Abstract
There is increasing interest in the potential health benefits of dietary flavonoids. Fruits and vegetables, tea, and cocoa are rich natural sources of flavonoids. Epidemiological studies have indicated that consumption of these foods is likely to be associated with a reduced risk of cardiovascular disease, but the etiology of this benefit is not yet clearly defined. Furthermore, in some acute interventions, a positive effect of tea and cocoa on vascular function has been reported. An alternative source of flavonoids is dietary supplements, which have become increasingly popular in the recent past. In this context, it needs to be critically evaluated whether vascular health-promoting and other positive properties of flavonoid-rich diets can be replaced by purified flavonoids as dietary supplements. Plant sources of flavonoids contain a complex mixture of secondary plant metabolites and not only flavonoids per se. This complex mixture of secondary plant metabolites cannot be simply exchanged by single purified compounds as dietary supplements. If flavonoids are given as dietary supplements, toxicity issues as well as nutrient drug interactions need to be taken into account. Purified flavonoids given in high doses as dietary supplements may affect trace element, folate, and vitamin C status. Furthermore, they may exhibit antithyroid and goitrogenic activities. In this review article, the available literature on the safety issues surrounding high dose supplemental flavonoid consumption has been summarized.
Introduction
There is increasing evidence that a diet rich in fruit and vegetables may be associated with a reduced risk of cardiovascular diseases (CVD),4 with CVD representing the leading cause of death in Western societies (1). A meta-analysis of 9 cohort studies (comprising >220,000 men and women) showed that fruit and vegetable consumption was inversely associated with the risk of CVD. Importantly, the risk of CVD was dose dependent and decreased by 4% for each additional portion per day of fruit and vegetables and by 7% for fruit consumption (2). In the European Prospective Investigation into Cancer and Nutrition study (3), the intake of vegetables, legumes, and fruit was significantly associated with reduced risks of CVD mortality and mortality due to non-CVD/noncancer causes [RR = 0.88 (95% CI = 0.81–0.95) and 0.90 (0.82–0.99), respectively] in a diabetic population comprising >10,000 individuals. In a meta-analysis including >250,000 individuals, He et al. (4) quantitatively reported on the relation between fruit and vegetable intake and the incidence of stroke. Compared with individuals who consumed <3 servings/d of fruit and vegetables, the pooled RR of stroke was 0.89 (95% CI = 0.83–0.97) for those consuming 3–5 servings/d and 0.74 (0.69–0.79) for those consuming >5 servings/d. Overall, there seems to be strong epidemiological support for the recommendations to consume at least 5 servings/d of fruit and vegetables.
In addition to a number of potentially bioactive components such as fiber, folate, antioxidant vitamins, and potassium, fruits and vegetables are important sources of dietary flavonoids (5, 6). Furthermore, flavonoid-rich tea (Camellia sinensis) and cocoa represent relatively concentrated sources and their impact on vascular function is becoming apparent (7, 8). Due to their widespread occurrence, flavonoids are regularly ingested by humans. The individual intake of flavonoids, however, varies considerably depending on the type of diet consumed. A number of epidemiological studies have examined the impact of total or individual flavonoid intake. For example, Huxley and Neil (9) assessed the relation between dietary flavonol intake and coronary heart disease mortality in a meta-analysis comprising 7 prospective cohort studies. The authors concluded that high dietary intake of flavonols from fruits and vegetables as well as from tea and red wine may be associated with a decrease in CVD mortality in free-living populations (9). To the best of our knowledge, there is currently no meta-analysis reporting the effect of dietary flavonoid supplements on CVD risk. Nevertheless, the use of dietary flavonoid supplements is becoming increasingly popular (10).
Cell culture studies using endothelial cells, monocytes, smooth muscle cells, and platelets have helped us to identify the potential underlying cellular and molecular mechanisms by which dietary flavonoids may affect vascular health. These in vitro studies in cultured cells indicate that purified flavonoids may positively affect critical steps in atherogenesis, including LDL oxidation (11, 12), inflammation (13), chemotaxis (14), cell adhesion (15), foam cell formation (16), smooth muscle cell proliferation (17), and platelet aggregation (18). Furthermore, there is evidence that purified flavonoids activate endothelial NO synthetase (8, 19) and inhibit arginase (20), thereby increasing the concentration of vasodilatory NO.
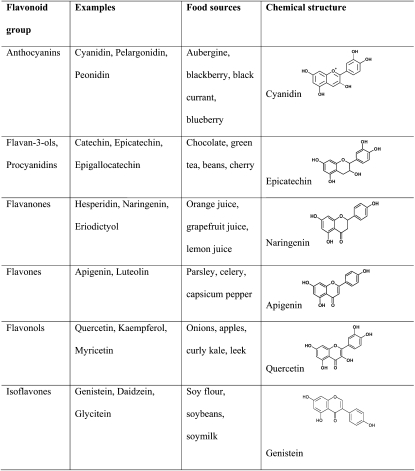
Based on cell culture studies, further in vivo studies in laboratory rodents, including mice (21) and rats (22), as well studies in (cholesterol-fed) rabbits (23) indicate potential positive effects of dietary flavonoids on vascular health. There are also several studies in humans that suggest a role of flavonoids as dietary supplements in CVD prevention (for review, see 24, 25). Overall, all subclasses of flavonoids (Fig. 1), including anthocyanidins (26), flavonols (27), flavones (28), flavanones (28), flavanols (8), and isoflavones (29), have been investigated in vitro and in vivo in terms of their potential to prevent CVD.
Figure 1.
Examples, chemical structure, and main dietary sources of major classes of flavonoids.
