Abstract
Stearoyl CoA desaturase 1 (SCD1) catalyzes the rate-limiting step in the production of MUFA that are major components of tissue lipids. Alteration in SCD1 expression changes the fatty acid profile of these lipids and produces diverse effects on cellular function. High SCD1 expression is correlated with metabolic diseases such as obesity and insulin resistance, whereas low levels are protective against these metabolic disturbances. However, SCD1 is also involved in the regulation of inflammation and stress in distinct cell types, including β-cells, adipocytes, macrophages, endothelial cells, and myocytes. Furthermore, complete loss of SCD1 expression has been implicated in liver dysfunction and several inflammatory diseases such as dermatitis, atherosclerosis, and intestinal colitis. Thus, normal cellular function requires the expression of SCD1 to be tightly controlled. This review summarizes the current understanding of the role of SCD1 in modulating inflammation and stress.
Introduction
Great diversity exists in the structures and functions of the vast array of lipid species. Lipids are essential for a number of processes that support cellular and tissue maintenance such as the synthesis of cellular membranes, signal transduction, energy storage, assembly of lipoprotein particles, protein modification, as well as many other important functions. Intracellular levels of lipids are tightly regulated by a network of metabolic pathways to sustain normal cellular functions. The regulated synthesis of major lipid classes, including phospholipids, TG, cholesterol esters (CE),5 and wax esters (WE), incorporates fatty acids, of which MUFA are preferred substrates (1, 2). These different lipids possess distinct biological functions and therefore disturbance of the cellular MUFA profile may produce diverse metabolic and systemic effects that include inflammation and stress.
The intracellular levels of MUFA are controlled by stearoyl-CoA desaturase (SCD), a family of enzymes that are Δ-9 fatty acid desaturases. Anchored in the membrane of the endoplasmic reticulum, SCD catalyzes the biosynthesis of MUFA from dietary or de novo synthesized SFA precursors (Fig. 1). Four SCD isoforms (SCD1–4) have been identified in the mouse genome and 2 SCD isoforms (hSCD1 and 5) have been reported in humans (3–7). The SCD isoforms exhibit different tissue distribution patterns but share the same enzymatic function. A number of articles have reviewed the SCD isoforms in detail (8, 9). Of these isoforms, SCD1 is the predominant one and is expressed ubiquitously among tissues, with constitutively high levels in adipose, meibomian gland, Harderian gland, and preputial glands and is highly induced in liver in response to a high-carbohydrate diet (2, 10). SCD1 contains a 33-amino acid sequence at the N terminus that leads to the rapid degradation of this enzyme via a ubiquitin-dependent proteasome mechanism (11, 12). In addition to post-translational control of SCD1 protein level, SCD1 gene expression is highly sensitive to a number of dietary, hormonal, and environmental factors. High-carbohydrate diets, glucose and fructose, cholesterol, and vitamins A and D induce SCD1 expression (13–18), whereas PUFA, especially the (n-3) and (n-6) families, and conjugated linoleic acid inhibit the expression of SCD1 (13, 19, 20). Furthermore, transcriptional control of SCD1 has been shown to be mediated by several transcription factors, including liver X receptor, sterol response element binding protein 1c, carbohydrate response element binding protein, PPAR, and estrogen receptor, as reviewed elsewhere (8, 9).
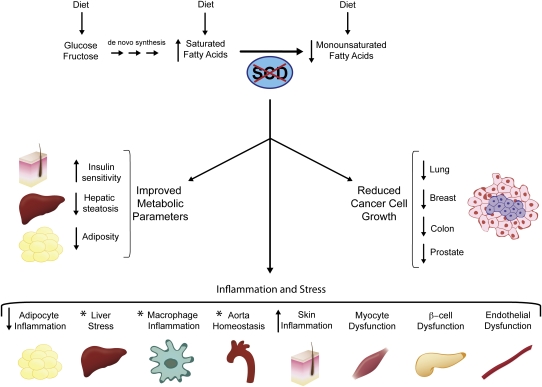
Figure 1.
Role of SCD in pathological processes. SCD1 mediates the synthesis of MUFA from dietary or endogenously synthesized SFA. Loss of SCD1 results in a favorable metabolic profile, including an increase in insulin sensitivity and a decrease in hepatic steatosis and adiposity. Inhibition of SCD1 is also associated with a reduction in cancer cell growth. Additionally, suppression of SCD1 alters cellular function by modulating inflammation and stress in a number of cell types and tissues, such as adipocytes, liver, macrophages, aorta, skin, myocyte, β-cell, and endothelial cell. The asterisk denotes mixed observation in a tissue or cell type.
