–45% of them achieved at least the recommended intake.
Figure 2.
Estimated median (131,132) or mean (82) legume intake of U.S. adults aged ≥19 y. The weighted average is based on the following sample sizes: n = 2005 men and 1904 women (132), n = 9317 adults (131), and n = 8229 adults (82). Estimates are based on either 1-d intake in NHANES 1999–2002 (82,131) or 2-d intake in NHANES 2003–2004 (132) (1 c = 240 mL).
There are numerous factors that determine food choices, including, taste, cost, convenience, and nutrition (134,135). In the case of pulses and soybeans, there are likely additional factors, including purported physiological effects (digestibility issues), cultural factors, habits (e.g. vegetarianism/veganism), and knowledge about how to incorporate them into everyday diets. A strong incentive to increase pulse consumption may be their low cost, particularly for a high nutrient-dense food. Drewnowski (136) recently reported that beans were among the top 5 classes of food having the highest micronutrient to price ratio. Indeed, according to U.S. national surveys, pulse consumption is highest among low-income populations. Households in the lowest quartile of income (<130% of poverty level) represent 19% of the U.S. population but consume 27% of all cooked, dried beans (137). Furthermore, there are differences in the type of bean consumed by income level, with lower-income individuals consuming primarily pinto and lima beans and high-income individuals consuming more black beans and garbanzos. Some of these differences may relate to cultural preference for certain beans among minority populations, who tend to have lower average income levels than Caucasians.
Palatability or taste preferences could be another factor determining the choice to incorporate pulses as a major part of one’s diet. Several studies have been conducted recently showing a general acceptability of foods incorporating pulses or pulse flours (59,62,138–140), although depending on the specific pulse and the population, the range of acceptability could vary widely (141). Simply increasing familiarity with pulses could also help to increase the likelihood they may be incorporated into a diet more regularly (142,143).
Digestibility issues and potentially adverse gastrointestinal effects of pulse consumption may also be of concern to many individuals. Few studies have included formal measures of gastrointestinal tolerance to pulse consumption. As reviewed by Veenstra et al. (144), moderate consumption tends to be well tolerated but tolerance decreases when pulse consumption reaches very high levels.
Conclusion and recommended future directions
Based on the few studies that have been conducted, there is some indication that pulses may help to increase satiety, at least in the short term, and weight loss during intentional energy restriction over a few weeks. However, additional, longer term (≥1 y), randomized controlled trials are needed in this area, including those that investigate the optimal dose of pulses for weight control balanced with other factors worth considering, such as any potential negative effects (e.g. gastrointestinal tolerance, phytate effects of mineral absorption). Studies on behavioral techniques to overcome barriers, perceived or real, to pulse consumption may be helpful to effectively increase pulse intake in individual diets and on a population-wide basis.
Acknowledgments
We thank Malinda Gehrke and Owen Holly Maroney for technical assistance. All authors have read and approved the final manuscript.
Footnotes
Author disclosures: M. A. McCrory, B. R. Hamaker, J. C. Lovejoy, and P. E. Eichelsdoerfer, no conflicts of interest.