There is currently an extensive range of flavonoid supplements on the market (30). Suppliers of such supplements recommend daily flavonoid intakes in amounts that are many times higher than those doses which can normally be achieved from a flavonoid-rich diet. For instance, the flavonol quercetin has been marketed as a dietary supplement with recommended daily doses of up to 1 g and more (31), whereas the daily quercetin intake from foods has been estimated to be 10–100 mg (32, 33). Another example are isoflavone-based nutraceuticals (e.g. pills, tablets, extracts), which are one of the most widely tested and used flavonoid supplements so far (30). The declared content of isoflavones is variable (e.g. 50, 135, and 500 mg) and different daily doses are recommended. However, at present, no specific dosage of isoflavones has been established to exert a beneficial effect (30). Isoflavones are scarce in Western diets compared with Asian diets, because the main isoflavone sources are soy-derived products.
The question arises whether supplements containing such supra-physiological flavonoid levels may exhibit adverse effects (Table 1). In addition, it is likely that a large proportion of individuals taking dietary flavonoid supplements are also taking conventional drugs (34). The concomitant intake of “supra-nutritional” flavonoid doses together with conventional drugs may lead to flavonoid-drug interactions (34). This additionally raises concerns about the safe use of dietary flavonoids.
Table 1.
Potential beneficial and adverse effect of flavonoid supplements1
| Beneficial effects | Adverse effects |
| Inhibition of LDL oxidation and induction of paraoxonase | Decrease in trace element bioavailability |
| Activation of endothelial NO synthetase and inhibition of arginase | Antithyroid and goitrogenic activity |
| Inhibition of chronic inflammatory processes | Impairment of folate uptake |
| Inhibition of chemotactic proteins (e.g. MCP1) and cell adhesion molecules (e.g. ICAM1, VCAM1, E-selectin) expression | Inhibition of vitamin C transport |
| Inhibition of smooth muscle cell proliferation | Nutrient–drug interactions |
| Inhibition of platelet aggregation | Estrogenicity |
MCP1, Monocyte chemotactic protein-1; E-selectin, endothelial-selectin; ICAM1, intracellular adhesion molecule-1; VCAM1, vascular cell adhesion molecule-1.
Current status of knowledge
Flavonoid-trace element interactions
Purified flavonoids (35) as well as flavonoid-rich extracts (36) have been reported to chelate iron in vitro. Ren et al. (37) studied the complexation mechanisms of several flavonoids (quercetin, luteolin, galangin, kaempferol, and chrysin) with iron. The most important chelation site was the 3-hydroxyl-4-carbonyl group followed by the 4-carbonyl-5-hydroxyl group. Quercetin and iron form orthogonal Fe-O bonds. Three quercetin molecules are required to saturate the bonds of a single Fe ion in vitro.
As far as green catechins are concerned, the galloyl group seems to be mainly responsible for iron binding, with the green tea catechin (GTC) epigallocatechin gallate (EGCG) containing 2 galloyl groups. In this context, in a randomized, double-blind, placebo-controlled trial, Ullmann et al. (38) studied nonheme iron absorption in response to pure cristalline EGCG. Nonheme iron absorption was dose-dependently decreased by 14 and 27% due to 150 and 300 mg/d EGCG relative to the placebo group. Furthermore, Sandstroem et al. (39) studied the effect of green tea or rosemary extract added to foods on nonheme iron absorption in humans. Iron absorption decreased from (mean ± SD) 12.1 ± 4.5% to 8.9 ± 5.2% (P < 0.01) in the presence of green tea extract (37 mg catechins) and from 7.5 ± 4.0% to 6.4 ± 4.7% (P < 0.05) in the presence of rosemary extract (32 mg of phenolic substances).
Ma et al. (40) have recently shown that polyphenols also inhibit heme iron absorption mainly by reducing basolateral iron exit rather than decreasing apical heme iron uptake in intestinal cells.
It should be considered that iron element bioavailability seems to be not only impaired by purified flavonoids or flavonoid extracts but also by flavonoid-rich food items such as green and black tea, coffee, cocoa, red wine, and legumes (41–44).
The flavonoid concentration of dietary supplements is likely to be many times higher than those consumed as part of a normal diet. Thus, for individuals at risk of iron deficiency, dietary flavonoid supplements may be problematic. In addition, interactions between flavonoids and copper (45) as well as manganese (46) have been reported.
Interactions between flavonoids and folate as well as vitamin C and vitamin E
GTC have been shown to inhibit the activities of enzymes involved in folate uptake. Using an in vitro enzyme activity assay, we observed a time- and dose-dependent inhibition of dihydrofolate reductase activity by EGCG and a green tea extract (47). A recent cell culture study showed that certain gallated derivatives of green tea polyphenols, namely EGCG, the predominant catechin in green tea, and epicatechin gallate, inhibited folic acid uptake in Caco-2 cells (48). In our studies in rats, green tea extract significantly decreased serum 5-methyl-tetrahydrofolate concentrations (47). In agreement with the cell culture studies, a study in humans suggests a potential risk of diminished folic acid bioavailability by both green and black tea extracts (49). However, in our human pilot study, no differences in plasma folate concentrations in response to dietary GTC supplementation were observed (47).
Song et al. (50) demonstrated that flavonoids, such as quercetin, inhibit sodium dependent vitamin C transporter 1 in cultured cells. Furthermore, quercetin significantly inhibited ascorbate absorption in rats (50). To the best of our knowledge, interactions between flavonoids and vitamin C in humans have not yet been reported.
