Abstract
Amino acids (AA) were traditionally classified as nutritionally essential or nonessential for animals and humans based on nitrogen balance or growth. A key element of this classification is that all nonessential AA (NEAA) were assumed to be synthesized adequately in the body as substrates to meet the needs for protein synthesis. Unfortunately, regulatory roles for AA in nutrition and metabolism have long been ignored. Such conceptual limitations were not recognized until recent seminal findings that dietary glutamine is necessary for intestinal mucosal integrity and dietary arginine is required for maximum neonatal growth and embryonic survival. Some of the traditionally classified NEAA (e.g. glutamine, glutamate, and arginine) play important roles in regulating gene expression, cell signaling, antioxidative responses, and immunity. Additionally, glutamate, glutamine, and aspartate are major metabolic fuels for the small intestine and they, along with glycine, regulate neurological function. Among essential AA (EAA), much emphasis has been placed on leucine (which activates mammalian target of rapamycin to stimulate protein synthesis and inhibit proteolysis) and tryptophan (which modulates neurological and immunological functions through multiple metabolites, including serotonin and melatonin). A growing body of literature leads to a new concept of functional AA, which are defined as those AA that regulate key metabolic pathways to improve health, survival, growth, development, lactation, and reproduction of organisms. Both NEAA and EAA should be considered in the classic “ideal protein” concept or formulation of balanced diets to maximize protein accretion and optimize health in animals and humans.
Introduction
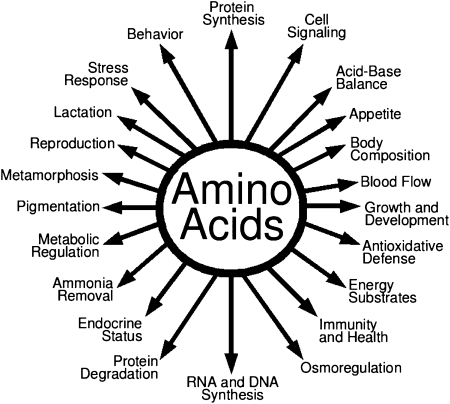
Based on growth or nitrogen balance (namely net synthesis of protein in the whole body), amino acids (AA)3 have traditionally been classified as nutritionally essential (indispensable) or nonessential (dispensable) for animals and humans (1, 2). Nutritionally essential AA (EAA) are those whose carbon skeletons are not synthesized by animal cells and, therefore, must be provided from the diet. Dietary essentiality of some AA (e.g. arginine, glycine, proline, and taurine) depends on species and developmental stage (2). In contrast, nonessential AA (NEAA) are those AA that are synthesized de novo in a species-dependent manner (3, 4). It was tactically assumed, without much evidence, that animals or humans could synthesize sufficient amounts of all NEAA and did not need them in diets for optimal nutrition or health. However, growing evidence from cell culture and animal studies shows that some of the traditionally classified NEAA (e.g. glutamine, glutamate, and arginine) play important roles in multiple signaling pathways, thereby regulating gene expression, intracellular protein turnover, nutrient metabolism, and oxidative defense (5–7). Additional work has also identified that young and gestating mammals cannot synthesize sufficient amounts of all NEAA to support maximum embryonic/fetal survival, neonatal growth, as well as vascular and intestinal health (8–11). Clearly, cell- and tissue-specific functions of AA beyond protein synthesis (Fig. 1) should be taken into consideration in the recommendation of nutrient requirements for animals and humans. Also, the long-standing classification of AA as EAA or NEAA has major conceptual limitations in protein nutrition.
Figure 1.
Roles of AA in nutrition and whole-body homeostasis. Besides serving as building blocks for proteins, AA have multiple regulatory functions in cells. These nutrients are crucial for growth, development, and health of animals and humans.
A growing body of literature has led to the development of the concept of functional AA (FAA), which are defined as those AA that regulate key metabolic pathways to improve health, survival, growth, development, lactation, and reproduction of organisms (2). A deficiency of a FAA (either EAA or NEAA) impairs not only protein synthesis but also whole-body homeostasis. Notably, supplementing a specific FAA (e.g. glutamine or arginine) to a conventional diet that was traditionally thought to provide adequate AA can maximize growth potential in young animals (2, 12, 13) and prevent diseases (e.g. obesity, diabetes, necrotizing enterocolitis, and intrauterine growth retardation) in both animals and humans (4). In view of the foregoing, the major objective of this article is to highlight recent advances in understanding the roles for FAA in nutrient metabolism, growth, reproduction, and health.