Literature Cited
- 1.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9:S228–33 [DOI] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–9 [DOI] [PubMed] [Google Scholar]
- 3.The National Heart, Lung, and Blood Institute Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. JAMA. 1998;98:1178–91 [Google Scholar]
- 4.Abete I, Astrup A, Martinez JA, Thorsdottir I, Zulet MA. Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev. 2010;68:214–31 [DOI] [PubMed] [Google Scholar]
- 5.FAO Definition and classification of commodities, 4. Pulses and derived products; 1994 [cited 2010 9 Sep]. Available from: http://www.fao.org/WAICENT/faoinfo/economic/faodef04e.htm
- 6.Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70:S439–50 [DOI] [PubMed] [Google Scholar]
- 7.Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007;4:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cope MB, Erdman JW, Jr, Allison DB. The potential role of soyfoods in weight and adiposity reduction: an evidence-based review. Obes Rev. 2008;9:219–35 [DOI] [PubMed] [Google Scholar]
- 9.Orgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood). 2008;233:1066–80 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Lorda P, Megias Rangil I, Salas-Salvado J. Nut consumption, body weight and insulin resistance. Eur J Clin Nutr. 2003;57 Suppl 1:S8–11 [DOI] [PubMed] [Google Scholar]
- 11.Natoli S, McCoy P. A review of the evidence: nuts and body weight. Asia Pac J Clin Nutr. 2007;16:588–97 [PubMed] [Google Scholar]
- 12.Mattes RD. The energetics of nut consumption. Asia Pac J Clin Nutr. 2008;17 Suppl 1:337–9 [PubMed] [Google Scholar]
- 13.Mattes RD, Kris-Etherton PM, Foster GD. Impact of peanuts and tree nuts on body weight and healthy weight loss in adults. J Nutr. 2008;138:S1741–5 [DOI] [PubMed] [Google Scholar]
- 14.Mattes RD, Dreher ML. Nuts and healthy body weight maintenance mechanisms. Asia Pac J Clin Nutr. 2010;19:137–41 [PubMed] [Google Scholar]
- 15.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6 [DOI] [PubMed] [Google Scholar]
- 16.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56 [DOI] [PubMed] [Google Scholar]
- 17.Rizkalla SW, Bellisle F, Slamma G. Health benefits of low glycemic index foods, such as pulses, in diabetic patients and healthy individuals. Br J Nutr. 2002;88:S255–62 [DOI] [PubMed] [Google Scholar]
- 18.Thorne MJ, Thompson LU, Jenkins DJ. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am J Clin Nutr. 1983;38:481–8 [DOI] [PubMed] [Google Scholar]
- 19.Jenkins DJ, Jenkins AL. Nutrition principles and diabetes. A role for “lente carbohydrate”? Diabetes Care. 1995;18:1491–8 [DOI] [PubMed] [Google Scholar]
- 20.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59:129–39 [DOI] [PubMed] [Google Scholar]
- 21.Yao M, Roberts SB. Dietary energy density and weight regulation. Nutr Rev. 2001;59:247–57 [DOI] [PubMed] [Google Scholar]
- 22.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105:S98–103 [DOI] [PubMed] [Google Scholar]
- 23.Schoeller DA, Buchholz AC. Energetics of obesity and weight control: does diet composition matter? J Am Diet Assoc. 2005;105:S24–8 [DOI] [PubMed] [Google Scholar]
- 24.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21:411–8 [DOI] [PubMed] [Google Scholar]
- 25.Gaesser GA. Carbohydrate quantity and quality in relation to body mass index. J Am Diet Assoc. 2007;107:1768–80 [DOI] [PubMed] [Google Scholar]
- 26.van Dam RM, Seidell JC. Carbohydrate intake and obesity. Eur J Clin Nutr. 2007;61 Suppl 1:S75–99 [DOI] [PubMed] [Google Scholar]
- 27.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87:S1558–61 [DOI] [PubMed] [Google Scholar]
- 28.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41 [DOI] [PubMed] [Google Scholar]
- 29.Acheson KJ. Carbohydrate for weight and metabolic control: where do we stand? Nutrition. 2010;26:141–5 [DOI] [PubMed] [Google Scholar]