We recently studied the effect of dietary quercetin, catechin, and genistein on tissue vitamin E levels in rats. The concentrations of α- and γ-tocopherol in the plasma, liver, lung, and cortex of flavonoid-fed rats were not significantly different from the concentrations measured in control rats receiving the flavonoid-free basal diet (51). Furthermore, dietary supplementation of growing pigs with green tea polyphenols did not affect tissue vitamin E levels and plasma antioxidant capacity in pigs (52). Similarly, in humans, urinary excretion of the α-/γ-tocopherol metabolites was not significantly affected by dietary intervention with an aqueous green tea supplement (53). Daily dietary quercetin supplementation did not affect concentrations of plasma α- and γ-tocopherols or plasma antioxidative capacity in humans (54). Based on our studies in mice, rats, and humans, it is therefore unlikely that the flavonoids investigated may impair plasma vitamin E status in vivo.
Antithyroid and goitrogenic activity of flavonoids
Flavonoids may exhibit antithyroid and goitrogenic activity. Ferreira et al. (55) studied the in vitro effects of various flavonoids on thyroid type 1 iodothyronine deiodinase activity in a murine thyroid microsome fraction and found type 1 iodothyronine deiodinase activity significantly inhibited by isoflavones, quercetin, and catechins.
In a rat study by Chandra and De (56), a decreased activity of thyroid peroxidase and 5′-deiodinase was reported in response to dietary green tea extracts. Serum thyroid hormone tri-iodothyronine and thyroxine levels were found to be significantly reduced and associated with a significant elevation of serum thyroid-stimulating hormone. The authors concluded that green tea extracts at high doses could adversely alter thyroid function (56).
Furthermore, isoflavones have been reported to inhibit thyroid hormone biosynthesis and may exert at high concentrations goitrogenic effects in humans. Milerova et al. (57) found a significant correlation between circulating isoflavone concentrations in blood and thyroid function. However, a recent long-term human study in almost 400 osteopenic, postmenopausal women suggested that genistein aglycone intake does not significantly increase the risk of clinical or subclinical hypothyroidism at the dose of 54 mg/d (58). There are also other safety issues associated with high-dose isoflavone intakes, mainly related to their estrogenicity (59). Due to the possible adverse effects of isoflavones and the lack of consensus regarding the health benefits derived from isoflavones consumption, the AHA does not recommend the use of isoflavone supplements (60).
Flavonoid-drug interactions
Flavonoids are subject to the same metabolic pathways as “classical” xenobiotics. Thus, flavonoids may interfere with the absorption, tissue distribution, metabolism, and excretion of drugs, altering their pharmacokinetics profiles (34). Indeed, many in vitro studies have shown effects of flavonoids on various cytochrome P450 monooxygenase (CYP) isoforms, Phase II conjugation enzymes, and drug transporters [reviewed in (34, 61, 62)]. In addition, flavonoids were able to alter the pharmacokinetics of concurrently administered drugs in some animal models (34).
Interactions of flavonoids with CYP.
A number of studies have shown inhibition of various CYP by flavonoids [reviewed in (34, 61)]. Among the various CYP isoforms (1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, and 3A5), isoforms belonging to the CYP3A subfamily like CYP3A4 and 3A5 play a prominent role. They are the major CYP isoforms in the liver and intestinal tract and are responsible for the metabolism of ∼50% of all prescribed drugs (63, 64). The flavonols quercetin and kaempferol were shown to inhibit the metabolism of the calcium channel blockers nifedipine and felodipine by CYP3A4 in human liver microsomes at concentrations > 10 μmol/L, with the flavanone naringenin being less potent (65). Similar results were obtained in other studies using 17 β-estradiol, felodipine, or nifedipine as substrates for CYP3A4 (65, 66). In addition to the 2 mentioned flavonols, the flavonol myricetin, the flavone apigenin, and the biflavone I3,II8-biapigenin were also reported to inhibit CYP3A4 activity at low micromolar concentrations in human liver microsomes (67).
Another important drug metabolized by CYP3A4 is the statin simvastatin (SV), a 3-hydroxy-3-methylglutaryl CoA reductase inhibitor primarily used for the treatment of hyperlipidemia. The lactone SV, which is an inactive prodrug like its analogue lovastatin, is transformed into the active form SV acid (SVA) in the plasma, liver, and probably intestinal mucosa by esterases with lactonase activity (68, 69). The major enzyme responsible for metabolism of the parent prodrug SV and its active form SVA is CYP3A4 (70). Oxidative products of CYP-dependent metabolism (e.g. 3′-hydroxy, 6′-hydroxy, 3′,5′-dihydrodiol metabolites) show no or only minimal inhibitory activity toward 3-hydroxy-3-methylglutaryl CoA reductase. CYP-dependent hydroxylation in turn increases the rate of subsequent conjugation and excretion; thus, potent CYP3A4 inhibitors are able to increase the plasma concentration and area under the plasma concentration-time curve of SV and SVA severalfold (71, 72). Because quercetin was shown to inhibit CYP3A4 activity in vitro (see above), it was speculated by Cermak et al. (73) that high doses of quercetin might be able to increase the bioavailability of SV and SVA. However, quercetin (10 mg/kg) coadministered with SV (0.25 mg/kg) in pigs decreased plasma concentrations of SV during the first 5 h after intake. Thus, the authors excluded an inhibitory effect of quercetin on CYP3A4-mediated metabolism of SV, although quercetin did inhibit CYP3A4 in human liver microsomes (see above). One explanation for the lack of CYP3A inhibition by the flavonol in vivo could be the fact that hepatic quercetin concentrations did not reach a level that was shown to be bioactive in in vitro experiments (73).