Substantial insights into the physiological functions of SCD have been gained through studying genetically engineered whole body and tissue specific SCD1 knockout models (21–23). Research using other models of SCD1 suppression has also provided important knowledge for SCD function, these models included Asebia mice that have a natural mutation in SCD1 and thus whole body deficiency of SCD1 protein, and mice treated with antisense oligonucleotides (ASO) against SCD1 (24, 25). With increasing prevalence of metabolic diseases such as obesity and type II diabetes, considerable research efforts have been dedicated to understanding the role of SCD in a number of metabolic diseases that are associated with abnormal lipid metabolism. It is well established by past studies that SCD1 deficiency protects against dietary (high-fat and high-carbohydrate induced) and genetic (leptin deficient and agouti induced) forms of obesity and liver steatosis (26–30). These results generated from studying mice with global deficiency of SCD1 led to the investigation of specific tissue(s) that might be responsible for the observed phenotypes. The treatment of wild type C57/B6 mice with ASO against SCD1, which reduced SCD1 expression in liver and adipose tissue, led to dramatic beneficial metabolic outcomes, including reduced weight gain and increased energy expenditure, when fed a high-fat diet compared with control mice (25). Targeted disruption of SCD1 in liver (SCD1 liver knockout) via cre-mediated elimination reduced weight gain and white adipose tissue fat mass caused by long term feeding of a high-carbohydrate diet (22). Furthermore, because SCD1 deficient mice exhibit a severe skin phenotype, including alopecia, atrophy of sebaceous glands, dermatitis, and increased permeability of the skin barrier (21, 24), the development of a skin specific knockout (SKO) mouse model allowed us to probe the role of skin MUFA synthesis in whole body metabolic homeostasis. With comparable skin defects to SCD1 global knockout mice, SCD1 SKO mice were unexpectedly shown to be hypermetabolic and completely protected from high-fat diet induced obesity (23). A catabolic state induced by reduced skin SCD1 expression and MUFA content is likely to mediate the protective effects against the unfavorable phenotypes caused by the long term consumption of a high-fat diet.
While normal functional cells require fatty acid metabolism for survival, cancer cells demand an even higher degree of metabolic flux to support their rapid growth, and SCD1 has been implicated in the pathology of cancer (31–33). Increased SCD expression has been detected in malignant human tissues such as colonic and esophageal carcinomas, as well as in hepatocellular adenoma (34), and elevated conversion of stearic to oleic acid has also been observed in transformed tumor cells (35). Due to the fundamental need of lipid biosynthesis by tumor cells, suppression of SCD1 may produce extensive consequences on several phenotypic features of cancer encompassing cell replication, enhanced cell survival, and increased tumor cell invasiveness (33). The impact of the inhibition of SCD1 on cancer cell growth also appeared to be universal among different neoplastic cells, including lung, breast, prostate, and colon cancer cells (33, 36–40). However, lower SCD expression was reported in a study that compared prostate carcinoma to normal prostate epithelium (41). Overall, these studies suggest that SCD activity and MUFA availability correlate with malignant cell survival and proliferation.
SCD1-deficient mice are characterized by a reduction in the percentage of fatty acids comprised of MUFA and an increase in the percentage of SFA. The reduction in MUFA levels significantly decreases the synthesis of neutral lipids such as TG and CE (2, 21, 42). Thus, the limited MUFA availability in SCD1-deficient mice may influence the partitioning of fatty acids into and out of neutral lipid species whose dysregulation might lead to a variety of cellular inflammatory and stress responses. In the pathology of obesity, chronic inflammation has been established as a causative factor for the associated disorders such as insulin resistance and cardiovascular disease (43, 44). Given the potent regulation of obesity by SCD1, it is conceivable that this enzyme is involved in modulating cellular inflammation and stress.
While studies aimed at understanding the role of SCD1 in regulating metabolic homeostasis have been pursued, more recent research suggests that the beneficial metabolic effects due to loss of SCD1 may be accompanied by detrimental outcomes such as skin alopecia and inflammation, pancreatic β-cell dysfunction, liver dysfunction, and increased atherosclerosis under certain conditions. SCD1 has also been implicated in the regulation of adipocyte inflammation, macrophage inflammation, and myocyte and endothelial cell function. In this review, we discuss recent findings of the function of SCD1 in the modulation of cellular inflammation and stress and its role in the related disorders.
Role for SCD1 in modulating cellular inflammation and stress
SCD1 is a key regulatory enzyme controlling the homeostasis of MUFA and SFA, 2 major types of fatty acids in mammalian cells. Modulation of cellular inflammation by fatty acids has long been recognized. Earlier studies demonstrated that dietary (n-6) PUFA such as arachidonic acid [20:4(n6)] promotes cellular inflammation after conversion to eicosanoids by cyclooxygenase enzymes (45). Whereas long-chain (n-3) PUFA such as EPA and DHA are able to incorporate into cell membrane phospholipids, they reduce the availability of arachidonic acid for the production of eicosanoids and thus suppress inflammatory responses in immune cells (46). More recent studies identified FFA, especially SFA, as potent proinflammatory factors in a variety of cell types such as macrophages, hepatocytes, and myocytes (47–49). SFA may directly promote inflammation by serving as ligands of immune receptors at the cell surface, such as members of the Toll-like receptor (TLR) family (47, 50). Subsequently, the downstream effectors, including mitogen-activated protein kinase 1 and NF-κB, initiate potent proinflammatory responses (43, 51). Additionally, fatty acids can be taken up by cells and metabolized to lipid intermediates, including diacylglycerols and ceramides, which are potent proinflammatory factors (44, 52, 53). Prolonged and unresolved inflammatory response due to abnormal levels of bioactive lipids leads to the onset of cellular stress response and dysfunction, eventually resulting in cell death and systemic degeneration in a process referred to as lipotoxicity (54). Lipotoxicity is regarded as one of the major causes for the pathology of obesity, insulin resistance, and cardiovascular disease (55, 56). The pathophysiological mechanism of metabolic diseases substantially overlaps with those pathways regulating inflammatory response (57, 58). Given its prominent regulation of fatty acid profile and strong association with metabolic diseases, SCD1 also exhibits potent regulatory roles in cellular inflammation and stress responses in a variety of cell types and disease conditions.