- 30.Hoover R, Hughes T, Chung HJ, Liu Q. Composition, molecular structure, properties, and modification of pulse starches: a review. Food Res Int. 2010;43:399–413 [Google Scholar]
- 31.Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H. Exceptionally low blood glucose response to dried beans: comparison with other carbohydrate foods. BMJ. 1980;281:578–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins DJ, Thorne MJ, Camelon K, Jenkins A, Rao AV, Taylor RH, Thompson LU, Kalmusky J, Reichert R, et al. Effect of processing on digestibility and the blood glucose response: a study of lentils. Am J Clin Nutr. 1982;36:1093–101 [DOI] [PubMed] [Google Scholar]
- 33.Brand JC, Snow BJ, Nabhan GP, Truswell AS. Plasma glucose and insulin responses to traditional Pima Indian meals. Am J Clin Nutr. 1990;51:416–20 [DOI] [PubMed] [Google Scholar]
- 34.Guillon F, Champ MM. Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr. 2002;88 Suppl 3:S293–306 [DOI] [PubMed] [Google Scholar]
- 35.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46 Suppl 2:S33–50 [PubMed] [Google Scholar]
- 36.Tovar J, Granfeldt Y, Björk IM. Effect of processing on blood glucose and insulin responses to starch in legumes. J Agric Food Chem. 1992;40:1846–51 [Google Scholar]
- 37.Sayago-Ayerdi SG, Tovar J, Osorio-Diaz P, Paredes-Lopez O, Bello-Perez L. In vitro starch digestibility and predicted glycemic index of corn tortilla, black beans, and tortilla-bean mixture: effect of cold storage. J Agric Food Chem. 2005;53:1281–5 [DOI] [PubMed] [Google Scholar]
- 38.Tappy L, Wursch P, Randin JP, Felber JP, Jequier E. Metabolic effect of pre-cooked instant preparations of bean and potato in normal and in diabetic subjects. Am J Clin Nutr. 1986;43:30–6 [DOI] [PubMed] [Google Scholar]
- 39.Wursch P, Del Vedovo S, Koellreutter B. Cell structure and starch nature as key determinants of the digestion rate of starch in legume. Am J Clin Nutr. 1986;43:25–9 [DOI] [PubMed] [Google Scholar]
- 40.Tosh SM, Yada S. Dietary fibres in pulse seeds and fractions: characterization, functional attributes, and applications. Food Res Int. 2010;43:450–60 [Google Scholar]
- 41.Boye J, Zare F, Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int. 2010;43:414–31 [Google Scholar]
- 42.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–8 [DOI] [PubMed] [Google Scholar]
- 43.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–33 [DOI] [PubMed] [Google Scholar]
- 44.Pai S, Ghugre PS, Udipi SA. Satiety from rice-based, wheat-based and rice-pulse combination preparations. Appetite. 2005;44:263–71 [DOI] [PubMed] [Google Scholar]
- 45.Sufian MKNB, Hira T, Asano K, Hara H. Peptides derived from dolicholin, a phaseolin-like protein in country beans (Dolichos lablab), potently stimulate cholecystokinin secretion from enteroendocrine STC-1 cells. J Agric Food Chem. 2007;55:8980–6 [DOI] [PubMed] [Google Scholar]
- 46.Campos-Vega R, Loarca-Piña G, Oomah BD. Minor components of pulses and their potential impact on human health. Food Res Int. 2010;43:461–82 [Google Scholar]
- 47.Lajolo FM, Genovese MI. Nutritional significance of lectins and enzyme inhibitors from legumes. J Agric Food Chem. 2002;50:6592–8 [DOI] [PubMed] [Google Scholar]
- 48.Amarowicz R, Troszynska A, Barylko-Pikielna N, Shahidi F. Polyphenolics extracts from legume seeds: correlations between total antioxidant activity, total phenolics content, tannins content and astrigency. J Food Lipids. 2004;11:278–86 [Google Scholar]
- 49.Welsch CA, Lachance PA, Wasserman BP. Dietary phenolic compounds: inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr. 1989;119:1698–704 [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K, Miyamoto Y, Shimizu M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000;48:5618–23 [DOI] [PubMed] [Google Scholar]