Flavonoids have also been shown to influence the activity and/or expression of other CYP isoforms. For example, some flavonoids are potent inhibitors of CYP1A2. This isoform is mainly expressed in the liver and metabolizes several important drugs such as caffeine, theophylline, warfarin (see below), or propranolol (64). The pentamethoxyflavone tangeretin, which occurs in the peel of oranges and tangerines, inhibited the activity of CYP1A2 in human liver microsomes in a competitive manner (74). The grapefruit flavanone naringenin inhibited CYP1A2-dependent 3-demethylation of caffeine in human liver microsomes (75). The activity of this CYP isoform was also inhibited at low micromolar concentrations by the flavonols galangin and quercetin as well as by the biflavone I3,II8-biapigenin (76–78).
Cranberry juice has been implicated in interacting with warfarin. Warfarin is used for anticoagulation to a target international normalized ratio of 2.0–3.0 for most indications or 2.5–3.5 for high-risk indications; however, many drugs and dietary supplements induce fluctuations in the international normalized ratio (79). Such fluctuations may lead to therapeutic failure or bleeding complications (79). Flavonoids in cranberry juice are suspected to inhibit CYP2C9, the enzyme responsible for metabolism of S-warfarin, which would increase warfarin levels (80, 81). S-warfarin is the more active enantiomer, whereas R-warfarin is mainly metabolized by CYP3A4 and CYP1A2. Alternatively, cranberry juice flavonoids might displace warfarin from albumin binding sites and transiently potentiate an anticoagulant response (82). Recent review and analysis of the literature revealed that ingestion of large volumes of cranberry juice (>600 mL/d) may destabilize warfarin therapy (79, 83). However, small to moderate amounts of juice are not expected to cause such an interaction (79, 83). It is not known how cranberry concentrates or supplements might interact with warfarin (83).
Interactions of flavonoids with phase II enzymes.
The conjugation of drugs to increase their hydrophilicity is a major pathway in drug metabolism. It is performed by phase II enzymes. UDP-glucuronosyltransferases (UGT) use UDP-glucuronic acid as a cosubstrate and transfer glucuronic acid to available substrates. Generally, glucuronidation is a detoxification process that converts xenobiotics into less or nontoxic and more water-soluble metabolites, thereby facilitating their excretion in the urine or bile (84). Another group of phase II enzymes are sulfotransferases (SULT) that catalyze the transfer of a sulfonate group to an appropriate substrate (85). Other phase II reactions are the conjugation of drugs with glutathione by glutathione-S-transferases (GST) or acetylation of xenobiotics by N-acetyltransferases (86). It has been shown that all these phase II enzymes are affected by flavonoids in cell and animal models. For example, the flavone chrysin (25 μmol/L) induced an increase in mRNA levels, protein expression, and enzyme activity of the UGT isoform 1A1 in human Hep G2 cells and human colon carcinoma cells (87, 88). At the same concentration, other flavones (e.g. apigenin, baicalein, and diosmetin) as well as the flavonols quercetin and kaempferol increased either the transcription or enzymatic activity of UGT1A1 in Hep G2 cells (89, 90). Feeding rats a diet containing 1% (wt:wt) quercetin for 2 wk increased UGT activity in the small intestine and liver (91). In the same study, flavone increased UGT activity in the large intestine and liver at a dietary concentration of 0.5% (wt:wt) (91). In vivo perfusion of human jejunum with an onion and broccoli extract containing 60 μmol/L quercetin significantly increased UGT1A1 mRNA in shed enterocytes. This was confirmed in Caco-2 cells with pure quercetin (92).
A number of studies have shown that flavonoids can be potent inhibitors of cytosolic SULT isoforms [reviewed in (34)]. In particular, quercetin exhibited a potent inhibitory impact on SULT activity in several in vitro investigations. Quercetin inhibited sulfation of 4-nitrophenol, a model substrate for SULT1A1, the major phenol SULT in human liver, and the sulfation of several drugs (e.g. salbutamol, minoxidil, paracetamol, and apomorphine) in human liver cytosol, with IC 50 concentrations in the nanomolar range (93–97).
We have previously shown that dietary quercetin significantly affected the activity of hepatic GST, whereas dietary catechin significantly changed NAD(P)H quinone oxidoreductase-1 activity (26%) in rats. Changes in GST and NAD(P)H quinone oxidoreductase-1 activity were partly reflected on mRNA levels. Our data indicate that dietary flavonoids modulate phase II enzymes in rat liver. This in turn may affect the ability of the organism to detoxify endogenous and exogenous xenobiotics (98).
Interactions of flavonoids with drug transporters.
A substantial number of studies demonstrate effects of flavonoids on transporters involved in drug metabolism (34, 61, 62). There are 2 main classes of transporters that are involved in the uptake and efflux of drugs and drug conjugates: 1) the ABC transporters, which belong to the ATP-binding cassette family; and 2) the transporters of the solute carrier family (34, 61). Among the ABC transporters, interactions have been shown with P-glycoprotein, multi-drug resistance associated proteins (MRP1 and MRP2), and with breast cancer resistance protein (BCRP) (99). Most of the studies have demonstrated inhibitory effects of flavonoids on the substrate efflux in cells that either endogenously expressed these transporters or that were transfected with them [reviewed in (34, 99)]. This flavonoid-ABC-transporter interaction could be beneficial for poorly absorbed drugs but could also result in severe drug intoxication, especially for drugs with a narrow therapeutic window. On the other hand, flavonoids are themselves substrates of ABC transporters. These proteins can affect the oral availability and tissue distribution of these compounds, modifying their beneficial effects (62, 100). In contrast to the well-described interactions of flavonoids with ABC transporters, evidence for potential flavonoid effects on transporters of the solute carrier family is less clear.
