Abstract
Epidemiological studies suggest an inverse relationship between tomato consumption and serum and tissue lycopene (LYC) levels with risk of some chronic diseases, including several cancers and cardiovascular disease. LYC, the red carotenoid found in tomatoes, is often considered to be the primary bioactive carotenoid in tomatoes that mediates health benefits, but other colorless precursor carotenoids, phytoene (PE) and phytofluene (PF), are also present in substantial quantities. PE and PF are readily absorbed from tomato foods and tomato extracts by humans. Animal models of carotenoid absorption suggest preferential accumulation of PE and PF in some tissues. The reasonably high concentrations of PE and PF detected in serum and tissues relative to the concentrations in foods suggest that absorption or metabolism of these compounds may be different from that of LYC. Experimental studies, both in vitro and in vivo, suggest that PE and PF exhibit bioactivity but little is known about their impact in humans. Methods for producing isotopically labeled PE, PF, and LYC tracers from tomato plant cell culture offer a unique tool for further understanding the differential bioavailability and metabolism of these 3 prominent tomato carotenoids and how they may affect health.
Introduction
Carotenoids are molecules found throughout nature that have unique chemical and physical properties that allow them to serve many functions in the vastly different organisms that either produce or consume them. Fruits and vegetables are the major sources of an assortment of carotenoids for the human diet. Some dietary carotenoids, such as β-carotene, are converted to vitamin A and contribute significantly to meeting nutritional requirements, whereas non-provitamin A carotenoids purportedly target diverse biological functions by a range of mechanisms that affect health and disease risk (1). In recent years, epidemiologic studies suggesting that those with greater intakes of carotenoid-containing foods have reduced risks for several chronic diseases have stimulated greater interest in carotenoids (2). For example, consumption of carotenoid-rich tomatoes is associated with decreased risks for several chronic diseases, most notably prostate cancer and cardiovascular disease (CVD)6 (2–4). Although the red carotenoid lycopene (LYC) is most intensely studied as a potential mediator of these relationships, other colorless carotenoids found in tomatoes, such as phytoene (PE) and phytofluene (PF), are postulated to have biological activity. Recent studies revealed potential mechanisms underlying the bioactivity of PE and PF. To determine the physiological relevance of these carotenoids, recent advances have been made in understanding the bioavailability (the proportion of intact carotenoid consumed that appears in the circulation), bioaccumulation (the amount of intact carotenoid consumed found in tissues), metabolism (the chemical modifications to a carotenoid for utilization, as a participant in biological processes, or clearance), and bioactivity of these red and colorless tomato carotenoids in humans, animal models, and in in vitro experiments. Recently, our laboratory enhanced techniques to produce isotopically labeled carotenoid tracers. The availability of new tools to investigate the dietary aspects of the tomato carotenoids, PE, PF, and LYC, will accelerate the expansion of our knowledge in this area of nutrition.
Current status of knowledge
Carotenoid biosynthesis in plants
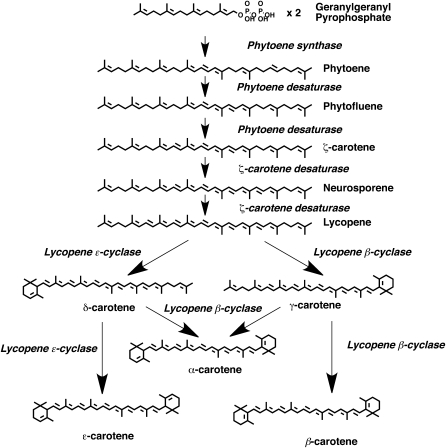
There are over 600 carotenoids found in nature and they are generally colorful orange, red, and yellow pigments synthesized by photosynthetic plants, bacteria, and fungi. Carotenoids are a class of hydrocarbon compounds that can be chemically subdivided into xanthophylls (oxygenated molecules) and carotenes (hydrocarbons lacking oxygen). These tetraterpenes usually consist of 8 isoprene units derived from isopentenyl diphosphate, the same precursor required for cholesterol synthesis in animals; however, in plants, carotenoids are synthesized in plastids via the 1-deoxy-D-xylulose-5-phosphate pathway rather than the mevalonic acid pathway of cholesterol biosynthesis (5) (Fig. 1). Isopentenyl diphosphate condenses with dimethylallyl diphosphate to yield geranylgeranyl diphosphate (GGPP), a C20 molecule, which subsequently condenses with another GGPP via PE synthase to give the basic carotenoid PE (5). As a result of the condensation of 2 GGPP molecules, PE has 3 conjugated double bonds in the center of the molecule and 6 other double bonds through the length of the molecule that are not conjugated (6). PE is next enzymatically desaturated in sequence, which effectively extends the central polyene chain by 2 double bonds, thus lengthening the chromophore and changing the spectral properties of PE, a molecule with a λmax of 286 nm, to first yield PF (λmax = 348 nm) and eventually LYC (λmax = 472 nm) (5). For more detailed information on spectral characteristics of carotenoids, please see reference (7). Tomatoes primarily contain carotenes with C40 structures with a series of conjugated double bonds. These double bonds confer electron delocalization, cis-trans isomerization, light absorption, and consequently pigmentation.
Figure 1.
Carotenoid biosynthetic pathway found in plants [reproduced from (88)].
The carotenoids present in plant foods occur in several different isomeric configurations. In plants, PE is generally synthesized as the 15-cis isomer and the intermediates leading to 7,9,7′,9′-tetracis-lycopene (prolycopene) are present in different cis conformations. Prolycopene is then isomerized by an isomerase, CrtISO, to yield all-trans-LYC. The presence of other LYC isomers in tomatoes or tomato food products is thought to be derived from isomerization induced by heat, light, or chemical reactions (6, 8).
Roles of carotenoids in nature
Will the greater understanding of the role carotenoids play in plants help us elucidate mechanisms of action and health impacts in humans? Through the course of evolution, carotenoids have proven to be tremendously versatile molecules, a concept presented in detail by Britton et al. (9) but that will be summarized here. From an evolutionary perspective, carotenoids likely first served the function for light harvesting for chlorophyll in antenna complexes in single-celled organisms. The carotenoids that accumulate in green plant tissues (lutein, β-carotene, violaxanthin, and neoxanthin) absorb light in the 400- to 500-nm range, effectively expanding the range of light that can be utilized for photosynthesis as plants expanded to new and varied environments around the planet.
As organisms adapted to aerobic environments, carotenoids could offer protection against oxidative damage by quenching singlet oxygen formed as a consequence of excess light and oxygen. The functions of carotenoids further expanded as organisms developed color vision, serving as color attractants in the fruits, flowers, and foliage of plants affecting a range of behaviors in a wide array of animals as they adapted to a given environment. In addition, some organisms adapted to utilize carotenoid derivatives (e.g. retinal in opsin proteins) for vision. Further, vitamin A is a well-known carotenoid derivative with widespread biological actions (1). Some organisms also utilize these dietary pigments, or endogenously produced pigments [as in the pea aphid (10)] as a means of communication with other organisms with color vision. Among other functions, carotenoids can serve as precursors to phytohormones and aroma compounds in plants. In short, carotenoids likely originated as molecules with narrow biological functions, but they now have a multitude of roles in nature, some of which are likely yet to be discovered.
Dietary sources of PE, PF, and LYC
As previously discussed, PE and PF are the common carotenoid precursors to the downstream carotenoid products found in plants. Therefore, PE and PF are conceivably present to some degree in a large array of carotenoid-containing foods. The presence of PE and PF has been reported for a variety of high carotenoid-containing fruits, including watermelon, apricots, cantaloupe, pink grapefruit, pumpkin, mango, papaya, peaches, prunes, and oranges, and are especially high in the aforementioned tomatoes (11, 12). When PE and PF content was analyzed in a variety of tomato products, the more processed and therefore more dehydrated tomato products had much greater concentrations of LYC, PE, and PF (e.g. 55.45, 8.36, and 3.63 mg/100 g, respectively, in tomato paste) than raw tomatoes, which had concentrations of LYC, PE, and PF of 9.27, 0.82, and 1.86 mg/100 g, respectively (13). Tomatoes are likely the prominent source of PE and PF as well as LYC in the Western diet.
Tomatoes contain a complex mixture of bioactive components and nutrients, serving as a dietary source of nutrients such as potassium, folate, and the vitamins A, C, and E (2). Additionally, they contain a mixture of carotenoids, including LYC, γ-carotene, PE, neurosporene, PF, ζ-carotene, β-carotene, and lutein (13). Because carotenogenesis is intimately tied to ripening processes, any biotic or abiotic factor that affects tomato ripening “such as genotype, fruit maturity level, cultivation practice, and environmental conditions” will affect the carotenoid profile present in the tomato (14). Carotenoids are not the only compounds affected by these factors that promote ripening, but other nutrients and bioactives such as tocopherols, folates, phenolics, glycoalkaloids, flavonoids, and vitamin C also vary by ripening stage (14–16). Thus, studies investigating the effect of tomato consumption on nutritional and health outcomes may have variable results partly due to the dynamic nature of phytochemical patterns and concentrations in these fruits.