- 51.Schlemmer U, Frolich W, Prieto RM, Grases F. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53 Suppl 2:S330–75 [DOI] [PubMed] [Google Scholar]
- 52.Thompson LU, Button CL, Jenkins DJ. Phytic acid and calcium affect the in vitro rate of navy bean starch digestion and blood glucose response in humans. Am J Clin Nutr. 1987;46:467–73 [DOI] [PubMed] [Google Scholar]
- 53.Dilawari JB, Kamath PS, Batta RP, Mukewar S, Raghavan KS. Exceptionally low blood glucose response to dried beans. BMJ. 1980;281:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collier G, McLean A, O'Dea K. Effect of co-ingestion of fat on the metabolic responses to slowly and rapidly absorbed carbohydrates. Diabetologia. 1984;26:50–4 [DOI] [PubMed] [Google Scholar]
- 55.Torsdottir I, Alpsten M, Andersson D, Brummer RJ, Andersson H. Effect of different starchy foods in composite meals on gastric emptying rate and glucose metabolism. I. Comparisons between potatoes, rice and white beans. Hum Nutr Clin Nutr. 1984;38:329–38 [PubMed] [Google Scholar]
- 56.Leathwood P, Pollet P. Effects of slow release carbohydrates in the form of bean flakes on the evolution of hunger and satiety in man. Appetite. 1988;10:1–11 [DOI] [PubMed] [Google Scholar]
- 57.Sparti A, Milon H, Di Vetta V, Schneiter P, Tappy L, Jequier E, Schutz Y. Effect of diets high or low in unavailable and slowly digestible carbohydrates on the pattern of 24-h substrate oxidation and feelings of hunger in humans. Am J Clin Nutr. 2000;72:1461–8 [DOI] [PubMed] [Google Scholar]
- 58.Morris KL, Zemel MB. Effect of dietary carbohydrate source on the development of obesity in agouti transgenic mice. Obes Res. 2005;13:21–35 [DOI] [PubMed] [Google Scholar]
- 59.Marinangeli CPF, Kassis AN, Jones PJH. Glycemic responses and sensory characteristics of whole yellow pea flour added to novel functional foods. J Food Sci. 2009;74:S385–7 [DOI] [PubMed] [Google Scholar]
- 60.Wong CL, Mollard RC, Zafar TA, Luhovy BL, Anderson GH. Food intake and satiety following a serving of pulses in young men: effect of processing, recipe, and pulse variety. J Am Coll Nutr. 2009;28:543–52 [DOI] [PubMed] [Google Scholar]
- 61.Bourdon I, Olson B, Backus R, Richter BD, Davis PA, Schneeman BO. Beans, as a source of dietary fiber, increase cholecystokinin and apolipoprotein B48 response to test meals in men. J Nutr. 2001;131:1485–90 [DOI] [PubMed] [Google Scholar]
- 62.Johnson SK, Thomas SJ, Hall RS. Palatability and glucose, insulin and satiety responses of chickpea flour and extruded chickpea flour bread eaten as part of a breakfast. Eur J Clin Nutr. 2005;59:169–76 [DOI] [PubMed] [Google Scholar]
- 63.Anderson GH, Woodend D. Effect of glycemic carbohydrates on short-term satiety and food intake. Nutr Rev. 2003;61:S17–26 [DOI] [PubMed] [Google Scholar]
- 64.Flint A, Gregersen NT, Gluud LL, Moller BK, Raben A, Tetens I, Verdich C, Astrup A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br J Nutr. 2007;98:17–25 [DOI] [PubMed] [Google Scholar]
- 65.Burton-Freeman BM, Keim NL. Glycemic index, cholecystokinin, satiety and disinhibition: is there an unappreciated paradox for overweight women? Int J Obes. 2005;32:1647–54 [DOI] [PubMed] [Google Scholar]
- 66.Niwano Y, Adachi T, Kashimura J, Sakata T, Sasaki H, Sekine K, Yamamoto S, Yonekubo A, Kimura S. Is glycemic index of food a feasible predictor of appetite, hunger, and satiety? J Nutr Sci Vitaminol (Tokyo). 2009;55:201–7 [DOI] [PubMed] [Google Scholar]
- 67.Roberts SB. High-glycemic index foods, hunger, and obesity: is there a connection? Nutr Rev. 2000;58:163–9 [DOI] [PubMed] [Google Scholar]
- 68.Ludwig DS, Roberts SB. Influence of glycemic index/load on glycemic response, appetite, and food intake in healthy humans. Diabetes Care. 2006;29:474, author reply 5–6 [DOI] [PubMed] [Google Scholar]