Concluding remarks
Flavonoids that derive from fruits and vegetables are consumed in relatively low quantities. Furthermore, fruits and vegetables contain a complex mixture of secondary plant metabolites and not only flavonoids per se. This complex mixture of secondary plant metabolites cannot be simulated by single purified compounds as dietary supplements. Fruits and vegetables may contain other micronutrients (e.g. vitamin C, potassium, and folate) as well as dietary fiber; the latter is known to positively affect satiety, lower the dietary energy density, and decrease circulating LDL-cholesterol.
In this context, it has been stated by Ross and Kasum (101) that “the sum of parts (total fruit and vegetable intake) is more important in providing health benefits than only 1 plant constituent.” There is no such thing as a “magic bullet” (102) and we have to be critical in supplying flavonoids as a supplement, which may exceed the nutritive flavonoid intake manifold compared with vegetarian diets without supplements. People may also take more than 1 dietary flavonoid supplement at the same time and almost nothing is known about flavonoid-flavonoid interactions. It has been criticized that consumers may have too little information about product safety, contraindications, interactions, or effectiveness of supplements (30, 103).
The consumer may have the misperception that dietary flavonoid supplements are devoid of toxicity and, therefore, they are safe to use because these compounds are “natural” (104). Also, when developing functional foods, the issue of the safety of flavonoids needs to be taken into account.
Acknowledgments
S. E. and G. R. conducted the literature research and wrote the manuscript. Both authors read and approved the final manuscript.
Footnotes
Author disclosures: S. Egert and G. Rimbach, no conflicts of interest.
Abbreviations used: ABC-transporter, ATP-binding cassette transporter; CVD, cardiovascular disease; CYP, cytochrome P450 monooxygenase; EGCG, epigallocatechin gallate; GTC, green tea catechin; GST, glutathione-S-transferase; SULT, sulfotransferase; SV, simvastatin; SVA, simvastatin acid; UGT, UDP-glucuronosyltransferase.
Literature Cited
- 1.WHO The atlas of heart disease and stroke. Mackay J, Mensah GA, Geneva: WHO; 2004 [Google Scholar]
- 2.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136:2588–93 [DOI] [PubMed] [Google Scholar]
- 3.Nöthlings U, Schulze MB, Weikert C, Boeing H, van der Schouw YT, Bamia C, Benetou V, Lagiou P, Krogh V, et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr. 2008;138:775–81 [DOI] [PubMed] [Google Scholar]
- 4.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367:320–6 [DOI] [PubMed] [Google Scholar]
- 5.Pascual-Teresa S, Moreno DA, Garcia-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J Agric Food Chem. 2000;48:5331–7 [DOI] [PubMed] [Google Scholar]
- 7.Hodgson JM. Effects of tea and tea flavonoids on endothelial function and blood pressure: a brief review. Clin Exp Pharmacol Physiol. 2006;33:838–41 [DOI] [PubMed] [Google Scholar]
- 8.Schewe T, Steffen Y, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys. 2008;476:102–6 [DOI] [PubMed] [Google Scholar]
- 9.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–8 [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, Wilkey SA, Kaptchuk TJ, Eisenberg DM. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 2001;135:262–8 [DOI] [PubMed] [Google Scholar]
- 11.Turner R, Baron T, Wolffram S, Minihane AM, Cassidy A, Rimbach G, Weinberg PD. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radic Res. 2004;38:209–16 [DOI] [PubMed] [Google Scholar]
- 12.Steffen Y, Jung T, Klotz LO, Schewe T, Grune T, Sies H. Protein modification elicited by oxidized low-density lipoprotein (LDL) in endothelial cells: protection by (-)-epicatechin. Free Radic Biol Med. 2007;42:955–70 [DOI] [PubMed] [Google Scholar]
- 13.Park YC, Rimbach G, Saliou C, Valacchi G, Packer L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000;465:93–7 [DOI] [PubMed] [Google Scholar]
- 14.Rimbach G, Valacchi G, Canali R, Virgili F. Macrophages stimulated with IFN-gamma activate NF-kappa B and induce MCP-1 gene expression in primary human endothelial cells. Mol Cell Biol Res Commun. 2000;3:238–42 [DOI] [PubMed] [Google Scholar]
- 15.Tribolo S, Lodi F, Connor C, Suri S, Wilson VG, Taylor MA, Needs PW, Kroon PA, Hughes DA. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–6 [DOI] [PubMed] [Google Scholar]
- 16.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–34 [DOI] [PubMed] [Google Scholar]
- 17.Ishizawa K, Izawa-Ishizawa Y, Ohnishi S, Motobayashi Y, Kawazoe K, Hamano S, Tsuchiya K, Tomita S, Minakuchi K, et al. Quercetin glucuronide inhibits cell migration and proliferation by platelet-derived growth factor in vascular smooth muscle cells. J Pharmacol Sci. 2009;109:257–64 [DOI] [PubMed] [Google Scholar]
- 18.Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–45 [DOI] [PubMed] [Google Scholar]
- 19.Leikert JF, Rathel TR, Wohlfart P, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation. 2002;106:1614–7 [DOI] [PubMed] [Google Scholar]