Current status of knowledge
Synthesis of AA
Milk has traditionally been thought to provide adequate amounts of all AA to neonates. However, results of recent studies with lactating sows indicate that milk provides at most only 40% of arginine for protein accretion in 7- to 21-d-old suckling pigs and that arginine deficiency is a major factor limiting their maximum growth (13). Besides arginine, the amount of milk-borne proline that enters the portal vein is inadequate to support proline requirements for protein synthesis in the piglet (14). Thus, a 7-d-old pig must synthesize daily at least 0.68 g arginine/kg body weight (13). Based on a degradation rate [0.93 g/(kg body weight ⋅ d)] of i.v. infused proline in young pigs, the de novo synthesis of proline must occur at a rate of at least 1.11 g/(kg body weight ⋅ d) or at least 60% of the proline need for protein accretion (14). Additionally, based on glycine and alanine content in sow milk, milk meets at most 23% and 66%, respectively, of the needs for protein synthesis in piglets, which must synthesize daily at least 0.71 g glycine and 0.18 g alanine/kg body weight (Table 1). Interestingly, although aspartate plus asparagine and glutamate plus glutamine represent 23 and 42%, respectively, of the total NEAA in sow milk (8), this food provides at most only 8 and 9% of aspartate and glutamate for protein deposition in suckling pigs, respectively. Considering the extensive utilization of arterial glutamine by enterocytes and other cell types (including kidneys and lymphocytes) (15), dietary glutamine is also substantially inadequate for protein synthesis in extra-intestinal tissues of piglets and the rate of de novo synthesis of glutamine is likely very high in the suckling piglet [at least 0.88 g/(kg body weight ⋅ d)]. Similarly, a typical corn- and soybean meal-based diet cannot provide sufficient amounts of arginine, proline, aspartate, glutamate, glutamine, or glycine for protein accretion in postweaning growing pigs (Table 1).
Table 1.
Use of dietary AA for protein accretion in sow-reared 14-d-old pigs and 30-d-old pigs weaned at 21 d of age1
| Sow-reared 14-d-old pigs |
Weaned 30-d-old pigs |
|||
| AA | AA in sow milk2 | AA accretion in the pig/AA intake from milk3 | AA in diet4 | AA accretion in the pig/AA intake from diet3 |
| % | ||||
| EAA | 27.3 | 65.9 (91.1) | 110 | 54.8 (92.2) |
| Arginine | 1.43 | 164 (203) | 13.2 | 59.5 (110) |
| Histidine | 0.92 | 78.6 (86.8) | 5.73 | 42.3 (62.5) |
| Isoleucine | 2.28 | 53.8 (79.4) | 8.91 | 46.0 (80.4) |
| Leucine | 4.46 | 53.1 (77.7) | 17.8 | 44.6 (76.9) |
| Lysine | 4.08 | 51.2 (61.8) | 14.2 | 49.4 (78.3) |
| Methionine | 1.04 | 63.2 (70.6) | 3.58 | 60.3 (85.4) |
| Phenylalanine | 2.03 | 59.5 (73.3) | 9.93 | 40.1 (59.1) |
| Proline | 5.59 | — | 15.8 | — |
| Hydroxyproline | 0.00 | — | 0.00 | — |
| Pro + OH-Pro5 | 5.59 | 75.1 (123) | 15.8 | 91.2 (174) |
| Threonine | 2.29 | 53.1 (84.1) | 8.52 | 47.8 (80.0) |
| Tryptophan | 0.66 | 58.3 (67.3) | 2.49 | 51.7 (75.0) |
| Valine | 2.54 | 57.8 (88.7) | 9.96 | 49.1 (88.7) |
| NEAA | 22.7 | 75.9 (154) | 99.2 | 56.4 (130) |
| Alanine | 1.97 | 116 (151) | 13.0 | 58.6 (76.6) |
| Asparagine | 2.53 | 48.5 (56.0) | 9.40 | 44.5 (60.2) |
| Aspartate | 2.59 | 57.2 (1227) | 13.2 | 37.7 (761) |
| Cysteine | 0.72 | 63.6 (84.0) | 3.74 | 40.9 (59.3) |
| Glutamate | 4.57 | 59.2 (1176) | 17.2 | 57.1 (1643) |
| Glutamine | 4.87 | 35.7 (112) | 18.4 | 32.4 (117) |
| Glycine | 1.12 | 350 (435) | 8.81 | 155 (217) |
| Serine | 2.35 | 65.1 (81.4) | 7.86 | 65.6 (88.7) |
| Tyrosine | 1.94 | 47.5 (58.7) | 7.62 | 41.4 (58.7) |
| Total AA | 50.0 | 69.6 | 209 | 55.4 |
Adapted from Wu et al. (14). Arginine and proline are EAA for young pigs because of inadequate synthesis (4).
g/L of whole milk.
Values for AA accretion are percent. Values in parentheses are percent of AA entering the portal vein from the small intestine.
g/kg diet (as-fed basis).
OH-Pro, hydroxyproline.