- 69.Bornet FR, Costagliola D, Rizkalla SW, Blayo A, Fontvieille AM, Haardt MJ, Letanoux M, Tchobroutsky G, Slama G. Insulinemic and glycemic indexes of six starch-rich foods taken alone and in a mixed meal by type 2 diabetics. Am J Clin Nutr. 1987;45:588–95 [DOI] [PubMed] [Google Scholar]
- 70.Jenkins DJ, Wolever TM, Taylor RH, Griffiths C, Krzeminska K, Lawrie J, Bennett CM, Goff DV, Sarson DL, et al. Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr. 1982;35:1339–46 [DOI] [PubMed] [Google Scholar]
- 71.Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr. 1988;48:1041–7 [DOI] [PubMed] [Google Scholar]
- 72.Torsdottir I, Alpsten M, Andersson H, Schweizer TF, Tolli J, Wursch P. Gastric emptying and glycemic response after ingestion of mashed bean or potato flakes in composite meals. Am J Clin Nutr. 1989;50:1415–9 [DOI] [PubMed] [Google Scholar]
- 73.Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, Jenkins DJ, Vantini I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83:817–22 [DOI] [PubMed] [Google Scholar]
- 74.Wolever TM, Brighenti F, Royall D, Jenkins AL, Jenkins DJ. Effect of rectal infusion of short chain fatty acids in human subjects. Am J Gastroenterol. 1989;84:1027–33 [PubMed] [Google Scholar]
- 75.Thorburn A, Muir J, Proietto J. Carbohydrate fermentation decreases hepatic glucose output in healthy subjects. Metabolism. 1993;42:780–5 [DOI] [PubMed] [Google Scholar]
- 76.Mayer J. Glucostatic mechanisms of the regulation of food intake. N Engl J Med. 1953;249:13–6 [DOI] [PubMed] [Google Scholar]
- 77.Flatt JP. The difference in storage capacities for carbohydrate and for fat, and its implication for the regulation of body weight. Ann N Y Acad Sci. 1987;499:104–23 [DOI] [PubMed] [Google Scholar]
- 78.Liljeberg HG, Lonner CH, Bjorck IM. Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J Nutr. 1995;125:1503–11 [DOI] [PubMed] [Google Scholar]
- 79.Holt SH, Miller JC, Petocz P, Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr. 1995;49:675–90 [PubMed] [Google Scholar]
- 80.McCrory MA, Lovejoy JL, Palmer PA, Eichelsdoerfer PE, Gehrke MM, Kavanaugh IT, Buesing SA, Rose TL. Effectiveness of legume consumption for facilitating weight loss: a randomized trial (abstract). FASEB J. 2008;22:1084.9 [Google Scholar]
- 81.Murty CM, Pittaway JK, Ball MJ. Chickpea supplementation in an Australian diet affects food choice, satiety and bowel health. Appetite. 2010;54:282–8 [DOI] [PubMed] [Google Scholar]
- 82.Papanikolaou Y, Fulgoni VL III. Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: results from the National Health and Nutrition Examination Survey 1999–2002. J Am Coll Nutr. 2008;27:569–76 [DOI] [PubMed] [Google Scholar]
- 83.Greenwood DC, Cade JE, Draper A, Barrett JH, Calvert C, Greenhalgh A. Seven unique food consumption patterns identified among women in the UK Women's Cohort Study. Eur J Clin Nutr. 2000;54:314–20 [DOI] [PubMed] [Google Scholar]
- 84.Haveman-Nies A, Tucker KL, de Groot LC, Wilson PW, van Staveren WA. Evaluation of dietary quality in relationship to nutritional and lifestyle factors in elderly people of the US Framingham Heart Study and the European SENECA study. Eur J Clin Nutr. 2001;55:870–80 [DOI] [PubMed] [Google Scholar]
- 85.Sichieri R. Dietary patterns and their associations with obesity in the Brazilian city of Rio de Janeiro. Obes Res. 2002;10:42–8 [DOI] [PubMed] [Google Scholar]
- 86.Newby PK, Muller D, Hallfrisch J, Andres R, Tucker KL. Food patterns measured by factor analysis and anthropometric changes in adults. Am J Clin Nutr. 2004;80:504–13 [DOI] [PubMed] [Google Scholar]
- 87.Roberts SB, Hajduk CL, Howarth NC, Russell R, McCrory MA. Dietary variety predicts low body mass index and inadequate macronutrient and micronutrient intakes in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2005;60:613–21 [DOI] [PubMed] [Google Scholar]