Liver dysfunction and stress
Due to the central role of liver as the hub of numerous metabolic pathways, studies targeting liver SCD1 have been conducted to elucidate the role of hepatic SCD1 in regulating metabolism. Although loss of SCD1 expression in liver provides beneficial metabolic effects such as reduced liver steatosis, several studies have found that SCD1 expression is strongly involved in the maintenance of liver function under a variety of stressful conditions. Hepatic SCD1 expression is substantially diminished in mice fed a methionine and choline deficient diet (MCD), a dietary model of steatohepatitis (59, 60). When SCD1 deficient mice were fed MCD, even though they had decreased liver steatosis, they exhibited increased hepatocellular stress response and liver injury compared with wild type mice through a mechanism of inefficient partitioning of SFA into MUFA for proper storage (60). Consistent with these reports, SCD1 deficient mice fed a high-sucrose very low-fat diet (HSVLF) also displayed severe liver stress response with liver injury despite reduced hepatic lipogenesis (61). This study reported that HSVLF stimulates an unfolded protein response with an acute induction of inflammation and macrophage recruitment in the liver of SCD1−/− mice. However, another study using a concanavalin A induced hepatitis mouse model demonstrated a favorable effect of SCD1 deficiency on steatohepatitis by reducing leptin production (62). Unlike MCD or HSVLF dietary models in which hepatocytes might be the primary cell type affected, treatment with concanavalin A, a T-cell mitogen, acts mainly at NK T cells and induces a set of proinflammatory cytokines leading to liver injury (63, 64). Thus, in addition to other variances, the different approaches inducing hepatic inflammation and injury in these studies may account for the different observations regarding the role of SCD1 in the regulation of hepatic function. These diverse inflammation regulatory effects of SCD1 deficiency warrant further studies to determine the role of SCD1 in liver function.
β-Cell dysfunction
Due to the proinflammatory activity of SFA, a potential pathological outcome in response to SCD1 deficiency is the induction of inflammatory and cellular stress responses. Indeed, using pancreatic β-cells as model systems, several studies have demonstrated that inhibition of SCD1 promotes cellular stress and β-cell dysfunction (65–67). β-Cells are highly sensitive to SFA induced lipotoxicity (67, 68). The ability to upregulate SCD1 expression in a subpopulation of β-cells rendered these cells resistant to SFA induced cell death, whereas inhibition of SCD1 activity increased their susceptibility to the detrimental effect of SFA (69). The conversion of lipotoxic SFA to MUFA by SCD1 is perhaps the mechanism responsible for maintenance of β-cell function (66, 70). Although inhibition of SCD1 was shown to be detrimental to β-cells in vitro, experiments using SCD1-deficient animal models have demonstrated metabolic beneficial effects from loss of SCD1, including improved insulin sensitivity (2, 9, 71). The observations from in vitro and in vivo studies regarding the function of SCD1 in diabetes appeared paradoxical. A more recent study using a ob/ob obesity mouse model uncovered an in vivo link between SCD1 and β-cell function. Even though protected from obesity, β-cells from ob/ob:SCD1−/− mice actually exhibited features of SFA induced lipotoxicity and substantial loss of insulin secretory function, thus resulting in severe diabetic condition (30, 72). Comparison of phenotypes of SCD1 deficiency in diet induced obesity and Agouti induced obesity with those observed in the ob/ob model further revealed that leptin gene expression was required for the improved insulin sensitivity by loss of SCD1 (30). The mechanism responsible for the leptin dependence of SCD1 deficiency in the regulation of insulin sensitivity remains to be determined.
Adipocytes and adipose tissue inflammation
Although SCD1 deficiency appears to be disadvantageous for β-cell function, this stress response is not universal for all cell types. Our recent study demonstrated that SCD1 deficiency protected mice from white adipose inflammation in both high-fat diet induced and Agouti induced obesity (73). Using isolated primary adipocytes, this study reported that SCD1 deficient adipocytes exhibited a reduced inflammatory response to treatment with LPS. They also elicited less paracrine stimulation of inflammation in macrophages and endothelial cells (73). Interestingly, the attenuated paracrine effects on inflammation due to SCD1 deficiency were attributed to the reduced levels of oleate, a major MUFA produced by SCD1. In fact, levels of SFA released by SCD1 deficient adipocytes were comparable to those released by wild type adipocytes. Consistently, in a separate study, oleate was also reported to promote inflammation when added to macrophage cells (50). These observations highlight an underappreciated proinflammatory activity of MUFA in specific contexts. Additionally, in a recent study using tissue inhibitor of metalloproteinase 3 knockout models, the authors reported that elevated SCD1 expression was one of the markers associated with the increased white adipose tissue and liver inflammation (74). The differential cellular inflammatory response in adipocytes and β-cells with loss of SCD1 might be associated with their different abilities to metabolize SFA, for which adipocytes have greater capacity than β-cells.