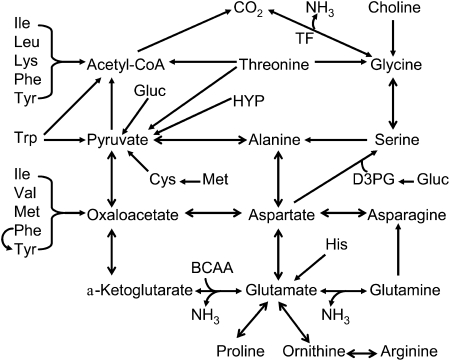
Pathways for the synthesis of arginine, glutamine, glutamate, and proline and alanine are now well documented and have important nutritional and physiological significance (2, 3, 14). In contrast, little is known about how glycine is produced in the body. Although biochemistry textbooks state that glycine is synthesized from serine, 81% of milk-born serine is utilized for protein deposition and the diet provides at most 0.32 g of serine for glycine formation in young pigs (14). Therefore, 90% of glycine must be synthesized from precursors other than serine. Currently, the underlying pathways (including substrates and reactions) for glycine synthesis are largely unknown. These pathways are expected to be nutritionally and physiologically important, because high rates of glycine utilization support the synthesis of protein, creatine, N5-N10-methylene-tetrahydrofolate, nucleotides, and other nitrogenous products (2, 16). Catabolism of EAA is necessary for the synthesis of NEAA in a cell- and tissue-specific manner (Fig. 2).
Figure 2.
Catabolism of nutritionally EAA for the synthesis of AA in animals. Dietary intake of most EAA exceeds their use for protein synthesis in the body. In contrast, the typical corn- and soybean meal-based diet cannot provide sufficient amounts of arginine, aspartate, glutamate, glutamine, glycine, and proline for protein accretion for young pigs, and these AA must be synthesized from EAA. BCAA, branched-chain AA; D3PG, D-3-phosphoglycerate; Gluc, glucose; HYP, hydroxyproline; TF, tetrahydrofolate.
Degradation of AA
It had been a long-standing belief that after digestion, dietary AA absorbed by enterocytes entered the portal vein intact. However, this concept has recently been challenged by findings from studies with young pigs that both EAA and NEAA in the enteral diet are degraded extensively by the small intestine in first pass, with <20% of the extracted AA being utilized for intestinal mucosal protein synthesis (17). Nearly all of glutamate and aspartate, 67–70% of glutamine, and 30–40% of proline in the enteral diet are catabolized by the small intestine of neonatal, weaned, and gestating swine (14). Thus, only 5% of glutamate and aspartate, 30–33% of glutamine, and 60–65% of proline in the diet enter the portal circulation. In postweaning pigs and humans, 40% of dietary arginine is catabolized by the small intestine in the first pass (4). Among dietary AA, the rate of degradation in the small intestine is the greatest for glutamate, followed by glutamine, aspartate, and proline. Of particular note, the small intestine takes up a large amount of glutamine, but no glutamate or aspartate, from the arterial blood. Consequently, the total rate of utilization of glutamine by the gut may be greater than that for glutamate. Bacteria in the intestinal lumen can degrade EAA and NEAA (18, 19), but EAA oxidation in enterocytes is limited (20, 21). Nitrogenous products of glutamate and glutamine include ornithine, citrulline, arginine, proline, aspartate, and alanine (2). Despite much work on glutamate oxidation in the gut, little is known about the effects of dietary glutamate supplementation on intestinal mucosal integrity and function, particularly under stressful conditions.
Intestinal metabolism of AA has profound impacts on nutrition and health. First, catabolism of glutamine, glutamate, and aspartate provides most of the ATP to maintain gut integrity and function (3). Second, because elevated levels of glutamine, glutamate, and aspartate in plasma exert a neurotoxic effect (2), their extensive catabolism by the small intestine is essential to the survival of organisms. Third, transformations of AA in the intestine play an important role in regulating endogenous synthesis of NEAA (e.g. citrulline, arginine, proline, and alanine) and modulating the availability of dietary AA to extra-intestinal tissues (18). Thus, the ratios of most AA in diets relative to lysine differ markedly from those entering the portal vein from the small intestinal lumen or appearing in plasma and body proteins (14). The discrepancies in the patterns of AA between diets and body proteins are particularly large for arginine, histidine, methionine, proline, glutamine, glycine, and serine (14). Therefore, ratios of these AA to lysine in body proteins are not accurate estimates of their optimal dietary requirements by rapidly growing animals or infants. This conceptual limitation also applies to gestating and lactating dams. The classic concept of “ideal protein” must be modified to include all NEAA.