- 20.Schnorr O, Brossette T, Momma TY, Kleinbongard P, Keen CL, Schroeter H, Sies H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch Biochem Biophys. 2008;476:211–5 [DOI] [PubMed] [Google Scholar]
- 21.Boesch-Saadatmandi C, Niering J, Minihane AM, Wiswedel I, Gardeman A, Wolffram S, Rimbach G. Impact of apolipoprotein E genotype and dietary quercetin on paraoxonase 1 status in apoE3 and apoE4 transgenic mice. Atherosclerosis. 2010;211:110–3 [DOI] [PubMed] [Google Scholar]
- 22.Terra X, Montagut G, Bustos M, Llopiz N, Ardevol A, Blade C, Fernandez-Larrea J, Pujadas G, Salvado J, et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20:210–8 [DOI] [PubMed] [Google Scholar]
- 23.Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007;193:86–93 [DOI] [PubMed] [Google Scholar]
- 24.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:S243–55 [DOI] [PubMed] [Google Scholar]
- 25.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50 [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Alonso M, Minihane AM, Rimbach G, Rivas-Gonzalo JC, Pascual-Teresa S. Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. J Nutr Biochem. 2009;20:521–9 [DOI] [PubMed] [Google Scholar]
- 27.Boesch-Saadatmandi C, Egert S, Schrader C, Coumol X, Barouki R, Muller MJ, Wolffram S, Rimbach G. Effect of quercetin on paraoxonase 1 activity: studies in cultured cells, mice and humans. J Physiol Pharmacol. 2010;61:99–105 [PubMed] [Google Scholar]
- 28.Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–205 [DOI] [PubMed] [Google Scholar]
- 29.Rimbach G, Weinberg PD, Pascual-Teresa S, Alonso MG, Ewins BA, Turner R, Minihane AM, Botting N, Fairley B, et al. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim Biophys Acta 2004;1670:229–37 [DOI] [PubMed] [Google Scholar]
- 30.Espin JC, Garcia-Conesa MT, Tomas-Barberan FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008 [DOI] [PubMed] [Google Scholar]
- 31.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–205 [DOI] [PubMed] [Google Scholar]
- 32.Erdman JW, Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, Harnly J, Hollman P, Keen CL, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. J Nutr. 2007;137:S718–37 [DOI] [PubMed] [Google Scholar]
- 33.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:S2073–85 [DOI] [PubMed] [Google Scholar]
- 34.Cermak R. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin Drug Metab Toxicol. 2008;4:17–35 [DOI] [PubMed] [Google Scholar]
- 35.Hider RC, Liu ZD, Khodr HH. Metal chelation of polyphenols. Methods Enzymol. 2001;335:190–203 [DOI] [PubMed] [Google Scholar]
- 36.Anghileri LJ, Thouvenot P. Natural polyphenols-iron interaction: its biological importance. Biol Trace Elem Res. 2000;73:251–8 [DOI] [PubMed] [Google Scholar]
- 37.Ren J, Meng S, Lekka C, Kaxiras E. Complexation of flavonoids with iron: structure and optical signatures. J Phys Chem B. 2008;112:1845–50 [DOI] [PubMed] [Google Scholar]
- 38.Ullmann U, Haller J, Bakker GC, Brink EJ, Weber P. Epigallocatechin gallate (EGCG) (TEAVIGO) does not impair nonhaem-iron absorption in man. Phytomedicine. 2005;12:410–5 [DOI] [PubMed] [Google Scholar]
- 39.Samman S, Sandstrom B, Toft MB, Bukhave K, Jensen M, Sorensen SS, Hansen M. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001;73:607–12 [DOI] [PubMed] [Google Scholar]
- 40.Ma Q, Kim EY, Han O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J Nutr. 2010;140:1117–21 [DOI] [PubMed] [Google Scholar]
- 41.Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81:289–95 [PubMed] [Google Scholar]
- 42.Cook JD, Reddy MB, Hurrell RF. The effect of red and white wines on nonheme-iron absorption in humans. Am J Clin Nutr. 1995;61:800–4 [DOI] [PubMed] [Google Scholar]
- 43.Zijp IM, Korver O, Tijburg LB. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr. 2000;40:371–98 [DOI] [PubMed] [Google Scholar]
- 44.Sandberg AS. Bioavailability of minerals in legumes. Br J Nutr. 2002;88 Suppl 3:S281–5 [DOI] [PubMed] [Google Scholar]
- 45.Frejnagel S, Wroblewska M. Comparative effect of green tea, chokeberry and honeysuckle polyphenols on nutrients and mineral absorption and digestibility in rats. Ann Nutr Metab. 2010;56:163–9 [DOI] [PubMed] [Google Scholar]
- 46.Record IR, McInerney JK, Dreosti IE. Black tea, green tea, and tea polyphenols. Effects on trace element status in weanling rats. Biol Trace Elem Res. 1996;53:27–43 [DOI] [PubMed] [Google Scholar]
- 47.Augustin K, Frank J, Augustin S, Langguth P, Ohrvik V, Witthoft CM, Rimbach G, Wolffram S. Green tea extracts lower serum folates in rats at very high dietary concentrations only and do not affect plasma folates in a human pilot study. J Physiol Pharmacol. 2009;60:103–8 [PubMed] [Google Scholar]
- 48.Alemdaroglu NC, Wolffram S, Boissel JP, Closs E, Spahn-Langguth H, Langguth P. Inhibition of folic acid uptake by catechins and tea extracts in Caco-2 cells. Planta Med. 2007;73:27–32 [DOI] [PubMed] [Google Scholar]
- 49.Alemdaroglu NC, Dietz U, Wolffram S, Spahn-Langguth H, Langguth P. Influence of green and black tea on folic acid pharmacokinetics in healthy volunteers: potential risk of diminished folic acid bioavailability. Biopharm Drug Dispos. 2008;29:335–48 [DOI] [PubMed] [Google Scholar]