Tomato consumption and chronic disease prevention in humans
In 2010, CVD remains the primary cause of death for Americans, followed by cancer. Prostate cancer was the most frequently diagnosed cancer in men, and the second leading cause of cancer death after lung cancer (17, 18). Risks for CVD and many cancers are affected by nutritional and environmental factors; therefore, public health dietary recommendations for disease prevention and attenuation is of great interest (19, 20). Studies specifically evaluating the roles of PE and PF intake in health and disease are minimal. Yet, because tomatoes can be viewed as the primary dietary source of PE and PF, research on tomatoes and disease risk can serve as a good starting point for evaluating the potential role of these carotenoids, either alone or in combination with LYC.
Strong epidemiological and experimental evidence implicates the risk factors for developing coronary heart disease include age, male gender, high serum LDL and low HDL cholesterol, cigarette smoking, and diabetes mellitus. In addition to these risk factors, emerging evidence indicates that additional lifestyle, diet, and genetic factors are important modulators of coronary heart disease risk (21). Over 40% of deaths in Western countries can be attributed to CVD and this increased mortality compared with non-Western countries may be partially attributable to differences in lifestyle (22).
The pathophysiology of CVD is thought to include processes such as oxidative stress, vascular inflammation, endothelial dysfunction, and vascular remodeling that leads to organ dysfunction. Thus, reduction of the primary oxidative insults that initiate the highly orchestrated cascade of events involved in vascular injury is important. Generally, oxidative damage occurs because of excessive reactive oxygen species (ROS). ROS are thought to oxidize LDL, which may eventually lead to atherosclerosis, the precursor condition for stroke and heart attack (21). Dietary antioxidants, such as carotenoids, are hypothesized to act in addition to endogenously produced antioxidants to diminish the initial oxidative stress that leads to CVD pathology (21). LYC is a powerful antioxidant in vitro and some studies examining tomato-based food consumption have shown short-term decreases in LDL oxidation (21, 23). Establishing the in vivo antioxidant role of carotenoids in animals has been elusive, however, and much more information is needed in this area (24). A supplementation trial with tomato extract reported a modest hypocholesterolemic effect (21), suggesting tomato consumption may reduce CVD risk via decreased cholesterol synthesis (25). Several epidemiological studies have suggested that increased LYC concentrations in adipose and serum are inversely related to intimal wall thickness, intima-media thickness, and the risk of myocardial infarction, CVD, and atherosclerosis of the carotid artery and the aorta. When dietary LYC was examined in the Women’s Health Study, there was a weak inverse association with CVD incidence; however, tomato product consumption had a stronger inverse association with CVD than dietary LYC did [these epidemiological studies are summarized in (25)]. To our knowledge, no epidemiological studies to date have examined serum or tissue levels of PE or PF and CVD risk, because they are not measured or reported in typical analyses. Whether LYC is the bioactive component or is simply a marker for tomato consumption and the array of phytochemicals consumed remains unanswered by these studies.
More robust evidence for a benefit of tomato products and their phytochemicals is likely derived from data regarding cancer, yet causative relationships have not been established. For example, the systematic review by the American Institute for Cancer Research (AICR) and World Cancer Research Fund (WCRF) summarizes considerable evidence linking tomato carotenoids, specifically LYC, and reduced cancer risk (19). A substantial literature of cohort and case-control studies indicates that foods rich in carotenoids may protect against tobacco-related cancers of the mouth, pharynx, larynx, and lung (19). Although data regarding lung cancer is controversial (26), substantial evidence suggests that lung cancer (18) is likely reduced with diets associated with increased circulating carotenoids (19), and a series of studies in the “smoking ferret” model suggest that LYC or metabolites may have chemopreventive properties against lung carcinogenesis (27). Others have developed hypotheses suggesting that LYC and/or tomato phytochemicals may be involved in the prevention of skin, liver, and breast cancer (19).
Perhaps the strongest data exist for a relationship among tomatoes, their carotenoids, and prostate cancer risk. Epidemiological evidence from the Health Professionals Follow-up Study supports a hypothesis that tomatoes and tomato product consumption is correlated with a decreased risk of prostate cancer (3). Clinical intervention studies with various tomato products or components evaluating biomarkers of cancer risk have been conducted (4, 28), but due to limited power and short interventions, the mostly favorable results cannot be considered as definitive. Based on a review of all available human studies, AICR/WCRF deemed “foods containing LYC probably protect against prostate cancer” yet a FDA review was far less encouraging (19, 29).
Damage to DNA and dysregulation of epigenetic processes results in disruption of cell growth, differentiation, and survival during carcinogenesis. Various studies have examined the impact of tomatoes and LYC on various components of this process, with intriguing results suggesting that tomato products protect DNA, induce phase II detoxification enzymes, modulate hormone and growth factor regulation of cell growth and function, normalize cell cycle controls, and affect gap junction communication [reviewed in (2)]. In contrast to the measured statement above by the WCRF/AICR specifying foods containing LYC, emphasis is often placed on LYC alone in research and product marketing. It is important to note that many studies reporting on LYC and health outcomes actually provide human participants with a supplement representing a tomato extract or concentrate that contains a mixture of tomato carotenoids but refer to it only as “LYC supplementation.” We hope that future investigators recognize the array of components present and also consider the potential biological activity of PE and PF.
Prostate cancer risk and tomato consumption in rats
Based on initial epidemiological associations regarding tomatoes and prostate cancer risk, our laboratory sought to determine whether LYC alone was responsible for disease prevention or if the mixture of bioactives found in tomatoes is important. We found tomato powder to be more effective than purified LYC beadlets in both slowing implanted prostate tumor growth and decreasing chemically induced tumor incidence in rats (30, 31). These findings suggest that LYC alone is not solely responsible for disease prevention and amelioration but that other components in tomatoes are bioactive. Yet recent studies in the TRAMP murine model support a major role for LYC (32).
Some recent studies provide some novel insight into the role of LYC. Gitenay et al. (33, 34) employed ex vivo assays and compared the effect of feeding LYC beadlets (a commercial, micellarized source of LYC) to the effect of feeding rats red (LYC-rich) or yellow (LYC-devoid) tomato-containing diets on biomarkers of oxidative stress and induction of connexin 43, a gap junction protein. In both studies, rats were fed diets for 6 wk that were supplemented with LYC beadlets (50 mg LYC/kg diet), red tomato powder (50 mg LYC/kg diet), yellow tomato powder (0 mg LYC/kg diet), or the basal diet, which contained one-third of the vitamin E requirement. The sera of the rats were added to the media of human prostate cancer cells, and the sera from rats fed yellow or red tomatoes significantly stimulated connexin 43 protein levels compared with the sera from LYC beadlets and control-fed animals (34). Similarly, when heart tissue homogenates from these rats were subjected to lipid peroxidation, yellow and red tomato diets were more protective compared with LYC beadlet and control powder diets (33). Taken together, these studies suggest that a combination of tomato phytochemicals is important in putative anticancer mechanisms compared with LYC alone and that LYC-free tomato powder is as effective as LYC-containing powder for gap-junction protein stimulation and antioxidant protection. These studies further suggest that other components, including the 2 colorless carotenoids, PE and PF, warrant evaluation. These carotenoids have been largely overlooked because they are colorless; however, they can be present in amounts similar to LYC in fresh tomatoes with concentrations of 2.6 mg LYC, 1.9 mg PE, and 0.8 mg PF/100 g (2), although the ratio of these carotenoids varies by variety, ripening stage, and growing conditions.
Absorption and bioavailability of PE, PF, and LYC in humans
As associations between carotenoid consumption and disease risk have emerged, investigators have directed greater attention toward understanding absorption and metabolism. Several studies have demonstrated that carotenoids are absorbed more efficiently with dietary fats; TG with long-chain fatty acids particularly enhance carotenoid absorption (35–37). Bile enhances micellarization and promotes subsequent absorption of carotenoids. Thus, because dietary fat stimulates bile secretion, efficient carotenoid absorption is mediated by this mechanism (38). Currently, it is thought that carotenoid absorption into the enterocyte is facilitated via the scavenger receptor B type 1 as well as by cluster determinant 36 (39, 40). Within the enterocyte, carotenoids are packaged into chylomicrons and secreted into the lymph, entering the systemic circulation primarily via the thoracic duct. They are repackaged by hepatic lipoprotein production and shuttled in the plasma to tissues by lipoproteins. Thus, their ultimate biodistribution to tissues is dependent on apolipoprotein metabolism (41).