Macrophage inflammation and atherosclerosis
Macrophages are another model system that has received substantial attention due to their inflammatory response with respect to SCD1 deficiency. In an earlier study of the effect of β-amyloid peptide (Aβ) on macrophage inflammation, an oligonucleotide microarray screening identified that SCD1 was specifically and significantly upregulated by Aβ in addition to a set of proinflammatory genes (75). Although SCD1 expression was implicated in Aβ induced macrophage inflammation, no mechanism was proposed for its action. We recently demonstrated that the inflammatory response of peritoneal macrophages isolated from SCD1 deficient mice and treated with LPS did not differ from those isolated from wild type mice (73). This observation is in agreement with another recent study using isolated peritoneal macrophages from Asebia mice (76). In contrast to these findings, the reduction of SCD1 in liver, adipose tissue, and macrophages by ASO elevated the LPS induced inflammatory response in peritoneal macrophages through increased activation of TLR4 (77). Additionally, knockdown of SCD1 with specific small interfering RNA in macrophages isolated from adipocyte/macrophage fatty acid binding protein aP2 knockout mice also abolished the protective effects of aP2 deficiency on macrophage inflammation and endoplasmic reticulum stress (78). A possible explanation for the discrepancies might be that SCD2, another SCD isoform, is compensating for the loss of SCD1 in immune cells. In certain immune cell types, such as lymphocytes, SCD2 is constitutively expressed, whereas SCD1 expression is not detected (32, 79). Macrophages and lymphocytes derive from common bone marrow precursor cells. We have observed higher SCD2 expression in macrophages than in SCD1 (X. Liu, unpublished data). This differential expression pattern of SCD isoforms in immune cells might explain the comparable inflammation response in macrophages from SCD1 deficient mice to that from wild type mice.
Macrophage inflammation is a key cellular event in the pathology of atherosclerosis, a life-threatening cardiovascular disease. The complexity of SCD1 in regulating inflammation of macrophages extends to its regulation of atherosclerosis in vivo. Studies of an elderly Swedish population identified that SCD activity, as estimated by the ratio of product to substrate (16:1 to 16:0), was positively correlated with the serum C-reactive protein level, an acute-phase protein that is associated with inflammation and cardiovascular disease (80). High SCD activity appeared to be a significant predictor of elevated C-reactive protein levels in a large cohort study of middle-aged Swedish men followed for 20 y (81). In contrast to what would be expected for cardiovascular disease based on the large population based human studies, SCD1 deficiency in certain rodent models of atherosclerosis was reported to have a detrimental effect. In the LDL receptor knockout (LDLR−/−) mouse model of atherosclerosis, Asebia mice exhibited increased atherosclerosis in aorta despite the improved metabolic parameters, such as decreased adiposity, reduced liver steatosis, and increased insulin sensitivity (76, 82). Macrophages in these SCD1 deficient mice were not causatively involved in the increased atherosclerosis, as shown by a comparable inflammatory response to LPS as well as similar atherosclerotic plaque formation in aorta after transplantation of bone marrow from SCD1 deficient mice to wild type mice. The elevated systemic inflammation due to skin defects in SCD1 deficient mice was further proposed as the reason for increased atherosclerosis (76). Studies in LDLR−/− mice overexpressing human ApoB100 also identified significantly more severe atherosclerosis in aorta after SCD1 was knocked down by ASO against SCD1 in multiple tissues such as liver, adipose, and macrophages (77). Whereas skin in these mice exhibited no detectable difference from mice that received control ASO, the macrophages with SCD1 knockdown displayed hypersensitivity to LPS through activation of the TLR4 pathway (77). Although the 2 studies reported similar outcomes of atherosclerosis due to inhibition of SCD1 expression, there was a discrepancy in macrophage inflammation, which will require more studies to elucidate. In addition to genetic models of atherosclerosis, obstructive sleep apnea was found to be a novel model of atherosclerosis, which is featured by induction of chronic intermittent hypoxia and dyslipidemia (83–85). Interestingly, in contrast to the exacerbated atherosclerosis in the above 2 LDLR−/− models, knockdown of SCD1 expression by ASO against SCD1 in the obstructive sleep apnea model identified a preventative effect against atherosclerosis (86). The opposite outcomes of atherosclerosis in response to suppression of SCD1 expression in different models may depend on the tipping of a balance between the beneficial metabolic effects and detrimental inflammatory action due to SCD1 deficiency under different contexts. In the 2 formerly mentioned LDLR−/− models, plasma total cholesterol levels were consistently very high, ranging from 1000 to 2000 mg/dL (77, 82). Deficiency of SCD1 in these models had only modest effects of lowering plasma total cholesterol levels (77, 82). Thus, the beneficial metabolic effects of SCD1 deficiency such as hypermetabolic rate and reduced lipogenesis did not counteract its proinflammatory action in the context of high cholesterol levels, leading to the balance tipping to increase atherosclerosis. However, in the obstructive sleep apnea model, the plasma total cholesterol levels ranged from 200 to 300 mg/dL, substantially lower than that in LDLR−/− models, allowing the beneficial effects of SCD1 deficiency to overcome the detrimental actions and therefore resulting in improved atherosclerosis. Nonetheless, given the complex regulation of SCD1 in metabolism and inflammation, other explanations for its role in atherosclerosis may also exist.
Endothelial cell function
SCD1 has been implicated in regulating inflammation in endothelial cells, an important cell type in the integral network of pathological responses in metabolic morbidities (87, 88). Endothelial cells comprise the inner lining of blood supplying vessels, including arteries in the heart. Disruption of endothelial cell homeostasis due to lipotoxicity under metabolic disease conditions is one of the major causative factors for the induction of systemic inflammation and downstream detrimental effects associated with metabolic disorders such as cardiovascular disease (89–91). As a key enzyme in balancing fatty acid desaturation profile, SCD has begun to receive more attention for its role in endothelial functions. In a recent report (92), expression of hSCD1 was detected in human arterial endothelial cells (HAEC). Unlike other cell types, HAEC did not exhibit enhanced hSCD1 expression when exposed to SFA, thus rendering these cells more susceptible to lipotoxicity (92). In this study, when the liver X receptor signaling pathway was activated by TO-901317 to induce more hSCD1 expression, HAEC became resistant to palmitate-induced lipotoxicity through increased desaturation of SFA followed by efficient esterification and storage of these toxic lipids. Interestingly, in addition to SFA induced lipotoxicity, SCD1 expression in endothelial cells has also been found to be elevated in response to mechanical shear stress applied onto human umbilical vein endothelial cells through a PPARγ dependent manner (93). This observation implied that the expression of SCD1 in human umbilical vein endothelial cells may contribute to the protective effects against shear stress induced from abnormal blood flow in vivo. Thus, SCD1 in endothelial cells might be required for a general maintenance of their function not only under lipotoxic conditions but also in other types of stressful situations such as shear stress.