- 88.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Legume and isoflavone intake and prostate cancer risk: The Multiethnic Cohort Study. Int J Cancer. 2008;123:927–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stamler J, Dolecek TA. Relation of food and nutrient intakes to body mass in the special intervention and usual care groups in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr. 1997;65:S366–73 [DOI] [PubMed] [Google Scholar]
- 90.Mendez MA, Covas MI, Marrugat J, Vila J, Schroder H. Glycemic load, glycemic index, and body mass index in Spanish adults. Am J Clin Nutr. 2009;89:316–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berkow SE, Barnard N. Vegetarian diets and weight status. Nutr Rev. 2006;64:175–88 [DOI] [PubMed] [Google Scholar]
- 92.Tohill BC, Seymour J, Serdula M, Kettel-Khan L, Rolls BJ. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62:365–74 [DOI] [PubMed] [Google Scholar]
- 93.Alinia S, Hels O, Tetens I. The potential association between fruit intake and body weight–a review. Obes Rev. 2009;10:639–47 [DOI] [PubMed] [Google Scholar]
- 94.Karlstrom B, Vessby B, Asp NG, Boberg M, Lithell H, Berne C. Effects of leguminous seeds in a mixed diet in non-insulin-dependent diabetic patients. Diabetes Res. 1987;5:199–205 [PubMed] [Google Scholar]
- 95.Institute of Medicine Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids, Part I. Washington, DC: National Academies of Science; 2002 [DOI] [PubMed] [Google Scholar]
- 96.Sichieri R, Condo AN, Saura SKI, Albino CC. Reducao de peso com dieta de baixo teor de gordura baseada em arroz e feijao. Arq Bras Endocrinol Metabol. 1993;37:135–8 [Google Scholar]
- 97.Rolls BJ, Miller DL. Is the low-fat message giving people a license to eat more? J Am Coll Nutr. 1997;16:535–43 [PubMed] [Google Scholar]
- 98.Chandon P, Wansink B. The biasing health halos of fast food restaurant health claims: lower calorie estimates and high side-dish consumption intentions. J Consum Res. 2007;34:301–14 [Google Scholar]
- 99.Fantini N, Cabras C, Lobina C, Colombo G, Gessa GL, Riva A, Donzelli F, Morazzoni P, Bombardelli E, et al. Reducing effect of a Phaseolus vulgaris dry extract on food intake, body weight, and glycemia in rats. J Agric Food Chem. 2009;57:9316–23 [DOI] [PubMed] [Google Scholar]
- 100.Abete I, Parra D, Martinez JA. Legume-, fish-, or high-protein-based hypocaloric diets: effects on weight loss and mitochondrial oxidation in obese men. J Med Food. 2009;12:100–8 [DOI] [PubMed] [Google Scholar]
- 101.Hermsdorff HH, Zulet MA, Abete I, Martinez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. Epub 2010 May 25 [DOI] [PubMed] [Google Scholar]
- 102.Contaldo F, Di Biase G, Giacco A, Pacioni D, Moro CO, Grasso L, Mancini M, Fidanza F. Evaluation of the hypocholesterolemic effect of vegetable proteins. Prev Med. 1983;12:138–43 [DOI] [PubMed] [Google Scholar]
- 103.Anderson JW, Story L, Sieling B, Chen WJ, Petro MS, Story J. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am J Clin Nutr. 1984;40:1146–55 [DOI] [PubMed] [Google Scholar]
- 104.Nervi F, Covarrubias C, Bravo P, Velasco N, Ulloa N, Cruz F, Fava M, Severin C, Del Pozo R, et al. Influence of legume intake on biliary lipids and cholesterol saturation in young Chilean men. Identification of a dietary risk factor for cholesterol gallstone formation in a highly prevalent area. Gastroenterology. 1989;96:825–30 [PubMed] [Google Scholar]
- 105.Anderson JW, Gustafson NJ, Spencer DB, Tietyen J, Bryant CA. Serum lipid response of hypercholesterolemic men to single and divided doses of canned beans. Am J Clin Nutr. 1990;51:1013–9 [DOI] [PubMed] [Google Scholar]
- 106.Cobiac L, McArthur R, Nestel PJ. Can eating baked beans lower plasma cholesterol? Eur J Clin Nutr. 1990;44:819–22 [PubMed] [Google Scholar]