Recent progress has been made in understanding tomato carotenoid bioavailability and bioaccumulation via several feeding studies. To evaluate LYC absorption from different LYC sources (LYC beadlets, tomato oleoresin, and high LYC tomato juice), one can examine the resulting carotenoid content of serum and in buccal mucosal cells (42, 43). An array of carotenoids including PE, PF, and LYC were provided by a tomato oleoresin (LYC, 74.9; PE, 5.8; PF, 5.1 mg/d), a LYC-rich tomato juice (LYC, 75.4; PE, 4.4; PF, 4.9 mg/d), and LYC beadlets (LYC, 70.2, PE, 2.5, and PF, 3.7 mg/d); therefore, the investigators were able to evaluate PE and PF absorption from the 4-wk-long treatments in addition to LYC absorption (42). The investigators utilized a complete crossover design with 15 participants with a 6 wk-long washout between the treatments. PE and PF plasma concentrations increased the greatest amount from baseline in response to tomato juice consumption, although the juice was not the most concentrated source of PE and PF. When plasma LYC concentrations were measured weekly during the 4-wk dietary treatments, LYC concentrations seemed to plateau after 2 wk of any treatment and did not significantly differ by treatment from that point on. It is conceivable that the high amount of LYC present in the supplements was great enough to rapidly reach the absorptive capacity for LYC. The authors suggested that either the absorption efficiency or the metabolism of these tomato carotenoids differ (42). However, it could also be that the absorption mechanisms are saturated for LYC at the 75-mg/d level as evidenced by pharmacokinetic research (44).
In another study, a standard, commercially available (lower LYC) tomato juice (18 mg LYC, 2.1 mg PE, and 1.1 mg PF/d) was provided for 3 wk to humans, and PF and LYC plasma concentrations increased by similar amounts (138 and 125%), whereas the increase in PE was lower (110%) (11). It is currently unknown at what plasma concentrations PE and PF plateau in response to chronic, high intakes, but perhaps with longer treatment duration in this study the plasma PE and PF would also have reached a maximum. Perhaps some of the differences in PE, PF, and LYC absorption and biodistribution reported by Paetau et al. (42) and Edwards et al. (11) can be explained by carotenoid distribution among the lipoproteins. At baseline, LYC was primarily associated with LDL. PE and PF were also largely found in the LDL fraction but were proportionately higher in the VLDL than the HDL fractions. When the different carotenoid treatments were administered, LYC distribution in lipoproteins was not altered; however, PE distribution increased in VLDL and HDL and decreased in LDL (42). Although the amounts of LYC in the 3 treatments were similar, buccal mucosal cell LYC concentrations significantly increased in response only to the oleoresin (141% increase) and beadlet regimens (95%), but not from the tomato juice. Alternatively, PF, which was present at nearly 7% of the level of LYC in the tomato juice and in the oleoresin, also significantly increased (133 and 76%, respectively) in response to these treatments up to 2.26 ± 0.26 and 1.48 ± 0.17 μg PF/g protein, respectively. PE was not detected in the buccal mucosal cells, although it was present at nearly equivalent levels to PF in the juice (43). Potentially, PE is not taken up or retained by as many tissues, explaining elevated HDL PE concentrations after carotenoid feeding. From the buccal mucosal cell data, it appears that LYC was more bioavailable to the tissue from oleoresin and beadlet treatments. PE and PF were not matched across the dietary treatments and small amounts of PE and PF may have been present in the diet from other fruits and vegetables, so PE and PF bioavailability from each product should be cautiously evaluated. However, it is interesting to note that although PF was present at much lower concentrations than LYC in the tomato juice, it accumulated at a similar μg carotenoid/g protein concentration as LYC. PE, which was present at similar levels to PF in the juice, was not detected in buccal mucosal cells. These findings raise the question of whether the results are due to differential bioavailability or preferential metabolic breakdown of certain carotenoids compared with others. Plasma and buccal mucosal cell concentrations of PF did correlate to a greater degree than LYC, such that samples from participants receiving the oleoresin or placebo treatments had correlated concentrations (P < 0.05) (43). An important observation is that the carotenoid profile of the food or dietary supplement does not adequately predict relative plasma or tissue amounts of carotenoids. It is therefore important to evaluate different tissue concentrations of tomato carotenoids to get a better picture of absorption and biodistribution. Lastly, the different lipoprotein distributions of PE and PF compared with LYC potentially suggest differences in tissue uptake and tissue metabolism. Future studies examining the absorption of PE and PF in comparison with LYC will be instructive.
Pharmacokinetic modeling of LYC absorption (transfer of carotenoids from the gastrointestinal lumen to the enterocyte and then circulation) and biodistribution from a tomato drink has been informative. One human study (44) indicated that regardless of LYC content in the drink (10 mg/79 mL, 30 mg/238 mL, 60 mg/476 mL, 90 mg/769 mL, or 120 mg/797 mL), the same amount of LYC was absorbed (4.69 ± 0.55 mg), suggesting a saturation of absorptive mechanisms for LYC. Different tissue compartments were included in a mathematical model developed to describe LYC absorption and biodistribution. Based on the model generated, the liver was predicted to be a fast turnover tissue, whereas other tissues that were more resistant to LYC depletion served as the slow turnover pool (44). Outright pharmacokinetic studies on PE and PF in humans have not yet been performed, but one may hypothesize that based on the differential tissue levels and lipoprotein partitioning, the pharmacokinetics of PE and PF may well differ from that of LYC.
Beyond what is known about tomato carotenoid accumulation in buccal mucosal cells, limited information on human tissue LYC, PE, and PF concentrations is available. Khachik (13) reported detection of PE and PF in the same tissues where LYC accumulates, including in liver, lung, breast, prostate, colon, and skin tissue. The liver is often enriched in carotenoids, and in autopsy samples, LYC was present at a greater concentration (0.66 nmol/g) than PE and PF (0.31 and 0.48 nmol/g, respectively) (13). LYC, notably, has been reported to be the most prominent carotenoid detected in prostate tissue (45) and when all 3 carotenoids were evaluated, LYC was found to be greater (374 ng/g) than PF (201 ng/g) and PE (45 ng/g) (13). Of the tissues examined, breast had the greatest concentration of PF (416 ng/g) with lesser amounts of LYC and PE, whereas the pulmonary tissue had markedly higher concentrations of PE (1275 ng/g) with lesser amounts of LYC and PF (300 and 195 ng/g, respectively) (13). These differences in PE, PF, and LYC ratios in human tissues raise many questions, but the data are too limited at present to draw meaningful conclusions. Future studies elucidating the underlying mechanisms of this differential bioaccumulation will be highly informative to understanding the bioactivity of these carotenoids.
Carotenoid metabolism and breakdown products
It is possible to speculate that a source of differential plasma and tissue concentrations of PE, PF, and LYC may be tissue-specific metabolic or oxidative metabolism. LYC metabolites may be the result of oxidation reactions. In vitro oxidation studies utilizing m-chloroperbenzoic acid have provided a series of potential LYC epoxides, diols, and an alcohol (46). Of these potential products, 5 were detected in tomato food products: LYC 1,2-epoxide; LYC 1,2;1′,2′-diepoxide, 2,6-cyclolycopene-1,5-epoxide A and B; and 2,6-cyclolycopene-1,5-diol A and B, and only the diols were detected in human serum (46).
In addition to oxidative mechanisms, there are enzymes that are postulated to be responsible for PE, PF, and LYC metabolism, including cytochrome P450 enzymes or soybean lipoxygenase, and the recently characterized carotenoid cleavage enzymes (47). There are 2 specific mammalian carotenoid cleavage enzymes that metabolize carotenoids: carotenoid monooxygenase (CMO)-I (also referred to as CMO1, BCDO1, or BCMO1) is well known to centrally cleave provitamin A carotenoids, such as β-carotene, at the 15, 15' double bond to yield vitamin A (48) and CMO-II (also referred to as CMO2 and BCMO), which cleaves β-carotene eccentrically (49) and is hypothesized to lead to eccentric cleavage of other carotenoids such as LYC (47). In fact, ferret CMO-II expressed in Spodoptera frugiperda cells effectively cleaved all-trans-β-carotene as well as cis-LYC isomers (but not all-trans-LYC) at the 9′, 10' double bond [summarized in (50)]. A series of chain-shortened LYC aldehydes (apo-lycopenals) were also detected in the liver of 14C-LYC-fed rats, tomato products, and human plasma, and these are putative cleavage products of the CMO-II enzyme (51, 52). The LYC metabolites found in food products in amounts ranging from 0.1 to 34 μg/100 g wet weight included apo-6′-, 8′-, 10'-, 12'-, and 14'-lycopenals, and all 5 of these were detected in plasma ranging between 0.12 and 0.73 nmol/L (51). Whether the oxidative LYC products and putative LYC metabolites of CMO-II were present in serum as a consequence of absorption from foods or endogenous production is not clear and must be confirmed via isotopically labeled LYC studies. The CMO-II gene has been detected in an array of human tissues both co-occurring with CMO-I (small intestinal and stomach mucosa, Leydig and Sertoli cells in the testis, kidney tubules, adrenal gland, exocrine pancreas, and epithelium in the eye) as well as being expressed in tissues that do not express CMO-I (cardiac and skeletal muscle, prostate and endometrial connective tissue, and the endocrine pancreas), suggesting that CMO-II has a function independent of vitamin A production (53).