Myocyte function
Modulation of myocyte function by changes in SCD1 expression has been demonstrated in many studies. Earlier studies on the role of SCD1 in muscle insulin signaling and lipid metabolism implied metabolic beneficial effects of SCD1 deficiency on myocytes, such as downregulation of protein tyrosine phosphatase 1B, decreasing ceramide production through reduced expression serine palmitatoyltransferase, and partitioning more fatty acids into β oxidation (28, 94, 95). However, more recent studies have identified that SCD1 might have important roles in maintaining muscle function. In humans, SCD1 expression was found to be elevated in skeletal muscle after endurance exercise, which prevented fatty acid-induced insulin resistance (96). This effect was attributed to the enhanced lipogenic capacity that partitions FFA and their bioactive metabolites into neutralized lipids such as TG, thus reducing the proinflammatory potential in muscle. Additionally, overexpression of SCD1 in cultured rat L6 myocytes has been reported to enhance esterification of fatty acids through TG synthesis and attenuate ceramide and diacylglycerol accumulation, thus protecting myocytes from fatty acid-induced cellular toxicity (97). In a more recent study using primary human myotubes, the inducibility of SCD1 expression was negatively correlated with inflammation and endoplasmic reticulum stress and positively associated with insulin sensitivity (98). Future studies on the effects of SCD1 in regulating metabolic homeostasis in muscle using tissue specific SCD1 knockout or overexpression models will gain further insights into the physiological role of this enzyme in muscle biology.
Sebaceous gland hypoplasia and skin inflammation
SCD1 is ubiquitously expressed in various tissues, including the sebaceous gland in the skin (99). The sebaceous gland produces sebum that is enriched with lipids, such as cholesterol, CE, TG, and WE. These lipids are crucial for normal skin function, including skin lubrication and prevention of moisture loss (21). The importance of SCD1 expression and endogenous MUFA for normal skin development and maintenance has been demonstrated in both global SCD1 deficient and skin specific SCD1 knockout (SCD1 SKO) mice, which exhibit a severe skin phenotype, including alopecia, hypoplasia of sebaceous glands, dermatitis, and increased permeability of the skin barrier (21, 23, 24). The skin defects due to loss of SCD1 expression were associated with a dramatically altered skin lipid profile characterized by deficiency of WE, CE, and TG but substantially increased free cholesterol levels (21).
Although SCD1 deficient mice are hypermetabolic and completely protected from diet induced obesity, the skin abnormalities caused by mutation of SCD1 gene were found to compromise the immune function of skin and reduced the ability of the mice to fight against bacteria infection (100). In this study, there was a reduced production of sebum in SCD1 mutant mice and they were unable to synthesize adequate levels of palmitoleaate [16:1(n7)] and oleate [18:1(n9)], both of which possess bactericidal activity against gram-positive organisms. Gram-positive bacteria elicit innate immune response through TLR2 (100). Activation of TLR2 signaling induces SCD1 expression in sebocytes through the numerous NF-κB binding elements in the SCD1 gene promoter (100). It thus indicates that the ability to efficiently induce SCD1 expression in sebocytes might be required for the effective immune response to certain bacteria infection. Moreover, the abnormal skin function due to loss of SCD1 expression also leads to skin inflammation and dermatitis. In Asebia mice lacking LDLR, there was an ulcerative dermatitis with increased expression of a proinflammatory factor, intercellular adhesion molecule-1 in the skin, as well as elevated levels of plasma intercellular adhesion molecule-1 and IL-6 (76). Interestingly, dietary supplementation of oleate [18:1(n9)] or intradermal administration of MUFA was unable to completely rescue the skin defects (21, 100). Thus, it is suggested that the de novo synthesis of MUFA through endogenous SCD1 activity is indispensible for maintaining normal function of the sebaceous gland and skin. However, the mechanism behind the skin defects and inflammation due to loss of SCD1 is currently undetermined.
Intestinal colitis
In addition to aortic atherosclerosis and liver stress, SCD1 has also been reported in another type of disorder associated with inflammation, namely intestinal colitis. An LC-MS based metabolic profiling study identified that hepatic SCD1 expression was inhibited in dextran sulfate sodium (DSS) induced acute colitis mouse model (101). This study further showed that DSS induced acute colitis was exacerbated in SCD1 null mice, whereas a diet supplemented with oleate or restoration of in vivo SCD1 expression by adenoviral strategy alleviated the phenotype. The observations of the role of SCD1 expression in regulating DSS induced intestinal colitis extend our understanding of this enzyme in the scope of metabolic disease to inflammatory disorders that are associated with cellular detoxification process. However, the higher amount of water consumed by SCD1 deficient mice in this study complicated the conclusion, because DSS was provided via the water supply. After adjusting DSS intake based on their water consumption in a more recent study, SCD1 deficient mice exhibited comparable intestinal colitis to wild type mice (102). Thus, the role of SCD1 in acute colitis currently remains to be determined through further investigation.