- 50.Song J, Kwon O, Chen S, Daruwala R, Eck P, Park JB, Levine M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J Biol Chem. 2002;277:15252–60 [DOI] [PubMed] [Google Scholar]
- 51.Wiegand H, Boesch-Saadatmandi C, Wein S, Wolffram S, Frank J, Rimbach G. Dietary flavonoids do not affect vitamin E status in growing rats. J Anim Physiol Anim Nutr (Berl). 2010;94:307–18 [DOI] [PubMed] [Google Scholar]
- 52.Augustin K, Blank R, Boesch-Saadatmandi C, Frank J, Wolffram S, Rimbach G. Dietary green tea polyphenols do not affect vitamin E status, antioxidant capacity and meat quality of growing pigs. J Anim Physiol Anim Nutr (Berl). 2008;92:705–11 [DOI] [PubMed] [Google Scholar]
- 53.Frank J, George TW, Lodge JK, Rodriguez-Mateos AM, Spencer JP, Minihane AM, Rimbach G. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 2009;139:58–62 [DOI] [PubMed] [Google Scholar]
- 54.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, Rimbach G, Müller MJ. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–21 [DOI] [PubMed] [Google Scholar]
- 55.Ferreira AC, Lisboa PC, Oliveira KJ, Lima LP, Barros IA, Carvalho DP. Inhibition of thyroid type 1 deiodinase activity by flavonoids. Food Chem Toxicol. 2002;40:913–7 [DOI] [PubMed] [Google Scholar]
- 56.Chandra AK, De N. Goitrogenic/antithyroidal potential of green tea extract in relation to catechin in rats. Food Chem Toxicol. 2010;48:2304–11 [DOI] [PubMed] [Google Scholar]
- 57.Milerova J, Cerovska J, Zamrazil V, Bilek R, Lapcik O, Hampl R. Actual levels of soy phytoestrogens in children correlate with thyroid laboratory parameters. Clin Chem Lab Med. 2006;44:171–4 [DOI] [PubMed] [Google Scholar]
- 58.Bitto A, Polito F, Atteritano M, Altavilla D, Mazzaferro S, Marini H, Adamo EB, D'Anna R, Granese R, et al. Genistein aglycone does not affect thyroid function: results from a three-year, randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2010;95:3067–72 [DOI] [PubMed] [Google Scholar]
- 59.Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110 Suppl 3:349–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: a summary of a statement for professionals from the american heart association nutrition committee. Arterioscler Thromb Vasc Biol. 2006;26:1689–92 [DOI] [PubMed] [Google Scholar]
- 61.Cermak R, Wolffram S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr Drug Metab. 2006;7:729–44 [DOI] [PubMed] [Google Scholar]
- 62.Alvarez AI, Real R, Perez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99:598–617 [DOI] [PubMed] [Google Scholar]
- 63.Wojnowski L, Kamdem LK. Clinical implications of CYP3A polymorphisms. Expert Opin Drug Metab Toxicol. 2006;2:171–82 [DOI] [PubMed] [Google Scholar]
- 64.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34:83–448 [DOI] [PubMed] [Google Scholar]
- 65.Miniscalco A, Lundahl J, Regardh CG, Edgar B, Eriksson UG. Inhibition of dihydropyridine metabolism in rat and human liver microsomes by flavonoids found in grapefruit juice. J Pharmacol Exp Ther. 1992;261:1195–9 [PubMed] [Google Scholar]
- 66.Schubert W, Eriksson U, Edgar B, Cullberg G, Hedner T. Flavonoids in grapefruit juice inhibit the in vitro hepatic metabolism of 17 beta-estradiol. Eur J Drug Metab Pharmacokinet. 1995;20:219–24 [DOI] [PubMed] [Google Scholar]
- 67.von Moltke LL, Weemhoff JL, Bedir E, Khan IA, Harmatz JS, Goldman P, Greenblatt DJ. Inhibition of human cytochromes P450 by components of Ginkgo biloba. J Pharm Pharmacol. 2004;56:1039–44 [DOI] [PubMed] [Google Scholar]
- 68.Tang BK, Kalow W. Variable activation of lovastatin by hydrolytic enzymes in human plasma and liver. 4. Eur J Clin Pharmacol. 1995;47:449–51 [DOI] [PubMed] [Google Scholar]
- 69.Vree TB, Dammers E, Ulc I, Horkovics-Kovats S, Ryska M, Merkx I. Differences between lovastatin and simvastatin hydrolysis in healthy male and female volunteers: gut hydrolysis of lovastatin is twice that of simvastatin. ScientificWorldJournal. 2003;3:1332–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003;56:120–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–70 [DOI] [PubMed] [Google Scholar]
- 72.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81 [DOI] [PubMed] [Google Scholar]
- 73.Cermak R, Wein S, Wolffram S, Langguth P. Effects of the flavonol quercetin on the bioavailability of simvastatin in pigs. Eur J Pharm Sci. 2009;38:519–24 [DOI] [PubMed] [Google Scholar]
- 74.Obermeier MT, White RE, Yang CS. Effects of bioflavonoids on hepatic P450 activities. Xenobiotica. 1995;25:575–84 [DOI] [PubMed] [Google Scholar]
- 75.Fuhr U, Klittich K, Staib AH. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. 1993;35:431–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsyrlov IB, Mikhailenko VM, Gelboin HV. Isozyme- and species-specific susceptibility of cDNA-expressed CYP1A P-450s to different flavonoids. Biochim Biophys Acta. 1994;1205:325–35 [DOI] [PubMed] [Google Scholar]
- 77.Zhai S, Dai R, Wei X, Friedman FK, Vestal RE. Inhibition of methoxyresorufin demethylase activity by flavonoids in human liver microsomes. Life Sci. 1998;63:PL119–23 [DOI] [PubMed] [Google Scholar]