Although information on CMO-II activity and tissue distribution as well as LYC metabolite identification is expanding, it is unknown if PE or PF are cleaved by CMO-II. Recent findings from our laboratory suggest that PE and PF may not be preferred substrates of the CMO-II enzyme, but further studies would be necessary to confirm this (54). However, it is known that PF is quite susceptible to oxidation; thus, oxidative products of PF may be present in tomato foods and serum. The instability of PF was observed when PF was delivered in tetrahydrofuran (THF) to in vitro cell culture media for HL-60 cells (10 mmol/L PF in media) and was found to be completely absent after 5 h of incubation (55). Alternatively, PE was more stable than PF and ζ-carotene and was not fully degraded or metabolized until 70 h after injecting it into the media. Similarly, when PE and PF were incubated in toluene at 37°C for 24 h, this oxidative treatment led to a 95% decrease in intact PF, but there was no decrease in intact PE. Although LYC is commonly known to be a powerful antioxidant, it was more stable than PE in the media and after toluene incubation (55). Based on these experiments, PE and LYC may be more stable, or less susceptible to oxidative breakdown, than PF. Our laboratory found that when human prostate cancer cells were incubated with 14C-PE, the cells absorbed the tracer PE and polar 14C-breakdown products were detected in the cells as well as the media (56). Further studies should investigate enzymatic and oxidative product formation from PE, PF, and LYC. Understanding whether PE and PF are cleaved by CMO-II as well as their relative susceptibility to oxidation may help to explain differences observed in tissue carotenoid concentrations.
In vivo studies of relative tomato carotenoid bioavailability and metabolism
To better understand the bioavailability and metabolism of PE, PF, and LYC, several animal studies have been undertaken. In Fisher 344 rats fed a 10% tomato powder diet and dosed with PE, PF, or a carrier dose, PE and PF were absorbed, accumulated more in some tissues than in others, and PE was potentially absorbed more readily than LYC (57). The 10% tomato powder diet had carotenoid concentrations (mg/g) of PE, 0.015; PF, 0.012; LYC, 0.011; and ζ-carotene, 0.001. In response to 10% tomato powder feeding, the most prominent carotenoid in serum was PE, followed by PF, and then LYC, whereas the liver had more PF than PE or LYC, which were nearly equal. The adrenals had nearly equal amounts of PE and PF with lesser amounts of LYC. The spleen had primarily LYC, followed by PF, and the adipose had nearly equal levels of PE, PF, and LYC. LYC was the primary carotenoid in the prostate, while LYC and PF were at equal concentrations in the testes. Dosing the rats with either 2.7 mg PE or PF led to significant increases in serum, liver, spleen, and testes of each respective carotenoid 24 h postdosing (57).
To learn about the bioavailability of PE and PF, Sprague-Dawley rats were fed a diet consisting of 0.1% dried algal (Dunaliella bardawil) powder (the algal powder contained 2% PE and 0.1% PF) (58). These carotenoids were primarily present as the 9-cis and all-trans isomers in a ratio of 1:1. Rats were fed the carotenoid diet for 2 wk, after which their tissues were analyzed for PE content. The liver had the greatest accumulation of PE (0.440 ± 0.024 μmol/100 g tissue) followed by the adrenals and much lesser amounts in the other tissues measured. Also, it appeared that the all-trans was more prominent than the 9-cis isomer in the liver, kidney, and spleen tissues, whereas the adrenals and serum maintained the same 1:1 ratio observed in the diet (58). PF tissue concentration results were not included in this study. Interestingly, this observation differs from the observation that cis isomers of LYC are more prominent in tissues (59).
In another study utilizing Copenhagen rats, PE and PF bioavailabilities from different tomato powders were evaluated (60). This study showed that different carotenoid profiles and amounts present in tomato powders result in differential liver carotenoid accumulation. Two of the tomato powders had similar amounts of PE, PF, and β-carotene but 1 had more than twice the amount of LYC (LYC-enriched tomato powder) than that of the other, standard tomato powder. When the rats were fed diets containing 10% of these tomato powders for 7 d, rats fed the LYC-enriched powders accumulated significantly more LYC and significantly less PE and PF than the standard tomato powder. A 3rd tomato powder had overall greater carotenoids with significantly more PE, PF, and β-carotene, but not enhanced LYC. The rats fed this carotenoid-enriched powder accumulated significantly more hepatic PE, PF, and β-carotene but the same amount of LYC as the standard powder-fed rats. This study suggested an interaction of these acyclic carotenoids at the level of absorption or metabolism. Further studies that can shed more light on this relationship will be very informative.
The gerbil as a model for tomato carotenoid absorption, biodistribution, and metabolism
Bioavailability studies of PE and PF have thus far utilized the rat as an animal model, but other rodents may provide advantages for studying carotenoid absorption and metabolism of PE, PF, and LYC. When F344 rats, BALB/c mice, nude mice, and Mongolian gerbils (Meriones unguiculatus) were provided oral doses of LYC (20 mg/kg body weight) every 2 d for 10 d, gerbils accumulated the greatest hepatic and plasma LYC. The authors therefore concluded that the gerbil is the most appropriate model of the 4 tested for studying LYC absorption. Additionally, in a subsequent experiment, the authors found that gerbils provided with the previously specified dose achieved a steady-state plasma LYC concentration between 597 and 722 nmol/L after 6 d of supplementation, which was not exceeded over the 20-d dosing duration (61). Another gerbil study sought to compare LYC bioavailability from tomato paste powder, red carrot powder, and purified LYC in oil (62). Each treatment provided ∼60 μg LYC/d to the gerbils for 3 wk. LYC was most bioavailable from the tomato paste powder, followed by the red carrot powder, and lastly the LYC in oil. In addition to LYC absorption, gerbils have proven to be an excellent model for other carotenoids and for pro-vitamin A absorption and metabolism research. Similar to humans, gerbils absorb β-carotene intact and convert β-carotene to vitamin A at a similar efficiency to humans, making them a useful model of carotenoid bioconversion and bioavailability from foods (63–68). Although we understand a great deal about gerbil provitamin A carotenoid uptake and metabolism and are learning more about LYC, currently nothing is known about the absorption and distribution of PE and PF in gerbils.
Animal and in vitro studies of the potential bioeffects of PE, PF, and LYC
Because most LYC treatments utilized in trials are actually tomato based and not just pure LYC, one can hypothesize that PE and PF may be involved in many of the underlying mechanisms of disease attenuation and prevention observed in experiments. In this following section, we will address the literature that specifically addresses PE and PF in investigations of tomato carotenoid bioactivity.
Skin protection by nutritional interventions is garnering more attention (69). Carotenoids have properties that may interrupt the course of assaults involved with UV radiation that eventually lead to skin damage. UVA (320–400 nm) exposure leads to formation of ROS, especially singlet oxygen, causing oxidative stress that can damage cellular components (69). Carotenoids are potent singlet oxygen quenchers. The carotenoid PF absorbs light in the UVA range and therefore may dampen the effect of UVA exposure. The most harmful range of UV light is the B range (290–320 nm) and it, along with the C range (100–290 nm), are known to cause erythema (sunburn) as well as mutagenic lesions (69). PE absorbs light in the UVB and C ranges. Dietary sources of tomato carotenoids, including purified synthetic LYC (10.2 mg LYC/d), a tomato extract (9.8 mg LYC, 0.8 mg PF, and 2 mg PE/d), and a tomato extract-containing drink (8.2 mg LYC, 3.2 mg PF, and 4.6 mg PE/d) provided for 12 wk led to decreased erythema in response to a dose of UV irradiation (70). Synthetic LYC treatment led to significant increases in serum LYC and skin total carotenoids with no increases of PE or PF in the serum. When participants consumed the tomato extract, PE, PF, and LYC all nearly doubled in concentration in the serum, with PF being the prominent carotenoid after 12 wk (0.94 μmol/L) (70). The drink also stimulated substantial serum increases in PE, PF, and LYC, although not substantially different from the tomato extract group, even though the drink had more PE and PF than the extract (70). Consumption of the tomato extract and tomato extract-containing drink led to significantly less intense erythema after 12 wk compared with baseline, whereas the LYC treatment alone did not have this effect. These data suggest that PE and PF may be important components in the tomato extract and tomato extract-containing drink for UV protection.
The ability of PE and PF to protect fibroblasts from UV-induced damage was investigated in vitro (71). Cells were exposed to 50 μW of UV that included 35 μW of UVA light and after irradiation were then grown in experimental growth medium containing 15 mg/L total concentration of combined PE and PF. The cells were then exposed to IL-1 to simulate an inflammatory response and the prostaglandin E2 was subsequently measured. The carotenoid treatment led to a 47% decrease in prostaglandin E2 levels in the cells compared with the control (71). Future work can expand on this area by examining the effects of PE and PF on inflammatory response propagation.