Conclusion
Intensive studies have demonstrated the important role of SCD1 expression in lipid metabolism; this enzyme is not only involved in the pathology of obesity but also emerges as a key regulator for other types of serious diseases associated with inflammation and stress. The expression of SCD1 needs to be tightly controlled within a normal range to sustain cellular function. A dramatic increase in SCD1 expression favors fat accumulation, which causes obesity and insulin resistance, whereas substantial inhibition of SCD1 expression may promote fat catabolism but at the expense of increasing cellular inflammation and stress. Thus, the disruption of the control of SCD1 expression has been implicated in many types of serious diseases, such as obesity, insulin resistance, cancer, and the inflammatory diseases. However, the physiological roles of SCD1 in these disorders are complex (summarized in Fig. 1), which necessitates additional investigations to determine the mechanism.
Although SCD1 deficiency provides beneficial metabolic effects, it actually leads to pancreatic β-cell dysfunction in vitro and in vivo. Loss of SCD1 expression is also detrimental for the sebaceous gland in the skin and causes skin alopecia. However, in adipocytes, loss of SCD1 appears to reduce their proinflammatory potential and prevent the obesity associated inflammation in white adipose tissue. Studies on other cell types demonstrated that the presence of SCD1 is required for normal function of endothelial cells and myocytes. Macrophage inflammation in response to SCD1 deficiency varies depending on different approaches to manipulate SCD1 expression. The observations of the impact of SCD1 deficiency on liver stress and inflammatory diseases, including atherosclerosis and intestinal colitis, also diverge in different studies, probably due to variation in the disease models used as well as the methodologies for the suppression of SCD1 expression. Thus far, the mechanism of SCD1 in the regulation of different inflammatory diseases remains to be determined. Future studies on the role of SCD1 in metabolic homeostasis, inflammation, and stress using tissue specific SCD1 deletion or overexpression will enhance our understanding of the function of this enzyme.
Acknowledgments
We thank Mary Cantu for artwork. X. Liu and M. S. Strable wrote the paper. J. M. Ntambi had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grant RO1-DK62388 to James Ntambi.
Author disclosures: X. Liu, M. S. Strable, and J. M. Ntambi, no conflicts of interest.
Abbreviations used: Aβ, β-amyloid peptide; ASO, antisense oligonucleotide; CE, cholesterol ester; DSS, dextran sulfate sodium; HAEC, human arterial endothelial cell; HSVLF, high-sucrose very low-fat diet; MCD, methionine choline deficient diet; LDLR−/−, LDL receptor knockout; SCD, stearoyl CoA desaturase; SKO, skin specific knockout; TLR, Toll-like receptor; WE, wax ester.
Literature Cited
- 1.Ntambi JM, Miyazaki M. Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol. 2003;14:255–61 [DOI] [PubMed] [Google Scholar]
- 2.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104 [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S. Scd3–a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics. 2001;71:182–91 [DOI] [PubMed] [Google Scholar]
- 4.Tabor DE, Xia YR, Mehrabian M, Edwards PA, Lusis AJ. A cluster of stearoyl CoA desaturase genes, Scd1 and Scd2, on mouse chromosome 19. Mamm Genome. 1998;9:341–2 [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J Biol Chem. 2003;278:33904–11 [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, Cao G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun. 2005;332:735–42 [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM. Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem J. 1999;340:255–64 [PMC free article] [PubMed] [Google Scholar]
- 8.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki M, Kim HJ, Man WC, Ntambi JM. Oleoyl-CoA is the major de novo product of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. J Biol Chem. 2001;276:39455–61 [DOI] [PubMed] [Google Scholar]
- 11.Mziaut H, Korza G, Ozols J. The N terminus of microsomal delta 9 stearoyl-CoA desaturase contains the sequence determinant for its rapid degradation. Proc Natl Acad Sci USA. 2000;97:8883–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato H, Sakaki K, Mihara K. Ubiquitin-proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J Cell Sci. 2006;119:2342–53 [DOI] [PubMed] [Google Scholar]
- 13.Ntambi JM. Dietary regulation of stearoyl-CoA desaturase 1 gene expression in mouse liver. J Biol Chem. 1992;267:10925–30 [PubMed] [Google Scholar]
- 14.Waters KM, Ntambi JM. Insulin and dietary fructose induce stearoyl-CoA desaturase 1 gene expression of diabetic mice. J Biol Chem. 1994;269:27773–7 [PubMed] [Google Scholar]
- 15.Jones BH, Standridge MK, Claycombe KJ, Smith PJ, Moustaid-Moussa N. Glucose induces expression of stearoyl-CoA desaturase in 3T3–L1 adipocytes. Biochem J. 1998;335:405–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Miyazaki M, Ntambi JM. Dietary cholesterol opposes PUFA-mediated repression of the stearoyl-CoA desaturase-1 gene by SREBP-1 independent mechanism. J Lipid Res. 2002;43:1750–7 [DOI] [PubMed] [Google Scholar]
- 17.Miller CW, Waters KM, Ntambi JM. Regulation of hepatic stearoyl-CoA desaturase gene 1 by vitamin A. Biochem Biophys Res Commun. 1997;231:206–10 [DOI] [PubMed] [Google Scholar]
- 18.Katayama ML, Pasini FS, Folgueira MA, Snitcovsky IM, Brentani MM. Molecular targets of 1,25(OH)2D3 in HC11 normal mouse mammary cell line. J Steroid Biochem Mol Biol. 2003;84:57–69 [DOI] [PubMed] [Google Scholar]