- 107.Duane WC. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J Lipid Res. 1997;38:1120–8 [PubMed] [Google Scholar]
- 108.Fruhbeck G, Monreal I, Santidrian S. Hormonal implications of the hypocholesterolemic effect of intake of field beans (Vicia faba L.) by young men with hypercholesterolemia. Am J Clin Nutr. 1997;66:1452–60 [DOI] [PubMed] [Google Scholar]
- 109.Jarvi AE, Karlstrom BE, Granfeldt YE, Bjorck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22:10–8 [DOI] [PubMed] [Google Scholar]
- 110.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER III, Conlin PR, Erlinger TP, Rosner BA, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64 [DOI] [PubMed] [Google Scholar]
- 111.Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J, Miller CK, Bobe G, Colburn NH, et al. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140:60–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jenkins DJ, Wong GS, Patten R, Bird J, Hall M, Buckley GC, McGuire V, Reichert R, Little JA. Leguminous seeds in the dietary management of hyperlipidemia. Am J Clin Nutr. 1983;38:567–73 [DOI] [PubMed] [Google Scholar]
- 113.Oosthuizen W, Scholtz CS, Vorster HH, Jerling JC, Vermaak WJ. Extruded dry beans and serum lipoprotein and plasma haemostatic factors in hyperlipidaemic men. Eur J Clin Nutr. 2000;54:373–9 [DOI] [PubMed] [Google Scholar]
- 114.Pittaway JK, Ahuja KD, Robertson IK, Ball MJ. Effects of a controlled diet supplemented with chickpeas on serum lipids, glucose tolerance, satiety and bowel function. J Am Coll Nutr. 2007;26:334–40 [DOI] [PubMed] [Google Scholar]
- 115.Winham DM, Hutchins AM. Baked bean consumption reduces serum cholesterol in hypercholesterolemic adults. Nutr Res. 2007;27:380–6 [Google Scholar]
- 116.Winham DM, Hutchins AM, Johnston CS. Pinto bean consumption reduces biomarkers for heart disease risk. J Am Coll Nutr. 2007;26:243–9 [DOI] [PubMed] [Google Scholar]
- 117.Mackay S, Ball MJ. Do beans and oat bran add to the effectiveness of a low-fat diet? Eur J Clin Nutr. 1992;46:641–8 [PubMed] [Google Scholar]
- 118.Rolls BJ, Ello-Martin JA, Tohill BC. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr Rev. 2004;62:1–17 [DOI] [PubMed] [Google Scholar]
- 119.Preuss HG. Bean amylase inhibitor and other carbohydrate absorption blockers: effects on diabesity and general health. J Am Coll Nutr. 2009;28:266–76 [DOI] [PubMed] [Google Scholar]
- 120.Layer P, Carlson GL, DiMagno EP. Partially purified white bean amylase inhibitor reduces starch digestion in vitro and inactivates intraduodenal amylase in humans. Gastroenterology. 1985;88:1895–902 [DOI] [PubMed] [Google Scholar]
- 121.Vinson JA, Al Kharrat H, Shuta D. Investigation of an amylase inhibitor on human glucose absorption after starch consumption. Open Nutraceutical J. 2009;2:88–91 [Google Scholar]
- 122.Chokshi D. Toxicity studies of Blockol, a dietary supplement containing Phase 2 starch neutralizer (Phase 2), a standardized extract of the common white kidney bean (Phaseolus vulgaris). Int J Toxicol. 2006;25:361–71 [DOI] [PubMed] [Google Scholar]
- 123.Chokshi D. Subchronic oral toxicity of a standardized white kidney bean (Phaseolus vulgaris) extract in rats. Food Chem Toxicol. 2007;45:32–40 [DOI] [PubMed] [Google Scholar]
- 124.Thom E. A randomized, double-blind, placebo-controlled trial of a new weight-reducing agent of natural origin. J Int Med Res. 2000;28:229–33 [DOI] [PubMed] [Google Scholar]
- 125.Udani J, Hardy M, Madsen DC. Blocking carbohydrate absorption and weight loss: a clinical trial using Phase 2 brand proprietary fractionated white bean extract. Altern Med Rev. 2004;9:63–9 [PubMed] [Google Scholar]
- 126.Koike T, Koizumi Y, Tang L, Takahara K, Saitou Y. The anti-obesity effect and the safety of taking "PhaseolaminTM 1600 diet". J New Rem & Clin. 2005;54:1–16 [Google Scholar]