The ability of PE and PF to inhibit oxidation was examined further in vitro by examining the ability of these carotenoids to protect LDL particles from oxidation. LDL oxidation is a critical event in the pathogenesis of CVD, and the acyclic tomato carotenoids are found primarily in LDL lipoproteins. PE incorporation efficiency (28.7 nmol/mg LDL) from a PE and PF enriched fraction isolated from Dunaliella bardawil (an alga) exceeds that of all-trans-β-carotene (2.18 nmol/mg LDL) or α-tocopherol when human lipoprotein particles are incubated in 500 μmol/L of either all-trans-β-carotene, α-tocopherol, or a PE/PF mixture (72). All 3 treatments reduced the maximal rate of LDL oxidation due to the oxidant 2,2′-azobis(2-amidinopropane) hydrochloride as well as the maximal production of oxidation products compared with untreated lipoprotein. The investigators suggested that future studies should seek to match carotenoid levels in lipoproteins to examine a dose response effect for LDL protection (72). These findings show that PE and PF may act as antioxidants in lipoproteins (72). Future studies should also examine PE and PF incorporation efficiencies into LDL particles to better understand the physiological impact of these findings.
In addition to the potential antioxidant mechanisms of tomato carotenoids, others have focused on different mechanisms for cancer prevention by PE, PF, and LYC. Short-term exposure (4-d feeding) to PF, LYC, and tomato-powder feeding decreased androgen concentrations in rats, which could be important for hormone-dependent prostate cancer (73). These rats were provided with 0.7 mg/d of PF or LYC or a 10% tomato powder diet for 4 d and it was found that serum testosterone, but not dihydrotestosterone, levels were significantly lowered with carotenoid or tomato powder feeding. To elucidate the underlying mechanisms for differences in serum testosterone, steroidogenic enzyme expression was examined. No differences were detected by treatment group for 17β-hydroxysteroid dehydrogenase 3 nor for 5α-reductase II mRNA expression (73). The effect of androgen status on carotenoid tissue accumulation was also examined by comparing carotenoid-fed castrated rats with sham-operated rats. Castration, or lower androgen status, led to significantly increased serum and liver PF and LYC concentrations after the respective carotenoid doses, but differences were not apparent in response to tomato powder feeding. Prostate and seminal vesicle carotenoid concentrations were also elevated in castrated rats that were dosed with PF or LYC. At this point, it is not exactly clear why androgen status alters carotenoid accumulation, but the investigators suggested that it might be because androgen metabolism leads to oxidative stress, which may “consume" carotenoids. Alternatively, the investigators detected lower expression of CYP 3A1, a hepatic enzyme that may metabolize carotenoids, in castrated rats, which may also help explain the differences in serum and tissue PF and LYC by androgen status (73).
Two studies have utilized carotenoid biosynthetic gene transfection methods rather than animal feeding or carotenoid dosing protocols to examine the biological effects of PE. First, when mouse embryonic fibroblasts (NIH3T3) were transfected with the PE synthase encoding gene crtB from Erwinia uredovora, the PE producing mammalian cells were protected from oxidative stress as well as malignant transformation induced by oncogenic transfection (74). Enhancement of gap-junction or cell-to-cell communication is a putative mechanism of cancer prevention mediated by carotenoids. LYC has been reported to increase the expression of connexin 43 in vitro (75). To investigate the effect of PE on connexin proteins, a transgenic crtB mouse was engineered to endogenously produce PE. PE production was confirmed in the embryonic cells after transfection, although final tissue PE concentrations were not reported. This experiment showed an increase in connexin 26, but not connexin 32 or 43, expression, which may translate to increased cell-cell communication and contribute to the carcinogenic process (75).
One putative mechanism of cancer prevention by carotenoids is via the induction of phase II detoxification enzymes. A study was performed to analyze the effects of different carotenoids, including LYC, PE, and PF, on the antioxidant response element (ARE) and nuclear factor E2-related factor (Nrf2) (76). The ARE and Nrf2 control expression of phase II enzymes, so they were investigated as a target of carotenoids. When MCF-7 and HepG2 cells were treated with either LYC or a PE and PF mixture (70:30), it was determined that LYC induced expression of 2 phase II enzymes, NAD(P)H:quinone oxidoreductase and γ-gluatamylcysteine synthetase and the PE/PF mixture caused only a slight increase in transcription of these genes. Similarly, LYC significantly increased the protein levels of these 2 genes, whereas the PE/PF mixture led to a nonsignificant increase in protein levels. Further, an ethanolic extract of LYC, containing putative oxidative LYC metabolites but not measurable intact LYC, also activated these genes and induced protein expression. Both LYC and the PE/PF mixture lowered intracellular ROS by the same approximate amount. Further work elucidating the identity of these oxidative metabolites and the concentrations of the metabolites needed for ARE and Nrf2 activation should be performed. It would also be useful to isolate the effects of PE compared with PF in these experimental systems.
Other potential mechanisms of action by which tomato carotenoids may prevent cancer are by inducing apoptosis and inhibiting growth. A study investigated the effect of the acyclic carotenoids PE, PF, LYC, ζ -carotene, or oxidized carotenoids on these 2 outcomes in human promyelocytic leukemia cells in the in vitro HL-60 cell line (55). Cells were grown in media supplemented with the carotenoids at concentrations of 2, 6, or 10 μmol/L or of oxidized carotenoids at 10 μmol/L. Each carotenoid was oxidized by incubation of 1 mmol/L in toluene for 24 h at 37°C. The most effective growth inhibitor was ζ-carotene (cell density was 3.7% that of the control), followed by PF (22.6% cell density of the control) and LYC (71.7% cell density of the control) at the 10 μmol/L concentration for all 3 carotenoids. Incidentally, ζ-carotene and PF were also the most easily oxidized carotenoids in the media over time, with complete degradation after 5 h in culture. Neither PE nor PF nor ζ-carotene was detected in cells after incubation, whereas LYC was taken up. These results may suggest that the production of oxidative products of ζ-carotene and PF may be important for inhibiting growth in this cancer cell line. After THF treatment, PF was also most fully oxidized (only ∼5% intact remaining after 24 h), followed by ζ-carotene (25% intact remaining), and then LYC. To compare the effect of oxidized carotenoids and intact carotenoids on growth inhibition, THF-oxidation mixtures of 6 μmol/L PF, ζ -carotene, and LYC were more effective in inhibiting cell growth (8.3, 2.5, and 3.4% cell density of control growth, respectively) than the intact carotenoid at 6 μmol/L (68.4, 44.9, and 100% cell density of control, respectively) (55). Apoptosis was induced in cells treated with either 10 μmol/L PF oxidation mixture, ζ-carotene oxidation mixture, or the LYC oxidation mixture as indicated by analysis for apoptotic DNA fragmentation (55). These interesting results warrant further investigation into the physiological relevance of the oxidation products of the acyclic carotenoids PF, ζ-carotene, and LYC. It would be pertinent to determine whether these products are present in foods or in human serum. It is important to note that the effect of PE oxidation products on cell growth could not be evaluated, because PE was not readily oxidized by THF and, alternatively, the effect of intact PF on the cells was not sufficiently evaluated because PF was too unstable in the media (55). Although in vitro technology provides a remarkable tool for the evaluation of mechanisms of action for phytochemicals such as carotenoids, the issue of stability is a major obstacle to establishing definitive cellular targets of carotenoids. Additional efforts to define optimal cell culture systems that will provide reliable data with respect to carotenoids and metabolites are needed.
Isotopically labeled tomato carotenoid tracers
One of the developing tools with the potential to enhance our understanding of PE, PF, and LYC absorption and metabolism is isotopically labeled tracers. Isotopically labeled (13C or 14C) tracers allow a single dose of a dietary component to be followed within preexisting, endogenous pools of the previously ingested component. This is especially important in dietary carotenoid metabolic research, because these carotenoids are widely found in the Western diet. It is difficult to completely exclude carotenoids from the diet, as would be necessary to conduct conventional pharmacokinetic and metabolic studies. Previously, 13C (a stable, nonradioactive carbon isotope) or 14C (radioactive carbon) tracers of lutein and β-carotene have been utilized to study absorption, plasma appearance, and endogenous pools of these specific carotenoids in humans. Labeled lutein and β-carotene studies have provided information on these carotenoids regarding their absorption kinetics, isomerization of β-carotene during absorption, endogenous plasma pool quantification, and the conversion of β-carotene to vitamin A (77–83). Additionally, deuterium-labeled LYC obtained either by chemical synthesis or from intrinsically labeled tomatoes grown hydroponically were used to determine the relative bioavailability of LYC from steamed and pureed tomatoes compared with synthetic LYC in oil. The results showed that synthetic LYC in oil was 3 times more bioavailable than that from tomatoes (84). Some pharmacokinetic LYC tracer research has been previously performed in rodents. Two studies from our laboratory focused on the absorption and biodistribution of 14C-LYC in F344 rats (85, 86). When rats were fed 0.25 g LYC/kg diet for 30 d and then gavaged with a single oral dose of 14C-LYC with unlabeled LYC (0.246 mg in oil), 7% of the dose was absorbed and 6% was retained over 168 h. Radiolabeled LYC was also used to determine that prefeeding LYC, as opposed to a control diet, decreases the amount of absorbed LYC (85). To date, no tracer studies on PE or PF absorption kinetics, relative bioavailability, biodistribution, or metabolism have been performed in animals or humans and therefore we know little about these prevalent dietary carotenoids.