Regulatory role for AA in gene expression and protein turnover
Regulatory effects of certain AA on gene expression may be mediated by transcription factors (including the basic region/leucine zipper factors, activating transcription factors, and CCAAT/enhancer-binding protein), specific regulatory sequences (including AA response elements, nutrient sensing response elements, and multiple sites) in the promoter, and various cis elements distinct from AA response elements or nutrient sensing response elements (5, 6). Two of the most studied AA regarding regulation of gene expression are glutamine and arginine. For example, dietary glutamine supplementation increased intestinal expression (120–124%) of genes that are necessary for cell growth and removal of oxidants, while reducing (34–75%) expression of genes that promote oxidative stress and immune activation (22). These findings provide molecular mechanisms for the beneficial effects of dietary glutamine supplementation to improve nutritional status in young mammals. Also, dietary arginine supplementation reduced mRNA levels for fatty acid binding protein 1, glycogenin, protein phosphotase 1B, caspases 1 and 2, and hepatic lipase, but increased expression of PPAR-γ, heme oxygenase 3, glutathione synthetase, insulin-like growth factor II, sphingosine-1-phosphate receptor, AMP-activated protein kinase (AMPK), and stress-induced protein in white adipose tissue (WAT) of diet-induced obese rats (23). In the adult-rat WAT, there is no synthesis of fatty acids due to the lack of acetyl-CoA carboxylase, but arginine enhances lipolysis in this tissue (23–25). Therefore, enhanced expression of PPAR-γ does not contribute to adipogenesis in adult obese rats. Biochemical analysis has revealed that oxidative stress in the obese-rat WAT can be prevented by arginine supplementation (24). Also, upregulation of genes involved in mitochondrial biogenesis by arginine provides another mechanism for increased oxidation of long-chain fatty acids and glucose in insulin-sensitive tissues (25).
Histone modifications by methylation, acetylation, and phosphorylation, as well as DNA methylation (occurring in the 5′-positions of cytosine residues within CpG dinucleotides throughout the mammalian genome) play an important role in epigenetic regulation of gene expression and physiological functions (26). AA (e.g. methionine, histidine, serine, and glycine) are major donors of 1-carbon groups that may affect these events and related reactions, including activities of histone acetyltransferase (e.g. cAMP response element-binding protein) and acetylase, as well as specific DNA methyltransferases (27). Upon acetylation of histones, DNA is dissociated from histones so that transcription will proceed. In contrast, DNA methylation and histone deacetylation result in highly dense packing of DNA and, therefore, silence gene expression.
RNA polymerase catalyzes the transcription of a gene to mRNA. This process can be regulated by AA through 1 or more of the following mechanisms: 1) alteration of specificity of RNA polymerase for promoters; 2) binding of repressors to noncoding DNA sequences that are near or overlap the promoter region; and 3) transcription factors (e.g. upregulation, downregulation, coactivators, and corepressors) (27). Post-transcriptional regulation is mediated by capping (changing the 5′ end of the mRNA to a 3′ end, conferring protection of the mRNA from 5′ exonuclease), splicing (removing introns and joining of exons), and the addition of a poly(A) tail (poly-adenylation) to confer protection of mRNA from 3′ exonuclease (2). Covalent modifications of DNA and core histones provide a basis for epigenetics (stable alterations in gene expression without changes in the underlying DNA sequence) (26, 28). Epigenetic changes may remain through cell divisions and, therefore, may be carried forward to subsequent generations (28). This notion of transgenerational effects of AA has important implications for human health and animal production.
Initiation of mRNA translation is a key event in the regulation of protein synthesis. The mammalian target of rapamycin (mTOR), a highly conserved serine/threonine protein kinase, is the master regulator of mRNA translation (29); mTOR is also known as FK506 binding protein 12-rapamycin associated protein 1. The mTOR system consists of mTOR complex-1 (mTOR, raptor, and G protein β-subunit-like protein) and mTOR complex-2 (mTOR, rictor, mitogen-activated-protein-kinase-associated protein 1, and G protein β-subunit-like protein) (30). mTOR complex-1 is sensitive to inhibition by rapamycin. AA (e.g. glutamine, arginine, and leucine) stimulate the phosphorylation of mTOR in a cell-specific manner, leading to phosphorylation of ribosomal protein S6 kinase-1 and eukaryotic translation initiation factor 4E-binding protein-1 and, therefore, the formation of translation initiation complex (7, 31, 32). Activation of mTOR may also result in inhibition of intracellular protein degradation, possibly via mechanisms involving autophagy and other unknown pathways (2, 32). Inadequate content of arginine and glutamine as well as other NEAA in a low-protein (12.7% crude protein) diet may explain why dietary supplementation with deficient EAA (lysine, methionine, threonine, tryptophan, leucine, isoleucine, and valine) was ineffective in restoring protein synthesis or whole-body growth in weanling piglets (33).
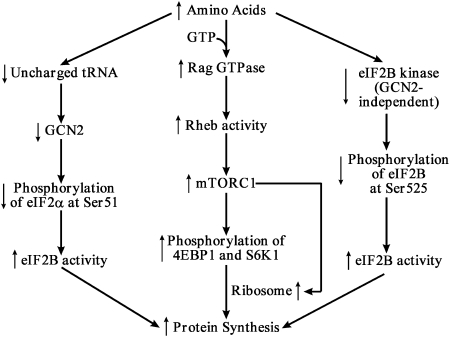
There are suggestions that some AA may directly act to phosphorylate mTOR or its down-stream target proteins (25, 34). Evidence also exists that certain AA may indirectly do so via the production of their metabolites (35) or interaction with Rag GTPases for binding raptor (36). Although results from cell culture studies led to 3 models to explain how AA can regulate protein synthesis in cells (Fig. 3), the physiological relevance of these models remains to be established. This is because in vitro experiments were conducted under conditions of either complete absence of an AA or its presence at a particularly high concentration (e.g. 2 mmol/L; at least 10 times the plasma concentrations for most AA) in culture medium (37, 38). Nonetheless, the potential for FAA (either EAA or NEAA) to stimulate protein synthesis indicates that these nutrients play important regulatory roles beyond serving as building blocks of proteins.