- 78.Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther. 2000;294:88–95 [PubMed] [Google Scholar]
- 79.Aston JL, Lodolce AE, Shapiro NL. Interaction between warfarin and cranberry juice. Pharmacotherapy. 2006;26:1314–9 [DOI] [PubMed] [Google Scholar]
- 80.Yamazaki H, Shimada T. Human liver cytochrome P450 enzymes involved in the 7-hydroxylation of R- and S-warfarin enantiomers. Biochem Pharmacol. 1997;54:1195–203 [DOI] [PubMed] [Google Scholar]
- 81.He M, Kunze KL, Trager WF. Inhibition of (S)-warfarin metabolism by sulfinpyrazone and its metabolites. Drug Metab Dispos. 1995;23:659–63 [PubMed] [Google Scholar]
- 82.Greenblatt DJ, von Moltke LL. Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol. 2005;45:127–32 [DOI] [PubMed] [Google Scholar]
- 83.Zikria J, Goldman R, Ansell J. Cranberry juice and warfarin: when bad publicity trumps science. Am J Med. 2010;123:384–92 [DOI] [PubMed] [Google Scholar]
- 84.Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106:97–132 [DOI] [PubMed] [Google Scholar]
- 85.Wang LQ, James MO. Inhibition of sulfotransferases by xenobiotics. Curr Drug Metab. 2006;7:83–104 [DOI] [PubMed] [Google Scholar]
- 86.Bosch TM, Meijerman I, Beijnen JH, Schellens JH. Genetic polymorphisms of drug-metabolising enzymes and drug transporters in the chemotherapeutic treatment of cancer. Clin Pharmacokinet. 2006;45:253–85 [DOI] [PubMed] [Google Scholar]
- 87.Galijatovic A, Otake Y, Walle UK, Walle T. Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in Caco-2 cells: potential role in carcinogen bioinactivation. Pharm Res. 2001;18:374–9 [DOI] [PubMed] [Google Scholar]
- 88.Walle T, Otake Y, Galijatovic A, Ritter JK, Walle UK. Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in the human hepatoma cell line Hep G2. Drug Metab Dispos. 2000;28:1077–82 [PubMed] [Google Scholar]
- 89.Walle UK, Walle T. Induction of human UDP-glucuronosyltransferase UGT1A1 by flavonoids-structural requirements. Drug Metab Dispos. 2002;30:564–9 [DOI] [PubMed] [Google Scholar]
- 90.Sugatani J, Yamakawa K, Tonda E, Nishitani S, Yoshinari K, Degawa M, Abe I, Noguchi H, Miwa M. The induction of human UDP-glucuronosyltransferase 1A1 mediated through a distal enhancer module by flavonoids and xenobiotics. Biochem Pharmacol. 2004;67:989–1000 [DOI] [PubMed] [Google Scholar]
- 91.van der Logt EM, Roelofs HM, Nagengast FM, Peters WH. Induction of rat hepatic and intestinal UDP-glucuronosyltransferases by naturally occurring dietary anticarcinogens. Carcinogenesis. 2003;24:1651–6 [DOI] [PubMed] [Google Scholar]
- 92.Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O'Leary KA, Kroon PA, et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–13 [DOI] [PubMed] [Google Scholar]
- 93.Walle T, Eaton EA, Walle UK. Quercetin, a potent and specific inhibitor of the human P-form phenosulfotransferase. Biochem Pharmacol. 1995;50:731–4 [DOI] [PubMed] [Google Scholar]
- 94.De Santi C, Pietrabissa A, Mosca F, Rane A, Pacifici GM. Inhibition of phenol sulfotransferase (SULT1A1) by quercetin in human adult and foetal livers. Xenobiotica. 2002;32:363–8 [DOI] [PubMed] [Google Scholar]
- 95.Marchetti F, De Santi C, Vietri M, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Differential inhibition of human liver and duodenum sulphotransferase activities by quercetin, a flavonoid present in vegetables, fruit and wine. Xenobiotica. 2001;31:841–7 [DOI] [PubMed] [Google Scholar]
- 96.Eaton EA, Walle UK, Lewis AJ, Hudson T, Wilson AA, Walle T. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab Dispos. 1996;24:232–7 [PubMed] [Google Scholar]
- 97.Vietri M, Vaglini F, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulfation of R(-)-apomorphine in the human liver and duodenum, and its inhibition by mefenamic acid, salicylic acid and quercetin. Xenobiotica. 2002;32:587–94 [DOI] [PubMed] [Google Scholar]
- 98.Wiegand H, Boesch-Saadatmandi C, Regos I, Treutter D, Wolffram S, Rimbach G. Effects of quercetin and catechin on hepatic glutathione-S transferase (GST), NAD(P)H quinone oxidoreductase 1 (NQO1), and antioxidant enzyme activity levels in rats. Nutr Cancer. 2009;61:717–22 [DOI] [PubMed] [Google Scholar]
- 99.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–30 [DOI] [PubMed] [Google Scholar]
- 100.Brand W, Schutte ME, Williamson G, van Zanden JJ, Cnubben NH, Groten JP, van Bladeren PJ, Rietjens IM. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed Pharmacother. 2006;60:508–19 [DOI] [PubMed] [Google Scholar]
- 101.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34 [DOI] [PubMed] [Google Scholar]
- 102.Duthie GG, Gardner PT, Kyle JA. Plant polyphenols: are they the new magic bullet? Proc Nutr Soc. 2003;62:599–603 [DOI] [PubMed] [Google Scholar]
- 103.Valli G, Giardina EG. Benefits, adverse effects and drug interactions of herbal therapies with cardiovascular effects. J Am Coll Cardiol. 2002;39:1083–95 [DOI] [PubMed] [Google Scholar]
- 104.Poppenga RH. Risks associated with the use of herbs and other dietary supplements. Vet Clin North Am Equine Pract. 2001;17:455–77, vi–vii [DOI] [PubMed] [Google Scholar]