Tools for studying PE, PF, and LYC metabolism
Further progress on determining the mechanisms of tomato carotenoid metabolism has been hampered, because there is no commercial source for isotopically labeled PE, PF, or LYC. Tomato cell suspension cultures are being developed as a tool for the production of [13C]- or [14C]-radiolabeled carotenoids, which can be used for pharmacokinetic studies (56, 87, 88). Plant cell culture has been applied for the radiolabeling of other bioactive compounds: grape polyphenols, berry anthocyanins, kudzu isoflavones, and red clover isoflavones (89–91). Several advantages are offered by using plant cell culture rather than whole plants to accumulate radiolabeled metabolites, such as the ability to target selected tissue sources based on desired secondary metabolite production profiles, shortened culture periods, relative ease of extraction, aseptic growth conditions, and uniform exposure to isotopically labeled carbon sources in the media (90).
Recent advances utilizing the VFNT cherry tomato cell line in suspension cultures grown with isotopically labeled carbohydrates and bleaching herbicides for production of 14C-PE, PF, and LYC have been made and are being applied to 13C-PE, PF, and LYC by our laboratory. Bleaching herbicides, utilized to enhance in vitro carotenogenesis (56, 92–95), are chemicals that interrupt the normal biosynthesis of carotenoids, either in vivo or in vitro, causing a fatal loss of photoprotection in the plant and leading to the destruction of chlorophyll upon light exposure (96). Previously, our laboratory utilized the bleaching herbicide norflurazon, a PE desaturase inhibitor (Fig. 1) (95), to promote [14C]-PE and PF accumulation in tomato cells, and the tracers were used for in vitro investigation of their uptake into prostate cancer cells (56). Campbell et al. (56) previously determined that VFNT cherry tomato cells could produce up to ∼634 μg PE/L culture and ∼88 μg PF/L culture with 0.03 mg norflurazon/40 mL culture.
To further increase labeled carotenoid yields and the mixture of carotenoids that could be produced from tomato cell cultures, it was determined that different herbicide treatments could be utilized to target production of specific tomato carotenoids. In addition to norflurazon, a second bleaching herbicide, 2-(4-chlorphenyl-thio) triethylamine (CPTA) was investigated. CPTA inhibits LYC cyclase, causing LYC accumulation and a lack of downstream cyclic carotenoid production (Fig. 1). When both CPTA and norflurazon were investigated in VFNT cherry tomato cell cultures, it was found that norflurazon treatment in the media promotes production of primarily PE and PF (2.06 and 0.18 mg/L, respectively), whereas CPTA treatment promotes primarily LYC production (2.49 mg/L). When both herbicides were administered, a mixture of all 3 carotenoids was produced (PE, 1.74 mg/L; PF, 0.3 mg/L; LYC, 1.24 mg/L). This combination treatment was then used for 14C-labeling. The cultures were grown with 14C-sucrose in the media, causing the media to be 45 mCi/L. The cells and carotenoids incorporated the 14C, and radiolabeled PE was the most prominent source of radioactive carotenoid (2.6 nCi/g cells), with lesser yields of LYC and PF (3.1 and 0.4 nCi/g cells, respectively) (88). As a result of these experiments, a source of 14C-carotenoids for absorption and metabolism studies in animals and cell cultures is available to make further strides in understanding biological mechanisms of PE, PF, and LYC.
With the basic methods in place for producing labeled carotenoids from plant cell cultures, we sought to develop a higher yielding source of stable isotopically labeled carotenoids for human metabolism research. Different high-carotenoid tomato lines were scanned in vitro for carotenoid production and of them, the hp-1 allelic variant was selected for further labeling studies, and it produced greater total carotenoids (PE, PF, and LYC) with varying herbicide treatments (3.6–5.2 mg/L) than the previously reported yields from VFNT cherry cell suspension cultures (2.3–3.3 mg/L) (87). The hp-1 cell line was then grown with CPTA and [U]-13C-glucose to promote accumulation of 13C-LYC. The LYC yield was 2.45 mg/L and the LYC molecules were very highly enriched with 13C. Eighty-eight percent of the LYC molecules contained 35–40 13C atoms, resulting in m/z = 571–576 (unlabeled LYC is m/z = 536), and no LYC with fewer than 20 labeled carbons were detected (87). This development is a major step in producing tracers for carotenoid metabolic studies. This technology should be applied to 13C-labeled PE and PF production for utilization in human tracer studies.
Conclusion
Tomato carotenoids, including LYC, PE, and PF, are hypothesized to play a preventative role in a variety of diseases; however, little is known about their biodistribution and metabolism in humans and tissue specific bioactivity. To make useful dietary recommendations to Americans regarding the potential protective roles of tomato products or specific carotenoids for human health, definitive human clinical trials are needed. Ongoing studies to expand our knowledge of tomato carotenoid bioavailability, biodistribution, and metabolism will help investigators design appropriate intervention studies of health outcomes. Recent strides in nutritional carotenoid research with LYC, PE, and PF indicate that the absorption, biodistribution, and metabolism of PE, PF, and LYC may differ substantially despite their similar structures. Research utilizing labeled tomato carotenoid tracers in rodent models and human participants will contribute to this important area of nutritional modification and disease prevention. Utilizing tomato plant cell culture methods as a source of isotopically labeled carotenoids will facilitate the study of PE, PF, and LYC bioavailability and metabolism. Thus, the research tools are now available to advance the knowledge of absorption, metabolism, and health aspects of PE and PF.
Acknowledgments
This manuscript was drafted, written, and reviewed by N.J.E., S.K.C., and J.W.E. All authors read and approved the final manuscript.
Footnotes
Supported by the NIH and the Center for Complementary and Alternative Medicine (NIH 1R21AT005166-01A1).
Author disclosures: N. J. Engelmann, S. K. Clinton, and J. W. Erdman Jr, no conflicts of interest.
Abbreviations used: AICR, American Institute for Cancer Research; ARE, antioxidant response element; CMO, carotenoid monooxygenase; CPTA, 2-(4-chlorphenyl-thio) triethylamine; CVD, cardiovascular disease; GGPP, geranylgeranyl diphosphate; LYC, lycopene; Nrf2, nuclear factor E2-related factor; PE, phytoene; PF, phytofluene; ROS, reactive oxygen species; THF, tetrahydrofuran; WCRF, World Cancer Research Fund.
Literature Cited
- 1.Britton G, Liaaen-Jensen S, Pfander H. SpringerLink. Nutrition and health. Basel, London: Birkhauser; 2009. p. 5. [Google Scholar]
- 2.Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW., Jr The tomato as a functional food. J Nutr. 2005;135:1226–30 [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E. Tomato products, lycopene, and prostate cancer: a review of the epidemiological literature. J Nutr. 2005;135:S2030–1 [DOI] [PubMed] [Google Scholar]
- 4.Etminan M, Takkouche B, Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340–5 [PubMed] [Google Scholar]
- 5.Bramley PM. Regulation of carotenoid formation during tomato fruit ripening and development. J Exp Bot. 2002;53:2107–13 [DOI] [PubMed] [Google Scholar]
- 6.Sandmann G. Evolution of carotene desaturation: the complication of a simple pathway. Arch Biochem Biophys. 2009;483:169–74 [DOI] [PubMed] [Google Scholar]
- 7.Britton G. UV/visible spectroscopy. : Britton G, Liaaen-Jensen S, Pfander H, Carotenoids: spectroscopy. Basel: Birkhauser; 1995. p. 13 [Google Scholar]
- 8.Xianquan S, Shi J, Kakuda Y, Yueming J. Stability of lycopene during food processing and storage. J Med Food. 2005;8:413–22 [DOI] [PubMed] [Google Scholar]
- 9.Britton G, Liaaen-Jensen S, Pfander H. SpringerLink. Natural functions. Basel, London: Birkhauser; 2007. p. 4 [Google Scholar]
- 10.Moran NA, Jarvik T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science. 2010;328:624–7 [DOI] [PubMed] [Google Scholar]
- 11.Edwards AJ, Vinyard BT, Wiley ER, Brown ED, Collins JK, Perkins-Veazie P, Baker RA, Clevidence BA. Consumption of watermelon juice increases plasma concentrations of lycopene and beta-carotene in humans. J Nutr. 2003;133:1043–50 [DOI] [PubMed] [Google Scholar]
- 12.Khachik F, Beecher GR, Goli MB, Lusby WR. Separation, identification, and quantification of carotenoids in fruits, vegetables and human plasma by high-performance liquid-chromatography. Pure Appl Chem. 1991;63:71–80 [Google Scholar]
- 13.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med (Maywood). 2002;227:845–51 [DOI] [PubMed] [Google Scholar]
- 14.Kacjan Marsic N, Sircelj H, Kastelec D. Lipophilic antioxidants and some carpometric characteristics of fruits of ten processing tomato varieties, grown in different climatic conditions. J Agric Food Chem. 2010;58:390–7 [DOI] [PubMed] [Google Scholar]
- 15.Jesus Periago M, Garcia-Alonso J, Jacob K, Belen Olivares A, Jose Bernal M, Dolores Iniesta M, Martinez C, Ros G. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int J Food Sci Nutr. 2009;60:694–708 [DOI] [PubMed] [Google Scholar]
- 16.Choi SH, Lee SH, Kim HJ, Lee IS, Kozukue N, Levin CE, Friedman M. Changes in free amino acid, phenolic, chlorophyll, carotenoid, and glycoalkaloid contents in tomatoes during 11 stages of growth and inhibition of cervical and lung human cancer cells by green tomato extracts. J Agric Food Chem. 2010;58:7547–56 [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. FastStats: leading causes of death. Version current December 31, 2009 [cited 2010 Jul 27]. Available from: http://www.cdc.gov/nchs/fastats/lcod.htm.