Figure 3.
Models for regulatory roles of AA in protein synthesis. Provision of sufficient amounts of AA promotes the binding of GTP to Rag GTPase leading to mTORC1 activation, while reducing concentrations of uncharged tRNA and activity of the GCN2-independent eIF2B kinase leading to increased activity of eIF2B. All of these changes stimulate the initiation of protein synthesis in cells. Abbreviations: eIF2α, eukaryotic translation initiation factor 2α; eIF2B, eukaryotic translation initiation factor 2B; GCN2, general control nonderepressible protein 2; mTORC1, mammalian target of rapamycin complex 1; S6K1, ribosomal protein S6 kinase-1; 4EBP1, eIF4E-binding protein-1. ↑, increase in concentration or activity; ↓, decrease in concentration or activity.
Roles of AA in gaseous signaling
Some AA (e.g. arginine and glutamine) modulate cellular signaling involving AMPK, extracellular signal-related kinase, Jun kinase, and mitogen-activated protein kinase (39). Emerging evidence shows that NO, CO, and H2S are physiologically important signaling gases (40). NO, CO, and H2S are synthesized from l-arginine, l-cysteine, and glycine (heme), respectively. Many AA have been reported to regulate the production of NO, CO, and H2S in a cell-dependent manner (40, 41). For example, arginine, citrulline, glutamate, glycine, taurine, and γ-aminobutyrate increase NO synthesis by constitutive NO synthase in endothelial cells or brain, whereas low-protein intake, glucosamine, glutamine, and lysine inhibit NO generation by both constitutive and inducible NO synthases (41). In addition, high-protein intake, arginine, glutamine, glutamate, alanine, taurine, methionine, and glycine promote CO synthesis by heme oxygenase in endothelial cells and nonvascular tissues, but N-acetyl-cysteine attenuates CO formation in injured brain and vascular smooth muscle cells (40). Furthermore, H2S production is stimulated by high-protein intake, arginine, cysteine, methionine, glycine, S-adenosylmethionine, N-acetyl-glutamate, and glutamate (0.1 mmol/L) but is inhibited by aspartate and higher levels of glutamate (1–3 mmol/L) in various cell types (40). Either imbalance or antagonism among AA affects the generation of 1 or more of these gaseous molecules and, therefore, protein nutrition in organisms.
NO is a highly reactive free radical (41). In contrast, CO, H2S, and SO2 are strong reducing agents. They are all water-soluble, colorless molecules that easily penetrate biological membranes. Therefore, these gases exert their effects on cells independent of membrane receptors. Because of their chemical properties, the effects of NO, CO, H2S, and SO2 depend on their respective concentrations in cells. At pathological levels, they are extremely destructive to all cell types (40, 41). At physiological levels, NO, CO, and SO2 activate guanylyl cyclase to generate cGMP, which elicits a variety of responses via cGMP-dependent protein kinases that include relaxation of vascular smooth muscle cells, hemodynamics, neurotransmission, and cell metabolism (40, 42). The actions of these gases may also involve cGMP-independent mechanisms (e.g. inhibition of ornithine decarboxylase, protein modification, and redox state). Emerging evidence shows that H2S is a crucial regulator of both neurological function and endothelium-dependent relaxation through cGMP-independent mechanisms involving stimulation of membrane KATP channels and intracellular cAMP signaling (43). Moreover, physiological levels of NO, CO, and H2S confer cytoprotective and immunomodulatory effects.
Translational research benefiting human health and animal production
AA are essential precursors for the synthesis of a wide array of nitrogenous substances with enormous biological importance (2). Some of these bioactive molecules include neurotransmitters (e.g. γ-aminobutyrate, dopamine, and serotonin), hormones (e.g. epinephrine, norepinephrine, triiodothyronine, and thyroxine), vasodilators, signaling gases (NO, CO, and H2S), antioxidants (glutathione, creatine, melatonin, melanin, and taurine), methyl-group donors, as well as key regulators of metabolism, growth, development, immune response, and health. Metabolism of AA is altered under various physiological and pathological conditions, leading to changes in whole-body homeostasis (14, 16, 44, 45).