- 19.Waters KM, Ntambi JM. Polyunsaturated fatty acids inhibit hepatic stearoyl-CoA desaturase-1 gene in diabetic mice. Lipids. 1996;31 Suppl:S33–6 [DOI] [PubMed] [Google Scholar]
- 20.Choi Y, Kim YC, Han YB, Park Y, Pariza MW, Ntambi JM. The trans-10,cis-12 isomer of conjugated linoleic acid downregulates stearoyl-CoA desaturase 1 gene expression in 3T3–L1 adipocytes. J Nutr. 2000;130:1920–4 [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–8 [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–96 [DOI] [PubMed] [Google Scholar]
- 23.Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Zhao M, Ntambi JM. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem. 2009;284:19961–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, Parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–70 [DOI] [PubMed] [Google Scholar]
- 25.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman SM, Dobrzyn A, Lee SH, Dobrzyn P, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases insulin signaling and glycogen accumulation in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E381–7 [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–71 [DOI] [PubMed] [Google Scholar]
- 28.Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci USA. 2003;100:11110–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99:11482–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki M, Sampath H, Liu X, Flowers MT, Chu K, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem Biophys Res Commun. 2009;380:818–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ntambi JM. The regulation of stearoyl-CoA desaturase (SCD). Prog Lipid Res. 1995;34:139–50 [DOI] [PubMed] [Google Scholar]
- 32.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–58 [PubMed] [Google Scholar]
- 33.Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31:1509–15 [DOI] [PubMed] [Google Scholar]
- 34.Li J, Ding SF, Habib NA, Fermor BF, Wood CB, Gilmour RS. Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. Int J Cancer. 1994;57:348–52 [DOI] [PubMed] [Google Scholar]
- 35.Habib NA, Wood CB, Apostolov K, Barker W, Hershman MJ, Aslam M, Heinemann D, Fermor B, et al. Stearic acid and carcinogenesis. Br J Cancer. 1987;56:455–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scaglia N, Caviglia JM, Igal RA. High stearoyl-CoA desaturase protein and activity levels in simian virus 40 transformed-human lung fibroblasts. Biochim Biophys Acta. 2005;1687:141–51 [DOI] [PubMed] [Google Scholar]
- 37.Scaglia N, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA carboxylase and impairs proliferation in cancer cells: role of AMPK. PLoS ONE. 2009;4:e6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess D, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS ONE. 2010;5:e11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avances C, Allory Y, de la Taille A, Culine S, et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther. 2010;9:1740–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan-Lappe SE, Tucker LA, Huang X, Zhang Q, Sarthy AV, Zakula D, Vernetti L, Schurdak M, Wang J, et al. Identification of Ras-related nuclear protein, targeting protein for xenopus kinesin-like protein 2, and stearoyl-CoA desaturase 1 as promising cancer targets from an RNAi-based screen. Cancer Res. 2007;67:4390–8 [DOI] [PubMed] [Google Scholar]
- 41.Moore S, Knudsen B, True LD, Hawley S, Etzioni R, Wade C, Gifford D, Coleman I, Nelson PS. Loss of stearoyl-CoA desaturase expression is a frequent event in prostate carcinoma. Int J Cancer. 2005;114:563–71 [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–8 [DOI] [PubMed] [Google Scholar]
- 43.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409 [DOI] [PubMed] [Google Scholar]
- 44.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:S343–8 [DOI] [PubMed] [Google Scholar]
- 46.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75:197–202 [DOI] [PubMed] [Google Scholar]
- 47.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94 [DOI] [PubMed] [Google Scholar]
- 49.Kadotani A, Tsuchiya Y, Hatakeyama H, Katagiri H, Kanzaki M. Different impacts of saturated and unsaturated free fatty acids on COX-2 expression in C(2)C(12) myotubes. Am J Physiol Endocrinol Metab. 2009;297:E1291–303 [DOI] [PubMed] [Google Scholar]
- 50.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92 [DOI] [PubMed] [Google Scholar]
- 51.Janeway CA., Jr A trip through my life with an immunological theme. Annu Rev Immunol. 2002;20:1–28 [DOI] [PubMed] [Google Scholar]
- 52.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–76 [DOI] [PubMed] [Google Scholar]
- 53.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture. Diabetologia. 2010;53:1270–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prieur X, Roszer T, Ricote M. Lipotoxicity in macrophages: evidence from diseases associated with the metabolic syndrome. Biochim Biophys Acta. 2010;1801:327–37 [DOI] [PubMed] [Google Scholar]
- 56.Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20:379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87:407–16 [DOI] [PubMed] [Google Scholar]
- 58.Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res. 2010;107:579–91 [DOI] [PubMed] [Google Scholar]
- 59.Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47:2280–90 [DOI] [PubMed] [Google Scholar]
- 60.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flowers MT, Keller MP, Choi Y, Lan H, Kendziorski C, Ntambi JM, Attie AD. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol Genomics. 2008;33:361–72 [DOI] [PubMed] [Google Scholar]
- 62.Feng D, Wang Y, Mei Y, Xu Y, Xu H, Lu Y, Luo Q, Zhou S, Kong X, et al. Stearoyl-CoA desaturase 1 deficiency protects mice from immune-mediated liver injury. Lab Invest. 2009;89:222–30 [DOI] [PubMed] [Google Scholar]