- 18.American Cancer Society. Cancer facts and figures 2009. Version current 2009 [2010 25 Oct]. Available from: http://www.cancer.org/Research/CancerFactsFigures/index2009.
- 19.WCRF/AICR Summary: food, nutrition, physical activity and the prevention of cancer: a global perspective. Second Expert Report ed. Washington, DC: American Institute for Cancer Research; 2007 [Google Scholar]
- 20.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49:1230–50 [DOI] [PubMed] [Google Scholar]
- 21.Riccioni G. Carotenoids and cardiovascular disease. Curr Atheroscler Rep. 2009;11:434–9 [DOI] [PubMed] [Google Scholar]
- 22.Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83:1265–71 [DOI] [PubMed] [Google Scholar]
- 23.Hadley CW, Clinton SK, Schwartz SJ. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J Nutr. 2003;133:727–32 [DOI] [PubMed] [Google Scholar]
- 24.Erdman JW, Jr, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys. 2009;483:229–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sesso HD. Carotenoids and cardiovascular disease: what research gaps remain? Curr Opin Lipidol. 2006;17:11–6 [DOI] [PubMed] [Google Scholar]
- 26.Goralczyk R. Beta-carotene and lung cancer in smokers: review of hypotheses and status of research. Nutr Cancer. 2009;61:767–74 [DOI] [PubMed] [Google Scholar]
- 27.Wang XD. Can smoke-exposed ferrets be utilized to unravel the mechanisms of action of lycopene? J Nutr. 2005;135:S2053–6 [DOI] [PubMed] [Google Scholar]
- 28.Miller EC, Giovannucci E, Erdman JW, Jr, Bahnson R, Schwartz SJ, Clinton SK. Tomato products, lycopene, and prostate cancer risk. Urol Clin North Am. 2002;29:83–93 [DOI] [PubMed] [Google Scholar]
- 29.Kavanaugh CJ, Trumbo PR, Ellwood KC. The U.S. Food and Drug Administration's evidence-based review for qualified health claims: tomatoes, lycopene, and cancer. J Natl Cancer Inst. 2007;99:1074–85 [DOI] [PubMed] [Google Scholar]
- 30.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67:836–43 [DOI] [PubMed] [Google Scholar]
- 31.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–86 [DOI] [PubMed] [Google Scholar]
- 32.Konijeti R, Henning S, Moro A, Sheikh A, Elashoff D, Shapiro A, Ku M, Said JW, Heber D, et al. Chemoprevention of prostate cancer with lycopene in the TRAMP model. Prostate. 2010;70:1547–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gitenay D, Lyan B, Rambeau M, Mazur A, Rock E. Comparison of lycopene and tomato effects on biomarkers of oxidative stress in vitamin E deficient rats. Eur J Nutr. 2007;46:468–75 [DOI] [PubMed] [Google Scholar]
- 34.Gitenay D, Lyan B, Talvas J, Mazur A, George S, Caris-Veyrat C, Rock E. Serum from rats fed red or yellow tomatoes induces Connexin43 expression independently from lycopene in a prostate cancer cell line. Biochem Biophys Res Commun. 2007;364:578–82 [DOI] [PubMed] [Google Scholar]
- 35.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80:396–403 [DOI] [PubMed] [Google Scholar]
- 36.Huo T, Ferruzzi MG, Schwartz SJ, Failla ML. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J Agric Food Chem. 2007;55:8950–7 [DOI] [PubMed] [Google Scholar]
- 37.Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135:431–6 [DOI] [PubMed] [Google Scholar]
- 38.van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000;130:503–6 [DOI] [PubMed] [Google Scholar]
- 39.van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, et al. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 2005;44:4517–25 [DOI] [PubMed] [Google Scholar]
- 40.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–12 [DOI] [PubMed] [Google Scholar]
- 41.Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent-Baudry S, Maillot M, Gastaldi M, Darmon M, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr. 2007;137:2653–9 [DOI] [PubMed] [Google Scholar]
- 42.Paetau I, Khachik F, Brown ED, Beecher GR, Kramer TR, Chittams J, Clevidence BA. Chronic ingestion of lycopene-rich tomato juice or lycopene supplements significantly increases plasma concentrations of lycopene and related tomato carotenoids in humans. Am J Clin Nutr. 1998;68:1187–95 [DOI] [PubMed] [Google Scholar]
- 43.Paetau I, Rao D, Wiley ER, Brown ED, Clevidence BA. Carotenoids in human buccal mucosa cells after 4 wk of supplementation with tomato juice or lycopene supplements. Am J Clin Nutr. 1999;70:490–4 [DOI] [PubMed] [Google Scholar]
- 44.Diwadkar-Navsariwala V, Novotny JA, Gustin DM, Sosman JA, Rodvold KA, Crowell JA, Stacewicz-Sapuntzakis M, Bowen PE. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J Lipid Res. 2003;44:1927–39 [DOI] [PubMed] [Google Scholar]
- 45.Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW., Jr Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–33 [PubMed] [Google Scholar]
- 46.Khachik F, Pfander H, Traber B. Proposed mechanisms for the formation of synthetic and naturally occurring metabolites of lycopene in tomato products and human serum. J Agric Food Chem. 1998;46:4885–90 [Google Scholar]
- 47.Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: are lycopene metabolites bioactive? Arch Biochem Biophys. 2007;458:136–40 [DOI] [PubMed] [Google Scholar]
- 48.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275:11915–20 [DOI] [PubMed] [Google Scholar]
- 49.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–6 [DOI] [PubMed] [Google Scholar]
- 50.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev. 2008;66:667–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopec RE, Riedl KM, Harrison EH, Curley RW, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12'-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136:1552–7 [DOI] [PubMed] [Google Scholar]
- 53.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9′,10'-monooxygenase in human tissues. J Histochem Cytochem. 2005;53:1403–12 [DOI] [PubMed] [Google Scholar]
- 54.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9′,10'-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr 2010;140:2134–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nara E, Hayashi H, Kotake M, Miyashita K, Nagao A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr Cancer. 2001;39:273–83 [DOI] [PubMed] [Google Scholar]
- 56.Campbell JK, Rogers RB, Lila MA, Erdman JW., Jr Biosynthesis of 14C-phytoene from tomato cell suspension cultures (Lycopersicon esculentum) for utilization in prostate cancer cell culture studies. J Agric Food Chem. 2006;54:747–55 [DOI] [PubMed] [Google Scholar]
- 57.Campbell JK, Engelmann NJ, Lila MA, Erdman JW., Jr Phytoene, phytofluene, and lycopene from tomato powder differentially accumulate in tissues of male Fisher 344 rats. Nutr Res. 2007;27:794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Werman MJ, Mokady S, Ben-Amotz A. Bioavailability of the isomer mixture of phytoene and phytofluene-rich alga Dunaliella bardawil in rat plasma and tissues. J Nutr Biochem. 2002;13:585–91 [DOI] [PubMed] [Google Scholar]
- 59.Boileau TW, Boileau AC, Erdman JW., Jr Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med (Maywood). 2002;227:914–9 [DOI] [PubMed] [Google Scholar]
- 60.Liu AG, Volker SE, Jeffery EH, Erdman JW., Jr Feeding tomato and broccoli powders enriched with bioactives improves bioactivity markers in rats. J Agric Food Chem. 2009;57:7304–10 [DOI] [PubMed] [Google Scholar]
- 61.Huang CS, Chuang CH, Hu ML. Effects of lycopene supplementation on plasma and tissue lycopene levels in various rodent strains. Int J Vitam Nutr Res. 2006;76:377–84 [DOI] [PubMed] [Google Scholar]
- 62.Mills JP, Simon PW, Tanumihardjo SA. Beta-carotene from red carrot maintains vitamin A status, but lycopene bioavailability is lower relative to tomato paste in Mongolian gerbils. J Nutr. 2007;137:1395–400 [DOI] [PubMed] [Google Scholar]