Historically, our understanding of human protein nutrition has been based largely on animal studies (1–3). The relative growth rates (percent change per day) and food intake (percent of body weight) of rodents, pigs, and poultry are higher than those for humans and primates (46–50). Because the composition of AA in the body is similar among species (2), requirements of intracellular AA (e.g. relative proportions of AA) for protein synthesis are not expected to vary substantially. Thus, new knowledge gained from animal models has important implications for human nutrition. First, as reported for sow-reared piglets (8), provision of arginine from human or primate milk is inadequate for optimal protein accretion in breast-fed infants (46). This necessitates endogenous synthesis of arginine from glutamate, glutamine, and proline in infants (4). Second, dietary supplementation with arginine prevents necrotizing enterocolitis (the most common and severe intestinal disease) in preterm infants (47), who have underdeveloped arginine-synthetic enzymes and insufficient arginine intake (48). Similarly, arginine ameliorates intestinal damage in early-weaned piglets with naturally-occurring gut atrophy (12). Third, a deficiency of dietary glutamine impairs cell signaling and results in intestinal atrophy in both piglets and infants (39).
Because of species differences in absolute requirements of dietary AA [expressed as g/(kg body weight ⋅ d)], cautions should be taken to extrapolate animal data to humans. Nonetheless, new knowledge about AA biochemistry and physiology has resulted in fruitful translational research to improve human and animal health, as well as animal production. For example, supplementation with AA (e.g. glutamine, arginine, and N-acetyl-cysteine) improves oxidative defense (51) and immune function (41) in both humans and animals. Studies with pigs, sheep, and rats have also demonstrated that glutamine and arginine enhance the survival, growth, and development of the embryo, fetus, and neonate (4, 14) as well as intestinal restitution, integrity, and function (39). Additionally, arginine ameliorates obesity, hyperglycemia, dyslipidemia, hypertension, cardiovascular dysfunction, and other problems of metabolic syndrome in humans and animals while enhancing milk production, mitochondrial biogenesis, growth of brown adipose tissue, wound healing, muscular strength and glycolysis, and spermatogenesis (4, 9, 14, 52). Meat quality can also be improved through supplementing arginine to growing-finishing pigs before slaughter (53, 54). Furthermore, proline supplementation stimulates small intestinal and whole-body growth in weanling pigs (10). Collectively, these findings not only greatly advance our knowledge of FAA but also exemplify the power of basic research in solving practical problems in both human health and animal production.
Perspectives
There is a dynamic equilibrium between free AA and protein-bound AA in the body (2). Concentrations of free individual AA in plasma and cells range from 0.01 to 1 and 0.05 to 20 mmol/L, respectively, depending on species, developmental stage, and cell type (24, 49). It should be borne in mind that physiological significance and functional effects of biochemical substances are not necessarily reflected by their concentrations. For example, hepatic concentrations of arginine in mammals are generally <0.1 mmol/L but play an essential role in ammonia detoxification via the urea cycle (4). Additionally, although concentrations of tryptophan are much lower than concentrations of valine in all cell types, tryptophan has more versatile roles than valine in the body (2).
It remains a subject of philosophical debate whether or not maximizing protein accretion in humans is desirable for health and longevity. However, maximum deposition of dietary AA as body proteins should be achieved for children who experience growth retardation due to inadequate intake of protein or disease. Although humans eat foods but not formulated diets, provision of AA in proper ratios is crucial for gastrointestinal, cardiovascular, and reproductive health (49, 50). It should also be recognized that not all NEAA carbons in the body are derived from dietary sources as pyruvate, oxaloacetate, and alpha-ketoglutarate can be synthesized from endogenous glucose. Indeed, data presented in Table 1 clearly indicate that large amounts of NEAA are formed from EAA in growing pigs (Fig. 1). This is also true for glutamate, aspartate, and glutamine in infants and adult humans (15).
The traditional classification of AA as nutritionally essential or nonessential has major conceptual limitations in protein nutrition. It is unfortunate that the current versions of NRC or Institute of Medicine books do not recommend dietary requirements of NEAA for animals or humans (55, 56). However, emerging evidence shows that traditionally classified NEAA, particularly glutamine and arginine, play important roles in regulating gene expression at both transcriptional and translational levels (57, 58). There is growing recognition that some NEAA participate in cell signaling via mTOR, AMPK, extracellular signal-related kinase, Jun kinase, mitogen-activated protein kinase, and gases (NO, CO, and H2S) (39, 43). Exquisite integration of these regulatory networks has profound effects on cell proliferation, differentiation, metabolism, homeostasis, survival, and function. Among EAA, much emphasis has been placed on leucine (which activates mTOR to stimulate protein synthesis and inhibit proteolysis) and tryptophan (which modulates neurological and immunological functions through multiple metabolites including serotonin and melatonin) (59–61). Because dietary AA are substantially catabolized by the small intestine, ratios of AA in the diet differ markedly from those in skeletal muscle or the body (2). Thus, the classic concept of “the ideal protein,” which is based solely on ratios of EAA in tissue proteins (1), should be revised to include all NEAA. In other words, both EAA and NEAA should be taken into consideration in: 1) formulation of balanced diets to maximize growth performance in livestock species, poultry, and fish; 2) recommendation of AA requirements for humans to prevent growth retardation and chronic diseases; and 3) optimization of immune and reproductive functions in all species. Unquestionably, recent advances in understanding FAA are expanding our basic knowledge of protein metabolism and transforming the practices of nutrition worldwide. Nutritionists must think out of the box and move forward to capitalize on the great potential of FAA in improving both human health and animal production.