- 127.Opala T, Rzymski P, Pischel I, Wilczak M, Wozniak J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects–a randomised double-blind placebo-controlled clinical trial. Eur J Med Res. 2006;11:343–50 [PubMed] [Google Scholar]
- 128.Celleno L, Tolaini MV, D'Amore A, Perricone NV, Preuss HG. A dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women. Int J Med Sci. 2007;4:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Udani J, Singh BB. Blocking carbohydrate absorption and weight loss: a clinical trial using a proprietary fractionated white bean extract. Altern Ther Health Med. 2007;13:32–7 [PubMed] [Google Scholar]
- 130.Wu X, Xu X, Shen J, Perricone NV, Preuss HG. Enhanced weight loss from a dietary supplement containing standardized Phaseolus vulgaris extract in overweight men and women. J Appl Res. 2010;10:73–9 [Google Scholar]
- 131.Mitchell DC, Lawrence FR, Hartman TJ, Curran JM. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J Am Diet Assoc. 2009;109:909–13 [DOI] [PubMed] [Google Scholar]
- 132.Kimmons J, Gillespie C, Seymour J, Serdula M, Blanck HM. Fruit and vegetable intake among adolescents and adults in the United States: percentage meeting individualized recommendations. Medscape J Med. 2009;11:26. [PMC free article] [PubMed] [Google Scholar]
- 133.USDA, Center for Nutrition and Policy Promotion. Inside the Pyramid: how many vegetables are needed daily or weekly? [OME no. 0584–0535]; September 11, 2008 [cited 2010 9 Sep]. Available from: http://www.mypyramidgov/pyramid/vegetables_amount_table.html.
- 134.Caltabiano ML, Shellshear J. Palatability versus healthiness as determinants of food preferences in young adults: a comparison of nomothetic and idiographic analytic approaches. Aust N Z J Public Health. 1998;22:547–51 [DOI] [PubMed] [Google Scholar]
- 135.Glanz K, Basil M, Maibach E, Goldberg J, Snyder D. Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc. 1998;98:1118–26 [DOI] [PubMed] [Google Scholar]
- 136.Drewnowski A. The Nutrient Rich Foods Index helps to identify healthy, affordable foods. Am J Clin Nutr. 2010;91:S1095–101 [DOI] [PubMed] [Google Scholar]
- 137.Lucier G, Lin B-H, Allshouse J, Kantor LS. Factors affecting dry bean consumption in the United States. Vegetables and Specialties S&O. 2000:26–34 Available from: www.ers.usda.gov/briefing/drybeans/pdfs/drybeanconsumption.pdf
- 138.Vorster HH, van Tonder E, Kotze JP, Walker AR. Effects of graded sucrose additions on taste preference, acceptability, glycemic index, and insulin response to butter beans. Am J Clin Nutr. 1987;45:575–9 [DOI] [PubMed] [Google Scholar]
- 139.Szafranski M, Whittington JA, Bessinger C. Pureed cannellini beans can be substituted for shortening in brownies. J Am Diet Assoc. 2005;105:1295–8 [DOI] [PubMed] [Google Scholar]
- 140.Ryland D, Vaisey-Genser M, Arntfield SD, Malcolmson LJ. Development of a nutrition acceptable snack bar using micronized flaked lentils. Food Res Int. 2010;43:642–9 [Google Scholar]
- 141.Gellar L, Rovner AJ, Nansel TR. Whole grain and legume acceptability among youths with type 1 diabetes. Diabetes Educ. 2009;35:422–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lacey JM. Improving familiarity with legumes in an introductory tertiary nutrition course in Pennsylvania, USA. Nutr Diet. 2004;61:159–61 [Google Scholar]
- 143.McCrory MA, Lovejoy JL, Gehrke MM, Palmer PA, Eichelsdoerfer PE, Kavanaugh IT, Schenck KE. Can liking of a healthy food increase with repeated exposure? [abstract]. Appetite. 2009;52:815 [Google Scholar]
- 144.Veenstra JM, Duncan AM, Cryne CN, Deschambault BR, Boye JI, Benali M, Marcotte M, Tosh SM, Farnworth ER, et al. Effect of pulse consumption on perceived flatulence and gastrointestinal function in healthy males. Food Res Int. 2010;43:553–9 [Google Scholar]