- 63.Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW., Jr Review of animal models in carotenoid research. J Nutr. 1999;129:2271–7 [DOI] [PubMed] [Google Scholar]
- 64.Howe JA, Maziya-Dixon B, Tanumihardjo SA. Cassava with enhanced beta-carotene maintains adequate vitamin A status in Mongolian gerbils (Meriones unguiculatus) despite substantial cis-isomer content. Br J Nutr. 2009;102:342–9 [DOI] [PubMed] [Google Scholar]
- 65.Davis CR, Howe JA, Rocheford TR, Tanumihardjo SA. The xanthophyll composition of biofortified maize (Zea mays Sp.) does not influence the bioefficacy of provitamin a carotenoids in Mongolian gerbils (Meriones unguiculatus). J Agric Food Chem. 2008;56:6745–50 [DOI] [PubMed] [Google Scholar]
- 66.Howe JA, Tanumihardjo SA. Carotenoid-biofortified maize maintains adequate vitamin a status in Mongolian gerbils. J Nutr. 2006;136:2562–7 [DOI] [PubMed] [Google Scholar]
- 67.Tanumihardjo SA, Howe JA. Twice the amount of alpha-carotene isolated from carrots is as effective as beta-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr. 2005;135:2622–6 [DOI] [PubMed] [Google Scholar]
- 68.Deming DM, Baker DH, Erdman JW., Jr The relative vitamin A value of 9-cis beta-carotene is less and that of 13-cis beta-carotene may be greater than the accepted 50% that of all-trans beta-carotene in gerbils. J Nutr. 2002;132:2709–12 [DOI] [PubMed] [Google Scholar]
- 69.Shapira N. Nutritional approach to sun protection: a suggested complement to external strategies. Nutr Rev. 2010;68:75–86 [DOI] [PubMed] [Google Scholar]
- 70.Aust O, Stahl W, Sies H, Tronnier H, Heinrich U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int J Vitam Nutr Res. 2005;75:54–60 [DOI] [PubMed] [Google Scholar]
- 71.Fuller B, Smith D, Howerton A, Kern D. Anti-inflammatory effects of CoQ10 and colorless carotenoids. J Cosmet Dermatol. 2006;5:30–8 [DOI] [PubMed] [Google Scholar]
- 72.Shaish A, Harari A, Kamari Y, Soudant E, Harats D, Ben-Amotz A. A carotenoid algal preparation containing phytoene and phytofluene inhibited LDL oxidation in vitro. Plant Foods Hum Nutr. 2008;63:83–6 [DOI] [PubMed] [Google Scholar]
- 73.Campbell JK, Stroud CK, Nakamura MT, Lila MA, Erdman JW., Jr Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in F344 rats. J Nutr. 2006;136:2813–9 [DOI] [PubMed] [Google Scholar]
- 74.Nishino H, Murakosh M, Ii T, Takemura M, Kuchide M, Kanazawa M, Mou XY, Wada S, Masuda M, et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:257–64 [DOI] [PubMed] [Google Scholar]
- 75.Satomi Y, Misawa N, Maoka T, Nishino H. Production of phytoene, a carotenoid, and induction of connexin 26 in transgenic mice carrying the phytoene synthase gene crtB. Biochem Biophys Res Commun. 2004;320:398–401 [DOI] [PubMed] [Google Scholar]
- 76.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–86 [PubMed] [Google Scholar]
- 77.Novotny JA, Kurilich AC, Britz SJ, Clevidence BA. Plasma appearance of labeled beta-carotene, lutein, and retinol in humans after consumption of isotopically labeled kale. J Lipid Res. 2005;46:1896–903 [DOI] [PubMed] [Google Scholar]
- 78.Kurilich AC, Britz SJ, Clevidence BA, Novotny JA. Isotopic labeling and LC-APCI-MS quantification for investigating absorption of carotenoids and phylloquinone from kale (Brassica oleracea). J Agric Food Chem. 2003;51:4877–83 [DOI] [PubMed] [Google Scholar]
- 79.Kelm MA, Flanagan VP, Pawlosky RJ, Novotny JA, Clevidence BA, Britz SJ. Quantitative determination of 13C-labeled and endogenous beta-carotene, lutein, and vitamin A in human plasma. Lipids. 2001;36:1277–82 [DOI] [PubMed] [Google Scholar]
- 80.Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Golden Rice is an effective source of vitamin A. Am J Clin Nutr. 2009;89:1776–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Wang Y, Wang Z, Li L, Qin J, Lai W, Fu Y, Suter PM, Russell RM, et al. Vitamin A equivalence of spirulina beta-carotene in Chinese adults as assessed by using a stable-isotope reference method. Am J Clin Nutr. 2008;87:1730–7 [DOI] [PubMed] [Google Scholar]
- 82.Parker RS, Brenna JT, Swanson JE, Goodman KJ, Marmor B. Assessing metabolism of beta-[13C]carotene using high-precision isotope ratio mass spectrometry. Methods Enzymol. 1997;282:130–40 [DOI] [PubMed] [Google Scholar]
- 83.Parker RS, Swanson JE, Marmor B, Goodman KJ, Spielman AB, Brenna JT, Viereck SM, Canfield WK. Study of beta-carotene metabolism in humans using 13C-beta-carotene and high precision isotope ratio mass spectrometry. Ann N Y Acad Sci. 1993;691:86–95 [DOI] [PubMed] [Google Scholar]
- 84.Tang G, Ferreira AL, Grusak MA, Qin J, Dolnikowski GG, Russell RM, Krinsky NI. Bioavailability of synthetic and biosynthetic deuterated lycopene in humans. J Nutr Biochem. 2005;16:229–35 [DOI] [PubMed] [Google Scholar]
- 85.Zaripheh S, Erdman JW., Jr The biodistribution of a single oral dose of [14C]-lycopene in rats prefed either a control or lycopene-enriched diet. J Nutr. 2005;135:2212–8 [DOI] [PubMed] [Google Scholar]
- 86.Zaripheh S, Boileau TW, Lila MA, Erdman JW., Jr 14C]-lycopene and [14C]-labeled polar products are differentially distributed in tissues of F344 rats prefed lycopene. J Nutr. 2003;133:4189–95 [DOI] [PubMed] [Google Scholar]
- 87.Engelmann NJ, Campbell JK, Rogers RB, Rupassara SI, Garlick PJ, Lila MA, Erdman JW., Jr Screening and selection of high carotenoid producing in vitro tomato cell culture lines for [(13)C]-carotenoid production. J Agric Food Chem. 2010;58:9979–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engelmann NJ, Rogers RB, Lila MA, Erdman JW., Jr Herbicide treatments alter carotenoid profiles for 14C tracer production from tomato (Solanum lycopersicum cv. VFNT cherry) cell cultures. J Agric Food Chem. 2009;57:4614–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yousef GG, Seigler DS, Grusak MA, Rogers RB, Knight CT, Kraft TF, Erdman JW, Jr, Lila MA. Biosynthesis and characterization of 14C-enriched flavonoid fractions from plant cell suspension cultures. J Agric Food Chem. 2004;52:1138–45 [DOI] [PubMed] [Google Scholar]
- 90.Reppert A, Yousef GG, Rogers RB, Lila MA. Isolation of radiolabeled isoflavones from kudzu (Pueraria lobata) root cultures. J Agric Food Chem. 2008;56:7860–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Engelmann NJ, Reppert A, Yousef GG, Rogers RB, Lila MA. In vitro production of radiolabeled red clover (Trifolium pratense) isoflavones. Plant Cell Tissue Organ Cult. 2009;98:147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishida BK, Mahoney NE, Ling LC. Increased lycopene and flavor volatile production in tomato calyces and fruit cultured in vitro and the effect of 2-(4-chlorophenylthio)triethylamine. J Agric Food Chem. 1998;46:4577–82 [Google Scholar]
- 93.Robertson GH, Mahoney NE, Goodman N, Pavlath AE. Regulation of lycopene formation in cell suspension culture of VFNT tomato (Lycopersicon esculentum) by CPTA, growth regulators, sucrose, and temperature. J Exp Bot. 1995;46:667–73 [Google Scholar]
- 94.Fosket DE, Radin DN. Induction of carotenogenesis in cultured cells of Lycopersicon Esculentum cultivar Ep-7. Plant Sci Lett. 1983;30:165–76 [Google Scholar]
- 95.Bramley PM. Carotenoid biosynthesis: a target site for bleaching herbicides. Biochem Soc Trans. 1994;22:625–9 [DOI] [PubMed] [Google Scholar]
- 96.Windhovel U, Sandmann G, Boger P. Genetic engineering of resistance to bleaching herbicides affecting phytoene desaturase and lycopene cyclase in cyanobacterial carotenogenesis. Pestic Biochem Physiol. 1997;57:68–78 [Google Scholar]