Acknowledgments
Contributions of colleagues and students to the recent development of the field are gratefully appreciated. G.W. wrote the paper and had sole responsibility for its final content.
Footnotes
Supported by National Research Initiative Competitive grants from the Animal Reproduction Program (2008-35203-19120) and Animal Growth and Nutrient Utilization Program (2008-35206-18764) of the USDA National Institute of Food and Agriculture, NIH (1R21 HD049449), AHA (10GRNT4480020), and Texas AgriLife Research (H-8200).
Author disclosure: G. Wu, no conflicts of interest.
Abbreviations used: AA, amino acid; AMPK, AMP-activated protein kinase; EAA, nutritionally essential amino acid; FAA, functional amino acid; mTOR, mammalian target of rapamycin; NEAA, nutritionally nonessential amino acid; WAT, white adipose tissue.
Literature Cited
- 1.Baker DH. Advances in protein-amino acid nutrition of poultry. Amino Acids. 2009;37:29–41 [DOI] [PubMed] [Google Scholar]
- 2.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17 [DOI] [PubMed] [Google Scholar]
- 3.Watford M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr. 2008;138:S2003–7 [DOI] [PubMed] [Google Scholar]
- 4.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasse-Lagnel C, Lavoinne A, Husson A. Control of mammalian gene expression by amino acids, especially glutamine. FEBS J. 2009;276:1826–44 [DOI] [PubMed] [Google Scholar]
- 6.Bruhat A, Cherasse Y, Chaveroux C, Maurin AC, Jousse C, Fafournous P. Amino acids as regulators of gene expression in mammals: molecular mechanisms. Biofactors. 2009;35:249–57 [DOI] [PubMed] [Google Scholar]
- 7.Yao K, Yin YL, Chu WY, Liu ZQ, Deng D, Li TJ, Huang RL, Zhang JS, Tan BE, et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008;138:867–72 [DOI] [PubMed] [Google Scholar]
- 8.Kim SW, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–30 [DOI] [PubMed] [Google Scholar]
- 9.Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J Nutr. 2007;137:652–6 [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li XL, McKnight JR, et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. Epub 2010 Aug 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng X, Wang F, Fan X, Yang W, Zhou B, Li P, Yin Y, Wu G, Wang J. Dietary arginine supplementation during early pregnancy enhances embryonic survival in rats. J Nutr. 2008;138:1421–5 [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Ruan Z, Gao YL, Yin YL, Zhou XH, Wang L, Geng MM, Hou YQ, Wu G. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids. 2010;39:831–9 [DOI] [PubMed] [Google Scholar]
- 13.Wu G, Knabe DA, Kim SW. Arginine nutrition in neonatal pigs. J Nutr. 2004;134:S2783–90 [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li XL, Satterfield MC, Smith SB, et al. Functional amino acids in swine nutrition and production. : Doppenberg J, editor Dynamics in animal nutrition. Wageningen (The Netherlands): Wageningen Academic Publishers; 2010. p. 69–98 [Google Scholar]
- 15.Bertolo RF, Burrin DG. Comparative aspects of tissue glutamine and proline metabolism. J Nutr. 2008;138:S2032–9 [DOI] [PubMed] [Google Scholar]
- 16.Brosnan JT, Brosnan ME. Creatine metabolism and the urea cycle. Mol Genet Metab. 2010;100:S49–52 [DOI] [PubMed] [Google Scholar]
- 17.Stoll B, Burrin DG. Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers. J Anim Sci. 2006;84:E60–72 [DOI] [PubMed] [Google Scholar]
- 18.Bergen WG, Wu G. Intestinal nitrogen recycling and utilization in health and disease. J Nutr. 2009;139:821–5 [DOI] [PubMed] [Google Scholar]
- 19.Dai ZL, Zhang J, Wu G, Zhu WY. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. Epub 2010 Mar 19 [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Yin Y, Jobgen WJ, Jobgen SC, Knabe DA, Hu W, Wu G. In vitro oxidation of essential amino acids by intestinal mucosal cells of growing pigs. Livest Sci. 2007;109:19–23 [Google Scholar]
- 21.Chen L, Li P, Wang J, Li X, Gao H, Yin Y, Hou Y, Wu G. Catabolism of essential amino acids in developing porcine enterocytes. Amino Acids. 2009;37:143–52 [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Chen L, Li P, Li X, Zhou H, Wang F, Li D, Yin Y, Wu G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. 2008;138:1025–32 [DOI] [PubMed] [Google Scholar]
- 23.Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, Spencer TE, Wu G. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids. 2009;37:187–98 [DOI] [PubMed] [Google Scholar]
- 24.Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr. 2009;139:230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39:349–57 [DOI] [PubMed] [Google Scholar]
- 26.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705 [DOI] [PubMed] [Google Scholar]
- 27.Oommen AM, Griffin JB, Sarath G, Zempleni J. Roles for nutrients in epigenetic events. J Nutr Biochem. 2005;16:74–7 [DOI] [PubMed] [Google Scholar]