- 63.Tiegs G. Experimental hepatitis and role of cytokines. Acta Gastroenterol Belg. 1997;60:176–9 [PubMed] [Google Scholar]
- 64.Tsutsui H, Adachi K, Seki E, Nakanishi K. Cytokine-induced inflammatory liver injuries. Curr Mol Med. 2003;3:545–59 [DOI] [PubMed] [Google Scholar]
- 65.Thorn K, Hovsepyan M, Bergsten P. Reduced levels of SCD1 accentuate palmitate-induced stress in insulin-producing beta-cells. Lipids Health Dis. 2010;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellemans KH, Hannaert JC, Denys B, Steffensen KR, Raemdonck C, Martens GA, Van Veldhoven PP, Gustafsson JA, Pipeleers D. Susceptibility of pancreatic beta cells to fatty acids is regulated by LXR/PPARalpha-dependent stearoyl-coenzyme A desaturase. PLoS ONE. 2009;4:e7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–42 [DOI] [PubMed] [Google Scholar]
- 68.Brown JM, Rudel LL. Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: considerations for future drug discovery. Curr Opin Lipidol. 2010;21:192–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, Hughes WE, Biden TJ. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes. 2005;54:2917–24 [DOI] [PubMed] [Google Scholar]
- 70.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–33 [DOI] [PubMed] [Google Scholar]
- 71.Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–39 [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Miyazaki M, Flowers MT, Sampath H, Zhao M, Chu K, Paton CM, Joo DS, Ntambi JM. Loss of stearoyl-CoA desaturase-1 attenuates adipocyte inflammation: effects of adipocyte-derived oleate. Arterioscler Thromb Vasc Biol. 2010;30:31–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menghini R, Menini S, Amoruso R, Fiorentino L, Casagrande V, Marzano V, Tornei F, Bertucci P, Iacobini C, et al. Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology. 2009;136:663–72 e4. [DOI] [PubMed] [Google Scholar]
- 75.Uryu S, Tokuhiro S, Oda T. beta-Amyloid-specific upregulation of stearoyl coenzyme A desaturase-1 in macrophages. Biochem Biophys Res Commun. 2003;303:302–5 [DOI] [PubMed] [Google Scholar]
- 76.MacDonald ML, van Eck M, Hildebrand RB, Wong BW, Bissada N, Ruddle P, Kontush A, Hussein H, Pouladi MA, et al. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:341–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, Zhu X, Duong MN, Wibley AL, et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tebbey PW, Buttke TM. Stearoyl-CoA desaturase gene expression in lymphocytes. Biochem Biophys Res Commun. 1992;186:531–6 [DOI] [PubMed] [Google Scholar]
- 80.Petersson H, Lind L, Hulthe J, Elmgren A, Cederholm T, Riserus U. Relationships between serum fatty acid composition and multiple markers of inflammation and endothelial function in an elderly population. Atherosclerosis. 2009;203:298–303 [DOI] [PubMed] [Google Scholar]
- 81.Petersson H, Basu S, Cederholm T, Riserus U. Serum fatty acid composition and indices of stearoyl-CoA desaturase activity are associated with systemic inflammation: longitudinal analyses in middle-aged men. Br J Nutr. 2008;99:1186–9 [DOI] [PubMed] [Google Scholar]
- 82.MacDonald ML, Singaraja RR, Bissada N, Ruddle P, Watts R, Karasinska JM, Gibson WT, Fievet C, Vance JE, et al. Absence of stearoyl-CoA desaturase-1 ameliorates features of the metabolic syndrome in LDLR-deficient mice. J Lipid Res. 2008;49:217–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2010;17:161–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706 [DOI] [PubMed] [Google Scholar]
- 86.Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103:1173–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol. 2008;93:158–63 [DOI] [PubMed] [Google Scholar]
- 89.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423–36 [DOI] [PubMed] [Google Scholar]
- 90.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335:165–89 [DOI] [PubMed] [Google Scholar]
- 91.Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat VA, Wheatcroft SB, Shah AM, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peter A, Weigert C, Staiger H, Rittig K, Cegan A, Lutz P, Machicao F, Haring HU, Schleicher E. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am J Physiol Endocrinol Metab. 2008;295:E339–49 [DOI] [PubMed] [Google Scholar]
- 93.Qin X, Tian J, Zhang P, Fan Y, Chen L, Guan Y, Fu Y, Zhu Y, Chien S, et al. Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells. Cardiovasc Res. 2007;74:506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E599–607 [DOI] [PubMed] [Google Scholar]
- 95.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinnamaneni SK, Southgate RJ, Febbraio MA, Watt MJ. Stearoyl CoA desaturase 1 is elevated in obesity but protects against fatty acid-induced skeletal muscle insulin resistance in vitro. Diabetologia. 2006;49:3027–37 [DOI] [PubMed] [Google Scholar]
- 98.Peter A, Weigert C, Staiger H, Machicao F, Schick F, Machann J, Stefan N, Thamer C, Haring HU, et al. Individual stearoyl-coa desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes. 2009;58:1757–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68:113–21 [DOI] [PubMed] [Google Scholar]
- 100.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, Hoebe K, Du X, Rutschmann S, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun. 2005;73:4512–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen C, Shah YM, Morimura K, Krausz KW, Miyazaki M, Richardson TA, Morgan ET, Ntambi JM, Idle JR, et al. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008;7:135–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Macdonald ML, Bissada N, Vallance BA, Hayden MR. Absence of stearoyl-CoA desaturase-1 does not promote DSS-induced acute colitis. Biochim Biophys Acta. 2009;1791:1166–72 [DOI] [PMC free article] [PubMed] [Google Scholar]