- 28.Chmurzynska A. Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutr Rev. 2010;68:87–98 [DOI] [PubMed] [Google Scholar]
- 29.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care. 2009;12:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw RJ. mTOR signaling: RAG GTPases transmit the amino acid signal. Trends Biochem Sci. 2008;33:565–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhoads JM, Niu XM, Surendran S, Liu YY, Wu G. Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and p70s6k signaling. J Nutr. 2008;138:1652–7 [DOI] [PubMed] [Google Scholar]
- 32.Tan B, Yin Y, Kong X, Li P, Li X, Gao H, Li X, Huang R, Wu G. L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids. 2010;38:1227–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng D, Yin YL, Chu WY, Yao K, Li TJ, Huang RL, Liu ZQ, Zhang JS, Wu G. Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low- protein diet. J Nutr Biochem. 2009;20:544–52 [DOI] [PubMed] [Google Scholar]
- 34.Yin YL, Yao K, Liu ZJ, Gong M, Ruan Z, Deng D, Tan BE, Liu ZQ, Wu G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids. Epub 2010 May 15 [DOI] [PubMed] [Google Scholar]
- 35.Pervin S, Singh R, Hernandez E, Wu G, Chaudhuri G. Nitric oxide in physiological concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Res. 2007;67:289–99 [DOI] [PubMed] [Google Scholar]
- 36.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of gene subjected to either complete or single amino acid deprivation. Amino Acids. 2009;37:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Fonseca BD, Tang H, Liu R, Elia A, Clemens MJ, Bommer UA, Proud CG. Re-evaluating the role of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem. 2008;283:30482–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marc Rhoads J, Wu G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37:111–22 [DOI] [PubMed] [Google Scholar]
- 40.Li X, Bazer FW, Gao H, Jobgen W, Johnson GA, Li P, McKnight JR, Satterfield MC, Spencer TE, et al. Amino acids and gaseous signaling. Amino Acids. 2009;37:65–78 [DOI] [PubMed] [Google Scholar]
- 41.Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002;22:61–86 [DOI] [PubMed] [Google Scholar]
- 42.Li P, Yin YL, Li DF, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–52 [DOI] [PubMed] [Google Scholar]
- 43.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–23 [DOI] [PubMed] [Google Scholar]
- 44.Braissant O. Current concepts in the pathogenesis of urea cycle disorders. Mol Genet Metab. 2010;100 Suppl 1:S3–12 [DOI] [PubMed] [Google Scholar]
- 45.Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57:56–63 [DOI] [PubMed] [Google Scholar]
- 46.Davis TA, Nguyen HV, Garciaa-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ. Amino acid composition of human milk is not unique. J Nutr. 1994;124:1126–32 [DOI] [PubMed] [Google Scholar]
- 47.Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–31 [DOI] [PubMed] [Google Scholar]
- 48.Wu G, Jaeger LA, Bazer FW, Rhoads JM. Arginine deficiency in premature infants: biochemical mechanisms and nutritional implications. J Nutr Biochem. 2004;15:442–51 [DOI] [PubMed] [Google Scholar]
- 49.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors. 2009;35:21–7 [DOI] [PubMed] [Google Scholar]
- 50.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134:2169–72 [DOI] [PubMed] [Google Scholar]
- 51.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92 [DOI] [PubMed] [Google Scholar]
- 52.Mateo RD, Wu G, Moon HK, Carroll JA, Kim SW. Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J Anim Sci. 2008;86:827–35 [DOI] [PubMed] [Google Scholar]
- 53.Ma X, Lin Y, Jiang Z, Zheng C, Zhou G, Yu D, Cao T, Wang J, Chen F. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids. 2010;38:95–102 [DOI] [PubMed] [Google Scholar]
- 54.Tan B, Yin YL, Liu ZQ, Li XG, Xu HJ, Kong XF, Huang RL, Tang WJ, Shinzato I, et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009;37:169–75 [DOI] [PubMed] [Google Scholar]
- 55.NRC Nutrient requirements of swine. 10th ed Washington, DC: National Academies Press; 1998 [Google Scholar]
- 56.Elango R, Ball RO, Pencharz PB. Amino acid requirements in humans: with a special emphasis on the metabolic availability of amino acids. Amino Acids. 2009;37:19–27 [DOI] [PubMed] [Google Scholar]
- 57.Haynes TE, Li P, Li XL, Shimotori K, Sato H, Flynn NE, Wang JJ, Knabe DA, Wu G. L-glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids. 2009;37:131–42 [DOI] [PubMed] [Google Scholar]
- 58.Stipanuk MH, Dominy JE, Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr. 2006;136:S1652–9 [DOI] [PubMed] [Google Scholar]
- 59.Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138:195–209 [DOI] [PubMed] [Google Scholar]
- 61.Suryawan A, O’Connor PMJ, Bush JA, Nguyen HV, Davis TA. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2009;37:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]